Abstract
Detection methods are necessary to quantify fullerenes in commercial applications to provide potential exposure levels for future risk assessments of fullerene technologies. The fullerene concentrations of five cosmetic products were evaluated using liquid chromatography with mass spectrometry to separate and specifically detect C60 and C70 from interfering cosmetic substances (e.g., castor oil). A cosmetic formulation was characterized with transmission electron microscopy, which confirmed that polyvinylpyrrolidone encapsulated C60. Liquid-liquid extraction of fullerenes from control samples approached 100% while solid-phase and sonication in toluene extractions yielded recoveries of 27–42%. C60 was detected in four commercial cosmetics ranging from 0.04 to 1.1 μg/g, and C70 was qualitatively detected in two samples. A single-use quantity of cosmetic (0.5 g) may contain up to 0.6 μg of C60, demonstrating a pathway for human exposure. Steady-state modeling of fullerene adsorption to biosolids is used to discuss potential environmental releases from wastewater treatment systems.
Keywords: Fullerenes, Cosmetics, Environmental transport, Nanomaterials, Liquid chromatography-mass spectroscopy, Radical Sponge®, Polyvinylpyrrolidone (PVP)
1. Introduction
Manufacturers are incorporating nanosized carbon particles, including fullerenes (e.g., C60 and C70), into consumer products such as cosmetics for their suggested “anti-aging” or radical scavenging properties (Halford, 2006; Woodrow Wilson International Center for Scholars, 2009; Xiao et al., 2006). Despite this commercial use, research has shown that fullerenes can cause adverse biological effects (Johansen et al., 2008; Kai et al., 2003; Miyata et al., 2000; Tsuchiya et al., 1996; Usenko et al., 2008), and uncertainty remains in regards to the environmental and human health effects of these molecules (Spohn et al., 2009). Fullerenes in cosmetics will increase human exposure through product use, and like other chemicals found in personal care products, can potentially be released into the environment after being rinsed “down the drain” (Benotti et al., 2009). In fact, a probabilistic study has predicted an increase in the fullerene concentration of sewage treatment plant effluent between 4 and 33 ng/L as a result of their use in new technologies (Gottschalk et al., 2009). The use of fullerenes in cosmetics is not trivial, as consumer products can be a means to introduce nanotechnologies to the public, who in turn make decisions whether or not to accept new technologies and to support nanotechnology development. An adequate evaluation of the environmental and human health impacts of nanoparticles (e.g., C60) in consumer products would be useful information for decision-making, and could therefore help prevent public distrust (Halford, 2006) and the slow economic development of nanotechnology (National Science and Technology Council, 2006). Such evaluations must use validated analytical techniques for fullerene detection in complex matrices like cosmetics.
Although some patent applications for the use of fullerenes in cosmetic formulations report fullerene contents of up to 9% by weight (Burangulov et al., 2005; Ito and Matsubayashi, 2006; Ziolo, 1993), the fullerene contents of off-the-shelf products need to be quantified. The “fullerenes” listed in cosmetic ingredient lists could encompass C60, C70, higher-order fullerenes, fullerene derivatives (i.e., C60-PVP), and fullerene-based molecules (i.e., C60O, C60O2, etc.), each of which might require separate and specific detection and quantification. Detection of fullerenes in cosmetics will indicate a possible environmental emission source of engineered fullerenes. One recent study has reported the occurrence of μg/L concentrations of fullerenes associated with the suspended solids of treated wastewater effluents (Farré et al., 2010). Although a correlation between increased effluent fullerene concentration and highly populated and industrialized areas was suggested, the source of these fullerenes was not determined. Detection of fullerenes in cosmetics would confirm commercial applications as fullerene emission sources and support the hypothesis that wastewater systems provide an environmental exposure route for these engineered nanomaterials.
Fullerenes can be modified in many ways to promote dispersion in water and subsequent integration into cosmetic matrices. In a formulation called Radical Sponge®, fullerenes are complexed with polyvinylpyrrolidone (PVP) (Xiao et al., 2007, 2006, 2005). It is hypothesized that PVP disperses C60 in water by forming a non-covalently bound C60-PVP complex or by encapsulation of C60 in the hydrophobic core of a PVP micelle. Previous work indicates that pure C60 can be qualitatively recovered from the Radical Sponge® aqueous formulation using a salt or non-polar solvent (toluene) (Yamakoshi et al., 1994). Quantifying recovery of C60 from this formulation will validate the potential detection of fullerenes in cosmetics.
High-performance liquid chromatography (HPLC) techniques have been used to detect fullerenes in various matrices (Becker et al., 1994; Benn et al., 2010; Chen et al., 2008; Fortner et al., 2005; Heymann et al., 1995; Santa et al., 1995) and are extensively reviewed elsewhere (Isaacson et al., 2009; Pycke et al., 2011). Briefly, HPLC coupled to mass spectrometry (LC-MS) has been used to quantify C60 in aqueous samples at environmentally relevant, ng/L concentrations (Chen et al., 2008). Additionally, MS can be used to distinguish between fullerene oxidation products (Lebedkin et al., 1995; Smith et al., 1995). Solvent extraction has been employed to recover fullerenes from complex geologic matrices (Heymann et al., 1995, 1994), although quantitative recovery has proven difficult (Jehlicka et al., 2005). Solvent extraction and LC-MS analysis have yet to be applied to detection of C60 in consumer products such as cosmetic matrices.
The goal of this research is to apply common extraction approaches and analytical techniques towards separating and detecting C60 in cosmetic products. First, the recovery of fullerenes from cosmetic matrices is investigated using three common extraction methods (liquid-liquid, toluene-sonication, and solid phase extraction). Second, the recovery of C60 from a common cosmetic fullerene formula (C60-PVP) is quantified using liquid-liquid (LLE) and solid phase (SPE) extractions. Third, LC-MS chromatograms are analyzed to confirm the detection of fullerenes in cosmetics as well as to identify possible fullerene-based molecules. Next, five commercial cosmetic samples are subjected to the extraction procedures to quantify C60 and qualitatively detect C70. Finally, a steady-state mass balance is used to model the fate of fullerenes released into a wastewater treatment plant (WWTP).
