Abstract
Enteric neuronal progenitor cells are neural crest-derived stem cells that can be isolated from fetal, post-natal and adult gut. Neural stem cell transplantation is an emerging therapeutic paradigm to replace dysfunctional or lost enteric neurons in several aganglionic disorders of the GI tract. The impetus to identify an appropriate microenvironment for enteric neuronal progenitor cells derives from the need to improve survival and phenotypic stability following implantation. Extracellular matrix composition can modulate stem cell fate and direct differentiation. Adult mammalian myenteric ganglia in vivo are surrounded by a matrix composed primarily of Collagen IV, Laminin and a Heparan sulfate proteoglycan. In these studies, adult mammalian enteric neuronal progenitor cells isolated from full thickness rabbit intestines were induced to differentiate when cultured on various combinations of neural ECM substrates. Neuronal and glial differentiation was studied as a function of ECM composition on coated glass coverslips. Poly- lysine coated coverslips (control) supported extensive glial differentiation but very minimal neuronal differentiation. Individual culture substrata (Laminin, Collagen I and Collagen IV) were conducive for both neuronal and glial differentiation. The addition of laminin or heparan sulfate to collagen substrates improved neuronal differentiation, significantly increased neurite lengths, branching and initiation of neuronal network formation. Glial differentiation was extensive on control poly lysine coated coverslips. Addition of laminin or heparan sulfate to composite collagen substrates significantly reduced glial immunofluorescence. Various neural ECM components were evaluated individually and in combination to study their effect of neuroglial differentiation of adult enteric neuronal progenitor cells. Our results indicate that specific ECM substrates that include type IV Collagen, laminin and heparan sulfate support and maintain neuronal and glial differentiation to different extents. Here, we identify a matrix composition optimized to tissue engineer transplantable innervated GI smooth muscle constructs to remedy aganglionic disorders.
Keywords: Neural stem cell, neural tissue engineering, extracellular matrix, adult stem cell, enteric glia
1. Introduction
Gastrointestinal motor function is intimately controlled by the intramural enteric nervous system. It is a complex interplay between the smooth muscle of the muscularis externa and the two enteric neuronal plexi [1]. Aganglionosis of varying lengths of distal gut is the central pathology in Hirschsprung’s disease [2]. Enteric neuropathy is also secondary to several other disorders (diabetes, Parkinson’s disease, inflammation) resulting in gastrointestinal dysfunction [3, 4]. Neural stem cell therapy is an emerging therapeutic paradigm that ideally aims to reinstate neuronal function and thus gastrointestinal motor function by repopulating the enteric plexi. The research is driven by two significant findings: i) neuroglial progenitor cells can be isolated from adult mammalian gut, including ganglionated colon of Hirschsprung’s patients [5, 6]; and ii) progenitor cells can be induced to differentiate into several neuronal subtypes and glia characteristic of the ENS upon transplantation into explant cultures of aganglionic/aneural gut [5–9], or in vivo into distal colo-rectums of adult rodents [10, 11]. However, phenotypic stability, long term survival, and post-transplant fate all remain to be optimized while moving forward with neural stem cell transplantation for clinical use [12, 13]. In order to provide trophic support and a permissive microenvironment, a more fundamental understanding of factors that affect and maintain differentiation of enteric neuronal progenitor cells is required. The studies described here focus on in vitro differentiation of adult mammalian enteric neuronal progenitor cells, particularly related to the effect of varying extracellular matrix composition of culture substrata.
The extracellular matrix (ECM) plays an enormous role in dictating stem cell fate. ECM composition, structure and mechanical properties can all modulate progenitor cell differentiation [14, 15]. The adult mammalian myenteric plexus is surrounded by an extracellular matrix primarily composed of Collagen IV, Laminin and a heparan sulfate proteoglycan, with enteric glia always in direct contact with the ECM. Enteric neurons also come in direct contact with this ECM, though much less frequently than glia [16, 17]. Laminin, fibronectin and proteoglycans are expressed within the embyonic gut to aid its colonization by vagal neural crest cells. Collagen IV is distributed in the developing nervous system along the neural crest. Additionally, laminin is implicated in both the central and peripheral nervous system in promoting neural cell adhesion and axonal outgrowth [18]. Heparan sulfate is required for GDNF signaling in the gut, and has been known to stabilize and influence neuronal differentiation in vitro [19–21].
In this paper, we describe the effect of components of neural ECM on the differentiation of gut-derived neuronal progenitor cells of neural-crest lineage in vitro. Two timepoints were defined to identify early and late differentiation events – day 5 (early) and day 15 (late) based on previous experiments [11]. Immunohistochemistry for β III Tubulin (neuron specific microtubule) and GFAP (Glial fibrillary acidic protein) was used to identify differentiated neurons and glia on coated culture substrata. The principal objective of this study was to identify the effect of extracellular matrix composition on the differentiation of adult enteric neuronal progenitor cells in vitro.
2. Materials and Methods
2.1 Reagents
All tissue culture reagents were purchased from Invitrogen (Carlsbad, CA) unless specified otherwise. Primary and fluorophore conjugated secondary antibodies were purchased from Abcam (Cambridge, MA). Rat tail type I Collagen and natural mouse type IV Collagen were purchased from BD Biosciences (Bedford, MA) and Laminin was from Invitrogen (Carlsbad, CA). Heparan sulfate was purchased from Celsus Labs (Cincinnati, OH).
2.2 Isolation and culture of rabbit enteric neuronal progenitor cells and rabbit intestinal smooth muscle cells
New Zealand white rabbits were euthanized using ketamine/xylazine. Intestinal smooth muscle cells were isolated and cultured using previously described protocols [22]. For the isolation of enteric neuronal progenitor cells, 5 cm2 biopsies were dissected from the jejunum, and retrieved in Hank’s Buffered Salt Solution (HBSS) with 2X antibiotics/antimycotics and 1X gentamicin sulfate. Luminal content was cleaned and tissues were washed extensively with HBSS. Enteric neuronal progenitor cells were isolated from these tissues using a collagenase/dispase digestion method, reported by Almond et al. [6]. Cells were plated on to bacterial petri dishes in neuronal growth media (Neurobasal + 1X N2 supplement + 1X antibiotics) following filtration through a 40μm mesh.
