Abstract
Aim
Peri-implant gingival healing following one-stage implant placement was investigated and compared to periodontal healing.
Methods
Healing at surgical sites (implant (I) and adjacent teeth (T+)) was compared to non-operated tooth (T-) in non-smokers receiving one-stage implant. Periodontal Indices (PI, GI) were recorded at surgery and up to 12 weeks postoperatively. Peri-implant (PICF) and gingival crevicular (GCF) fluids were analyzed for cytokines, collagenases and inhibitors. Data was analyzed by linear mixed model regression analysis and repeated measures ANOVA.
Results
40 patients (22 female; 21-74 yrs old) completed the study. Surgical site GI, increased at week 1, decreased significantly during early healing (weeks 1-3; p=0.0003) and continually decreased during late healing (weeks 6-12) for I (p<0.01). PICF volume decreased 3-fold by week 12 (p=0.0003). IL-6, IL-8, MIP-1β, and TIMP-1 levels significantly increased at surgical sites at week one, significantly decreasing thereafter (P<0.016). Week one IL-6, IL-8 and MIP-1β levels were ~3-fold higher, and TIMP-1 levels 63% higher, at I compared to T+ (p=0.001).
Conclusion
Peri-implant gingival healing, as determined by crevicular fluid molecular composition, differs from periodontal healing. The observed differences suggest that peri-implant tissues, compared to periodontal tissues, represent a higher pro-inflammatory state.
Keywords: dental implant, periodontal surgery, crevicular fluid, gingiva, wound healing, cytokines
INTRODUCTION
Peri-implant soft tissue healing is described as gingival seal formation (Berglundh et al., 2007). The implant-gingiva interface consists of sulcular and junctional epithelium and underlying connective tissue (Berglundh et al., 2007). Epithelial attachment occurs as early as week 1-2 postoperatively and epithelial barrier matures within 6-8 weeks (Berglundh et al., 2007). In contrast to teeth, connective tissue fibers do not penetrate the implant and are organized parallel to the implant surface, generating a cuff (Berglundh et al., 1996). Because of the lack of periodontal ligament (and its vascular anastomoses to periodontium and alveolar bone), supraperiosteal blood vessels consist the sole blood supply of peri-implant gingiva (Berglundh et al., 1994). Such anatomic differences have suggested that peri-implant gingiva may have impaired defenses compared to gingiva around teeth. Recent studies comparing dental implants and teeth without clinical signs of inflammation reported that peri-implant crevicular fluid (PICF) pro-inflammatory cytokine levels were much higher than corresponding gingival crevicular fluid levels (GCF) (Nowzari et al., 2008; 2010). These findings suggest that peri-implant gingiva may be characterized by a higher pro-inflammatory state under apparent homeostatic conditions (clinically healthy tissues). However, information on the molecular events occurring during early peri-implant wound healing, i.e., during dynamic tissue conditions, is scarce.
Detection of early peri-implant tissue changes through conventional clinical methods (e.g., probing) is limited. GCF, with its rich content of locally-derived mediators, mimics wound fluid following surgery. Among several molecules, the following have been reported to be involved in the acute inflammatory response to surgical trauma: interleukin-8 (IL-8), a potent chemotactic agent for neutrophils (Okada H & Murakami S., 1998); macrophage inflammatory protein-1 (MIP-1), another chemokine that selectively stimulates monocyte and lymphocyte migration to inflammation sites (Petkovic AB et al., 2010); interleukin-1 (IL-1), a pro-inflammatory polypeptide implicated in a wide range of biological processes including inflammation, tissue breakdown and tissue homeostasis (Tatakis DN 1993); interleukin-6 (IL-6), another pro-inflammatory cytokine associated with elevated neutrophil elastase levels (Baumann H & Gauldie J., 1994); matrix metalloproteinase-8 (MMP-8) and matrix metalloproteinase-9 (MMP-9), the two main collagen degrading enzymes in GCF (Ingman T et al., 1996), are secreted from neutrophils during activation and are responsible for extracellular matrix degradation; metallopeptidase inhibitor 1 (TIMP-1), a major inhibitor of MMPs, is released at higher levels in inflamed gingiva (Nomura T et al., 1998); and vascular endothelial growth factor (VEGF), a critical mediator of angiogenesis (Pathak AP et al., 2008). Modulation of these tissue byproducts plays a major role in soft tissue integrity (Griffiths 2003). The peri-implant wound created by surgical trauma is expected to exhibit an acute phase response that controls tissue damage and infection and stimulates repair; this fast and robust response should be detectable by studying PICF. Implant placement under one-stage protocol allows optimal access to evaluate such early phases of healing in peri-implant tissue and to compare with surgically accessed adjacent teeth in the same individual.
We hypothesized that the wound healing response of peri-implant gingival tissues following implant placement surgery differs from the healing response around surgically manipulated teeth. Thus, the purpose of this study was to assess and compare gingival wound healing, including clinical and biological parameters, around dental implants (following implant placement surgery) and periodontally healthy teeth (following access flap surgery).
MATERIAL AND METHODS
Study population and study design
Fifty-seven patients treatment planned for mandibular posterior sextant one-stage single implant placement were recruited (Graduate Periodontology Clinic, Ohio State University; March 2009-January 2011) for this prospective observational cohort study. Eligibility criteria were: non-smokers, aged 18-75 years; treatment planned to receive single implant on mandibular posterior sextant; implant site bounded by natural teeth; no systemic diseases/conditions affecting periodontal health or healing; no untreated periodontal disease; able and willing to provide informed consent for surgery and study. Exit criteria were: placement changed to two-stage protocol (Implant Stability Quotient (ISQ) ≤45 or concurrent bone grafting needed); voluntary withdrawal; non-compliance with study protocol; no longer meeting eligibility criteria (development of systemic/oral disease). Institutional Review Board (Ohio State University) approved study protocol and informed consent forms.
