Abstract
There is ample evidence to suggest that a dramatic decrease in mitochondrial Ca2+ retention may contribute to the cell death associated with stroke, excitotoxicity, ischemia and reperfusion, and neurodegenerative diseases. Mitochondria from all studied tissues can accumulate and store Ca2+, but the maximum Ca2+ storage capacity varies widely and exhibits striking tissue specificity. There is currently no explanation for this fact. Precipitation of Ca2+ and phosphate in the mitochondrial matrix has been suggested to be the major form of storage of accumulated Ca2+ in mitochondria. How this precipitate is formed is not known. The molecular identity of almost all proteins involved in Ca2+ transport, storage and formation of the permeability transition pore is also unknown. This review summarizes studies aimed at identifying these proteins, and describes the properties of a known mitochondrial protein that may be involved in Ca2+ transport and the structure of the permeability transition pore.
Keywords: brain mitochondria, Ca2+ accumulation, Ca2+ and Pi precipitate, calciphorin, calcium uniporter, calvectin, dense granules, gC1qR, liver mitochondria, permeability transition pore
The standard model
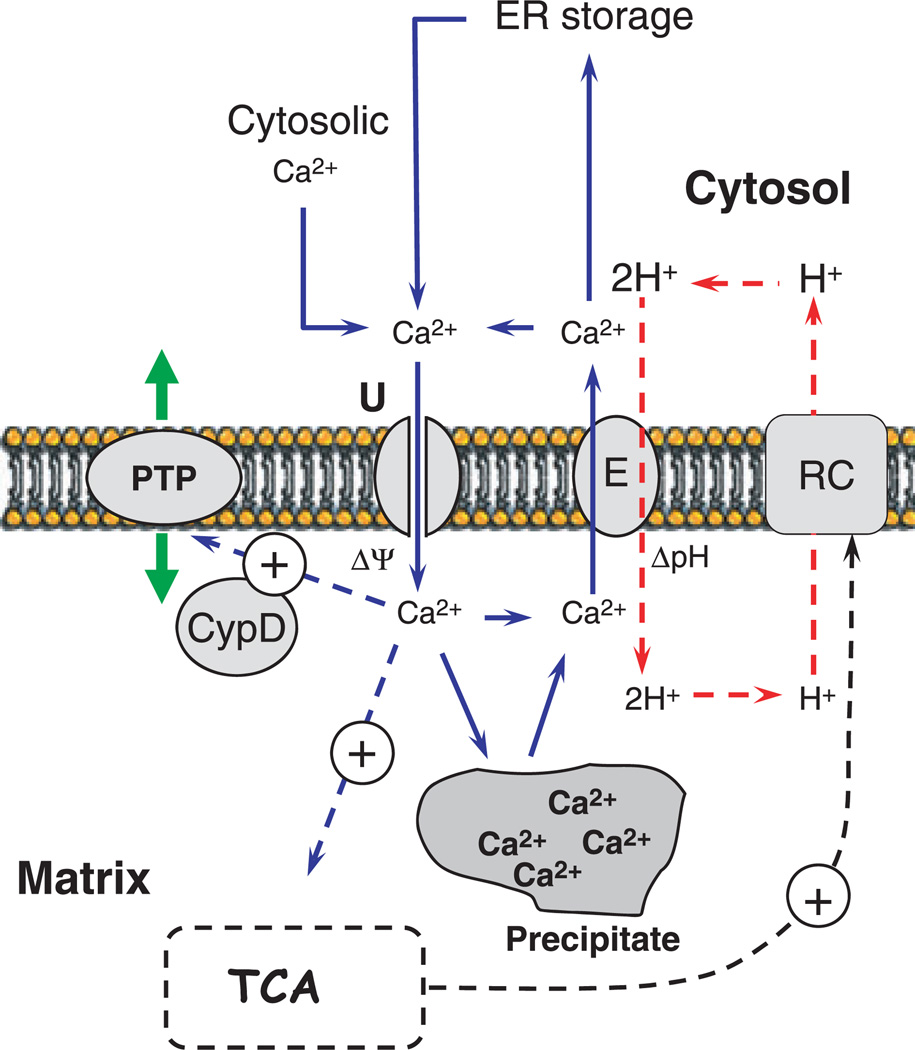
The ability to accumulate, retain and release Ca2+ is a fundamental ubiquitous function of animal mitochondria. Extensive research during the last 50 years has resulted in a consensus model of mitochondrial Ca2+ handling that adequately accommodates most if not all experimental data, referred to here as ‘the standard model of mitochondrial Ca2+ handling’. This model is shown in Fig. 1 in a greatly simplified form, and assumes that mitochondria accumulate exogenous Ca2+ by means of an electrogenic carrier that facilitates Ca2+ transport across the inner mitochondrial membrane (IM) into the matrix. The transport is coupled to simultaneous accumulation of inorganic phosphate. Inside the matrix, accumulated Ca2+ and phosphate are stored in the form of osmotically inactive precipitates, and eventually are slowly released back into the cytosol with the assistance of Ca2+/nNa+ and/or Ca2+/2H+ exchangers (Fig. 1) that are also situated in the IM. When accumulated above a certain threshold, Ca2+ triggers opening of the so-called permeability transition pore (PTP). This may also be mediated by matrix proteins such as cyclophilin D (CypD). Opening of the PTP is thought to have a detrimental effect on mitochondria and cell well-being in general. The Ca2+ uniporter system and the PTP structure are thought to consist of proteins, but the molecular identities of these proteins are unknown. The only two Ca2+ transport-related proteins that have been identified are the Ca2+/nNa+ exchanger and Ca2+/2H+ exchanger: the gene for the CGP37157-sensitive mitochondrial Ca2+/nNa+ exchanger has recently been identified as NCLX (SLC24A6 family) [1], and that for the Ca2+/2H+ exchanger has been identified as Letm1 [2]. These two proteins will not be reviewed here; please see the review by Chinopoulos & Adam-Vizi [3] and that by Pivovarova & Andrews [4] in this issue for details. Nevertheless, extensive studies to identify proteins involved in Ca2+ transport, storage and the PTP have been performed over the last 50 years. This review is not concerned with kinetic, biophysical channel-related, bioenergetic or pathophysiological aspects of Ca2+ handling in mitochondria; numerous excellent reviews on these subjects can be found elsewhere. Here, the present review describes some of the most prominent and followed-up research efforts to identify the proteins involved in Ca2+ transport, storage and PTP, and presents a hypothesis on this subject that somewhat modifies the ‘standard model’.
Fig. 1.
Standard model of mitochondrial Ca2+ handling. Mitochondria accumulate exogenous Ca2+ by means of an electrogenic carrier (calcium uniporter, ‘U’) that facilitates Ca2+ transport across the inner mitochondrial membrane (IM) into the matrix. The transport is coupled to simultaneous accumulation of inorganic phosphate (not shown). Inside the matrix, accumulated Ca2+ and phosphate are stored in the form of osmotically inactive precipitates (‘precipitate’), and eventually slowly released back into the cytosol through a Ca2+/nNa+ (not shown) and / or a Ca2+/2H+ exchanger that is also located in the IM. The process of Ca2+ uptake is driven by the membrane potential; the process of Ca2+ release is driven by the pH gradient, in the case of the Ca2+/2H+ exchanger. Elevated intramitochondrial Ca2+ can stimulate the activities of enzymes of the tricarboxylic acid cycle (TCA), thereby boosting energy production in the mitochondria. When it accumulates above a certain threshold, Ca2+ triggers PTP opening, and this is also modulated by matrix-located protein cyclophilin D (CypD). E, exchanger; RC, respiratory chain.