2. Methods and materials
2.1. Cosmetic and fullerene sources
Five cosmetics from four manufacturers were purchased based on labeling that indicated that the products contained “fullerenes” or “Radical Sponge®” (Table 1). The matrices of the cosmetics varied considerably: two were watery serums (medium water content), two were dense creams (low water content), and one was a water-based lotion (high water content). A sunscreen (SkinCeuticals, Garland, TX) that did not identify the use of fullerenes was used as a control to represent a common cosmetic cream matrix. A common cosmetic ingredient, castor oil (USP 100%, CVS Pharmacy, Inc., Woonsocket, RI), was tested for interference with fullerene detection at specific mass-to-charge ratios.
Table 1.
Cosmetic sample characterization.
| Sample ID | Commercial name | Intended use | Fullerene ingredient | Matrix appearance |
|---|---|---|---|---|
| 1 | Dr. Wu Whitening System | Antioxidant whitening face serum | “Radical Sponge®” | Clear, water-based serum |
| 2 | Jeudisurpris Essence | Clinical lift face serum | “Radical Sponge®” | Clear, water-based serum |
| 3 | Derma Science DP EST Charge Plus | Face cream | “Radical Sponge®” | Dense, opaque cream |
| 4 | Dr. Brandt Laser Lightning Day Lotion | Anti-aging face cream | “fullerene” | Dense, opaque cream |
| 5 | Dr. Brandt Laser Lightning Toner | Anti-aging skin toner | “fullerene” | Water-based lotion |
C60 and C70 (99% purity) were purchased from MER Corp. (Tucson, AZ). Standard fullerene solutions for LC-MS calibration and spike/recovery experiments were prepared by adding 13 mg of fullerene powder to 100 mL of HPLC-grade toluene (Mallinckrodt, Phillipsburg, NJ) and sonicating in a water bath (Branson Model 2510, Danbury, CT) for 15 min.
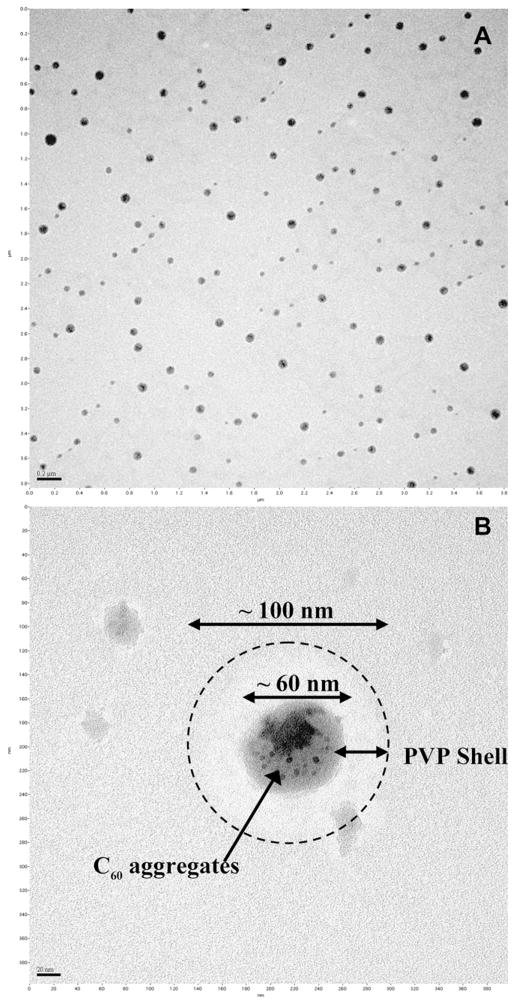
The C60-PVP suspension was prepared in nanopure water as described elsewhere (Xiao et al., 2006). Five mL of concentrated C60-toluene solution (920 mg/L) was mixed with 10 mL of 50,000 mg/L PVP solution (K30, Sigma Aldrich, St. Louis, MO) in chloroform (Mallinckrodt, Phillipsburg, NJ), and the solvents were evaporated under purified nitrogen. The residual solid was sonicated in 10 mL nanopure water (Millipore, Billerica, MA), and particulates were filtered (GF/F, Whatman, Kent, UK). A carbon-coated, copper TEM grid was immersed in the C60-PVP solution and air-dried in preparation for imaging using a JEOL 1200EX electron microscope (JEOL Ltd., Tokyo, Japan). TEM images of the C60-PVP solution indicate a dominant presence of spherical agglomerates around 100 nm in size (Fig. 1A) in which C60 appears to be wrapped in PVP (Fig. 1B). These agglomerates are similar to those found by SEM (Xiao et al., 2006).
Fig. 1.
A) TEM images of spherical C60-PVP agglomerations 50–100 nm in diameter (scale bar = 0.2 μm). B) Higher magnification shows that fullerenes (dark particles) appear to be wrapped within a PVP structure (scale bar = 20 nm).