With respect to any prior periodontal disease history, comprehensive periodontal examination was completed and periodontal diagnoses and related treatment plan were provided to potential study participants prior to recruitment; only patients who had successfully completed the recommended periodontal therapy were recruited. Following screening/recruitment visit and eligibility verification, surgery was scheduled. Immediately prior to surgery, plaque (PI) and gingival (GI) indices (Loe 1967) were recorded and GCF samples were collected from the two teeth bounding the implant site (T+: two teeth combined as single site) and from a non-operated contralateral tooth (T-). After implant placement, implant primary stability was assessed by resonance frequency analysis (RFA). At weeks 1, 2, 3, 6, 8, and 12, PI, GI measurements and GCF/PICF collection were obtained from T+, implant (I) and T- sites. Implant stability was again assessed at weeks 1, 3, 6, 8, 12 (data reported elsewhere). Therefore, the study included seven visits (surgery; weeks 1, 2, 3, 6, 8, 12).
Surgical and postoperative protocol
Following local anesthesia, sulcular incisions around teeth and crestal incision over edentulous site were performed. After full-thickness flap elevation, osteotomy site was prepared using custom-made surgical template. Screw type, root form implants(Astra Tech, Moindal, Sweden; Straumann USA, LLC, Andover, MA,USA; Zimmer Dental, Carsbal, CA, USA) were placed following manufacturer's recommendations. After implant placement and stability assessment, polished and platform-matched transgingival healing abutment (height=2-4 mm, dependent on tissue thickness, to avoid soft tissue covering the abutment during the early post-operative period) was screwed to implant and flaps were sutured (standard one-stage protocol). Routine post-operative care was not modified for the study. Post-operative instructions included 1-week abstinence from mechanical plaque control (surgical sites), antimicrobial rinse (0.12% chlorhexidine rinse) use and analgesics (prn). Prescription medication use was recorded through patient diaries. Sutures were removed at week 1.
Clinical Data and Sample Collection
A single trained and calibrated examiner obtained clinical parameters and GCF/PICF samples. PI and GI were recorded (six sites/tooth; T+ and T-) immediately prior to surgery and all postoperative visits (weeks 1, 2, 3, 6, 8, 12). Modified PI and GI (Mombelli et al., 1987) were recorded (four sites/implant; I sites) at weeks 1, 2, 3, 6, 8, 12.
For GCF/PICF collection (one strip/site; 4 sites/implant or tooth), sites were isolated (cotton rolls), supragingival plaque removed using probe, and tissues gently air-dried. Paper strips (Periopaper, Proflow Inc. Amityville, NY, USA) were sequentially introduced into sulci until mild resistance was felt, removed after 20 seconds, and collected fluid volume immediately determined by calibrated instrument (Periotron 8000, Ora Flow Inc, Plain View, NY, USA). Contaminated samples (blood, saliva) were discarded. GCF/PICF samples from each tooth/implant were pooled, placed into sterile cryovials kept on ice, and transferred to -80°C for storage.
Following manufacturer's guidelines, a transducer (implant system/diameter specific; Smartpeg™, Straumann USA, LLC, Andover, MA, USA) was hand-torqued into implant body to measure implant stability by RFA (Osstell™, Mentor, Straumann USA, LLC, Andover, MA, USA). Three ISQ measurements/implant were taken from buccal and lingual aspect and averaged.
Sample Preparation and Analysis
Paper strips were separated from waxed portion using sterile scissors and transferred into prepared sterile microtubes. After addition of sterile cold PBS (200 μl/sample; Cellgro® Dulbecco's Phosphate Buffered Saline Mediatech Inc., Manassas, VA, USA) and 15-minute incubation (occasional vortexing) on ice, samples were centrifuged (13,000 g; 10 min). Eluant was collected and centrifugation repeated once to collect additional volume.
Commercially available multiplex bead-based assay kits (human cytokine group I kit, Bioplex™ Cytokine Assay, Bio-Rad Laboratories, Inc., and MMP/TIMP kit, R&D Laboratories LTD., Antrim, Northern Ireland) were used to detect IL-1β, IL-1ra, IL-6, IL-7, IL-8, IL-10, IL-12, Eotaxin, FGF-b, MCP-1, TNF-α, MIP-1β, VEGF, MMP-8, MMP-9, TIMP-1, TIMP-2, TIMP-3 and TIMP-4 in GCF/PICF samples, following manufacturer's instructions. Both protein concentration and total amount per sample are reported; however, statistical analysis is based on total amount.
Statistical analysis
Sample size was determined by a-priori power analysis, to allow detection of 3-fold difference between baseline GCF IL-8 levels and post-operative week 1 GCF/PICF IL-8 levels, at alpha=0.05 with a 80% power, based on previously published data (Khoury et al., 2008). Because of anticipated individual variations in specific cytokine responses during wound healing and the specific study design (inclusion of additional variables and observation time points), the sample size was doubled (n=40), and the recruitment goal was increased (n=57) to account for anticipated attrition (30%).
Data analysis was conducted by expert consultant (VY) using computer software (SAS/STAT® version 9.2; SAS Institute Inc. Cary, North Carolina, USA). Data was analyzed to detect differences during early (weeks 1-3) and late (weeks 6-12) healing. A linear mixed model regression analysis was used for repeated measures fixed and random effects within and between groups. Initially a random effect (intercept and slope) regression analysis was conducted to estimate the slopes of the outcome over continuous time for I, T+ and T- groups. Repeated measure ANOVA was used to make the within group comparisons between week 1 and week 12 and between group comparisons at baseline and at week 12. Sandwich estimator was used to control the correlation due to dependence of the observations among repeated measurements. The variability of the outcome within and between subjects was used to estimate standard error that tests regression coefficients. Bonferroni correction was used to conserve the overall type I error at α=0.05. Secondary analyses compared first to last observation time for I, T+ and T-groups for each outcome.
RESULTS
Study population
Forty patients (54.4±2 yrs old; 55% female) completed the study having received 26 and 14 implants replacing mandibular molars and premolars, respectively. Patient exclusion reasons were: surgery modified to two-stage protocol (n=4); implant stability loss by week 3 (n=6); post-operative complications requiring additional treatment (n=2), and failure to comply with protocol (n=5).
Clinical Parameters
Average preoperative PI was <1.0 for periodontal (T+ and T-) sites (Table 1). Only T+ showed statistically significant (p=0.029) PI differences during early healing. At I, PI decrease during early healing was non-significant (p=0.08) while week 12 PI differed significantly from week 1 (p=0.0003). Between group comparisons revealed no significant differences.
Table 1. Clinical Parameters During Early and Late Healing.