2.3 Immunohistochemical characterization of rabbit enteric neurospheres
In order to characterize the initial phenotype of rabbit enteric neurospheres in culture, neurospheres were harvested by centrifugation at 1000g for 10 minutes in microfuge tubes. The growth media was gently aspirated, and neurospheres were fixed with 3.7% neutral buffered formaldehyde and blocked with 10% horse serum. Primary antibodies for p75 (Millipore, Billerica MA), Sox2 and Nestin were incubated for 30 minutes at room temperature. Unbound antibody was washed using phosphate buffered saline (PBS), and appropriate fluorophore-conjugated secondary antibodies were incubated for an additional 30 minutes. Neurospheres were mounted using Prolong Gold antifade mounting medium (Invitrogen, Carlsbad CA), and visualized using an inverted Nikon TiE fluorescent microscope.
2.4 Differentiation of rabbit enteric neurosphere differentiation as a function of extracellular matrix composition
22x11mm coverslips were washed in Neutrad (Decon Labs, King of Prussia PA) and rinsed extensively in deionized water. Coverslips were sterilized by 70% ethanol, and subsequent UV exposure for 45 minutes. Coverslips were coated with poly-L-lysine (pLL; 1 mg/ml), pLL+ 10μg/cm2 type I Collagen, pLL + 10μg/cm2 type IV Collagen or pLL + 10μg/cm2 Laminin. Composite coatings included:
5μg/cm2 Collagen I + 5 μg/cm2 type IV Collagen;
5 μg/cm2 Collagen I + 5 or 10 μg/cm2 Laminin;
5 μg/cm2 Collagen IV + 5 or 10 μg/cm2 Laminin;
5 μg/cm2 Collagen I + 5 μg/cm2 Collagen IV + 0.1 μg/cm2 Heparan Sulfate (HS);
5 μg/cm2 Collagen I + 5μg/cm2 Collagen IV + 5 μg/cm2 Laminin + 0.1 μg/cm2 HS.
Uncoated glass coverslips were seeded with rabbit colonic smooth muscle cells, and allowed to reach confluence. Rabbit enteric neurospheres were harvested and treated with Accutase to obtain a mixture of single cells as well as small neurospheres. 10,000 neuronal progenitor cells were harvested and plated on to coated coverslips. To stimulate differentiation induced via soluble smooth muscle factors, each plate was shared by one confluent smooth muscle coverslip along with a coated coverslip containing adhered neurospheres. Enteric neurospheres were allowed to differentiate for a period of fifteen days, with a supplementation of neuronal differentiation medium every 2 days (Neurobasal-A medium + 1X B27 supplement + 2% fetal calf serum + 1X antibiotics).
2.5 Immunohistochemical analysis of neuronal and glial differentiation of rabbit enteric neurospheres on coated coverslips
Neuronal and glial differentiation was analyzed at two time points– Day 5 and Day 15 post initiation of differentiation. Medium was aspirated and cells on coverslips were fixed with 3.7 neutral buffered formaldehyde. Cells were permeabilized with 0.15% Triton-X 100 and blocked with 10% horse serum. βIII Tubulin was used to stain neuronal cells, and glial fibrillary acidic protein (GFAP) was used to stain glial cells. Primary antibodies were incubated for 1 hour at room temperature and unbound antibody was washed with PBS. Fluorophore conjugated secondary antibodies (FITC-anti mouse and TRITC-anti rabbit) were used to visualize fluorescence using an inverted Nikon TiE fluorescent microscope. Staining with FITC-conjugated secondary antibody without the primary antibody was used as a negative control. Confluent smooth muscle coverslips were stained with neuronal or glial markers to avoid a false positive staining while identifying differentiated neurons or glia.
2.6 Data Analysis
Neurite lengths were measured from individual 10X micrographs obtained at the same amplifier gain and exposure. Neurites were identified primarily by expression of immunoreactivity for βIII Tubulin concurrently with neuronal morphology. Up to five sequential fields of view were measured on each coverslip starting from one edge to the other, covering the area of the coverslip. All cells were measured on each coverslip, covering the entire area of the neuronal coverslip. Number of neurites measured for each coverslip coating varied between 20–50 readings and is indicated next to a reported measurement. The length of the longest neurite from each cell was measured using NIH Image J using the freeform tool. Neurite lengths between coatings were compared using one way ANOVA, with Bonferroni post-test to identify a significant difference (p<0.05) in neurite lengths by varying culture substrata. GFAP immunofluorescence was quantified using the Nikon Elements imaging software. Mean red (TRITC) fluorescence was calculated from 10X micrographs, using a constant rectangular area tool that covered 100% of the field of view. Multiple (at least 5) sequential fields of view at the same magnification were chosen for each sample to obtain mean fluorescence. Mean red fluorescence indicated the presence of GFAP expressing cells. One way ANOVA with Bonferroni post-test was used to identify a significant difference in red fluorescent intensity between coated culture substrata. GraphPad Prism 5.1 for Windows (San Diego, CA) was used to perform statistical analysis. All statistics are from experiments between 3–5 individual sets, with multiple micrographs within each set. Reported numbers are mean ± standard error of the mean.
3. Results
3.1 Initial phenotype of rabbit enteric neurospheres
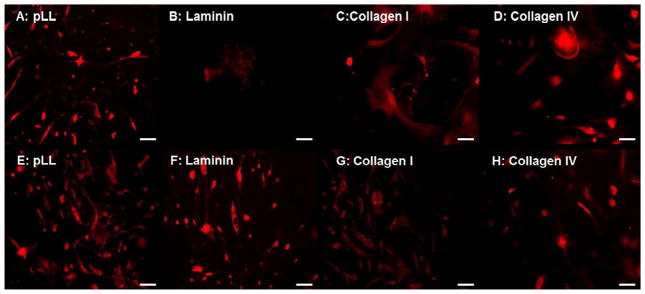
Upon digestion of rabbit jejunal biopsies with collagenase/dispase, near single cell suspensions were obtained by filtration through 70μm and 40μm meshes. Single cells were approximately 7μm in diameter. These cells were plated in non-adherent culture dishes. Over the course of two weeks post plating, rabbit enteric neuronal progenitor cells aggregated and proliferated in culture and formed floating spherical structures, called enteric neurospheres (Figure 1A). Average neurospheres were 98.2 ± 8.3 μm (n=34) two weeks post plating. The neurospheres continued to grow and aggregate, approaching 200–300μm, whereupon they were broken down by trituration. Upon immunohistochemical examination, the cells within enteric neurospheres were positive for the low affinity nerve growth factor receptor p75NTR (Figure 1B). They were additionally also positive for Sox2 (Figure 1C, SRY related homeobox factor 2) and Nestin (Figure 1D), a neuroepithelial stem cell marker. Our results indicate that neurospheres derived from the rabbit intestine following this procedure are comprised of neural crest-derived cells that are capable of differentiating in to enteric neurons and/or enteric glia.