2.2. Extraction methods
Fullerenes were extracted from cosmetics using three methods. 1) Liquid–liquid extraction was performed patterned after previous research (Xia et al., 2006) by sonicating 0.5–1.5 g of the cosmetic in 5 mL of nanopure water followed by addition of 2 mL of 100 mM Mg(ClO4)2 (Fisher, Fair Lawn, NJ) and 11.3 mL of glacial acetic acid (GAA) to control emulsions. Five mL of toluene were added, and the two-phase solution was mixed on an orbital shaker table (New Brunswick Scientific, Edison, NJ) at 35 RPM for at least 1 h. The two-phase solution was allowed to separate for at least 20 min. The toluene phase was sampled (1 mL) and evaporated under purified (Ultrapure) nitrogen to dryness to remove GAA, which interferes with mass spectrometric detection using atmospheric pressure chemical ionization (APCI). The sample was reconstituted in 1 mL of toluene and briefly sonicated (3–5 min) to dissolve fullerenes. 2) The toluene-sonication extraction method was performed by sonicating 0.5–1.5 g of cosmetic in 10 mL of toluene for at least 4 h. The toluene extract was centrifuged at F = 12,000 G (Thermo IEC, Waltham, MA) for 10 min and filtered (Qualitative 2 or GF/F, Whatman, Kent, UK) to remove particulates. Control experiments showed negligible removal of fullerenes from pure toluene during filtration and centrifugation. The toluene filtrate was then evaporated to a measured volume less than 2 mL prior to LC-MS quantification. 3) SPE was performed after sonicating 0.5–1.5 g of cosmetic in nanopure water for at least 4 h. Highly particulate samples were filtered (GF/F) prior to SPE. The aqueous solutions were passed through SPE cartridges using a vacuum pump (Buchi Labortechnik, Flawil, Switzerland) at approximately 1 mL/min. Strata C-18 E SPE cartridges (Phenomenex, Torrance, CA) were conditioned with 5 mL of HPLC-grade methanol (Fisher, Fair Lawn, NJ) followed by 5 mL of nanopure water. The sample was loaded and the column allowed to air-dry prior to washing with 5 mL of methanol. Samples were eluted with 10 mL of toluene and evaporated to a measured volume less than 2 mL prior to LC-MS quantification.
2.3. Quantification of fullerenes
Fullerenes (C60 and C70) were quantified in the toluene extracts using LC-MS with APCI. The method is a variation of a procedure reported elsewhere (Chen et al., 2008). An LC-MS system was used consisting of 2 Varian Prostar 210 pumps coupled to a Varian 1200 triple quadrupole mass spectrometer (Varian, Inc., Palo Alto, CA). Samples were eluted isocratically using toluene and acetonitrile at a ratio of 55/45 on a Nova-Pak C-18 column (3.9 × 150 mm) (Waters, Milford, MA) with a constant flow rate of 1 mL/min. Using a 200 μL sample loop, 50 μL of sample was loaded followed by 75 μL of acetonitrile to bring the sample matrix close to the eluent mixture and facilitate a resolved C60 peak. The temperatures for the APCI housing, N2 drying gas, and APCI torch were set at 50 °C, 200 °C, and 350 °C, respectively. Shield voltage was set at −200 V, and the mass detector was operated at a fixed voltage of −1200 V. Negatively-charged m/z 720 and 840 were used for the identification and quantification of C60 and C70, respectively, with a scan width of 0.70 amu and scan time of 0.3 s. Calibration was achieved by using dilutions from stock solutions of solid C60 and C70 dissolved in toluene. Fig. 2 shows representative calibration curves for C60 and C70, which elute at 4 and 6 min, respectively. Instrument detection limits based on 3× the background signal were 3 and 12 μg/L for C60 and C70 in toluene, respectively.
Fig. 2.
LC-MS calibration curves for C60 and C70 standards in toluene. Instrument detection limits based on 3× the background signal are 3 ppb and 12 ppb for C60 and C70, respectively. Error bars represent three analyses of each standard. Inset: C60 (−720 m/z) and C70 (−840 m/z) elution peaks at 4.0 and 6.0 min., respectively.
Standard addition was used to determine any cosmetic matrix interference on LC-MS detection using fullerene-toluene solutions. C60 was introduced at 55 μg/L into the toluene extractions (LLE and SPE) of cosmetic samples. The percentage decrease from the expected signal was compensated for in the spike/recovery of C70 and the quantification of C60 in cosmetic samples.
2.4. Spike/recovery experiments
Spike-recovery experiments were used to evaluate the efficiency of three methods for extracting fullerenes. Cosmetic samples #2 and #4 were chosen as representative serum and cream matrices, respectively. Because these sample matrices were expected to contain C60, C70 was chosen as a surrogate to spike fullerene quantities relevant to the C60 concentration of the cosmetics. C70 (4.2 μg) was administered in toluene (1 mL) to the serum (~0.6 g of cosmetic #2) and the cream (~1.1 g of cosmetic #4), the toluene was evaporated, and C70 was recovered using SPE, LLE, and toluene-sonication extraction methods. The spiked quantity (4–7 μg-C70/g-cosmetic) was within one order of magnitude of the C60 detected in the cosmetic samples (see Results).
It has been hypothesized that allowing fullerene-toluene solutions to evaporate to dryness may inhibit recovery in trace analysis owing to adsorption to glassware (Perez et al., 2009; Xia et al., 2006). To test this possibility, triplicate samples of the C70 spike alone were evaporated in a glass vial under N2 and subsequently recovered in toluene with sonication. Nearly complete (94 ±14%) recovery of the spike (4.1 μg-C70) suggests that adsorption of C70 to glassware for this spiked concentration is not inhibiting recovery in these experiments.
2.5. Modeling fullerene transport within a WWTP
A steady-state mass balance with non-linear adsorption can be applied to model the transport of nanomaterials in a WWTP (Benn and Westerhoff, 2008) as,
| (1) |
where C is the effluent concentration of fullerenes, C0 is the influent fullerene concentration, and K and 1/n are the Freundlich adsorption parameters. Variables X, τ, and θ, are operational parameters of a WWTP (mixed liquor suspended solids, hydraulic and solids retention time, respectively), and were set as 2000 mg/L, 720 min, and 7200 min, respectively, for a one million gallon per day (MGD) WWTP. Freundlich adsorption parameters were estimated from previous research where the adsorptive removal of n-C60 (3 mg/L) and C60-PVP (2 mg/L) with 50 mg/L of biosolids was 36 and 6%, respectively (Kiser et al., 2010). From prior work 1/n was set at 0.5 while K was estimated for C60-PVP as 1/6th (6%/36%, K = 0.17 (μg-C60/g-biomass) (L/μg- C60)1/n) of the relative capacity of n-C60 which has an experimental K value of 1 (μg-C60/g-biomass)(L/μg- C60)1/n.