PI: Plaque Index*; GI: Gingival Index*; CF: Crevicular fluid volume; I: Implant site; T+: Surgically treated tooth: T-: non-treated control tooth.
| mean±se median(range) |
|||||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 week | 2 weeks | 3 weeks | 6 weeks | 8 weeks | 12 weeks | |
| PI | |||||||
| I | 0.6±0.1 0.5 (0-2) |
0.5±0.1 0.4 (0-3) |
0.4±0.1 0.3 (0-2) |
0.4±0.1 0.35 (0-1) |
0.3±0.1 0.2 (0-2) |
0.3±0.1◇ 0.2 (0-2) |
|
| T+ | 0.8±0.1 0.8 (0.2-3) |
0.7±0.1 0.7 (0-3) |
0.6±0.1 0.5 (0-2) |
0.6±0.1• 0.5 (0-2.5) |
0.7±0.1 0.6 (0.2-1.8) |
0.6±0.1 0.5 (0.2-2) |
0.7±0.1 0.5 (0-2) |
| T- | 0.7±0.1 0.5 (0-3) |
0.4±0.1 0.5 (0-2) |
0.4±0.1 0.2 (0-1.5) |
0.5±0.1 0.5 (0-1) |
0.5±0.1 0.5 (0-2) |
0.5±0.1 0.5 (0-2) |
0.5±0.1 0.5 (0-1.2) |
| GI | |||||||
| I | 1.1±0.1 1 (0-3) |
0.6±0.1 0.5 (0-2) |
0.4±0.1•* 0.3 (0-2) |
0.2±0.1 0 (0-1) |
0.1±0.0 0 (0-1) |
0.1±0.0□◇ 0 (0-0.5) |
|
| T+ | 0.6±0.1 0.5 (0-2) |
0.9±0.1 1 (0-3) |
0.6±0.1 0.5 (0-2) |
0.5±0.1•* 0.5 (0-2) |
0.4±0.1 0.2 (0-2) |
0.3±0.1 0.2 (0-2) |
0.3±0.1◇ 0.2 (0-1.2) |
| T- | 0.4±0.1 0.2 (0-1.5) |
0.2±0.0 0 (0-1) |
0.1±0.0 0 (0-1) |
0.2±0.1 0 (0-1.5) |
0.2±0.1 0 (0-2) |
0.2±0.1 0 (0-1) |
0.2±0.1 0 (0-1) |
| CF volume (μl) | |||||||
| I | 1.8±0.2 1.6(0.2-4.1) |
1.2±0.1 0.8(0.3-3.1) |
1±0.1•* 0.8(0.2-4.6) |
0.7±0.1 0.6(0.2-2.2) |
0.5±0.0 0.5(0.2-1.1) |
0.6±0.1◇ 0.5(0.02-2) |
|
| T+ | 1.4±0.1 1.1(0.5-3.6) |
1.7±0.1 1.5(0.7-4) |
1.5±0.1 1.3(0.4-5.3) |
1.3±0.1•* 1(0.4-3.7) |
1.4±0.1 1.2(0.5-3.8) |
1.1±0.1 1.2(0.3-2.5) |
1.3±0.1◇ 1.1(0.4-3.7) |
| T- | 1.2±0.1 0.95(0.2-3.6) |
1.2±0.1 0.9(0.1-3.7) |
1.2±0.1 1.1(0.3-4.3) |
1.2±0.1 0.8(0.2-3.3) |
0.9±0.1 0.84(0.2-4) |
0.9±0.1 0.7(0.2-2.3) |
1.1±0.1 0.9(0.2-4) |
| ISQ | |||||||
| I | 69.5±1.3 68(52-83) |
69.2±1.2 69.3 (51-81) |
70±1.3• 73 (51-85) |
75±1 75 (62-85) |
75±1.1 76 (62-87) |
75±1.3◇ 77 (54-86) |
|
Early healing (week 1 through week 3) statistically significant differences
Late healing (week 6 through week 12) statistically significant differences
between groups I vs T-
between groups T+ vs T-
Statistically significant difference between week 1 and week 12
PI and GI were obtained from six surfaces from T+ and T- sites while they were obtained from four surfaces from I site.
At surgical (T+ and I) sites, week 1 GI was increased significantly (p=0.01) compared to T+ baseline GI (Table 1). Surgical site GI declined significantly between weeks 1 and 12 (p=0.0003). Between group comparisons showed that non-surgical controls had significantly lower GI than either surgical site (p=0.0003), only during early healing.
GCF/PICF volume changes (Table 1) were based on total volume (pooled 4 strips/site). GCF/PICF volume decreased significantly during early healing for surgical sites (p<0.001). Volume differences between weeks 1 and 12 were also significant for surgical sites (I:p=0.0003; T+:p=0.02). T- GCF volume changes were non-significant throughout. During early healing, T-sites exhibited significantly less volume than either surgical site (I: p=0.0003; T+: p=0.0006).
Biological Parameters
All molecules, except TIMP-3 and TIMP-4, were detectable in GCF/PICF. However, IL-7, IL-10, IL-12, Eotaxin, FGF-b, TNF-α, and MCP-1 were found at low levels throughout, while consistently high IL-1ra levels showed no significant differences within/between sites (data not shown).
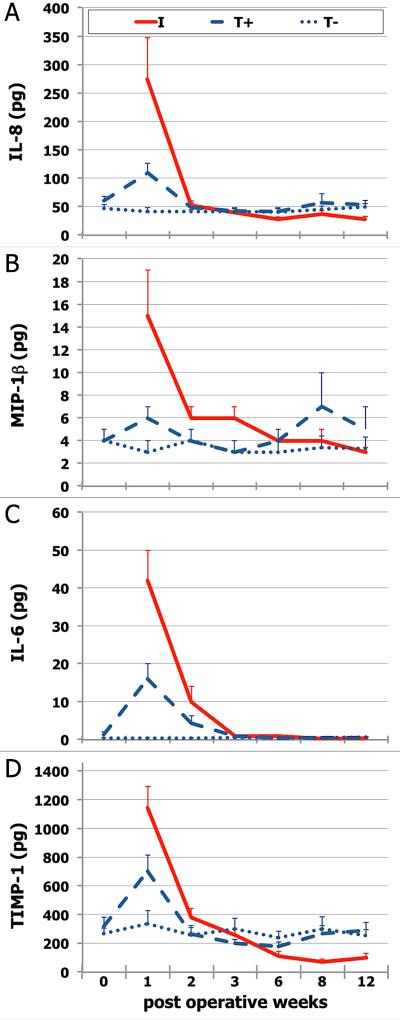
IL-8 and MIP-1β were differentially released at surgical sites with higher levels during early wound healing (Figure 1; Table 2). Week one IL-8 was high at surgical sites, decreasing 7-fold at I (p=0.002) and ~3-fold at T+ (p=0.0002) by week 3. IL-8 decrease at I, between weeks 1 and 12, was significant (p=0.0003). Early healing IL-8 levels were significantly different between T- and surgical sites (I, p=0.007; T+, p=0.003). At late healing, slight but significant increase was detected at T+ (p=0.02), without significant difference between T+ and I (p=0.1). At 12 weeks, IL-8 levels at I were lower than either periodontal site (p>0.05).