Figure 1.
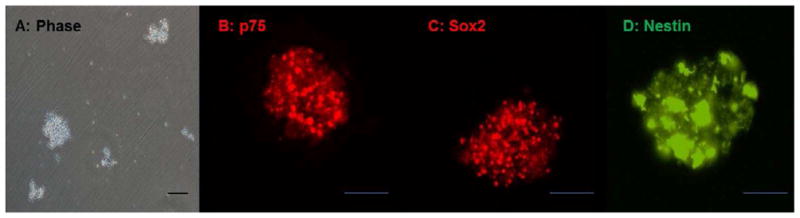
Rabbit enteric neurospheres- (A) Phase contrast micrograph of rabbit enteric neurospheres in culture. Upon primary isolation and culture, progenitor cells proliferate and aggregate to form neurosphere-like bodies (enteric neurospheres). Immunohistochemistry for initial phenotype (B–D): Rabbit enteric neurospheres are p75NTR (A), Sox2 (B) and Nestin (C) positive–indicating that they are comprised of neural crest-derived neuronal and glial progenitor cells. Scale bar 100μm.
3.2 Neuronal differentiation on individual ECM substrates (Collagen I, Collagen IV or Laminin)
Poly-lysine (pLL) coating was a pre-requisite to enteric neurosphere adhesion to glass coverslips. Glass coverslips that lacked any coating did not support enteric neurosphere adhesion sufficiently to differentiate into neurons or glia. In order to maintain uniformity, all coverslips were initially coated with pLL and additionally with Laminin, Collagen I or Collagen IV. All coated coverslips required between 2–4 hours for enteric neurospheres to attach.
Enteric neurospheres on coated coverslips were allowed to differentiate initially using neuronal differentiation medium alone. Several sets of experiments demonstrated that these cells did not undergo any differentiation under these conditions. Thereby, in order to render the soluble environment conducive to differentiation, a confluent coverslip containing colonic smooth muscle cells was placed in the same culture dish (Figure 2). The neuronal coverslip (coated with ECM substrate and containing enteric neurospheres) and the smooth muscle coverslips thereby shared soluble factors. The addition of the smooth muscle coverslip marked the initiation of differentiation (day 0).
Figure 2.
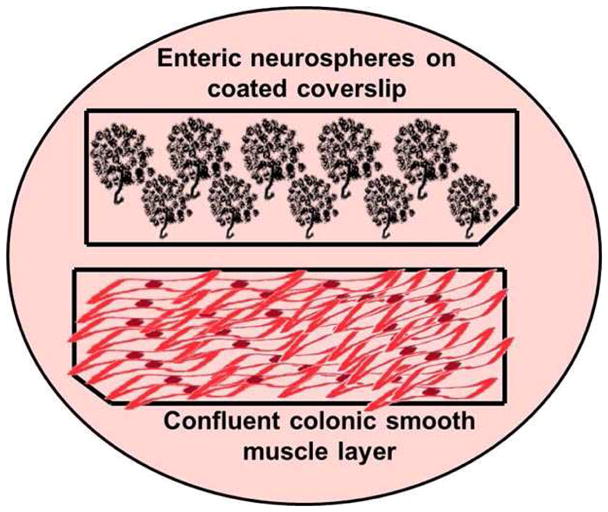
Schematic of enteric neurosphere differentiation as a function of ECM composition: 22x11mm coverslips were coated with pLL, Collagen I, Collagen IV, Laminin or Heparan sulfate individually or in combination. Enteric neurospheres were allowed to adhered to the coated coverslips for 6 hours. Separately, uncoated glass coverslips were seeded with colonic smooth muscle cells, and allowed to grow to confluence. In order to stimulate differentiation of enteric neurospheres, a coverslip containing confluent smooth muscle was placed within the same dish, so the two coverslips shared soluble factors.
Morphological evidence of neuronal or glial differentiation was readily visible by day 5. A later time point (day 15) was identified to study the development of mature neurons or glia in vitro as a function of ECM composition. During the differentiation process, the culture dishes remained undisturbed till the early time point (day 5) or the late time point (day 15), except for medium supplementation. Neuronal differentiation was identified by immunofluorescent staining of the neuronal coverslip at either day 5 or day 15 with an antibody directed against βIII Tubulin.
Day 5 timepoint
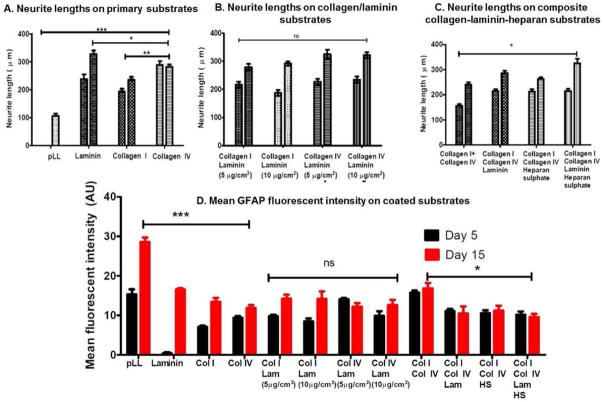
Even in the presence of smooth muscle, enteric neurospheres on pLL remained undifferentiated, with some progenitor cells within neurospheres expressing low levels of βIII Tubulin (Figure 3A). However, with the addition of either laminin, Collagen I or Collagen IV to pLL on the culture substrata, neuronal differentiation was evident by day 5 (Figure 3B–D). Neurite lengths varied non-significantly between 193.2 ± 9.9 μm and 288.2 ± 14. 5μm on ECM substrata at the early time point (Figure 9A). Neurons on Collagen IV and Laminin coated coverslips demonstrated a higher level of branching (two or more neurites per cell; Figure 3B,D).
Figure 3.
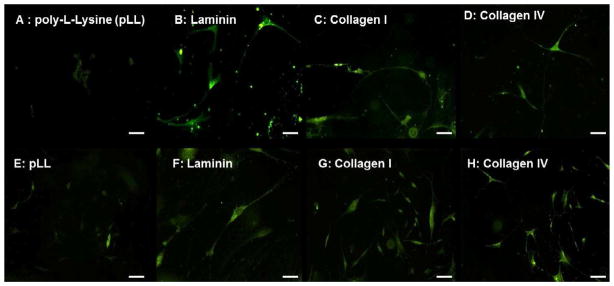
Neuronal differentiation on individual coated coverslips - βIII Tubulin antibody (green) was used to visualize neurons on Day 5 (A–D) and Day 15 (E–H) coverslips coated with pLL (A,E), Laminin (B,F), type I Collagen (C,G) and type IV Collagen (D,H). Enteric neurospheres on pLL barely initiate neuronal differentiation even at day 15. Neurospheres on Laminin, Collagen I and Collagen IV showed branching and several neuronal processes both at the early and late time points in vitro.