3. Results
3.1. Evaluating extraction method efficiencies
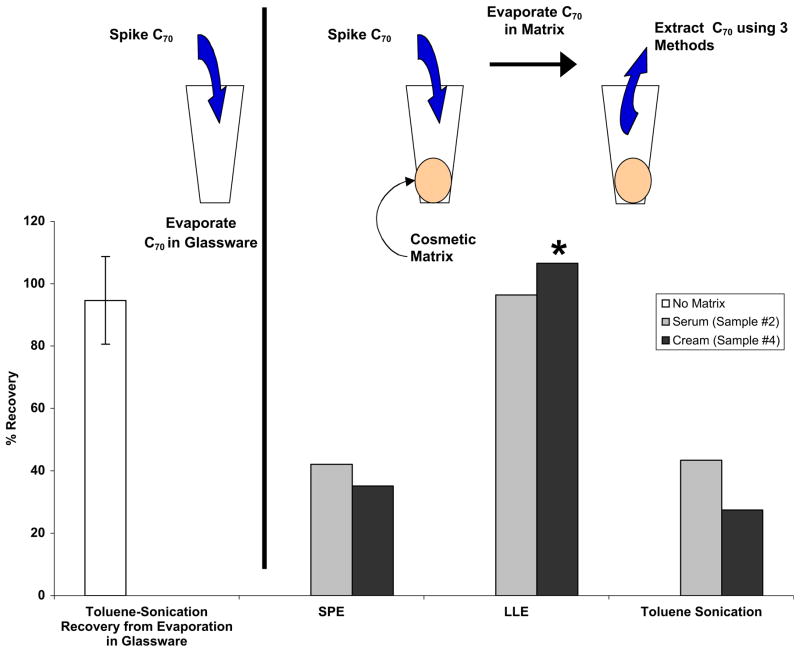
C70 was spiked into a serum and a cream cosmetic matrix and recovered using SPE, LLE, and sonication in toluene (Fig. 3). LLE was used to recover 96 and 107% of the C70 from the serum and cream, respectively. SPE and sonication in toluene achieved much lower fullerene recovery efficiencies than LLE, ranging from 27 to 42%. These lower recoveries could be due to inefficient extraction from the cosmetic or matrix interferences on LC-MS detection.
Fig. 3.
Comparison of three extraction methods to recover C70 (4–7 μg-C70/g-cosmetic) spiked into two types of cosmetic matrices (serum and cream). C70 was administered in toluene, allowed to deposit in the matrix, then extracted. The first column represents triplicate recoveries of C70 allowed to deposit in a glass vial with no matrix. * Recovery adjusted for 47% matrix inhibition of LC-MS detection of fullerenes.
Fullerene standard addition was applied to determine any matrix interference on LC-MS detection. The LLE of the cream (sample #4) caused a 47% reduction in fullerene detection signal. When this interference was accounted for, recovery of the C70 spike was 107%. In contrast to the cream matrix, no LC-MS interference was observed for the LLE of the serum, indicating the role that specific components in cosmetic formulations play in suppressing the LC-MS detection of fullerenes under these experimental conditions. No matrix interferences on LC-MS detection were observed for the SPEs of the serum or cream. SPE produced a toluene extract that did not interfere with LC-MS detection unlike LLE, but SPE was less efficient than LLE at recovering fullerenes.
It is important to note that this spike/recovery experiment was designed only to compare extraction methods and identify matrix interferences, not to quantify actual fullerene recovery from cosmetics. Evaporating fullerenes into a cosmetic matrix from an organic solvent does not necessarily represent how fullerenes are incorporated into commercial cosmetics. For example, fullerenes are often added to cosmetics with a dispersant (i.e., PVP), which may influence fullerene recovery by these methods. Therefore, the recovery of C60 from an aqueous C60-PVP formulation using LLE and SPE was assessed.
3.2. Recovery of C60 from C60-PVP with LLE and SPE
Since three of the cosmetic samples (1, 2 and 3) contained fullerenes in the Radical Sponge® formulation (Xiao et al., 2007, 2006, 2005), the recovery of C60 from a C60-PVP formula prepared in-house was investigated. C60 was recovered in triplicate from 5 mL of a stock solution of C60-PVP. The concentration of C60 in the C60-PVP stock solution as determined by LLE and SPE was 800 ± 30 and 860 ± 20 μg/L, respectively. When C60-PVP was exposed to a cosmetic cream sample not advertised to contain fullerenes (sunscreen) C60 recovery was 50 ± 13% and 70 ± 13% for LLE and SPE, respectively. The presence of cosmetic cream impairs the recovery of fullerenes compared to extraction of C60-PVP in water.
The cosmetic matrix can interfere with the extraction and/or the detection of fullerenes via LC-MS. Standard addition was conducted to investigate the interference in LC-MS detection. Toluene solutions from LLE and SPE of blank sunscreen samples (i.e., containing no C60-PVP) were spiked with 110 μg/L of a C60-toluene standard. The detection of this standard in the LLE and SPE toluene solution was impaired by 28 and 33%, respectively. Accounting for these interferences, recoveries of C60 from C60-PVP spiked into the sunscreen using LLE and SPE could be as high as 78 ± 13% and 103 ± 13%, respectively.
These results suggest that SPE is more efficient than LLE at recovering C60 from the C60-PVP formula in contrast to the recovery of C70 spiked into cosmetics. Therefore, multiple extraction methods were used to quantify C60 in commercial cosmetics where the matrices vary.