Figure 1. Peri-implant and gingival crevicular fluid biomarkers following implant placement.
IL-8 (A), MIP-1β (B), IL-6 (C), and TIMP-1 (D) total amount/site (mean+SE). Each data point represents crevicular fluid samples (4 strips/site; 20 sec/strip) from 40 patients. Each patient was sampled at peri-implant crevice (I), gingival crevice of adjacent tooth (T+), and gingival crevice of contralateral, non-operated tooth (T-). I and T+ were surgical sites (full-thickness flap elevated to place one-stage dental implant). Week 0 (T+ and T-) refers to immediately pre-operative (baseline) samples. Week 1 values differed significantly from week 12 for IL-8 (I, p=0.0003), MIP1-β (I, p=0.0003), IL-6 (I, p=0.0003; T+, p=0.01), and TIMP-1 (I, p=0.0003; T+, p=0.004). At early healing (weeks 1-3), surgical sites differed significantly from T- sites for IL-8, IL-6, and TIMP-1 (adjusted p≤0.05); as did I from T+ for IL-6 (p≤0.05). At late healing (weeks 6-12), I differed from T+ for MIP1-β (p≤0.03). Additional details provided in text.
Table 2. Crevicular fluid IL-8, MIP-1β, IL-1β, IL-6 and VEGF content.
I: Implant site; T+: Surgically treated tooth: T-: non-treated control tooth. Each value represents crevicular fluid samples (4 strips/site; 20 sec/strip) from 40 patients.
| PICF/GCF Cytokine Concentration (ng/ml) PICF/GCF Cytokine Total amount (pg) |
|||||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 week | 2 weeks | 3 weeks | 6 weeks | 8 weeks | 12 weeks | |
| IL-8 | |||||||
| I | 168±36 274±74 |
58±10 52±8 |
48±7 39±7•* |
46±8 28±4 |
77±11 37±6 |
52±8 28±5◇ |
|
| T+ | 54±9 60±8 |
67±10 109±17 |
40±5 49±7 |
43±8 42±6•* |
34±5 41±7 |
49±9 57±16 |
47±6 53±8□ |
| T- | 47±6 47±7 |
44±7 41±7 |
48±7 41±4 |
46±6 41±5 |
56±10 40±6 |
64±9 45±6 |
63±10 49±6 |
| MIP-1β | |||||||
| I | 9±2 15±4 |
7±1 6±1 |
6±1 6±1• |
6±1 4±1 |
7±1 4±1 |
5±1 3±1⊗*◇ |
|
| T+ | 4±1 4±1 |
4±0.4 6±1 |
3±0.5 4±1 |
3±1 3.3±1• |
3±1 4±1 |
6±3 7±3 |
5±1.4 5±2□ |
| T- | 4±1 4±1 |
3±0.3 3±1 |
4±1 4±1 |
3±1 3±1 |
4±1 3±1 |
4±1 3±1 |
4±0.5 3±1 |
| IL-1β | |||||||
| I | 13±3 19±3 |
8±1 7±1 |
12±2 10±2•* |
11±2 8±1.3 |
14±2 7±1 |
13±3 7±2*◇ |
|
| T+ | 17±3 18±3 |
10±1 16±2 |
13±3 16±4 |
17±4 15±3 |
13±2 14±2 |
20±4 19±3 |
25±4 26±4□◇ |
| T- | 11±2 9±2 |
9±2 8.3±2 |
9.5±2 9±1 |
9±1 8.2±1 |
9±2 6.4±1 |
11±2 8±1 |
15±4 11±3 |
| IL-6 | |||||||
| I | 28±6 42±8 |
9±3 10±4 |
1±0.2 1±0.3•*⊗ |
1±0.3 0.6±0.3 |
0.5±0.1 0.2±0.1 |
1±0.2 0.4±0.1◇ |
|
| T+ | 1.2±0.6 1.3±1 |
11±2 16±4 |
3±1 4±2 |
0.6±0.2 1±0.3•* |
0.4±0.1 0.4±0.1 |
0.7±0.1 0.6±0.1 |
0.8±0.3 0.5±0.1◇ |
| T- | 0.3±0 0.3±0.1 |
0.4±0.1 0.3±0.1 |
0.4±0.1 0.3±0.1 |
0.4±0.1 0.4±0.2 |
0.5±0.1 0.3±0.1 |
0.7±0.2 0.4±0.1 |
0.8±0.3 0.5±0.2 |
| VEGF | |||||||
| I | 25±3 41±7 |
24.±3 22±3 |
37±5 31±4 |
42±6 26±4 |
68±13 29±4 |
45±6 22±3◇ |
|
| T+ | 36±5 40±4 |
26±3 43±6 |
26±4 32±6 |
29±4 32±5• |
23±3 32±5 |
36±5 38±6 |
43±6 47±6□ |
| T- | 34±6 30±4 |
28±4 30±5 |
29±4 27±3 |
32±6 28±4 |
38±9 24±3 |
39±6 29±4 |
36±5 29±4 |
Early healing (week 1 through week 3) statistically significant differences
Late healing (week 6 through week 12) statistically significant differences
between groups I vs T-
between groups T+ vs T-
between groups I and T+
Statistically significant difference between week 1 and week 12
A sharp increase in MIP-1β levels (Figure 1; Table 2) at I at week one was followed by 2-fold decrease by week 3 (p=0.016). MIP-1β decreases between weeks 1 and 12 were also significant for I (p=0.0003). The decrease observed at T+ was moderate but still significant at early healing (p=0.007). All sites reached baseline values by week 12 with statistically significant difference between I and T+ at late healing (p=0.03).