Figure 9.
Neurite lengths were measured on coated culture substrata and compared using one way ANOVA. Two bars for each substrate show mean neurite lengths at day 5 and day 15. (A) Neurite lengths on pLL were significantly (***p<0.001) shorter than any primary coating substrate. Laminin substrates had the longer neurites (*p<0.05). (B): No significant difference was observed in neurite lengths with the addition of 5 or 10 μg/cm2 laminin. (C): The addition of laminin or heparan sulphate significantly increased neurite lengths over composite collagen substrata (*p<0.05). (D) Mean GFAP immunofluorescence was quantified: pLL substrates (***p<0.001) and composite collagen substrates (*p<0.05) supported extensive glial differentiation.
Day 15 timepoint
At the day 15 timepoint, neurospheres on pLL coverslips barely initiated neuronal differentiation, evidenced by a flatter morphology and the appearances of faint tubulin-positive extensions (Figure 3E). With the addition of either laminin, Collagen I or Collagen IV, neurite lengths were significantly longer compared to pLL (p<0.001). Differentiated neurons on laminin coverslips demonstrated the longest neurite extensions (326.9 ± 13.3μm, n=27; Figure 3F), significantly longer than Collagen I or Collagen IV (p<0.05, Figure 9A). By day 15, neurons on Collagen I-coated coverslips still had no significant branching compared to those on Collagen IV-coated coverslips (Figures 3G–H).
3.3 Neuronal differentiation on collagen-laminin substrates
In the next set of experiments, combinations of collagens and laminin were evaluated. Two concentrations of laminin were evaluated to identify the minimum amount of laminin required to influence neuronal differentiation. Coverslips were coated with either collagen I or collagen IV with 5μg/cm2 or 10μg/cm2 of laminin. The addition of laminin enhanced neuronal differentiation when compared to individual collagen substrates (compare Figure 4 with Figure 3), but no significant difference was observed in neurite length between the two concentrations of laminin.
Figure 4.
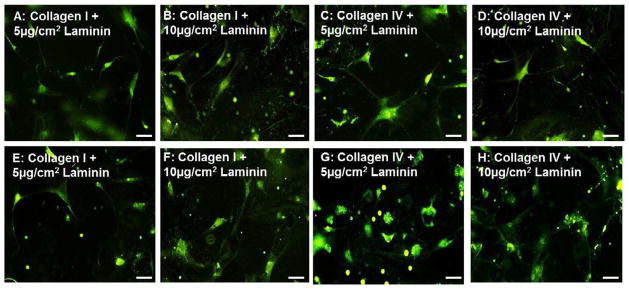
Neuronal differentiation on collagen-laminin substrates–Neurons stained with βIII Tubulin (green) on Day 5 (A–D) and Day 15 (E–H) coverslips coated with type I Collagen and 5μg/cm2 Laminin (A,E) or 10μg/cm2 Laminin (B,F) or type IV Collagen with 5 (C,G) or 10 (D,H) μg/cm2 of Laminin. Addition of laminin to collagen substrates enhanced early and late neuronal differentiation, but no significant difference was observable between 5 and 10 μg/cm2 of laminin. Type IV Collagen substrates (C,D,G,H) demonstrated enhanced neuronal branching and differentiation compared to type I collagen (A,B,E,F) substrates.
Collagen I and Laminin
At the day 5 timepoint, addition of laminin to Collagen I increased the number of progenitor cells undergoing neuronal differentiation, but did not alter neuronal branching or neurite lengths significantly (Figure 4A,B,E,F). At the day 15 timepoint, significantly enhanced neuronal differentiation (p<0.05) was observed compared to Collagen I only. Neurite lengths on collagen-laminin substrates at day 15 (279.7 ± 10.8 μm – 291.9 ± 8.1 μm; n=27–34) were longer than Collagen I substrates (235.5 ± 10.1 μm; Figure 9B).
Collagen IV and Laminin
The addition of laminin to Collagen IV enhanced neuronal differentiation when compared to coverslips coated individually with Collagen IV only (compare Figure 4C,D,G,H to Figure 3D,H). At the day 5 timepoint, the addition of laminin increased the number of cells undergoing neuronal differentiation. No significant difference was observed in neurite lengths at day 5 (247 ± 12.9 μm – 263.7 ± 9.7 μm; Figure 9B). At the day 15 time point, coverslips coated with both collagen IV and laminin had significantly (p<0.05) longer neurites compared to Collagen IV only (324.6 ± 16.5 μm compared to 281.7 ± 8.6μm, Figure 4G–H). Initiation of inter-neuronal networking was also observed. There was no observable or significant difference in neurite lengths between the two concentrations of laminin used (5μg/cm2 or 10μg/cm2; Figure 9B).
3.4 Neuronal differentiation on composite ECM substrates with laminin and heparan sulfate
In this additional set, the effect of a combination of collagens on neuronal differentiation was investigated. Composite coatings were evaluated with a 2:1 mix of Collagen I/Collagen IV as the base. This composite collagen base was evaluated first. Additionally, neuronal differentiation was evaluated on substrates that included laminin and/or heparan sulfate in combination with composite collagen.
Composite Collagen I/Collagen IV
Several cells underwent neuronal differentiation (Figure 5A, E; similar to individual coatings of either Collagen I or Collagen IV), but neurite lengths were significantly shorter on composite collagen substrates at day 5. Neurite lengths measured at 156.1 ± 7.2 μm on day 5. At day 5, neuronal differentiation progressed and individual neurons had multiple branches and long neurites (Figure 5E). Neurite lengths averaged at 241.2 ± 9.4 μm on day 15 (Figure 9C).
Figure 5.