Ca2+ uniporter system
Although the molecular identity of the mitochondrial calcium uniporter is still unknown, experimental data have suggested that it is a highly selective, inward-rectifying ion channel [5], a ‘gated’ pore containing a Ca2+ binding site on the cytosolic side of the inner mitochondrial membrane that activates Ca2+ transport [6,7]. It has been suggested that mitochondrial calcium uniporter contains at least two subunits, one of which is a dissociable intermembrane factor that is glycoprotein in nature, and that the mitochondrial calcium uniporter is regulated by association and dissociation of this factor, activated by calcium binding [8]. It has been shown that mitochondria depleted of endogenous Ca2+ exhibited low initial rate of energy-dependent Ca2+ uptake. Pre-incubation of de-energized mitochondria with added Ca2+ stimulated their energy-dependent Ca2+ uptake up to 10-fold, with strong cooperativity in the velocity–substrate curves for Ca2+-depleted mitochondria. To explain these and other kinetic peculiarities of Ca2+ transport, a model has been proposed in which the Ca2+-transporting system is present in a de-activated state in the absence of cytosolic Ca2+, and formation of the active Ca2+ uniporter is triggered by an increase in external Ca2+. The uniporter is formed by oligomerization of two or more protomers, resulting in formation of the ruthenium- and lanthanides-sensitive Ca2+-conducting gated channel [9].
As already mentioned, the molecular identity of the mitochondrial calcium uniporter remains unknown, despite considerable efforts by many prominent researchers. Since the pioneering reports of Sottocasa et al. [10] and Lehninger [11], numerous attempts have been made to isolate the calcium uniporter [12–23]. Various Ca2+ binding proteins and peptides have been isolated and characterized, all of which are able to bind Ca2+ in a ruthenium red- and La3+-inhibited fashion, and some of which are able to transport bound Ca2+ through artificial bilayer membranes. Reviewing all this literature is beyond the scope of the present review: Lars Ernster’s [23a] and Saris and Carafoli’s [24] recent review provide comprehensive literature surveys on the history of Ca2+ transport and attempts to isolate the Ca2+ uniporter. The present review covers only the most followed-up and detailed studies.
Calvectin
The earliest extensively studied preparations of mitochondrial Ca2+ binding protein(s) were isolated by Sottocasa et al. from intermembrane space of rat liver mitochondria [10] and ox liver mitochondria [14]. These preparations were capable of high-affinity Ca2+ binding that was inhibited by ruthenium red and La3+. These preparations showed a single band of approximately 30 kDa on PAGE, and contained sialic acid and neutral and amino sugars, typical of glycoproteins, a high content of dicarboxylic amino acids, and some bound Ca2+ and Mg2+. This preparation was capable of binding Ca2+ with high affinity (Kd of approximately 100 nm), and also contained a number of low-affinity Ca2+ binding sites. It was named ‘calvectin’ [25], and was suggested to represent the mitochondrial Ca2+ carrier or a major component thereof. Similarly isolated glycoprotein increased the conductance of artificial lipid bilayers in the presence of Ca2+, and the conductance was sensitive to ruthenium red [22], implying that it may be the Ca2+ uniporter or part thereof. Further studies revealed a set of unique features for this preparation. One of them was that the glycoprotein was found primarily in the inter-membrane space in both a free soluble form and also tightly bound to the inner membrane, but was absent in the matrix of mitochondria [26]. Binding to inner and outer membranes apparently required Mg2+ and/or Ca2+ [27]. Moreover, calvectin appeared to be able to move reversibly between mitochondrial compartments in the presence of Ca2+. The binding to the membrane could further be modulated by pyridine nucleotides, which also bind to calvectin; bound NAD+ decreased the association of calvectin with the membrane [28]. Mitochondria could be depleted of calvectin by treating them with uncoupling concentrations of pentachlorophenol in the presence of phosphate and acetate. This treatment affected the ability of mitochondria to release pre-loaded Ca2+ in response to the addition of pentachlorophenol, with an almost linear correlation between the amount of released glycoprotein and the rate of Ca2+ efflux [29]. Adding the glycoprotein back to mitoplasts (mitochondria stripped of their outer membrane) depleted of it by swelling in oxaloacetate/EDTA restored the Ca2+ uptake if Mg2+ was also included in the mixture [28]. Antibodies raised against calvectin were able to inhibit Ca2+ transport in mitoplasts, indicating that this glycoprotein is a required part of the mitochondrial Ca2+ transport machinery [30], (to note, a review by Saris and Carafoli mentions that “Saris found that the antiserum formed four precipitation bands in Ouchterlony immunodiffusion tests and did not inhibit Sr2+ uptake by the uniporter” [24]. We were not able to find another published record of that finding which is important because mitochondria are known to accumulate both Ca2+ and Sr2+ with about similar efficiency and ruthenium red sensitivity. Hence, this finding might imply that a conformation of the “uniporter” that transports Ca2+ is different from that transporting Sr2+). The authors suggested an interesting but rather simple ‘two-step’ model of calvectin involvement in Ca2+ transport: first, soluble calvectin in the intermembrane space binds Ca2+ and associates spontaneously with the inner membrane; second, it carries Ca2+ through the membrane and somehow returns back to the outer surface of the inner membrane [31]. Eventually, a single protein was purified from these crude preparations that migrated at approximately 14 kDa on SDS/PAGE and had a minimum molecular weight of 15 577 calculated on the basis of its amino acid composition. However, no sugars were found in this protein, although it had a high content of glutamic and aspartic acids. This protein also carried fewer low-affinity Ca2+ binding sites than the original ‘calvectin’ [23a].
Calciphorin
An integral low-molecular-weight membrane protein with the properties of a Ca2+ ionophore was isolated from calf heart inner mitochondrial membrane [15– 17,32,33]. It was characterized as a 3000 Da high-affinity calcium carrier and named ‘calciphorin’ [16]. In contrast to hydrophilic calvectin, the calciphorin was hydrophobic and lacked phospholipids, sugars and free fatty acids. Calciphorin was able to extract Ca2+ into an organic solvent phase and to transport Ca2+ through a bulk organic phase in the presence of a lipophilic anion (picrate), indicating the electrophoretic nature of the calciphorin–Ca2+ complex. The Ca2+ extraction was strongly inhibited by ruthenium red and lanthanum. The selectivity of ion extraction by calciphorin was Zn2+ > Ca2+, Sr2+ > Mn2+ > Na+ > K+ [32]. The Ca2+ binding site had a dissociation constant of 5.2 pm, with 1 mole Ca2+ bound per mole of calciphorin [32]. Calciphorin was shown to transport Ca2+ in a lipid bilayer membrane model such as reconstituted phospholipid vesicles. Furthermore, calciphorin-mediated Ca2+ transport across the vesicle membrane was toward the negatively charged side of the membrane. This calciphorin-mediated calcium transport in vesicles was also strongly inhibited by ruthenium red and La3+ [33].
The role of calciphorin as the Ca2+ ionophore was subsequently challenged by Sokolove and Brenza [34], who isolated a mixed protein–lipid fraction from rat liver mitochondria that had properties similar to those of calciphorin. They attributed all the Ca2+-binding and transporting ability of that fraction to its lipid components. In another study, these authors demonstrated that cardiolipin binds Ca2+ with high affinity (apparent Kd = 0.70 ± 0.17 µm) and can extract Ca2+ into a bulk organic phase. The interaction of cardioli-pin with Ca2+ was insensitive to Na+, but was inhibited by divalent cations (Mn2+ > Zn2+ > Mg2+). In addition, La3+ and ruthenium red were found to be strong inhibitors of Ca2+ binding by cardiolipin [35]. However, it should be noted that the isolation procedure used by Sokolove and Brenza was similar but not identical to that originally reported by Shamoo’s group who later successfully isolated ‘calciphorin’ from rat liver mitochondria [36]. Nevertheless, it is still not clear whether ‘liver calciphorin’ and ‘heart calciphorin’ are the same proteins, or indeed whether the procedure described by Jeng and Shamoo is reproducible. As can be seen from Table 1 in [36], the ‘calciphorin’ isolated from liver and two ‘calciphorin’ isolates from calf heart were quite different in terms of their estimated molecular mass and other parameters. To the best of our knowledge, there were no new reports on calciphorin after 1984.