IL-1β was higher at surgical sites compared to T- even prior to surgery (baseline T+ vs T, p=0.003) (Table 2). The decrease observed at I between weeks 1 and 12 was significant (p=0.0006). Most of this PICF IL-1β decrease occurred during early healing (p=0.003). Comparisons between groups showed that I sites were significantly different from T- site during early (p=0.03) and T+ site during late (p=0.004) healing. During late healing, periodontal site IL-1β increased, becoming significant for T+ by week 12 (p<0.001; vs. week 1).
Surgical manipulation induced significant IL-6 levels at surgical sites at week 1 (Figure 1; Table 2). IL-6 levels decreased significantly (p≤0.0001) for both surgical sites during early healing. IL-6 level decreases between weeks 1 and 12 were also significant (I, p=0.0003; T+, p=0.01). Between group comparisons revealed that I sites had higher IL-6 levels than periodontal sites (T+, p=0.01; T-, p=0.0003) during early healing. Similarly, IL-6 level difference between T+ and T- was significant during early healing (p=0.0003). By week 12, IL-6 levels returned to the low baseline values for all sites.
PICF VEGF levels at week 1 reached baseline GCF levels and were significantly decreased by week 12 (p=0.0001). Fluctuations in VEGF levels from week 1 through 12 were minimal for periodontal sites. VEGF differences between I and T+ became significant (p=0.001) at 12 weeks due to continuous decrease at I (Table 2).
MMP-8 and MMP-9 were highly expressed in GCF at baseline (Table 3). Surgical manipulation caused significant decreases in GCF MMP-8 levels during early healing (T+, p=0.001; T+ vs T-, p=0.008). PICF MMP-8 and MMP-9 levels reached baseline GCF levels by week 1 and started decreasing, without significant differences over time. The continuous MMP-9 decrease at I resulted in significant differences between I and periodontal sites (p=0.0003) at early healing (Table 3). There were no significant differences between groups for either MMP at late healing.
Table 3. Crevicular fluid MMP-8, MMP-9, TIMP-1 and TIMP-2 content.
I: Implant site; T+: Surgically treated tooth: T-: non-treated control tooth. Each value represents crevicular fluid samples (4 strips/site; 20 sec/strip) from 40 patients.
| PICF/GCF Protein Concentration (μg/ml) PICF/GCF Protein Total amount (ng)†† |
|||||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 week | 2 weeks | 3 weeks | 6 weeks | 8 weeks | 12 weeks | |
| MMP8 | |||||||
| I | 19.3±6 27±7 |
12.4±2 15±4 |
16.2±3 18±5 |
24.2±7 19±6 |
19.3±4 9±2 |
22±6 14±5 |
|
| T+ | 21±3 25±4 |
16.4±2 28±4 |
12±1.6 17±2 |
14.1±2 16±3•* |
13.7±2 23±7 |
21.4±3 23±4 |
25.1±4 27±5 |
| T- | 21±4 20±4 |
16.2±3 17±4 |
20.7±4 21±5 |
18±2.6 18±4 |
22.9±6 20±6 |
18.1±3 15±3 |
25±5 20±4 |
| MMP9 | |||||||
| I | 42.2±5 59±6 |
31.8±4 35±5 |
37.3±5 34±5•* |
45.4±7 34±5 |
54.8±10 29±5 |
50±6.3 29±5◇ |
|
| T+ | 44±6 52±7 |
37.2±4 57±5 |
30.3±4 41±6 |
35.2±5 40±5•* |
33.1±5 39±5 |
50.8±6 51±6 |
51.4±8 51±6□ |
| T- | 50±6 45±6 |
38.7±6 36±6 |
47.1±6 45±5 |
50.6±8 44±5 |
53.8±8 42±6 |
52.5±7 44±7 |
59.1±11 45±5 |
| TIMP-1 | |||||||
| I | 1.3±4 1142±151 |
0.5±0.1 380±61 |
0.3±0.1 256±42•* |
0.1±0.0 110±34 |
0.2±0.1 70±20 |
0.2±0.1 99±30⊗◇ |
|
| T+ | 0.3±0.1 318±64 |
0.5±0.1 702±114 |
0.4±0.1 265±59 |
0.6±0.3 201±24•* |
0.2±0.0 180±28 |
0.3±0.1 270±52 |
0.4±0.1 291±54□◇ |
| T- | 0.3±0.0 267±40 |
1.1±0.8 335 ± 93 |
0.3±0.1 256±53 |
0.4±98 302±0.73 |
0.3±0.1 240±43 |
0.5±0.2 300±83 |
0.5±0.1 254±40 |
| TIMP-2 | |||||||
| I | 2.3±0.3 2756±457 |
2.2±0.4 1429±221 |
2±0.4 1559±295•* |
2.5±0.5 1562±304 |
3.8±0.8 1734±289 |
5±1.5 1495±255◇ |
|
| T+ | 2±1 2708±610 |
1.9±0.3 2440±365 |
2.2±0.5 1803±317 |
2.2±0.4 1828±317•* |
1.7±0.3 1756±241 |
2.8±0.5 1904±240 |
2.8±0.5 2099±221□ |
| T- | 2±0.4 1485±243 |
2.3±0.4 1548±203 |
2.6±0.6 1714±268 |
2.6±0.5 1727±278 |
2.8±0.9 1472±269 |
4.5±1.9 1881±316 |
3.5±0.7 2034±233 |
TIMP-1 and TIMP-2 total amount in pg
Early healing (week 1 through week 3) statistically significant differences
Late healing (week 6 through week 12) statistically significant differences
between groups I vs T-
between groups T+ vs T-
between groups I and T+
Statistically significant difference between week 1 and week 12
At week 1, surgical sites had high TIMP-1 levels (Figure 1; Table 3), which decreased significantly by week 3 (p<0.0001) and week 12 (p≤0.004). TIMP-1 PICF levels at late healing were significantly lower than T+ GCF levels (p=0.03). TIMP-2 GCF/PICF levels showed similar temporal patterns (Table 3). Surgical site TIMP-2 levels decreased significantly between weeks 1 and 3 (I, p<0.0001; T+, p=0.0004), while PICF TIMP-2 levels also decreased significantly between weeks 1 and 12 (p=0.0003). In contrast, T+ showed slight but significant increase during late healing (p=0.01).