3.3. Detection of C60 extracted from cosmetic formulations
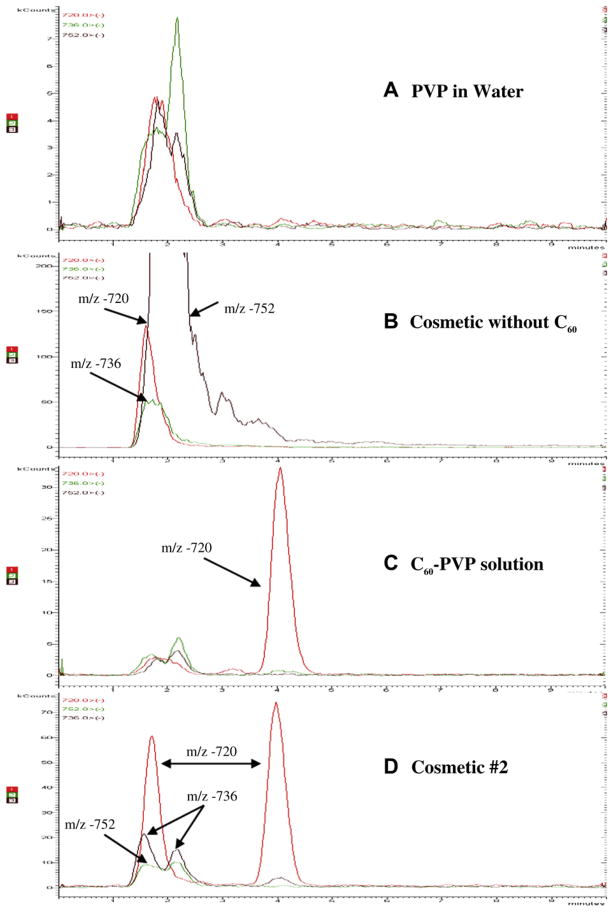
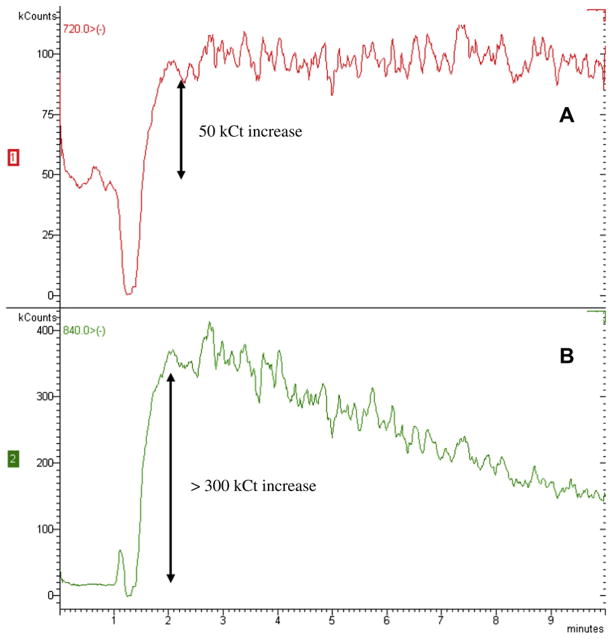
Fig. 4 shows the chromatograms from SPEs of A) aqueous PVP, B) a cosmetic cream (sunscreen) without C60, C) aqueous C60-PVP, and D) cosmetic #2 with Radical Sponge®. SPE of aqueous PVP results in a low LC-MS response between 1 and 2.5 min retention times for −720 m/z (Fig. 4A), but chromatography efficiently separates these materials from times at which C60 or C70 elute (4 and 6 min., respectively). This small signal (<8 kCts) is considered background noise from SPE and is not attributed to fullerenes. SPE of a cosmetic matrix without C60 (sunscreen) does not yield a −720 m/z peak at 4 min (Fig. 4B), indicating that these methods yield no false-positive C60 detection. However, significant responses occur at m/z ratios −720, −736, and −752 between 1 and 4 min, which inhibits the identification of fullerene-based compounds such as C60O and C60O2 in fullerene-containing cosmetics. Castor oil, a common triglyceride ingredient in other cosmetic samples, was found to increase background signal at fullerene mass-to-charge ratios −720 and −840 (Fig. 5). While these background signals from cosmetic matrices inhibit the identification of fullerene-based compounds that would be retained in the column for less than 4 min, they do not interfere with quantification of pure C60. The chromatogram of a SPE of aqueous C60-PVP (Fig. 4C) shows insignificant background noise at 1–2.5 min similar to Fig. 4A, and C60 is detected at 4 min. This indicates that these methods can recover and identify fullerenes from cosmetics containing the Radical Sponge® formulation. Finally, the peak at −720 m/z in the chromatogram from SPE of cosmetic #2, reported to contain the Radical Sponge® formulation, demonstrates the extraction of pure C60 (Fig. 4D). The chromatogram also shows peaks for −720, −736, and −752 m/z between 1 and 2.5 min, which are potentially explained by 1) background interferences in the cosmetic matrix, 2) fullerene-epoxide molecules, or 3) functionalized/derivatized C60 molecules. More research using standard solutions of fullerene-based molecules would be necessary to investigate any chemical change to the fullerenes used in cosmetics.
Fig. 4.
Chromatograms of m/z −720, −736, and −752 for solid phase extractions of A) PVP aqueous solution, B) a cosmetic (sunscreen) without C60, C) C60-PVP aqueous solution, and D) cosmetic #2 with Radical Sponge®. Elution time is plotted on the x-axis up to 10 min. The y-axis (MS signal in kilocounts) maximum is 8, 200, 35, and 75 for A, B, C, and D, respectively.
Fig. 5.
Chromatograms showing the increase in background signal at A) −720 (50 kCt) and B) −840 m/z (>300 kCt) due to toluene sample containing 1% castor oil.