Mironova’s glycoprotein and peptide
Mironova’s group worked on isolation and identification of Ca2+-transporting substances in mitochondria for almost two decades since approximately 1976, but most of the earlier results were published in hard-to-access Russian journals. The authors isolated a component capable of inducing selective Ca2+ transport in artificial bilayer lipid membranes from mitochondria and homogenates of various animal and human tissues. The Ca2+-transporting properties of this component were ascribed to the presence of a glycoprotein and a peptide. The 40 kDa glycoprotein and 2 kDa peptide from beef heart homogenate and mitochondria induced highly selective Ca2+ transport through bilayer lipid membranes. The glycoprotein contained 60–70% and 30–40% protein and carbohydrate, respectively. Sulfur-containing amino acids (1 mole per 1 mole of glycoprotein) and sialic acids (2 or 3 moles per 1 mole of glycoprotein) were also detected in the glycoprotein, and it was enriched in asparagine and glutamine [21], similar to calvectin. Lipids were not essential for the Ca2+-transporting activity of glycoprotein. Micromolar concentrations of the glycoprotein and the peptide were found to greatly increase the conductivity of bilayer lipid membranes. Ruthenium red abolished the glycoprotein- and peptide-induced Ca2+ transport in bilayer lipid membranes. A transmembrane Ca2+ gradient induced an electric potential difference whose magnitude was close to the theoretical value for optimum Ca2+ selectivity. The authors also identified thiol groups that were essential for Ca2+ transport in both the glycoprotein and the peptide. On the basis of these studies, the authors proposed a model in which the peptide is an active Ca2+-transporting portion of the glycoprotein, which lacks Ca2+-transporting activity when the peptide is detached. Ca2+ moves through special channels in the membrane formed by the peptide, and the glycoprotein, which has many Ca2+-binding centers, creates a high concentration of Ca2+ near the channel mouth. Functioning of the channels is controlled by thiol-disulfide transitions of sulfur-containing groups of the glycoprotein–peptide complex [21].
A decade later, the same group (in collaboration with Saris) generated polyclonal rabbit antibodies against a ‘Ca2+-binding mitochondrial glycoprotein’ (presumably the former glycoprotein). These antibodies were found to inhibit the uniporter-mediated transport of Ca2+ in mitoplasts prepared from rat liver mitochondria. Spermine, a modulator of the uniporter, decreased the inhibition [37]. The peptide was isolated and purified to homogeneity and shown to form a Ca2+-transporting channel in bilayer lipid membranes, requiring addition of the peptide from both sides of the membrane, [20]. This suggested that the channel is formed by two or more subunits, as in formation of the gramicidin D channel [38]. The authors also demonstrated that the Ca2+-binding 40 kDa glycoprotein previously reported as a precursor of the peptide may in fact be an irrelevant contaminant, as it was immunologically indistinguishable from beef plasma orosomucoid protein. However, antibody raised against the orosomucoid was not able to inhibit mitochondrial Ca2+ uptake [20], in contrast to the antibodies derived against mitochondrial glycoprotein in the previous study [37]. Nevertheless, the authors concluded that the presence of the 40 kDa glycoprotein in association with a channel-forming peptide [39] was due to co-purification.
Most recent ‘Ca2+ uniporter’ isolations
Chavez’s group isolated a semi-purified extract of proteins from rat kidney cortex mitochondria that conferred Ca2+-transporting capacity to energized cytochrome oxidase-containing proteoliposomes, and generated a mouse hyperimmune serum that inhibited Ca2+ transport in mitoplasts and proteoliposomes. The serum recognized three major proteins of 75, 70 and 20 kDa. The purified antibody recognizing the 20 kDa component inhibited Ca2+ transport by approximately 70% in mitoplasts, suggesting that this 20 kDa protein is a necessary component of the Ca2+ uniporter [23]. In a follow-up study, the same group isolated an 18 kDa protein that binds Ru360 (an inhibitor of Ca2+ uniporter) with high affinity, and proposed that it is part of the uniporter [40]. Most recently, these authors isolated a Ca2+-transporting protein fraction and separated it further by preparative electrofocusing. After incorporating the separated fractions into cytochrome oxidase containing proteoliposomes, they recovered two Ca2+-transporting activities, only one of which was inhibited by Ru360. On the basis of these results, the authors suggested that the Ca2+ uniporter is composed of at least two different subunits that become partially dissociated at low pH. The Ru360-resistant proteins are dissociated at low pH and represent the Ca2+ channel, whereas the subunit that binds to Ru360 remains linked to the channel at higher pH [41]. The same group also showed that glycosyl residues on the putative Ca2+ uniporter are not required for Ca2+ transport activity: deglycosylation of mitoplasts using glycosidase F removed the ruthenium red sensitivity of Ca2+ uptake but did not inhibit it [42].
It is very surprising that so much effort spanning several decades of research did not result in molecular identification of any of the isolated putative Ca2+-transporting proteins. Even the most recent studies by Zazueta et al. [40], performed when the majority of the new proteomics approaches, sequencing techniques and a wealth of genetic information were already available, did not identify the isolated proteins. Unfortunately, the chances of reproducing the older research and isolating the same proteins are low. Protein purification from mitochondrial membranes that carry hundreds of proteins is akin to magic: unless a spell is cast precisely (in this case a step-by-step isolation protocol listing all the reagents, procedures and conditions), the result could be just a sore throat. It may be more productive to adopt a targeted approach, selecting a few known mitochondrial proteins fitting the required ‘profile’ and using genetic approaches to prove their involvement in Ca2+ transport. What kind of ‘profile’ for a putative Ca2+ uniporter can be deduced from these older studies? The structure of this protein should accommodate the following features: the protein should be of moderate to low molecular mass, approximately 15–40 kDa, it should be capable of binding Ca2+, the binding should be inhibited by ruthenium red and other known Ca2+ uniporter inhibitors, it also should be able to bind to the inner mitochondrial membrane from at least the cytosolic side, preferably in the presence of Ca2+ (like calvectin), and, according to all the hypotheses reviewed above and a wealth of other known characteristics regarding Ca2+ transport, should be able to form a gated pore comprising several identical protomers or as a complex with other proteins. A known protein that mostly fits this profile is discussed below.
Storage of Ca2+ in mitochondria
Net Ca2+ uptake into mitochondria requires co-transport of an IM-permeable anion such as acetate or phosphate. In the latter case, the accumulated Ca2+ forms a precipitate in the matrix of mitochondria in an apparently spontaneous process. The precipitate can store large amounts of Ca2+ and is readily observed in isolated mitochondria by electron microscopy [4,43]. The precipitates appear in the form of large (50– 100 nm diameter) electron-dense granules with a hollow electron transparent core, and are always found in immediate proximity to the IM [43]. Formation of the Ca2+ and phosphate precipitates is thought to be the major mechanism of Ca2+ storage in mitochondria [4,43,44]. It has been suggested that a protein or other matrix constituents may serve as a nucleation center facilitating formation of the Ca2+ precipitates [43]. Indeed, the presence of a protein may explain the always amorphous nature of Ca2+-phosphate precipitates, which is somewhat puzzling because hydroxyapatite [Ca5(PO4)3(OH)], the most commonly found composition of mitochondrial Ca2+ and Pi precipitates, is crystalline. In blood, where high levels of Ca2+ and Pi are standard, a protein called ‘fetuin’ had been shown to inhibit the precipitation of hydroxyapatite from supersaturated solutions of calcium and phosphate [45]. Perhaps a similar protein serves the same function in the mitochondrial matrix. The presence of substantial amounts of the Ca2+-binding proteins mitocalcin [46], calbindin-28k and calbindin-30k (calretinin) in a particulate fraction of rat brain [47] and in brain mitochondria [48,49] has been demonstrated previously, and annexin I [50] and annexin VI [51] were found in liver mitochondria. At least some of these proteins (annexin VI) serve as nucleation factors in vitro [52]. However, the contribution of these proteins to mitochondrial Ca2+ storage has not been examined. Although Ca2+-binding matrix-located proteins are the main candidates for the role of nucleation factors, non-protein factors such as mitochondrial DNA cannot be ruled out, as the Ca2+-binding ability of DNA is well known [53].