DISCUSSION
The purpose of this study was to assess gingival wound healing responses following implant placement, by comparing relevant clinical and biological parameters between implants and teeth. The results indicate that, in the absence of significant differences in clinical parameters between peri-implant and periodontal tissues during early healing phases, crevicular fluid content exhibits differences in the expression of specific pro-inflammatory mediators between implants and teeth during postoperative healing, revealing a more robust response to surgical trauma in peri-implant compared to periodontal tissues. Therefore, these results support the hypothesis that the wound healing response of peri-implant gingival tissues differs from the healing response around surgically manipulated teeth. To our knowledge, this is the first study to compare peri-implant and periodontal wound healing, at either clinical or molecular level.
Plaque accumulation was well controlled throughout the study and gingiva had minimal inflammation. Periodontal and peri-implant surgical site week-1 GI increases were expected (Cattermole and Wade 1978; DeAngelo et al., 2007; Khoury et al., 2008). Reported peri-implant tissue clinical responses to plaque accumulation or loading (Baldi et al., 2009; Salvi et al., 2012) are not relatable to postoperative healing changes.
GCF/PICF strip collection was limited to 20 sec to minimize contamination risk. This methodological difference may explain the lower volume and mediator levels than previously reported in periodontal health (Okada and Murakami 1998). GCF/PICF volume increases observed at surgical sites at week one gradually declined. Early postoperative GCF volume increases are consistent with previous reports (Cattermole and Wade 1978; Kuru et al., 2004; Kuru et al., 2005). However, early postoperative PICF volume changes are reported for the first time.
GCF/PICF content was reported in both concentration and total amount, but statistical analysis was based on total protein. This approach was chosen since sampling crevicular fluid from a wound site, especially at late time points during healing, results in collecting the entire volume of fluid at the specific site (Lamster and Ahlo 2007). In addition, statistical analysis using concentration values revealed identical results in terms of significance (data not shown). IL-6, IL-8, MIP-1β, and TIMP-1 responses appeared to be surgical trauma-specific with differential several fold increases at both peri-implant and periodontal sites. The sharp, transient GCF/PICF IL-6 increases following surgery, as reported herein, likely represent the acute response of both soft and hard tissues to surgical trauma (Jawa et al., 2011a; Jawa et al.,2011b). We previously reported postoperative PICF IL-8 increases (Khoury et al., 2008), but postoperative GCF/PICF MIP-1β levels have not been previously reported. The exaggerated IL-6 and IL-8 early postoperative responses in I sites (vs. T+ sites), mirror the elevated PICF IL-6 and IL-8 levels (vs. GCF levels) around implants (vs. teeth) without clinical signs of inflammation (Nowzari et al., 2008; 2010). Collectively, these findings suggest that peri-implant gingiva may be characterized by a higher pro-inflammatory state, in the absence of concomitant PI or GI increases. This sub-clinical pro-inflammatory status of the peri-implant gingiva likely reflects unique aspects of peri-implant tissue anatomy and physiology, as indicated by the reported differences between periodontal and peri-implant tissues in extracellular matrix composition (Romanos GE et al., 1995), epithelial cell characteristics (Carmichael RP et al., 1991), and immune cell distribution in both health (Horewicz VV et al., 2013) and disease (Berglundh T et al., 2011). To what extent, if any, this higher pro-inflammatory state confers greater protection against bacterial pathogens or increases the susceptibility of peri-implant gingiva to tissue breakdown following bacterial challenge remains to be determined.
Matrix metalloproteinases (MMPs) are primarily responsible for tissue turnover (matrix degradation/remodeling) in physiological and pathological conditions (Siefert and Sarkar 2012). The balance between local catabolic (MMP) and anti-catabolic (TIMP) activities determines tissue degradation and remodeling (Baker and Leaper 2003). Consistent with previous reports on implant placement surgery (Nomura et al. 2000), TIMP-1 levels increased significantly during postoperative week one as a response to surgical trauma in general and, gradually decreased; the postoperative TIMP-1 level increase could be important in limiting the acute post-trauma tissue destruction.
The present study is the first one to provide extensive data on GCF molecular changes occurring in healthy periodontal sites following access flap surgery. Kuru et al. (2004, 2005) also studied GCF composition during early postoperative healing following surgery in “control” teeth (T+ equivalent) receiving crown lengthening; however, their GCF analysis was limited to TGFβ1 and specific adhesion molecules, thus precluding comparisons to the results reported here.
In the present study, wound size and location were limited to tooth-bounded mandibular posterior single implant sites. The limited wound size and restricted anatomical location minimized variations in extent and severity of surgical trauma; the control of implant positioning by use of a custom surgical stent designed for a screw-retained crown further minimized variation. This clinical model allows control of relevant variables and inclusion of intra-individual periodontal control (positive/negative) sites. Fourteen implant sites had received prior bone grafting for ridge reconstruction (missing tooth) or preservation (at tooth extraction), with grafting performed at least 4 months prior to study surgery and all 14 cases receiving the same treatment (freeze-dried bone allograft plus collagen membrane). Grafting history did not affect either clinical or PICF parameters (data not shown), consistent with earlier reports of previously grafted peri-implant sites having clinical characteristics similar to non-grafted sites (Sjostrom et al., 2005; Degidi et al., 2007).
Three different implant systems were used in the present study, based on case-specific treatment plan; the systems differ in surface characteristics (especially roughness/porosity) and thread morphology. However, all implants, regardless of system, were root form, screw type, and placed at bone level, and all received a smooth (polished) healing abutment of matching diameter. Therefore, the peri-implant soft tissues were always sutured against the same metallic abutment material, regardless of implant system used; thus, any effects on soft tissue healing potentially stemming from diverse implant system characteristics were minimized, if not eliminated altogether.
Based on patient diaries, 10 of the 40 patients took systemic antibiotics and 30 took analgesics during postoperative days 1-3, with no association between medication intake and clinical or PICF/GCF parameter changes during week one (data not shown). Pre- or post-operative antibiotic or NSAID use apparently has limited effect on PICF biomarkers, early implant failure, or wound healing (Khoury et al., 2008; Alissa et al., 2009; Waasdorp et al., 2010); nevertheless, evidence suggests that pre-operative antibiotics may contribute to a slight decrease in overall implant failure (Sharaf et al., 2011). Post-operative instructions following surgery included soft diet, chlorhexidine mouth rinse (0.12%) use, and abstinence from performing mechanical plaque control at surgery site for one week; this protocol was applied to all patients. This approach has been shown to effectively control postoperative plaque levels and inflammation (Grusovin MG et al., 2010), and the clinical outcomes of the present study (Table 1) corroborate this finding.