3.4. Quantification of C60 extracted from cosmetics
C60 was detected in four out of five cosmetic samples using LLE, toluene-sonication, and SPE (Table 2). Fullerene concentrations in the cosmetics ranged from 0.04 to 1.1 ppm (μg-C60/g-product). Standard additions of C60 were performed on LLE and SPE samples. No matrix interference on detection was observed for LLE of cosmetics 1, 2, and 3. No matrix interference was observed for SPE of cosmetics 2 and 4. SPE resulted in the highest detection of fullerenes for samples 2 and 4, while LLE yielded the highest detection for sample 1 and 3. Neither LLE nor SPE yielded the highest recoveries for both serum and cream matrices. This suggests that varied cosmetic compositions (serum versus cream) and/or cosmetic ingredients require specific extraction techniques to achieve the highest fullerene recoveries.
Table 2.
Mass of C60 detected in cosmetics [μg-C60/g-product].
| Sample ID | Matrix | LLE | Toluene sonication | SPE |
|---|---|---|---|---|
| 1 | Serum | 1.0 ± 0.3a | 0.40 | 0.42a |
| 2 | Serum | ND | 0.04 | 0.24a |
| 3 | Cream | 1.1 ± 0.2 | 0.48 | 0.04 |
| 4 | Cream | ND | ND | 0.04 |
| 5 | Water | ND | NA | ND |
ND – No Detection.
NA – Not Analyzed.
C70 also qualitatively detected at a retention time of 6 min but was below quantification limits.
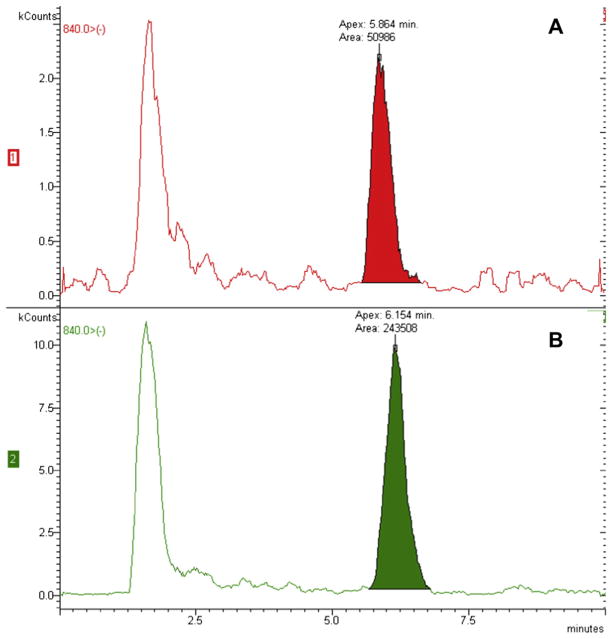
C60 was not detected in the water-based lotion (sample 5). The fullerenes of this cosmetic may be functionalized differently (i.e., not with PVP) such that recovery of the C60 molecule by these extraction/detection methods is inefficient. C70 was also detected in samples 1 and 2 (Fig. 6) but was below quantification limits (<70 ng-C70/g-cosmetic). These small quantities of C70 could be impurities from the manufacturing process of C60 (Taylor et al., 1990).
Fig. 6.
Chromatograms of A) cosmetic #2 and B) cosmetic #1 showing qualitative detection of C70 at ~6 min for m/z −840.
3.5. Modeling environmental releases through WWTP
The experimental results confirm that cosmetics 1–4 contain fullerenes (sample 5 remains unconfirmed) detectable as C60. These products are intended for skin application and consequently would result in dermal exposure to fullerenes. Using the highest concentration of C60 detected of 1.1 μg-C60/g-cosmetic, an approximate single-use quantity (0.5 g) of cosmetic might contain up to 0.6 μg of C60. This type of usage information can be used for estimating human exposures and releases of engineered C60 into wastewater systems.
Assuming that 10,000 people release 0.6 μg-C60/day into a 3.79 million liter per day capacity WWTP, the averaged influx of C60 would be 1.6 ng/L (assuming 379 L/capita/day of wastewater flow). The fate of these fullerenes in a WWTP is primarily a function of their adsorption to biosolids, which then settle and are removed from the water prior to discharge of the treated effluent to streams, rivers or lakes. Biosolids adsorption of n-C60 can be six times more efficient than that of C60-PVP (Kiser et al., 2010). Using these adsorption data, and methodology described in the methods section, for a steady-state mass balance model for a 3.79 million liter per day WWTP, the predicted effluent concentration for n-C60 fullerenes is 6 × 10−8 ng/L compared to 2 × 10−6 ng/L if the fullerenes are in C60-PVP form. These effluent concentrations are at least 8 orders of magnitude lower than predicted no effect concentrations previously calculated for fullerenes (Gottschalk et al., 2009, Supporting Information). The concentration of n-C60 or C60-PVP in the biosolids would be 9 × 10−4 mg/kg-biosolids, more than 4 orders of magnitude lower than the predicted no effect concentration (1 mg/kg-soil) for fullerenes in soils (Gottschalk et al., 2009, Supporting Information). While this analysis started with a conservative assumption that everyone in a population uses cosmetics containing fullerenes, the model suggests that environmental release of fullerenes through WWTP effluent and biosolids disposal will be minimal as a result of their use in cosmetics. The model also illustrates that hydrophilic fullerene formulations (i.e., C60-PVP) used in some products may be more difficult to remove during activated sludge wastewater treatment than n-C60 aggregates. Therefore, the use of fullerenes in a wide range of applications in addition to cosmetics may lead to higher concentrations in WWTPs. In fact, modeling efforts that account for many applications of fullerenes have predicted ng/L and μg/kg concentrations in WWTP effluent and biosolids, respectively (Gottschalk et al., 2009).
