Abstract
BACKGROUND & AIMS
Pancreatic β-cell mass increases in response to increased demand for insulin, but the factors involved are largely unknown. Glial cell line-derived neurotrophic factor (GDNF) is a growth factor that plays a role in the development and survival of the enteric nervous system. We investigated the role of GDNF in regulating β-cell survival.
METHODS
Studies were performed using the β-TC-6 pancreatic β-cell line, isolated mouse pancreatic β-cells, and in vivo in transgenic mice that overexpress GDNF in pancreatic glia. GDNF receptor family αl and c-Ret receptor expression were assessed by RT-PCR and immunofluorescence microscopy. Apoptosis was evaluated by assessing caspase-3 cleavage. Phosphoinositol-3-kinase signaling pathway was analyzed by Akt phosphorylation. Glucose homeostasis was assessed by performing intraperitoneal glucose tolerance tests. Insulin sensitivity was assessed using intraperitoneal injection of insulin.
RESULTS
We demonstrate the presence of receptors for GDNF, GFR-α1 and c-Ret on β-cells. GDNF promoted β-cell survival, proliferation and protected them from thapsigargin-induced apoptosis (P<0.0001). GDNF resulted in phosphorylation of Akt and GSK3β. Transgenic mice that overexpress GDNF in glia exhibit increased β-cell mass, proliferation and insulin content. No differences in insulin sensitivity and c-peptide levels were noted. Compared to wild-type mice GDNF-transgenic mice have significantly lower blood glucose levels and improved glucose tolerance (P<0.01). GDNF-transgenic mice are resistant to streptozotocin-induced β-cell loss (P<0.001) and subsequent hyperglycemia.
CONCLUSIONS
We demonstrate that overexpression of GDNF in pancreatic glia improves glucose tolerance and that GDNF may be a therapeutic target for improving β-cell mass.
Keywords: GDNF, β-cells, enteric neurons, hyperglycemia, diabetes, apoptosis, PI-3-kinase, Akt, GFR1α
Type 1 and type 2 diabetes are characterized by loss or dysfunction of β-cells. The rate of β-cell proliferation, hyperplasia, neogenesis, and death combine to determine β-cell mass1, and are influenced by exposure to insulin, IGF-1, glucose, glucagon like peptide-1, growth hormone and several other growth factors2. Among signaling cascades, the significance of the PI-3-kinase/Akt pathway in enhancing β-cell proliferation and survival has been recognized 3, 4.
Pancreatic β-cells share several biological properties with neuronal cells including expression of the neuronal differentiation factor neuroD/β2 and dependence on nerve growth factor (NGF) in vitro5. Moreover, multipotent precursors that generate both neural and pancreatic lineages have been isolated from pancreatic ductal cells and islets6 suggesting that neurotrophic factors such as GDNF might play a role in β-cell growth and survival
GDNF is a neurotrophic factor that plays a critical role in the development and survival of the enteric nervous system 7. GDNF mediates its functions through binding to a multi-component receptor complex consisting of the Ret receptor tyrosine kinase and the glycosylphosphoinositol (GPI)-anchored co-receptor, GFRα18. Although the role of GDNF in pancreatic β-cell growth and survival is not known, reports of increased expression of GDNF and other neurotrophic factors in the proximity of pancreatic β-cells following islet injury 9 raises the possibility that GDNF and other neurotrophic factors might be involved in islet repair. In the present study we examined the role of GDNF in the regulation of β-cell proliferation and survival and the physiologic consequence on glucose homeostasis. We show that pancreatic β-cells express receptors for GDNF, and that GDNF supports the survival and proliferation of β-cells in vitro. Using transgenic mice that over express GDNF in glial cells we show that GDNF promotes increased β-cell mass, improves glycemic control, and protects against β-cell destruction in vivo.