The Ca2+ and phosphate granules have been isolated from Ca2+-loaded rat liver mitochondria and their composition assessed [54]. The granules contained significant amount of carbon and nitrogen, indicating the presence of protein(s). However, the protein was not isolated or identified because the focus of that study was identifying the molecular form of the Ca2+ and phosphate precipitate. In addition, protein identification techniques were much more time-consuming and costly in 1967 when the study was performed than they are now. The Ca2+-phosphate precipitates are discussed in more detail elsewhere [3].
Proteins implicated in PTP formation
One of the most dramatic manifestations of abnormal Ca2+ homeostasis in mitochondria is the opening of a large channel called the ‘permeability transition pore’ (PTP) in the inner membrane that renders them incapable of energy production and can result in cell death by either apoptosis or necrosis. The functional and physiological aspects of PTP and mitochondrial Ca2+ transport have been reviewed extensively [55–61].
After a certain tissue-dependent threshold for the accumulated Ca2+ is reached, the permeability of IM to solutes abruptly increases due to opening of the PTP, a protein-mediated pore in the IM. It has been suggested that opening of the PTP is probably triggered by the increase in the free matrix Ca2+ concentration [55–61], although the evidence for this is ambiguous. The free matrix Ca2+ does increase somewhat upon loading mitochondria with Ca2+, but does not exhibit any abrupt changes immediately before PTP opening [44]. On the other hand, it increases significantly upon loading of brain mitochondria with Ca2+ if opening of the PTP is inhibited [44]. Therefore, it is not clear whether opening of the PTP is triggered by the free matrix Ca2+ or bound matrix Ca2+, or both together. In either case, factors affecting formation of the Ca2+ and phosphate precipitates are expected to influence the Ca2+ threshold for PTP activation. Changes in the precipitation characteristics are expected to have a strong effect on the overall Ca2+ retention and storage in mitochondria.
The molecular identity of the protein(s) that actually form the PTP channel remains a mystery. Past studies identified several proteins involved in the PTP formation or modulation, such as the voltage-dependent anion channel, the adenine nucleotide translocator (ANT), and, more recently, the mitochondrial phosphate transporter PIC [59], although none of these proteins are currently thought to directly form the PTP channel [59,60]. Until recently, mitochondrial ANT was viewed as the most likely PTP-forming protein [57,59,60]. ANT may also interact with another matrix protein, cyclophilin D (CypD) [62]. The latter is a target of cyclosporin A, a peptide inhibitor of PTP. Although the role of CypD in modulating the Ca2+ threshold for PTP activation had recently been strongly confirmed [63–66], the role of ANT in PTP formation was strongly challenged [67]. The PTP in mitochondria isolated from CypD-ablated mice is insensitive to cyclosporin A and exhibits a much higher Ca2+ threshold [63–66], whereas the PTP is activated by Ca2+ accumulation in ANT-deficient liver mitochondria isolated from mice that were genetically ablated of ANT in the liver [67].
Experimental evidence supporting a role for the voltage-dependent anion channel in PTP formation has been discussed in detail [60,61]. However, the recent finding that genetic ablation of any of the three mammalian voltage-dependent anion channel isoforms or all of them together does not affect Ca2+-induced PTP opening strongly suggests that voltage-dependent anion channels are not involved in PTP channel formation [68]. While the molecular identity of the PTP channel-forming protein remains unknown, a role for the mitochondrial phosphate transporter PIC in PTP formation cannot be ruled out [59].
An alternative model of PTP implicates no specific proteins in the role of the PTP channel [69]. According to this model, the pore is formed by aggregation of some misfolded integral membrane proteins; transport through these proteins is normally be blocked by cyclophilin D or other chaperones but Ca2+ accumulation and or oxidative stress increase the number of misfolded proteins. When the number of protein clusters exceeds the number of chaperones available to block transport, opening of ‘unregulated pores’ that are no longer sensitive to PTP inhibitors such as cyclosporin A would occur [69]. Although interesting, this model fails to account for approximately half of the known PTP features, such as its fast reversibility by Ca2+ chelation, its sensitivity to regulation by matrix pH, transmembrane voltage, fixed pore size, etc. [60].
Overall, literature analysis [55–61] allow us to formulate a minimum set of requirements to be fulfilled by a plausible candidate for the role of PTP channel-forming protein. First, it has to be able to bind to the IM. Although it has always been presumed that the PTP-forming protein is an integral protein embedded in the IM, there is no evidence to support this presumption. The PTP-forming protein does not have to be located in the IM before it forms a channel; it may simply bind to the IM and move into the IM upon activation. There are numerous examples of proteins moving between various cellular compartments and membranes upon activation. Second, it has to be able to form a large (approximately 2–3 nm diameter) transmembrane channel to allow the passage of charged and uncharged solutes up to 1500 Da. Third, it has to be able to form the channel in a fully reversible, fast and Ca2+-dependent fashion, as the full reversibility of PTP opening upon Ca2+ chelation and its fast transition between an open and a closed state are well known [55]. Finally, the molecular structure of this putative protein should ideally feature Ca2+-binding sites, reactive thiol groups to facilitate channel formation upon oxidation, and conformationally critical β-sheets, as suggested by the effect of cyclophilin D, which is a peptidyl-prolyl-cis/trans isomerase.
The above features are a minimum set of features that, if present in a single protein, would strongly implicate it as a plausible candidate for the role of PTP channel-forming protein. Other PTP features such as regulation by adenine nucleotides and effectors of ANT may be due to other proteins that interact with this putative PTP channel and modulate its activity.
gC1qR
Although there may be a number of unknown mitochondrial proteins that fulfil these requirements, at least one ubiquitous and evolutionary conserved eukaryotic protein, gC1qR, meets these requirements in full. As mentioned earlier, the molecular identity of the protein(s) that actually form the PTP channel remains the most intriguing question. On the basis of structural and other information, we hypothesize that the gC1qR protein, also known as p32, gC1QR/33, splicing factor 2 (SF2) and hyoluronan-binding protein 1 (HABP1), is the most plausible candidate for the role of PTP channel-forming protein. gC1qR is a 23.8. kDa multifunctional cellular protein (although it migrates at 33 kDa in SDS/PAGE, probably due to glycosylation and strong charges [70]) that was originally isolated and characterized as a plasma membrane protein with high affinity for the globular ‘heads’ of the complement component C1q, but was actually just one of its diverse binding partners. gC1qR is synthesized with an N-terminal mitochondrial targeting sequence that is cleaved after import into mitochondria. The matrix location of this protein is firmly established [71–73]; however, it is also found in other cellular compartments [74]. In humans, it is encoded by the C1qBP gene [75]. The function of this protein in mitochondria is not known. gC1qR is an evolutionarily conserved eukaryotic protein. Homologous genes have been identified in a number of eukaryotic species, ranging from fungi to mammals [74], compatible with its potential role in PTP as the latter is also ubiquitous among species. Its mitochondrial location and unique structural features make gC1qR protein a highly plausible candidate for the role of PTP channel, as discussed below.