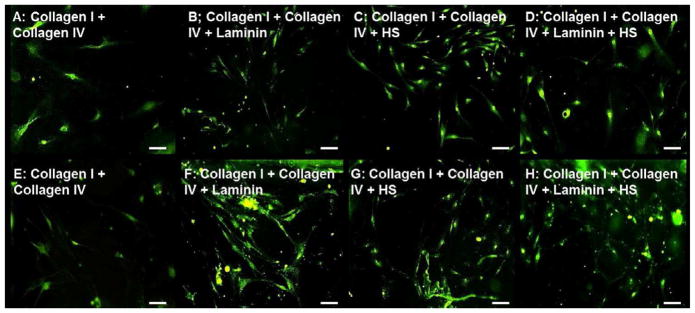
Neuronal differentiation on composite collagen-laminin-heparan sulfate (HS) substrates–Neurons stained with βIII Tubulin (green) on Day 5 (A–D) and Day 15 (E–H) coverslips coated with type I and IV Collagen with either 5μg/cm2 Laminin (B,F), 0.1μg/cm2 Heparan Sulfate (HS; C,G), both (D,H) or none (A,E). Addition of laminin or HS to collagen substrates enhanced early and late neuronal differentiation, with visible networking by day 15. Substrates without laminin or HS demonstrated minimal neuronal differentiation (A,E).
Addition of Laminin
Addition of laminin to composite collagen substrates increased the number of differentiated neurons visible by day 5 (Figure 5B). Neurite lengths were significantly (p<0.05) longer on substrates containing laminin (215.1 ± 7.6 μm, n=43). At the day 15 time point, substrates containing composite collagen and laminin demonstrated significant clustering of neurons (Figure 5F), with increased neurite lengths averaging at 286.8 ± 9.5 μm, n=50.
Addition of heparan sulfate
Addition of heparan sulfate also dramatically increased the number of progenitor cells undergoing neuronal differentiation by day 5 (Figure 5C). Average neurite lengths on substrates containing heparan sulfate along with composite collagen was 212.8 ± 9.5 μm, n=43 at day 5. At day 15, the initiation of neuronal networking was visible with βIII Tubulin staining (Figure 5G).
Addition of laminin and heparan sulfate
The addition of laminin and heparan sulfate together with the composite collagen increased the number of differentiated neurons as well as the length of the individual neuronal processes and neurite branching (Figure 5D). At day 15, initiation of neuronal networking with significant clustering of neurons was observed (Figure 5H). Neurite lengths were significantly longer (325.5 ± 19.4μm, n=36) compared to composite collagen alone (Figure 9C).
3.5 Glial differentiation on individual ECM coatings (Collagen I, Collagen IV or Laminin)
In addition to neuronal differentiation studies described above, glial differentiation was also studied as a function of ECM composition of culture substrata. Enteric neurospheres were plated on to coated coverslips in duplicate, and one coverslip was used to evaluate neuronal differentiation while a duplicate coverslip was used to evaluate glial differentiation. A primary antibody directed against Glial fibrillary acidic protein (GFAP) was utilized to identify glial differentiation. Fluorescent microscopy was used to visualize differentiated glia, using a TRITC fluorophore conjugated secondary antibody. The Nikon documentation software was used to calculate mean red fluorescence indicating the number of differentiated glia in a field of view of constant area.
Day 5 timepoint
In the presence of smooth muscle, enteric neurospheres on pLL coated coverslips demonstrated significant GFAP staining by day 5 (15.3 ± 1.3 AU; Figure 6A). In contrast, enteric neurospheres on pLL coverslips did not demonstrate significant neuronal differentiation at day 5, indicating the preferential differentiation in to glia at the early time point on pLL coverslips. With the addition of laminin, enteric neurospheres demonstrated highly significantly reduced GFAP staining (0.4 ± 0.2 AU). Undifferentiated neurospheres on the laminin coverslips contained several progenitor cells that were positive for GFAP (Figure 6B). On the same laminin coated coverslips, neuronal differentiation was extensive at the early timepoint, indicating an early preference for neuronal differentiation in the presence of laminin (Figure 3B). Minimal glial differentiation was observed on either of the collagen substrates (Figure 6C–D).
Figure 6.
Glial differentiation on primary coated substrates–Glia stained with GFAP (red) on Day 5 (A–D) and Day 15 (E–H) coverslips coated with pLL (A,E), Laminin (B,F), type I Collagen (C,G) and type IV Collagen (D,H). Enteric neurospheres on pLL demonstrated maximal glial differentiation starting at day 5 up to day 15. While neurospheres on collagen substrates demonstrated good early and late glial differentiation, laminin coated substrates showed no early glial differentiation by day 5 (B), but differentiated subsequently by day 15 (F).
Day 15 timepoint
By the late day 15 timepoint, pLL coated coverslips had the highest number of glia, indicated by a highly significant (p<0.0001) GFAP fluorescent intensity averaging at 28. 6 ± 1.1 AU (Figure 6E, 9D). Glia were apparent on ECM coated coverslips as well, but to a lower extent than on pLL. In contrast to day 5, laminin coated coverslips demonstrated the presence of several glia at the day 15 time point, and a robust GFAP fluorescent intensity was observed (Figure 5F, 16.5 ± 0.3 AU). Several glia were observed by day 15 on either of the collagen substrates, with fluorescence ranging from 11.8 to 13.4 AU. (Figure 6G–H).
3.6 Glial differentiation on collagen-laminin substrates
Similar to neuronal differentiation, glial differentiation was evaluated on substrates that were coated with either Collagen I or Collagen IV with laminin. The addition of laminin to collagen coated coverslips did not inhibit glial differentiation. Several differentiated glia were observed on day 5 (8.4 ± 0.8 – 14.1 ± 0.3 AU) on collagen-laminin substrates (Figure 7A–D). There was no significant difference in the number of GFAP positive cells at the early time point with the addition of laminin (5μg/cm2 or 10μg/cm2) to either Collagen I or Collagen IV substrates Figure 9D). Robust GFAP expression (12.6 ± 1.3 – 14.2 ± 1.0 AU) was observed at the day 15 time point on all collagen-laminin substrates, not significantly different from one another (Figure 7E–H).
Figure 7.
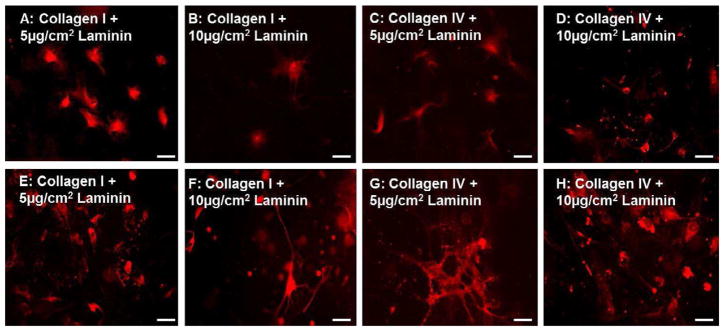
Glial differentiation on collagen-laminin substrates–Glia stained with GFAP (red) on Day 5 (A–D) and Day 15 (E–H) coverslips coated with type I Collagen and 5μg/cm2 Laminin (A,E) or 10μg/cm2 Laminin (B,F) or type IV Collagen with 5 (C,G) or 10 (D,H) μg/cm2 of Laminin. Addition of laminin to collagen substrates promoted glial differentiation both at days 5 and 15. No significant difference was observable in GFAP+ glial differentiation between 5 and 10 μg/cm2 of laminin.