The present study results indicate the feasibility of detecting signs of acute surgical trauma at newly placed dental implant sites and surgically manipulated teeth, by analyzing PICF and GCF, respectively. The robust molecular responses specific to surgical trauma are much more pronounced at peri-implant gingiva compared to surgically-accessed adjacent periodontal tissues; these responses likely represent unique aspects of peri-implant tissue anatomy and physiology (e.g., healing processes). Given the reported effects of surgical technique (flapless placement versus flap surgery) on healing peri-implant soft tissue gene expression and inflammation levels (Mueller CK et al., 2011), additional studies are necessary to determine whether the observed differences between teeth and implants can be attributed, at least in part, to the potential differences between routine healing (reformation of severed periodontal attachment existing around teeth) and healing following wounding of previously intact masticatory mucosa (edentulous site) and de novo contact with foreign (smooth metallic) material. The present investigation model could serve as basis for future studies to differentiate physiological from delayed or compromised peri-implant wound healing.
Clinical Relevance.
Scientific Rationale for the Study: The major anatomical differences (blood supply; nature of connective tissue attachment) between gingiva around teeth and implants may impact wound healing and result in differential crevicular fluid content.
Principal Findings: Inflammation- and tissue degradation/remodeling-specific mediators were detected at high levels in both peri-implant and adjacent periodontal surgically manipulated gingiva, demonstrating a robust acute response to surgical trauma. Some mediators were selectively highly released around implants compared to adjacent teeth during early healing.
Practical Implications: The observed molecular differences between teeth and implants likely reflect unique aspects of peri-implant tissue physiology and anatomy. The present experimental model could serve as basis for future studies to differentiate physiological from delayed/compromised peri-implant wound healing.
ACKNOWLEDGEMENTS
The authors thank Drs. John Sheridan and Clay Marsh for generous access to laboratory space and equipment and Dr. John Walters for guidance. This study was supported by NIDCR (R21 DE018718) and the Division of Periodontology, College of Dentistry, The Ohio State University.
Footnotes
Disclosure of COI: Authors report no conflict of interest.
REFERENCES
- Alissa R, Sakka S, Oliver R, Horner K, Esposito M, Worthington HV, Coulthard P. Influence of ibuprofen on bone healing around dental implants: a randomized double-blind placebo controlled clinical study. Eur J Oral Implantol. 2009;2:185–199. [PubMed] [Google Scholar]
- Baker EA, Leaper DJ. Profiles of matrix metalloproteinases and their tissue inhibitors in intraperitoneal drainage fluid: relationship to wound healing. Wound Repair Regen. 2003;11:268–74. doi: 10.1046/j.1524-475x.2003.11406.x. [DOI] [PubMed] [Google Scholar]
- Baldi D, Menini M, Pera F, Ravera G, Pera P. Plaque accumulation on exposed titanium surfaces and peri-implant tissue behavior. A preliminary 1 year clinical study. Int J Prosthodont. 2009;22:447–55. [PubMed] [Google Scholar]
- Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Abrahamsson I, Welander M, Lang NP, Lindhe J. Morphogenesis of the peri-implant mucosa: an experimental study in dogs. Clin Oral Implants Res. 2007;18:1–8. doi: 10.1111/j.1600-0501.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Lindhe J. Dimension of the peri-implant mucosa. Biological width revisited. J.Clin.Periodontol. 1996;23:971–973. doi: 10.1111/j.1600-051x.1996.tb00520.x. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Lindhe J, Jonsson K, Ericsson I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J Clin Periodontol. 1994;21:189–193. doi: 10.1111/j.1600-051x.1994.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Zitzmann NU, Donati M. Are peri-implantitis lesions different from periodontitis lesions? J Clin Periodontol. 2011;38(Suppl 11):188–202. doi: 10.1111/j.1600-051X.2010.01672.x. [DOI] [PubMed] [Google Scholar]
- Carmichael RP, McCulloch CA, Zarb GA. Quantitative immunohistochemical analysis of keratins and desmoplakins in human gingiva and peri-implant mucosa. J Dent Res. 1991;70:899–905. doi: 10.1177/00220345910700050701. [DOI] [PubMed] [Google Scholar]
- Cattermole AE, Wade AB. A comparison of the scalloped and linear incisions as used in the reverse bevel technique. J Clin Periodontol. 1978;5:41–49. doi: 10.1111/j.1600-051x.1978.tb01905.x. [DOI] [PubMed] [Google Scholar]
- DeAngelo SJ, Kumar PS, Beck FM, Tatakis DT, Leblebicioglu B. Early soft tissue healing around one-stage dental implants: clinical and microbiologic parameters. J. Periodontol. 2007;78:1878–1886. doi: 10.1902/jop.2007.070122. [DOI] [PubMed] [Google Scholar]
- Degidi M, Daprile G, Piattelli A, Carinci F. Evaluation of factors influencing resonance frequency, at insertion surgery, of implants placed in sinus-augmented and nongrafted sites. Clin Implant Dent Relat Res. 2007;9:144–149. doi: 10.1111/j.1708-8208.2007.00042.x. [DOI] [PubMed] [Google Scholar]
- Ersanli S, Karabuda C, Beck F, Leblebicioglu B. Resonance frequency analysis of one-stage dental implant stability during the osseointegration period. J Periodontol. 2005;76:1066–1071. doi: 10.1902/jop.2005.76.7.1066. [DOI] [PubMed] [Google Scholar]
- Griffiths GS. Formation, collection and significance of gingival crevice fluid. Periodontol 2000. 2003;31:32–42. doi: 10.1034/j.1600-0757.2003.03103.x. [DOI] [PubMed] [Google Scholar]