Structural features of gC1qR as related to PTP and Ca2+ uniporter
There are striking structural features of this protein that make it perfect for the roles of PTP channel and calvectin-like ‘Ca2+ uniporter’. The putative role of gC1qR in PTP formation was suggested previously [76], but this hypothesis did not attract much interest, mostly due to then dominant view that the PTP is formed by ANT, which has now been disproved [67]. gC1qR is a doughnut-shaped trimer with an outer diameter of approximately 7.5 nm, a mean inner diameter of approximately 2 nm, and a thickness of approximately 3 nm. Each monomer consists of seven consecutive β-strands forming a highly twisted antiparallel β-sheet. The channel wall is formed by the β-sheets from all three subunits. This makes gC1qR a potential target for cyclophilin D, a well-known modulator of PTP sensitivity to Ca2+ [55,58–60,63–66]. The latter belongs to a class of enzymes called peptidyl-prolyl-cis /trans-isomerases that act upon prolines in β-sheets, resulting in a conformation change in the target protein. The gC1qR is a very acidic protein with a highly asymmetric negative charge distribution on the protein surface. One side of the doughnut and the inside portion of the channel possess a high number of negatively charged residues; the opposite side is much less negatively charged. These features permit the gC1qR trimer to interact with other charged surfaces such as phospholipid membranes or other proteins, and the ability of gC1qR trimer to bind to the plasma membrane surface is well documented [74]. Moreover, these interactions are inherently sensitive to modulation by divalent cations such as Mg2+ and Ca2+, which can bind to gC1qR and compensate its surface charges. As gC1qR is acidic, its interactions with other proteins may be weakened by increasing the acidity of the medium. It is well known that PTP is inhibited at low pH, probably due to release of cyclophilin D from its putative binding site on the PTP [77]. Thus, it is not unreasonable to suggest that gC1qR has a putative binding site for cyclophilin D. Each monomer of gC1qR has one cysteine at residue 186 (Cys186). This residue does not form inter-chain disulfide bonds between the monomers of a single gC1qR trimer [74]. However, under oxidative conditions, it forms a disulfide bond between monomers of different gC1qR trimers, thereby forming a hexameric structure consisting of two trimers. This complex has a much higher hydrodynamic radius and altered ligand-binding properties [78]. Lastly, a very important feature of the gC1qR trimer is that its inner channel is very large (approximately 2 nm diameter in the compact trimeric crystal), allowing easy passage of solutes with molecular mass 0.4–3 kDa [76], irrespective of their nature and electric charge, which is compatible with the necessary PTP channel properties. Moreover, the primary sequence of gC1qR predicts three putative N-linked glycosylation sites, and the protein was indeed found to be strongly glycosylated [70]. Considering that gC1qR has to be present at the cytosolic side of the inner mitochondrial membrane (see below) for its many activities to be possible, the presence of glycosyl residues should render it a target for ruthenium red binding, as it would be expected form a putative Ca2+ uniporter.
Interaction of gC1qR with pro-apototic and other Ca2+-dependent cell signaling cascades
Both the mitochondrial PTP and Ca2+ uniporter are implicated in so many pathological and physiological scenarios that it would be virtually impossible for the proteins involved in these systems to avoid multiple interactions with other signaling and regulatory proteins. It has been shown that gC1qR is a partner of the pro-apoptotic protein Hrk [79], a mammalian BH3-only protein. Multiple lines of experimental evidence verified a specific interaction and co-localization of Hrk and gC1qR, both of which depended on the presence of the highly conserved C-terminal region of gC1qR. Hrk-induced apoptosis was suppressed by expression of gC1qR mutants lacking the N-terminal mitochondrial signal sequence (gC1qR74–282) or the conserved C-terminal region (gC1qR1–221), which inhibit competitive binding of Hrk to the gC1qR protein and disrupt the channel function of gC1qR, respectively [79]. Another recently discovered pro-apototic protein, smARF, also binds to mitochondrial gC1qR. This protein is known to induce autophagic cell death. gC1qR physically interacts with both human and murine smARF, and co-localizes with them to the mitochondria. Remarkably, knock-down of gC1qR levels significantly reduced the steady-state levels of smARF by increasing its turnover. As a consequence, the ability of ectopically expressed smARF to induce autophagy was significantly reduced. gC1qR stabilizes the smARF [80]. Mitochondrial gC1qR has also been shown to be a substrate for ERK and an integral part of the MAP kinase cascade [81]. It is also a general protein kinase C (PKC)-binding protein [70]; it binds to and regulates the activity of PKC isoforms PKC-α, PKC-ζ, PKC-δ, and PKC-µ (the latter being constitutively associated with gC1qR at mitochondrial membranes) without being their substrate [82]. Several lines of evidence suggest that mitochondrial PKC may directly regulate PTP status, at least in heart [83], and the involvement of PTP in cell apoptosis is suggested in so many publications that it is difficult to provide a key reference. The most recent data strongly linking gC1qR to Ca2+-related mitochondrial dysfunction and apoptosis was obtained by Chowdhury et al. [84], who demonstrated that constitutively expressing gC1qR in a normal murine fibroblast cell line induced growth perturbation, swelling and derangements of cristae in cell mitochondria, release of cytochrome c and formation of apoptosome complexes. They also showed that mitochondrial dysfunction was due to a gradual increase in ROS generation in cells over-expressing gC1qR. Together with ROS generation, they found an increased Ca2+ influx in mitochondria, resulting in a decreased membrane potential and severe inhibition of the respiratory chain complex I [84].
How could gC1qR participate in both the PTP channel formation and in Ca2+ uniport?
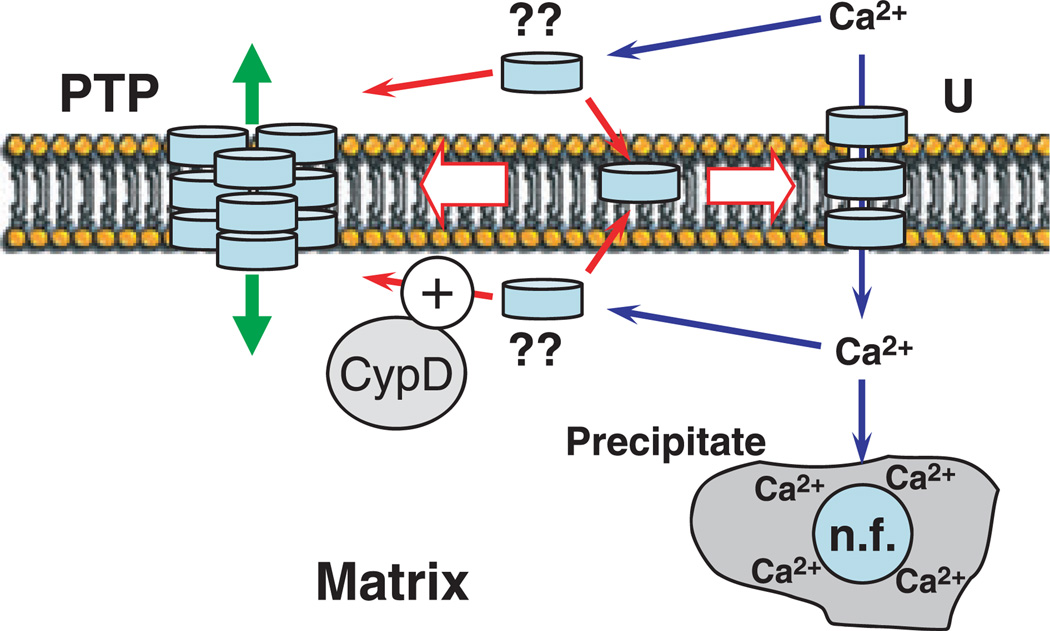
On the basis of the structural features of gC1qR, a novel mechanism of PTP formation and Ca2+ transport can be proposed that involves the same protein in both systems (Fig. 2). According to this model, gC1qR ‘protomers’ are present in the intermembrane space, the inner membrane and the matrix of mitochondria, but the ‘Ca2+ uniporter’, consisting in this case of IM-and perhaps matrix-located gC1qR protomers, is not assembled to its fully active form. When a threshold of Ca2+ is reached, a few protomers of gC1qR migrate from the intermembrane space to the IM and bind to the protomer located in the IM. This creates a fully functional Ca2+ uniporter. The binding does not occur in the presence of ruthenium red, which blocks it by interacting with the glycosyl residues of IM-embedded protomers. Prolonged accumulation of Ca2+ results in its concentration in the matrix and the intermembrane space exceeding another Ca2+ threshold, triggering the formation of a larger, multi-component PTP channel (Fig. 2). Both the Ca2+ transport system and the PTP have to be pre-assembled for their full activity, and our hypothesis is that they are two stages of the same process of Ca2+-dependent assembly of gC1qR protomers, perhaps in co-operation with some other regulatory proteins. The PTP channel in the IM may be formed by a gC1qR multimer comprising several (e.g. three in Fig. 2) identical gC1qR trimers stacked onto each other. Formation of the multimer means that the structure acquires sufficient hydrophobicity to move into the IM and form a transmembrane channel. Formation of this structure is caused by Ca2+ accumulation in the matrix, but not necessarily an increase in free Ca2+ concentration. For example, gC1qR trimers, which are inherently capable of binding Me2+ ions, may initially be sequestered by another Me2+-binding protein in the matrix as a gC1qR*Mg2+ complex, and the Me2+-binding protein may also be capable of serving as a nucleation factor catalyzing the formation of Ca2+-phosphate precipitates. When the latter accumulate, they displace gC1qR from the complex, thereby priming it to PTP. The next step may be a change in gC1qR conformation that would increase the probability of its interaction with another gC1qR trimer to form a hexamer. This conformational change may be facilitated by binding to cyclophilin D. The last step is a further Ca2+-dependent change in conformation of this newly formed hexameric gC1qR–cyclophilin D complex to allow it to translocate into the IM and form the PTP. In this model, chelating free Ca2+ by EGTA would force the structure to leave the IM, suppressing channel formation, but will not reverse the entire process because it could not quickly remove the Ca2+-phosphate precipitates that have already formed. Therefore, the pore-forming complex will remain primed and ready for repeated cycles of PTP opening and closing. On the other hand, Cys186 mediated formation of disulfide bridges between the two gC1qR trimers would render the channel structure permanent and insensitive to modulation by Ca2+ or cyclophilin D, thereby producing an ‘unregulated’ pore. This PTP model can easily accommodate most if not all known data on PTP activation and regulation, including its sensitivity to a wide variety of chemically dissimilar compounds and even to the changes in the conformation of major IM proteins that are capable of modifying the surface charge of the IM, such as ANT.