3.7 Glial differentiation on composite ECM substrates with laminin and heparan sulfate
Glial differentiation was evaluated by varying the culture substratum with a combination of collagen I and IV. Additionally, the effect of the addition of laminin and/or heparan sulfate was also studied on glial differentiation.
Composite Collagen I/Collagen IV
Glial differentiation peaked on day 5, on coverslips coated with the collagen I/IV mixture (Figure 8A). Red fluorescence (15.8 ± 0.5 AU) was comparable to that on pLL coated coverslips at day 5 (Figure 9D). In contrast, neuronal differentiation on composite collagen coated coverslips was poor at the early time point, indicating a preferential differentiation into glia early on. By day 15, initiation of clustering of glial cells was observable (Figure 8E), with no significant increase in red fluorescence.
Figure 8.
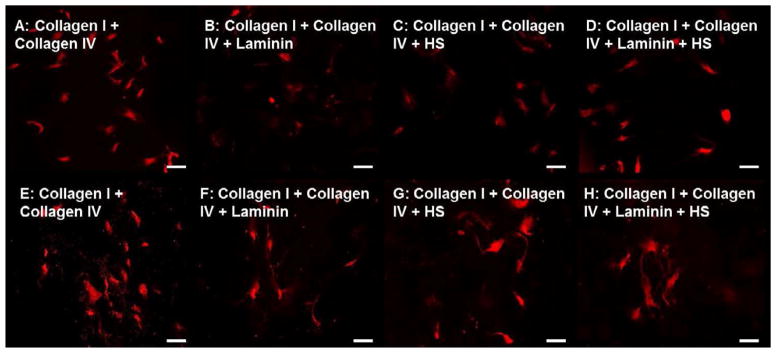
Glial differentiation on composite collagen-laminin-heparan sulfate (HS) substrates Glia stained with GFAP (red) on Day 5 (A–D) and Day 15 (E–H) coverslips coated with type I and IV Collagen with either 5μg/cm2 Laminin (B,F), 0.1μg/cm2 Heparan Sulfate (HS; C,G), both (D,H) or none (A,E). Addition of laminin or HS to composite collagen substrates demonstrated glial differentiation that peaked at day 5(B–D) that was sustained at the later time point (E–H).
Addition of laminin and/or heparan sulfate
Early glial differentiation at day 5 was reduced (10.2 ± 0.8 to 11.1 ± 0.5 AU) with the addition of laminin and/or heparan sulfate to composite collagen substrates (Figure 8B–D). In contrast, these substrates supported neuronal differentiation extensively (compare Figure 8A–D to Figure 5A–D), indicating a preferential neuronal differentiation at the early time point. At the later day 15 time point, a non-significant increase in the number of glia was observed (Figure 8E–H, Figure 9D).
4. Discussion
Enteric neuronal progenitor cells have been identified in the adult mammalian gut, and have been isolated from humans >80 years of age [5, 23]. Previously, several groups have shown that a self-renewing population of Sox 2 [8], Sox10 [23], Nestin [24] and p75 [25] positive neural-crest derived progenitor cells can be isolated either from full-thickness, muscularis or mucosal biopsies of the adult mammalian gut [26–28]. These cells have been demonstrated to have the potential to differentiate in to several neuronal subtypes including inhibitory and excitatory motor neurons and glia. We have isolated neuronal progenitor cells from full thickness biopsies of adult rabbit jejunums that aggregate in culture to form floating spherical colonies, dubbed enteric neurospheres (Figure 1A). We confirmed that these enteric neurospheres were comprised of cells positive for p75, Sox2 and Nestin (Figure 1B–D). The presence of p75NTR confirms the neural-crest lineage of the isolated cells. The presence of Sox2 and Nestin confirm the progenitor status of the isolated cells, indicating that these cells are similar to enteric neuronal progenitor cells previously isolated from the gut that have the potential to differentiate into both neurons and glia.
The feasibility of transplantation of various types of neuronal progenitor cells (CNS-derived, neural tube-derived, embryonic and adult ENS-derived) in explant cultures of aneural gut is well established [5, 7, 9]. Conditions required for successful engraftment and long-term phenotype maintenance, focusing on a permissive environment are yet to be satisfactorily identified. Alterations in the extracellular matrix of the gut mesenchyme has been documented in aganglionic regions of rodent gut, suggesting the importance of a permissive extracellular environment to promote effective in utero colonization and differentiation of neural crest cells in the developing gut [29–32]. Since transplantation and subsequent functional neo-innervation is the clinical goal of neural stem cell transplantation, in vitro studies must mimic developmental conditions in vivo, in terms of providing a permissive and favorable ECM (preferably three-dimensional). A focus on the role of the ECM in affecting neuroglial differentiation of adult enteric neuronal progenitor cells can optimize the survivability and maintenance of a stable phenotype upon transplantation. Previous studies by Schäfer et al. demonstrate that fetal rodent enteric neurospheres differentiate forming neurites on laminin coated culture surfaces, and secondary ganglion like structures in composite ECM gels within 7 days in vitro [33].
Mammalian myenteric ganglia in vivo are surrounded by a matrix comprised predominantly of type IV Collagen, laminin, heparan sulphate proteoglycan, and entactin [17, 34]. The enteric plexus lacks large connective tissue spaces for blood vessels like the peripheral nervous system. The studies in this paper sought to address the hypothesis that a two-dimensional culture substratum will modulate neuronal and glial differentiation solely based on ECM composition. In this study, glass coverslips were coated with combinations of three different ECM components that enteric glia and neurons come in to contact with in vivo in the adult myenteric plexus: Collagen IV, Laminin and Heparan sulfate [17]. Collagen I was evaluated additionally, because of its extensive use in neural tissue engineering of the peripheral nervous system [35, 36]. Poly-lysine (pLL) coated coverslips were used a control because a coating was a prerequisite to cell adhesion and subsequent differentiation.