- Grusovin MG, Coulthard P, Worthington HV, George P, Esposito M. Interventions for replacing missing teeth: maintaining and recovering soft tissue health around dental implants. Cochrane Database Syst Rev. 2010;8:CD003069. doi: 10.1002/14651858.CD003069.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lulic M, Lang NP. Factors influencing resonance frequency analysis assessed by Osstell mentor during implant tissue integration: II. Implant surface modifications and implant diameter. Clin Oral Implants Res. 2010;21:605–11. doi: 10.1111/j.1600-0501.2009.01909.x. [DOI] [PubMed] [Google Scholar]
- Horewicz VV, Ramalho L, dos Santos JN, Ferrucio E, Cury PR. Comparison of the distribution of dendritic cells in peri-implant mucosa and healthy gingiva. Int J Oral Maxillofac Implants. 2013;28:97–102. doi: 10.11607/jomi.2487. [DOI] [PubMed] [Google Scholar]
- Ingman T, Tervahartiala T, Ding Y, Tshesche H, Haerian A, Kinane DF, Konttinen YT, Sorsa T. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J Clin Periodontol. 1996;23:1127–1132. doi: 10.1111/j.1600-051x.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med. 2011;26:73–87. doi: 10.1177/0885066610384188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Interleukin-6 in surgery, trauma, and critical care: part I: basic science. J Intensive Care Med. 2011;26:3–12. doi: 10.1177/0885066610395678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury SB, Thomas L, Walters JD, Sheridan JF, Leblebicioglu B. Early wound healing following one-stage dental implant placement with and without antibiotic prophylaxis: a pilot study. J Periodontol. 2008;79:1904–1912. doi: 10.1902/jop.2008.070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru L, Griffiths GS, Petrie A, Olsen I. Changes in TGF-β1 in GCF following periodontal surgery. J.Periodontol. 2004;31:527–533. doi: 10.1111/j.1600-051x.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- Kuru L, Kirby AC, Griffiths GS, Petrie A, Olsen I. Changes in soluble adhesion molecules in GCF following periodontal surgery. J.Periodontol. 2005;76:526–533. doi: 10.1902/jop.2005.76.4.526. [DOI] [PubMed] [Google Scholar]
- Lamster IB, Ahlo JK. Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann NY Acad Sci. 2007;1098:216–29. doi: 10.1196/annals.1384.027. [DOI] [PubMed] [Google Scholar]
- Loe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;386(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- Mombelli A, Van Ossten MA, Schurch E, Jr., Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145–151. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Mueller CK, Thorwarth M, Schultze-Mosgau S. Histomorphometric and whole-genome expression analysis of peri-implant soft tissue healing: a comparison of flapless and open surgery. Int J Oral Maxillofac Implants. 2011;26:760–767. [PubMed] [Google Scholar]
- Nomura T, Ishii A, Oishi Y, Kohma H, Hara K. Tissue inhibitors of metalloproteinases levels and collagenase activity in gingival crevicular fluid: the relevance to periodontal diseases. Oral Dis. 1998;4:231–240. doi: 10.1111/j.1601-0825.1998.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Nomura T, Ishii A, Shimizu H, Taguchi N, Yoshie N, Kusakari H, Hara K. Tissue Inhibitor of Metalloproteinases-1, matrix metalloproteinases-1 and -8, and collagenase activity levels in peri-implant crevicular fluid after implantation. Clin Oral Implants Res. 2000;11:430–440. doi: 10.1034/j.1600-0501.2000.011005430.x. [DOI] [PubMed] [Google Scholar]
- Nowzari H, Botero JE, DeGiacomo M, Villacres MC, Rich SK. Microbiology and cytokine levels around healthy dental implants and teeth. Clin Implant Dent Relat Res. 2008;10:166–173. doi: 10.1111/j.1708-8208.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- Nowzari H, Phamduong S, Botero JE, Villacres MC, Rich SK. The profile of inflammatory cytokines in gingival crevicular fluid around healthy osseointegrated implants. Clin Implant Dent Relat Res. 2010 Jul 17; doi: 10.1111/j.1708-8208.2010.00299.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9:248–266. doi: 10.1177/10454411980090030101. [DOI] [PubMed] [Google Scholar]
- Pathak AP, Hochfeld WE, Goodman SL, Pepper MS. Circulating and imaging markers for angiogenesis. Angiogenesis. 2008;11:321–335. doi: 10.1007/s10456-008-9119-z. [DOI] [PubMed] [Google Scholar]
- Petkovic AB, Matic SM, Stamatovic NV, Vojrodic DV, Todorovic TM, Lazic ZR, Kozomara RJ. Proinflammatory cytokines (IL-1beta and TNF-alpha) and chemokines (IL-8 and MIP-1alpha) as markers of peri-impant tissue condition. Int J Oral Maxillofac Surg. 2010;39:478–485. doi: 10.1016/j.ijom.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Romanos GE, Schröter-Kermani C, Weingart D, Strub JR. Health human periodontal versus peri-implant gingival tissues: an immunohistochemical differentiation of the extracellular matrix. Int J Oral Maxillofac Implants. 1995 Nov-Dec;10(6):750–8. [PubMed] [Google Scholar]
- Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23:182–90. doi: 10.1111/j.1600-0501.2011.02220.x. [DOI] [PubMed] [Google Scholar]
- Sharaf B, Jandali-Rifai M, Susarla SM, Dodson TB. Do perioperative antibiotics decrease implant failure? J Oral Maxillofac Surg. 2011;69:2345–2350. doi: 10.1016/j.joms.2011.02.095. [DOI] [PubMed] [Google Scholar]
- Siefert SA, Sarkar R. Matrix metalloproteinases in vascular physiology and disease. Vascular. 2012;20:210–216. doi: 10.1258/vasc.2011.201202. [DOI] [PubMed] [Google Scholar]
- Sjostrom M, Lundgren S, Nilson H, Sennerby L. Monitoring of implant stability in grafted bone using resonance frequency analysis. A clinical study from implant placement to 6 months of loading. Int J Oral Maxillofac Surg. 2005;34:45–51. doi: 10.1016/j.ijom.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Tatakis DN. Interleukin-1 and bone metabolism: a review. J Periodontol. 1993;64:416–431. [PubMed] [Google Scholar]
- Waasdorp JA, Evian CI, Mandracchia M. Immediate placement of implants into infected sites: a systematic review of the literature. J. Periodontol. 2010;81:801–806. doi: 10.1902/jop.2010.090706. [DOI] [PubMed] [Google Scholar]