Fig. 2.
Proposed model of CA2+ uniporter and PTP assembly. The ‘protomers’ (flat disks) of a putative protein forming the ‘Ca2+ uniporter’ (‘U’) and PTP (e.g. gC1qR as discussed in the text) are present in the intermembrane space, the inner membrane and the matrix of mitochondria, but the ‘Ca2+ uniporter’ consisting of IM- and matrix-located protomers, is not assembled to its fully active form. When a threshold amount of Ca2+ is reached, a few protomers migrate from the intermembrane space to the IM and bind to the protomer located in the IM. This creates a fully functional ‘Ca2+ uniporter’. Such binding does not occur in the presence of ruthenium red, which blocks it by interacting with the glycosyl residues of IM-embedded protomers. Accumulated Ca2+ and phosphate bind to an unidentified ‘nucleation factor’ (‘n.f.’) that prevents the formation of crystalline Ca2+-phosphate precipitates. Upon prolonged accumulation of Ca2+, the storage capacity of this ‘nucleation factor’ is exceeded, and the Ca2+ concentration in the matrix and the intermembrane space rises above the threshold for PTP assembly, thereby triggering formation of a larger multi-component PTP channel (see text for further details).
Concluding remarks
Obviously, this model is highly speculative as there are no data that directly support its key features. However, there are three predictions about this mechanism that can be verified experimentally. First, gC1qR has to physically move into the IM to form a PTP, i.e., upon accumulation of Ca2+ and phosphate, the distribution of free gC1qR between the mitochondrial compartments should change dramatically towards the IM, preceding opening of the PTP. Second, knocking out the gC1qR protein should prevent Ca2+-induced PTP formation or at least severely increase its Ca2+ threshold. Third, antibodies against gC1qR should inhibit Ca2+ uptake in mitoplasts. We are currently trying to verify these predictions experimentally.
Acknowledgement
This work was supported by National Institutes of Health grant number NS065396 to A.S.
Abbreviations
- ANT
adenine nucleotide translocase
- CGP37157
7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one
- CypD
cyclophilin D
- EKR
extracellular-signal-regulated kinase
- Hrk
a product of harakiri gene
- IM
inner mitochondrial membrane
- PTP
permeability transition pore
- Ru360
C2H26Cl3N8O5Ru2
- smARF
“short mitochondrial ARF”, a short isoform of p19ARF protein
References
- 1.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, et al. NCLX is an essential component of mitochondrial Na+ /Ca2+ exchange. Proc Natl Acad Sci USA. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinopoulos C, Adam-Vizi V. Mitochondrial Ca2+ sequestration and precipitation revisited. FEBS J. 2010;277:3637–3651. doi: 10.1111/j.1742-4658.2010.07755.x. [DOI] [PubMed] [Google Scholar]
- 4.Pivovarova NB, Andrews SB. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 2010;277:3622–3636. doi: 10.1111/j.1742-4658.2010.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 6.Litsky ML, Pfeiffer DR. Regulation of the mitochondrial Ca2+ uniporter by external adenine nucleotides: the uniporter behaves like a gated channel which is regulated by nucleotides and divalent cations. Biochemistry. 1997;36:7071–7080. doi: 10.1021/bi970180y. [DOI] [PubMed] [Google Scholar]
- 7.Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem. 1995;270:27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- 8.Igbavboa U, Pfeiffer DR. Regulation of reverse uniport activity in mitochondria by extramitochondrial divalent cations. Dependence on a soluble intermembrane space component. J Biol Chem. 1991;266:4283–4287. [PubMed] [Google Scholar]
- 9.Kasparinsky FO, Vinogradov AD. Slow Ca2+-induced inactive / active transition of the energy-dependent Ca2+ transporting system of rat liver mitochondria: clue for Ca2+ influx cooperativity. FEBS Lett. 1996;389:293–296. doi: 10.1016/0014-5793(96)00606-0. [DOI] [PubMed] [Google Scholar]
- 10.Sottocasa GL, Sandri G, Panfili E, De Bernard B. A glycoprotein located in the intermembrane space of rat liver mitochondria. FEBS Lett. 1971;17:100–105. doi: 10.1016/0014-5793(71)80574-4. [DOI] [PubMed] [Google Scholar]
- 11.Lehninger AL. A soluble, heat-labile, high-affinity Ca2+-binding factor extracted from rat liver mitochondria. Biochem Biophys Res Commun. 1971;42:312–318. doi: 10.1016/0006-291x(71)90104-5. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Puyou A, De Gomez-Puyou MT, Becker G, Lehninger AL. An insoluble Ca 2+-binding factor from rat liver mitochondria. Biochem Biophys Res Commun. 1972;47:814–819. doi: 10.1016/0006-291x(72)90565-7. [DOI] [PubMed] [Google Scholar]
- 13.Blondin GA. Isolation of a divalent cation ionophore from beef heart mitochondria. Biochem Biophys Res Commun. 1974;56:97–105. doi: 10.1016/s0006-291x(74)80320-7. [DOI] [PubMed] [Google Scholar]
- 14.Sottocasa G, Sandri G, Panfili E, De Bernard B, Gazzotti P, Vasington FD, Carafoli E. Isolation of a soluble Ca2+ binding glycoprotein from ox liver mitochondria. Biochem Biophys Res Commun. 1972;47:808–813. doi: 10.1016/0006-291x(72)90564-5. [DOI] [PubMed] [Google Scholar]
- 15.Jeng AY, Ryan TE, Shamoo AE. Isolation of a low molecular weight Ca2+ carrier from calf heart inner mitochondrial membrane. Proc Natl Acad Sci USA. 1978;75:2125–2129. doi: 10.1073/pnas.75.5.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeng AY, Shamoo AE. Isolation of a Ca2+ carrier from calf heart inner mitochondrial membrane. J Biol Chem. 1980;255:6897–6903. [PubMed] [Google Scholar]
- 17.Shamoo AE, Jeng AY. Possible calcium carrier from the inner membrane of calf heart mitochondria. Ann NY Acad Sci. 1978;307:235–237. doi: 10.1111/j.1749-6632.1978.tb41951.x. [DOI] [PubMed] [Google Scholar]
- 18.Panfili E, Sottocasa GL, Sandri G, Liut G. The Ca2+-binding glycoprotein as the site of metabolic regulation of mitochondrial Ca2+ movements. Eur J Biochem. 1980;105:205–210. doi: 10.1111/j.1432-1033.1980.tb04490.x. [DOI] [PubMed] [Google Scholar]
- 19.Makhmudova EM, Gagel’gans AI, Mirkhodzhaev UZ, Tashmukhamedov BA. Effect of the mitochondrial ionophore for divalent cations on bilayer phospholipid membranes. Biofizika. 1975;20:225–227. In Russian. [PubMed] [Google Scholar]
- 20.Mironova GD, Baumann M, Kolomytkin O, Krasichkova Z, Berdimuratov A, Sirota T, Virtanen I, Saris NE. Purification of the channel component of the mitochondrial calcium uniporter and its reconstitution into planar lipid bilayers. J Bioenerg Biomembr. 1994;26:231–238. doi: 10.1007/BF00763072. [DOI] [PubMed] [Google Scholar]