4. Summary and implications
It is important to note that these methods quantify individual C60 molecules (via extraction into toluene) whereas human and environmental exposures to C60 may occur as fullerene agglomerations (such as in Fig. 1) and/or as other fullerene-based molecules following chemical transformation processes such as oxidation. This possibility must be considered when evaluating fullerene exposure and toxicity studies.
Similar to other chemicals in cosmetics or personal care products that can be found in treated wastewater and downstream water sources, fullerene molecules currently used in cosmetics could be released into wastewater by rinsing the skin after product use. The increased water solubility of some commercial fullerenes may increase their environmental persistence. For example, C60-PVP is more difficult to remove by adsorption to biosolids during wastewater treatment than is aqueous n-C60 (Kiser et al., 2010). The results presented here suggest that commercial applications provide a pathway for engineered fullerenes to enter the environment.
If some commercial fullerenes are likely to be released and persist in aquatic environments, products can be designed to use fullerenes with discretion. With the ability to quantify fullerenes in products and various environmental matrices (e.g., water, soil, biological samples), research can evaluate the minimum quantity of fullerenes necessary to produce a desired function in products. This will help minimize the excess environmental release of fullerenes.
Acknowledgments
This work has been funded through the Urban Ecology IGERT at Arizona State University (NSF Grant #0504248), the Paul L. Busch Award from the Water Environment Research Foundation (Westerhoff recipient), and the EPA STAR Grant (#RD833322). This work was also supported by the NIH Grand Opportunities (RC2) program through NIEHS grant DE-FG02-08ER64613. TEM images were acquired by David Lowry at the Bioimaging Facility within the School of Life Sciences at Arizona State University. James Hutchings provided support with LC-MS operation. The authors thank Frank von der Kammer for useful discussions during the development of these experiments. The work presented here reflects the views of the authors only.
Footnotes
Fullerenes were detected in cosmetics with liquid chromatography-mass spectrometry up to 1.1 μg/g, demonstrating a source for human/environmental exposure.
Author disclosure statement
No competing financial interests exist.
Contributor Information
Paul Westerhoff, Email: p.westerhoff@asu.edu.
Pierre Herckes, Email: pierre.herckes@asu.edu.
References
- Becker L, Bada JL, Winans RE, Hunt JE, Bunch TE, French BM. Fullerenes in the 1.85-billion-year-old Sudbury impact structure. Science. 1994;265:642–645. doi: 10.1126/science.11536660. [DOI] [PubMed] [Google Scholar]
- Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol. 2008;42:4133–4139. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- Benn TM, Pycke BFG, Herckes P, Westerhoff P, Halden RU. Evaluation of extraction methods for quantification of aqueous fullerenes in urine. Anal Bioanal Chem. 2010 doi: 10.1007/s00216-010-4465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD, Snyder S. Pharmaceuticals and endocrine disrupting compounds in US drinking water. Environ Sci Technol. 2009;43:597–603. doi: 10.1021/es801845a. [DOI] [PubMed] [Google Scholar]
- Burangulov N, Moravsky A, Kulikova Y, Dyachuk G, Loutfy R. Patent application number 20050136079. Cosmetic compositions containing fullerene clusters. 2005 Available at: http://www.freepatentsonline.com/20050136079.html.
- Chen Z, Westerhoff P, Herckes P. Quantification of C-60 fullerene concentrations in water. Environ Toxicol Chem. 2008;27:1852–1859. doi: 10.1897/07-560.1. [DOI] [PubMed] [Google Scholar]
- Farré M, Pérez S, Gajda-Schrantz K, Osorio V, Kantiani L, Ginebreda A, Barceló D. First determination of C60 and C70 fullerenes and N-methylfulleropyrrolidine C60 on the suspended material of wastewater effluents by liquid chromatography hybrid quadrupole linear ion trap tandem mass spectrometry. J Hydrol. 2010;383:44–51. [Google Scholar]
- Fortner JD, et al. C60 in water: nanocrystal formation and microbial response. Environ Sci Technol. 2005;39:4307–4316. doi: 10.1021/es048099n. [DOI] [PubMed] [Google Scholar]
- Gottschalk F, Sonderer T, Scholz R, Nowack B. Modeled environmental concentrations of engineered nanomaterials. Environ Sci Technol. 2009;43:9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- Halford B. Fullerene for the face. Chem Eng News. 2006;84:47. [Google Scholar]
- Heymann D, Chibante LPF, Brooks RR, Wolbach WS, Smalley RE. Fullerenes in the cretaceous-tertiary boundary-layer. Science. 1994;265:645–647. doi: 10.1126/science.265.5172.645. [DOI] [PubMed] [Google Scholar]
- Heymann D, Chibante LPF, Smalley RE. Determination of C60 and C70 fullerenes in geologic materials by high-performance liquid chromatography. J Chromatogr A. 1995;689:157–163. [Google Scholar]
- Isaacson C, Kleber M, Field J. Quantitative analysis of fullerene nano-materials in environmental systems: a critical review. Environ Sci Technol. 2009;43:6463–6474. doi: 10.1021/es900692e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Matsubayashi K. USA. Patent Application Number 20060134095. Antioxidative composition and composition for external use. 2006 Available at: http://www.freepatentsonline.com/20060134095.html.