Neurospheres and neuronal progenitor cells attached to the pLL coated coverslips, and stayed attached at day 5, but did not initiate neuronal differentiation. However, glial differentiation was readily visible by day 5, and improved by day 15 (Figure 3A,E; Figure 6A,E). Enteric neurospheres on pLL substrates indicated a clear preference towards glial differentiation versus neuronal differentiation. It is likely that progenitor cells or neurons were only weakly attached to pLL in the absence of cellular adhesion recognition sequences/molecules, as reported previously for hippocampal neurons and dorsal root ganglion neurons [37, 38]. The severely reduced neurite outgrowth could have also been caused by losing undifferentiated progenitor cells during medium supplementation or immunohistochemical staining.
Addition of laminin to collagen substrates improved neurite outgrowth with longer neurite lengths (compare 156.1 ± 7.2 μm to 215.1 ± 7.6 μm). Laminin has been long known to stimulate neural cell attachment and neurite outgrowth [18]. Indeed, migratory neural crest cells have been shown to acquire a neurally-related laminin receptor upon entering the gut mesenchyme, that facilitates differentiation [39]. Our studies showed that while there was an overall enhancement in neuronal differentiation as well as neurite outgrowth, there was no significant difference between the additions of 5 or 10 μg/cm2 of laminin. This empirical determination was important in determining a minimal amount of laminin that can influence neuroglial differentiation without affecting neurite outgrowth adversely in a situation that requires neo-innervation of denervated tissues.
Addition of heparan sulfate to composite collagen mixtures improved neuronal differentiation as well. Neuronal networking and neuronal clustering was visible at the later time point. Heparan sulfate and its interaction with GDNF and other neurotrophic factors stabilizes and makes these factors locally available, possibly modulating neurite outgrowth and neuronal differentiation [19, 20]. Heparan sulfate interacts with both Collagen IV and with Laminin, to positively modulate neuronal differentiation, evidenced by the enhanced neurite lengths and initiation of neuronal networking (Figure 4A–H). Mammadov et al. recently demonstrated that self-assembled peptide nanofibers with heparan sulphate and laminin mimetic sequences promoted neurite outgrowth of a PC12 cell line [20]. Composite collagen substrates with laminin and/or heparan sulfate all maintained a low level of GFAP positive glial cells, with initiation of astrocytic networking becoming more obvious at the later time point. In general, substrates that supported neuronal differentiation demonstrated a bare minimum of glial cells required to possibly support neuronal cell phenotype or survival. Several studies in literature suggest that the presence of several axolemmal fragments can arrest the proliferation of glia [40]. This is consistent with our observations of low levels of GFAP immunofluorescence observed on substrates that supported extensive neuronal differentiation. The only substrates that supported extensive differentiation of enteric neuroglial progenitor cells into glia were pLL and individual coatings of Collagen I/IV. Neuronal differentiation was present on these substrates, but not as extensively as any of the other composite coatings that included laminin and heparan sulfate.
5. Conclusions
Individually, all evaluated culture substrates in these studies supported neuronal and glial differentiation to varying degrees by day 15. Enteric neurospheres demonstrated a clear tendency to differentiate into glia on pLL coated substrates as well as on composite collagen substrates in the absence of laminin and heparan sulfate. In contrast, culture substrates with laminin and heparan sulfate promoted extensive neuronal differentiation while simultaneously supporting only a minimal glial cell population. Laminin and collagen IV coated coverslips positively modulated neuronal differentiation by increased number of neurites per neuron and longer neurite lengths compared to fibrillar Collagen I. These results identify suitable 3D matrix compositions to deliver neuronal progenitor cells. Future studies will focus on evaluating neuroglial differentiation within 3D matrices with variable ECM composition, with an aim to tissue engineer intrinsically innervated sheets of smooth muscle suitable for transplantation. Three dimensional hydrogel environments also provide the mechanical cues for neural differentiation, more readily translatable to in vivo conditions than infinitely stiff glass substrates.
Acknowledgments
This work was supported by NIH/NIDDK research grants RO1DK071614 and RO1DK042876.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hansen MB. The enteric nervous system I: organisation and classification. Pharmacol Toxicol. 2003;92:105–13. doi: 10.1034/j.1600-0773.2003.t01-1-920301.x. [DOI] [PubMed] [Google Scholar]
- 2.Arshad A, Powell C, Tighe MP. Hirschsprung’s disease. BMJ. 2012;345:e5521. doi: 10.1136/bmj.e5521. [DOI] [PubMed] [Google Scholar]
- 3.De Giorgio R, Stanghellini V, Barbara G, Corinaldesi R, De Ponti F, Tonini M, et al. Primary enteric neuropathies underlying gastrointestinal motor dysfunction. Scand J Gastroenterol. 2000;35:114–22. doi: 10.1080/003655200750024263. [DOI] [PubMed] [Google Scholar]
- 4.De Giorgio R, Guerrini S, Barbara G, Stanghellini V, De Ponti F, Corinaldesi R, et al. Inflammatory neuropathies of the enteric nervous system. Gastroenterology. 2004;126:1872–83. doi: 10.1053/j.gastro.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Metzger M, Bareiss PM, Danker T, Wagner S, Hennenlotter J, Guenther E, et al. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology. 2009;137:2063–73. e4. doi: 10.1053/j.gastro.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56:489–96. doi: 10.1136/gut.2006.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natarajan D, Grigoriou M, Marcos-Gutierrez CV, Atkins C, Pachnis V. Multipotential progenitors of the mammalian enteric nervous system capable of colonising aganglionic bowel in organ culture. Development. 1999;126:157–68. doi: 10.1242/dev.126.1.157. [DOI] [PubMed] [Google Scholar]
- 8.Heanue TA, Pachnis V. Prospective identification and isolation of enteric nervous system progenitors using Sox2. Stem Cells. 2011;29:128–40. doi: 10.1002/stem.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauch U, Hansgen A, Hagl C, Holland-Cunz S, Schafer KH. Isolation and cultivation of neuronal precursor cells from the developing human enteric nervous system as a tool for cell therapy in dysganglionosis. Int J Colorectal Dis. 2006;21:554–9. doi: 10.1007/s00384-005-0051-z. [DOI] [PubMed] [Google Scholar]