- 21.Mironova GD, Sirota TV, Pronevich LA, Trofimenko NV, Mironov GP, Grigorjev PA, Kondrashova MN. Isolation and properties of Ca2+-transporting glycoprotein and peptide from beef heart mitochondria. J Bioenerg Biomembr. 1982;14:213–225. doi: 10.1007/BF00751016. [DOI] [PubMed] [Google Scholar]
- 22.Prestipino G, Ceccarelli D, Conti F, Carafoli E. Interactions of a mitochondrial Ca2+-binding glycoprotein with lipid bilayer membranes. FEBS Lett. 1974;45:99–103. doi: 10.1016/0014-5793(74)80820-3. [DOI] [PubMed] [Google Scholar]
- 23.Zazueta C, Masso F, Paez A, Bravo C, Vega A, Montano L, Vazquez M, Ramirez J, Chavez E. Identification of a 20-kDa protein with calcium uptake transport activity. Reconstitution in a membrane model. J Bioenerg Biomembr. 1994;26:555–562. doi: 10.1007/BF00762740. [DOI] [PubMed] [Google Scholar]; 23a Lars Ernster. “Bioenergetics” New comprehensive biochemistry v9. Amsterdam, New York: Elsevier; 1984. pp. 274–277. New York, NY, USA. [Google Scholar]
- 24.Saris NE, Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry (Mosc) 2005;70:187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- 25.Panfili E, Sandri G, Liut G, Stancher B, Sottocasa GL. In: Calcium-Binding Proteins. de Bernard B, Sottocasa GL, Sandri G, Carafoli E, Taylor AN, Vanaman TC, Williams RJP, editors. Amsterdam: Elsevier Science Publishers; 1983. pp. 347–354. [Google Scholar]
- 26.Sandri G, Panfili E, Sottocasa GL. Intramitochondrial location of the calcium binding glycoprotein. Acta Vitaminol Enzymol. 1974;28:317–322. [PubMed] [Google Scholar]
- 27.Sandri GP, Panfili E, Sottocasa GL. Specific association of the Ca2+ binding glycoprotein to inner mitochondrial membrane. Bull Mol Biol Med. 1978;3:179–188. [Google Scholar]
- 28.Sandri G, Sottocasa G, Panfili E, Liut G. The ability of the mitochondrial Ca2+-binding glycoprotein to restore Ca2+ transport in glycoprotein-depleted rat liver mitochondria. Biochim Biophys Acta. 1979;558:214–220. doi: 10.1016/0005-2736(79)90061-0. [DOI] [PubMed] [Google Scholar]
- 29.Sandri G, Panfili E, Sottocasa GL. The calcium-binding glycoprotein and mitochondrial calcium movements. Biochem Biophys Res Commun. 1976;68:1272–1279. doi: 10.1016/0006-291x(76)90334-x. [DOI] [PubMed] [Google Scholar]
- 30.Panfili E, Sandri G, Sottocasa GL, Lunazzi G, Liut G, Graziosi G. Specific inhibition of mitochondrial Ca2+ transport by antibodies directed to the Ca2+-binding glycoprotein. Nature. 1976;264:185–186. doi: 10.1038/264185a0. [DOI] [PubMed] [Google Scholar]
- 31.Sottocasa G, Panfili E, Sandri G. The problem of mitochondrial calcium transport. Bull Mol Biol Med. 1977;2:1–28. [Google Scholar]
- 32.Jeng AY, Shamoo AE. The electrophoretic properties of a Ca2+ carrier isolated from calf heart inner mitochondrial membrane. J Biol Chem. 1980;255:6904–6912. [PubMed] [Google Scholar]
- 33.Shamoo AE, Brenza JM. Calciphorin structure and function. Ann NY Acad Sci. 1980;358:73–82. doi: 10.1111/j.1749-6632.1980.tb15387.x. [DOI] [PubMed] [Google Scholar]
- 34.Sokolove PM, Brenza JM. Isolation of a fraction with Ca2+ ionophore properties from rat liver mitochondria. Arch Biochem Biophys. 1983;221:404–416. doi: 10.1016/0003-9861(83)90159-5. [DOI] [PubMed] [Google Scholar]
- 35.Sokolove PM, Brenza JM, Shamoo AE. Ca2+-cardiolipin interaction in a model system. Selectivity and apparent high affinity. Biochim Biophys Acta. 1983;732:41–47. doi: 10.1016/0005-2736(83)90184-0. [DOI] [PubMed] [Google Scholar]
- 36.Ambudkar IS, Kima PE, Shamoo AE. Characterization of calciphorin, the low molecular weight calcium ionophore, from rat liver mitochondria. Biochim Biophys Acta. 1984;771:165–170. doi: 10.1016/0005-2736(84)90528-5. [DOI] [PubMed] [Google Scholar]
- 37.Saris NE, Sirota TV, Virtanen I, Niva K, Penttila T, Dolgachova LP, Mironova GD. Inhibition of the mitochondrial calcium uniporter by antibodies against a 40-kDa glycoprotein. J Bioenerg Biomembr. 1993;25:307–312. doi: 10.1007/BF00762591. [DOI] [PubMed] [Google Scholar]
- 38.Urry DW. A molecular theory of ion-conductng channels: a field-dependent transition between conducting and nonconducting conformations. Proc Natl Acad Sci USA. 1972;69:1610–1614. doi: 10.1073/pnas.69.6.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mironova GD, Utesheva ZhA. Molecular mechanism of calcium ion transport in mitochondria. I. Glycoprotein-peptide complex as a component of the electron transport system. Ukr Biokhim Zh. 1989;61:48–54. In Russian. [PubMed] [Google Scholar]
- 40.Zazueta C, Zafra G, Vera G, Sanchez C, Chavez E. Advances in the purification of the mitochondrial Ca2+ uniporter using the labeled inhibitor 103Ru360. J Bioenerg Biomembr. 1998;30:489–498. doi: 10.1023/a:1020546331217. [DOI] [PubMed] [Google Scholar]
- 41.Zazueta C, Correa F, Garcia N, Garcia Gde J. Different subunit location of the inhibition and transport sites in the mitochondrial calcium uniporter. J Bioenerg Biomembr. 2004;36:439–445. doi: 10.1023/B:JOBB.0000047326.30536.86. [DOI] [PubMed] [Google Scholar]
- 42.Correa F, Zazueta C. Mitochondrial glycosidic residues contribute to the interaction between ruthenium amine complexes and the calcium uniporter. Mol Cell Biochem. 2005;272:55–62. doi: 10.1007/s11010-005-6754-1. [DOI] [PubMed] [Google Scholar]
- 43.Greenawalt JW, Rossi CS, Lehninger AL. Effect of active accumulation of calcium and phosphate ions on the structure of rat liver mitochondria. J Cell Biol. 1964;23:21–38. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 45.Price PA, Lim JE. The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin-mineral complex. J Biol Chem. 2003;278:22144–22152. doi: 10.1074/jbc.M300744200. [DOI] [PubMed] [Google Scholar]
- 46.Tominaga M, Kurihara H, Honda S, Amakawa G, Sakai T, Tomooka Y. Molecular characterization of mitocalcin, a novel mitochondrial Ca2+-binding protein with EF-hand and coiled-coil domains. J Neurochem. 2006;96:292–304. doi: 10.1111/j.1471-4159.2005.03554.x. [DOI] [PubMed] [Google Scholar]
- 47.Hubbard MJ, McHugh NJ. Calbindin28kDa and calbindin30kDa (calretinin) are substantially localised in the particulate fraction of rat brain. FEBS Lett. 1995;374:333–337. doi: 10.1016/0014-5793(95)01135-2. [DOI] [PubMed] [Google Scholar]