- Jehlicka J, Frank O, Hamplova V, Pokorna Z, Juha L, Bohacek Z, Weishauptova Z. Low extraction recovery of fullerene from carbonaceous geological materials spiked with C-60. Carbon. 2005;43:1909–1917. [Google Scholar]
- Johansen A, Pedersen AL, Jensen KA, Karlson U, Hansen BM, Scott-Fordsmand JJ, Winding A. Effects of C-60 fullerene nanoparticles on soil bacteria and protozoans. Environ Toxicol Chem. 2008;27:1895–1903. doi: 10.1897/07-375.1. [DOI] [PubMed] [Google Scholar]
- Kai Y, Komazawa Y, Miyajima A, Miyata N, Yamakoshi Y. [60]Fullerene as a novel photoinduced antibiotic. Fullerene Nanot Carbon Nanostruct. 2003;11:79–87. [Google Scholar]
- Kiser M, Ryu H, Jang H, Hristovski K, Westerhoff P. Biosorption of nanoparticles to heterotrophic wastewater biomass. Water Res. 2010;44:4105–4114. doi: 10.1016/j.watres.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Lebedkin S, Ballenweg S, Gross J, Taylor R, Kratschmer W. Synthesis of C120O - a new dimeric [60]fullerene derivative. Tetrahedron Lett. 1995;36:4971–4974. [Google Scholar]
- Miyata N, Yamakoshi Y, Nakanishi I. Reactive species responsible for biological actions of photoexcited fullerenes. Yakugaku Zasshi-J Pharmaceut Soc Jap. 2000;120:1007–1016. doi: 10.1248/yakushi1947.120.10_1007. [DOI] [PubMed] [Google Scholar]
- National Science and Technology Council Committee on Technology, Subcommettee on Nanoscale Science, engineering and Technology (NSET), Nanotechnology Environmental and Health Implications (NEHI) Working Group. Environmental, health, and Safety research needs for engineered Nanoscale Materials. Arlington, VA: 2006. Available at: http://www.nano.gov/NNI_EHS_research_needs.pdf. [Google Scholar]
- Perez S, Farre M, Barcelo D. Analysis, behavior and ecotoxicity of carbon-based nanomaterials in the aquatic environment. Trac-Trend Anal Chem. 2009;28:820–832. [Google Scholar]
- Pycke BFG, Benn TM, Herckes P, Westerhoff P, Halden RU. Strategies for quantifying C60 fullerenes in environmental and biological samples and implications for studies in environmental health and ecotoxicology. Trac-Trend Anal Chem. 2011;30:44–57. doi: 10.1016/j.trac.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa T, Yoshioka D, Homma H, Imai K, Satoh M, Takayanagi I. High-performance liquid-chromatography of fullerene (C-60) in plasma using ultraviolet and mass-spectrometric detection. Biol Pharm Bull. 1995;18:1171–1174. doi: 10.1248/bpb.18.1171. [DOI] [PubMed] [Google Scholar]
- Smith AB, Tokuyama H, Strongin RM, Furst GT, Romanow WJ, Chait BT, Mirza UA, Haller I. Synthesis of oxo-bridged and methylene-bridged C-60 dimers, the first well-characterized species containing fullerene-fullerene bonds. J Am Chem Soc. 1995;117:9359–9360. [Google Scholar]
- Spohn P, Hirsch C, Hasler F, Bruinink A, Krug HF, Wick P. C-60 fullerene: a powerful antioxidant or a damaging agent? The importance of an in-depth material characterization prior to toxicity assays. Environ Pollut. 2009;157:1134–1139. doi: 10.1016/j.envpol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Taylor R, Hare JP, Abdulsada AK, Kroto HW. Isolation, separation and characterization of the fullerenes C-60 and C-70-the 3rd form of carbon. J Chem Soc-Chem Commun. 1990:1423–1424. [Google Scholar]
- Tsuchiya T, Oguri I, Yamakoshi YN, Miyata N. Novel harmful effects of [60] fullerene on mouse embryos in vitro and in vivo. FEBS Lett. 1996;393:139–145. doi: 10.1016/0014-5793(96)00812-5. [DOI] [PubMed] [Google Scholar]
- Usenko CY, Harper SL, Tanguay RL. Fullerene C-60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol Appl Pharmacol. 2008;229:44–55. doi: 10.1016/j.taap.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow Wilson International Center for Scholars. Nanotechnology Consumer Product Inventory. Washington, DC: Project on Emerging Nanotechnologies; 2009. Available at: http://www.nanotechproject.org/inventories/consumer/analysis_draft/ [Google Scholar]
- Xia XR, Monteiro-Riviere NA, Riviere JE. Trace analysis of fullerenes in biological samples by simplified liquid-liquid extraction and high-performance liquid chromatography. J Chromatogr A. 2006;1129:216–222. doi: 10.1016/j.chroma.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Xiao L, Takada H, Maeda K, Haramoto M, Miwa N. Antioxidant effects of water-soluble fullerene derivatives against ultraviolet ray or peroxylipid through their action of scavenging the reactive oxygen species in human skin keratinocytes. Biomed Pharmacother. 2005;59:351–358. doi: 10.1016/j.biopha.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Xiao L, Takada H, Gan XH, Miwa N. The water-soluble fullerene derivative ‘Radical Sponge (R)’ exerts cytoprotective action against UVA irradiation but not visible-light-catalyzed cytotoxicity in human skin keratinocytes. Bioorg Med Chem Lett. 2006;16:1590–1595. doi: 10.1016/j.bmcl.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Xiao L, Matsubayashi K, Miwa N. Inhibitory effect of the water-soluble polymer-wrapped derivative of fullerene on UVA-induced melanogenesis via downregulation of tyrosinase expression in human melanocytes and skin tissues. Arch Dermatol Res. 2007;299:245–257. doi: 10.1007/s00403-007-0740-2. [DOI] [PubMed] [Google Scholar]
- Yamakoshi YN, Yagami T, Fukuhara K, Sueyoshi S, Miyata N. Solubilization of fullerenes into water with polyvinylpyrrolidone applicable to biological tests. J Chem Soc-Chem Commun. 1994:517–518. [Google Scholar]
- Ziolo RF. United States: Xerox Corporation; Stamford, CT: Patent application number 5188918. Toner and developer compositions comprising fullerene. 1993 Available at: http://www.freepatentsonline.com/5188918.html.