- 10.Hanani M, Ledder O, Yutkin V, Abu-Dalu R, Huang TY, Hartig W, et al. Regeneration of myenteric plexus in the mouse colon after experimental denervation with benzalkonium chloride. J Comp Neurol. 2003;462:315–27. doi: 10.1002/cne.10721. [DOI] [PubMed] [Google Scholar]
- 11.Raghavan S, Gilmont RR, Miyasaka EA, Somara S, Srinivasan S, Teitelbaum DH, et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology. 2011;141:310–9. doi: 10.1053/j.gastro.2011.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schäfer Kh, Micci Ma, Pasricha PJ. Neural stem cell transplantation in the enteric nervous system: roadmaps and roadblocks. Neurogastroenterology & Motility. 2009;21:103–12. doi: 10.1111/j.1365-2982.2008.01257.x. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni S, Becker L, Pasricha PJ. Stem cell transplantation in neurodegenerative disorders of the gastrointestinal tract: future or fiction? Gut. 2012;61:613–21. doi: 10.1136/gut.2010.235614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 15.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabella G. Innervation of the intestinal muscular coat. J Neurocytol. 1972;1:341–62. doi: 10.1007/BF01102939. [DOI] [PubMed] [Google Scholar]
- 17.Bannerman PG, Mirsky R, Jessen KR, Timpl R, Duance VC. Light microscopic immunolocalization of laminin, type IV collagen, nidogen, heparan sulphate proteoglycan and fibronectin in the enteric nervous system of rat and guinea pig. J Neurocytol. 1986;15:733–43. doi: 10.1007/BF01625191. [DOI] [PubMed] [Google Scholar]
- 18.Martin GR, Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- 19.Barnett MW, Fisher CE, Perona-Wright G, Davies JA. Signalling by glial cell line-derived neurotrophic factor (GDNF) requires heparan sulphate glycosaminoglycan. J Cell Sci. 2002;115:4495–503. doi: 10.1242/jcs.00114. [DOI] [PubMed] [Google Scholar]
- 20.Mammadov B, Mammadov R, Guler MO, Tekinay AB. Cooperative effect of heparan sulfate and laminin mimetic peptide nanofibers on the promotion of neurite outgrowth. Acta Biomaterialia. 2012;8:2077–86. doi: 10.1016/j.actbio.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somara S, Bitar KN. Phosphorylated HSP27 modulates the association of phosphorylated caldesmon with tropomyosin in colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2006;291:G630–9. doi: 10.1152/ajpgi.00350.2005. [DOI] [PubMed] [Google Scholar]
- 23.Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130:6387–400. doi: 10.1242/dev.00857. [DOI] [PubMed] [Google Scholar]
- 24.Suarez-Rodriguez R, Belkind-Gerson J. Cultured nestin-positive cells from postnatal mouse small bowel differentiate ex vivo into neurons, glia, and smooth muscle. Stem Cells. 2004;22:1373–85. doi: 10.1634/stemcells.2003-0049. [DOI] [PubMed] [Google Scholar]
- 25.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–69. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belkind-Gerson J, Carreon-Rodriguez A, Benedict LA, Steiger C, Pieretti A, Nagy N, et al. Nestin-expressing cells in the gut give rise to enteric neurons and glial cells. Neurogastroenterol Motil. 2012 doi: 10.1111/nmo.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva AT, Wardhaugh T, Dolatshad NF, Jones S, Saffrey MJ. Neural progenitors from isolated postnatal rat myenteric ganglia: expansion as neurospheres and differentiation in vitro. Brain Res. 2008;1218:47–53. doi: 10.1016/j.brainres.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 28.Becker L, Kulkarni S, Tiwari G, Micci MA, Pasricha PJ. Divergent fate and origin of neurosphere-like bodies from different layers of the gut. Am J Physiol Gastrointest Liver Physiol. 2012;302:G958–65. doi: 10.1152/ajpgi.00511.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson DJ, Copp AJ. Role of the extracellular matrix in neural crest cell migration. J Anat. 1997;191 ( Pt 4):507–15. doi: 10.1046/j.1469-7580.1997.19140507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young HM, Anderson RB, Anderson CR. Guidance cues involved in the development of the peripheral autonomic nervous system. Auton Neurosci. 2004;112:1–14. doi: 10.1016/j.autneu.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Parikh DH, Leibl M, Tam PK, Edgar D. Abnormal expression and distribution of nidogen in Hirschsprung’s disease. J Pediatr Surg. 1995;30:1687–93. doi: 10.1016/0022-3468(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 32.Gershon MD. Transplanting the enteric nervous system: a step closer to treatment for aganglionosis. Gut. 2007;56:459–61. doi: 10.1136/gut.2006.107748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schafer KH, Hagl CI, Rauch U. Differentiation of neurospheres from the enteric nervous system. Pediatr Surg Int. 2003;19:340–4. doi: 10.1007/s00383-003-1007-4. [DOI] [PubMed] [Google Scholar]
- 34.Rauch U, Schafer KH. The extracellular matrix and its role in cell migration and development of the enteric nervous system. Eur J Pediatr Surg. 2003;13:158–62. doi: 10.1055/s-2003-41265. [DOI] [PubMed] [Google Scholar]
- 35.Stang F, Fansa H, Wolf G, Reppin M, Keilhoff G. Structural parameters of collagen nerve grafts influence peripheral nerve regeneration. Biomaterials. 2005;26:3083–91. doi: 10.1016/j.biomaterials.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 36.Dewitt DD, Kaszuba SN, Thompson DM, Stegemann JP. Collagen I-matrigel scaffolds for enhanced Schwann cell survival and control of three-dimensional cell morphology. Tissue Eng Part A. 2009;15:2785–93. doi: 10.1089/ten.TEA.2008.0406. [DOI] [PubMed] [Google Scholar]
- 37.Liu B, Ma J, Gao E, He Y, Cui F, Xu Q. Development of an artificial neuronal network with post-mitotic rat fetal hippocampal cells by polyethylenimine. Biosens Bioelectron. 2008;23:1221–8. doi: 10.1016/j.bios.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Orr DJ, Smith RA. Neuronal maintenance and neurite extension of adult mouse neurones in non-neuronal cell-reduced cultures is dependent on substratum coating. J Cell Sci. 1988;91 ( Pt 4):555–61. doi: 10.1242/jcs.91.4.555. [DOI] [PubMed] [Google Scholar]
- 39.Pomeranz HD, Sherman DL, Smalheiser NR, Tennyson VM, Gershon MD. Expression of a neurally related laminin binding protein by neural crest-derived cells that colonize the gut: relationship to the formation of enteric ganglia. J Comp Neurol. 1991;313:625–42. doi: 10.1002/cne.903130408. [DOI] [PubMed] [Google Scholar]
- 40.Eccleston PA, Bannerman PG, Pleasure DE, Winter J, Mirsky R, Jessen KR. Control of peripheral glial cell proliferation: enteric neurons exert an inhibitory influence on Schwann cell and enteric glial cell DNA synthesis in culture. Development. 1989;107:107–12. doi: 10.1242/dev.107.1.107. [DOI] [PubMed] [Google Scholar]