- 48.Winsky L, Kuznicki J. Distribution of calretinin, calbindin D28k, and parvalbumin in subcellular fractions of rat cerebellum: effects of calcium. J Neurochem. 1995;65:381–388. doi: 10.1046/j.1471-4159.1995.65010381.x. [DOI] [PubMed] [Google Scholar]
- 49.Pasteels JL, Pochet R, Surardt L, Hubeau C, Chirnoaga M, Parmentier M, Lawson DE. Ultrastructural localization of brain ‘vitamin D-dependent’ calcium binding proteins. Brain Res. 1986;384:294–303. doi: 10.1016/0006-8993(86)91165-0. [DOI] [PubMed] [Google Scholar]
- 50.Yoshii K, Sugimoto K, Tai Y, Konishi R, Tokuda M. Purification, identification and phosphorylation of annexin I from rat liver mitochondria. Acta Med Okayama. 2000;54:57–65. doi: 10.18926/AMO/32286. [DOI] [PubMed] [Google Scholar]
- 51.Rainteau D, Mansuelle P, Rochat H, Weinman S. Characterization and ultrastructural localization of annexin VI from mitochondria. FEBS Lett. 1995;360:80–84. doi: 10.1016/0014-5793(95)00087-p. [DOI] [PubMed] [Google Scholar]
- 52.Genge BR, Wu LN, Wuthier RE. In vitro modeling of matrix vesicle nucleation: synergistic stimulation of mineral formation by annexin A5 and phosphatidylserine. J Biol Chem. 2007;282:26035–26045. doi: 10.1074/jbc.M701057200. [DOI] [PubMed] [Google Scholar]
- 53.Dobi A, v Agoston D. Submillimolar levels of calcium regulates DNA structure at the dinucleotide repeat (TG/AC)n . Proc Natl Acad Sci USA. 1998;95:5981–5986. doi: 10.1073/pnas.95.11.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinbach EC, Von Brand T. Formation, isolation and composition of dense granules from mitochondria. Biochim Biophys Acta. 1967;148:256–266. doi: 10.1016/0304-4165(67)90301-7. [DOI] [PubMed] [Google Scholar]
- 55.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 56.Forte M, Bernardi P. Genetic dissection of the permeability transition pore. J Bioenerg Biomembr. 2005;37:121–128. doi: 10.1007/s10863-005-6565-9. [DOI] [PubMed] [Google Scholar]
- 57.Halestrap AP, Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10:1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- 58.Nicholls DG. Mitochondria and calcium signaling. Cell Calcium. 2005;38:311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Leung AW, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta. 2008;1777:946–952. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. Febs J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 61.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 62.Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J. 1998;336:287–290. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 64.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 65.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 67.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Mol-kentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 70.Robles-Flores M, Rendon-Huerta E, Gonzalez-Aguilar H, Mendoza-Hernandez G, Islas S, Mendoza V, Ponce-Castaneda MV, Gonzalez-Mariscal L, Lopez-Casillas F. p32 (gC1qBP) is a general protein kinase C (PKC)-binding protein; interaction and cellular localization of p32–PKC complexes in ray hepatocytes. J Biol Chem. 2002;277:5247–5255. doi: 10.1074/jbc.M109333200. [DOI] [PubMed] [Google Scholar]
- 71.Muta T, Kang D, Kitajima S, Fujiwara T, Hamasaki N. p32 protein, a splicing factor 2-associated protein, is localized in mitochondrial matrix and is functionally important in maintaining oxidative phosphorylation. J Biol Chem. 1997;272:24363–24370. doi: 10.1074/jbc.272.39.24363. [DOI] [PubMed] [Google Scholar]
- 72.Soltys BJ, Kang D, Gupta RS. Localization of P32 protein (gC1q-R) in mitochondria and at specific extramitochondrial locations in normal tissues. Histochem Cell Biol. 2000;114:245–255. doi: 10.1007/s004180000191. [DOI] [PubMed] [Google Scholar]
- 73.Ghebrehiwet B, Peerschke EI. Structure and function of gC1q-R: a multiligand binding cellular protein. Immunobiology. 1998;199:225–238. doi: 10.1016/S0171-2985(98)80029-6. [DOI] [PubMed] [Google Scholar]
- 74.Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol. 2004;41:173–183. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 75.Tye AJ, Ghebrehiwet B, Guo N, Sastry KN, Chow BK, Peerschke EI, Lim BL. The human gC1qR/p32 gene, C1qBP. Genomic organization and promoter analysis. J Biol Chem. 2001;276:17069–17075. doi: 10.1074/jbc.M009064200. [DOI] [PubMed] [Google Scholar]
- 76.Jiang J, Zhang Y, Krainer AR, Xu RM. Crystal structure of human p32, a doughnut-shaped acidic mitochondrial matrix protein. Proc Natl Acad Sci USA. 1999;96:3572–3577. doi: 10.1073/pnas.96.7.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J Biol Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 78.Jha BK, Salunke DM, Datta K. Disulfide bond formation through Cys186 facilitates functionally relevant dimerization of trimeric hyaluronan-binding protein 1 (HABP1)/p32/gC1qR. Eur J Biochem. 2002;269:298–306. doi: 10.1046/j.0014-2956.2001.02654.x. [DOI] [PubMed] [Google Scholar]
- 79.Sunayama J, Ando Y, Itoh N, Tomiyama A, Sakurada K, Sugiyama A, Kang D, Tashiro F, Gotoh Y, Kuchino Y, et al. Physical and functional interaction between BH3-only protein Hrk and mitochondrial pore-forming protein p32. Cell Death Differ. 2004;11:771–781. doi: 10.1038/sj.cdd.4401418. [DOI] [PubMed] [Google Scholar]
- 80.Reef S, Shifman O, Oren M, Kimchi A. The autophagic inducer smARF interacts with and is stabilized by the mitochondrial p32 protein. Oncogene. 2007;26:6677–6683. doi: 10.1038/sj.onc.1210485. [DOI] [PubMed] [Google Scholar]
- 81.Majumdar M, Meenakshi J, Goswami SK, Datta K. Hyaluronan binding protein 1 (HABP1)/C1QBP/p32 is an endogenous substrate for MAP kinase and is translocated to the nucleus upon mitogenic stimulation. Biochem Biophys Res Commun. 2002;291:829–837. doi: 10.1006/bbrc.2002.6491. [DOI] [PubMed] [Google Scholar]
- 82.Storz P, Hausser A, Link G, Dedio J, Ghebrehiwet B, Pfizenmaier K, Johannes FJ. Protein kinase Cl is regulated by the multifunctional chaperon protein p32. J Biol Chem. 2000;275:24601–24607. doi: 10.1074/jbc.M002964200. [DOI] [PubMed] [Google Scholar]
- 83.Budas GR, Mochly-Rosen D. Mitochondrial protein kinase Ce (PKCe): emerging role in cardiac protection from ischaemic damage. Biochem Soc Trans. 2007;35:1052–1054. doi: 10.1042/BST0351052. [DOI] [PubMed] [Google Scholar]
- 84.Chowdhury AR, Ghosh I, Datta K. Excessive reactive oxygen species induces apoptosis in fibroblasts: role of mitochondrially accumulated hyaluronic acid binding protein 1 (HABP1/p32/gC1qR) Exp Cell Res. 2008;314:651–667. doi: 10.1016/j.yexcr.2007.10.033. [DOI] [PubMed] [Google Scholar]