Methods
Materials
The following antibodies were used for immunocytochemistry: rabbit polyclonal antibodies to GDNF (D-20) and GFRα1 (H-70) (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), human c-Ret (R787: IBL, Japan), S-100β (BD Pharmingen, San Diego, CA, U.S.A.), cleaved caspase-3 (Cell Signaling Technologies, Danvers, MA, U.S.A.), and guinea pig anti-insulin (Zymed Laboratories, San Francisco, CA, U.S.A.) were used at 1:50 dilution. Biotin-conjugated donkey anti-rabbit IgG secondary antibody and peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Westgrove, PA, U.S.A.), Alexa Fluor 488 and 594 donkey anti-guinea pig and rabbit IgG (Molecular Probes, Eugene, OR, U.S.A) and Ki67 (Novocastra, Newcastle, U.K.) were used at a 1:500 dilution.
For Western blotting, rabbit polyclonal antibodies to phospho-Akt (ser473), Akt, and phospho-GSK3β, GSK3β and cleaved caspase-3 (Cell Signaling Technologies) were used at a 1:1000 dilution, streptavidin-horseradish peroxidase conjugated anti-rabbit and anti mouse IgG (Cell Signaling Technologies) at 1:2500 dilution, and mouse monoclonal antibody to β-actin (Sigma, St. Louis, MO, U.S.A.) at 1:5000 dilution.
Animals
In vivo studies were conducted in littermates obtained from crossing CF1 WT mice with GFAP-GDNF transgenic mice on a CF1 background generated at Washington University, in St. Louis, MO, U.S.A. GFAP-GDNF transgenic mice are engineered to overexpress GDNF in glial cells under the control of the glial fibrillary acidic protein promoter10. The genotypes of the mice were determined by PCR using DNA extracted from mouse tail using the REDExtract-N-Amp Tissue PCR Kit (Sigma-Aldrich CO, St. Louis, MO, USA) according to recommended procedure. Forward (5'-AGACGCATCACCTCCGCT-3') and reverse (5'-TGACGTCATCAAACTGGTCAGG-3') primers designed to only amplify the transgene sequence were used.
MIP-GFP transgenic mice 11 were used to isolate primary β-cells for in vitro studies. These mice express green fluorescent protein (GFP) in pancreatic β-cells under the control of the mouse insulin I promoter (MIP). The mice were used at 8–10 weeks of age. All animal studies were approved by the Emory University Animal care and use committee.
Islet isolation and cell culture
Islets were isolated using a modification of the procedure described by Bernal-Mizrachi et al 3. Pancreata were digested for 40 min at 37°C with 2 mg/ml collagenase (Type IV, Gibco BRL, Grand Island, NY, U.S.A.), and islets isolated by density gradient centrifugation using 1.108, 1.096, and 1.037 islet gradient (Mediatech Inc., Herndon, VA, U.S.A). To obtain single cells, islets were digested with 0.01% trypsin in modified Eagle's medium and dispersed by gently pipetting up and down 3. β-TC-6 cells (ATCC, Manassas, VA, U.S.A.) were cultured in Dulbecco's Modified Eagles' Medium (DMEM) (ATCC) supplemented with 15% fetal bovine serum. For GDNF stimulation studies cells were serum deprived for 48 hours, followed by incubation with DMEM alone or DMEM supplemented with different concentrations of GDNF.
PCR
First-strand cDNA synthesized using the Omniscript reverse transcription kit (Qiagen GmbH, Hilden, Germany) from RNA isolated using the RNeasy Mini kit (Qiagen) was subjected to 40 cycles of PCR amplification. The primers used included mouse GFRα1 forward (5'<ATGAAGAACGAGAGAGGCCCAA>3') and reverse (5'<ACTCTGGCTGGCAGTTGGTAAA>3') primers, Ret forward (5'<TACCGTACACGGCTGCATGAGAAT>3') and reverse (5'<ATGTGGAAGTGGTAGAAGGTGCCA>3') primers, and GDNF forward (5'<TCGATATTGCAGCGGTTCCTGT>3') and reverse (5'<ACATCCACACCGTTTAGCGGAA>3') primers. All primers used were designed to span at least an intron and thus are able to discriminate between products amplified from mRNA and genomic DNA.
Immunocytochemistry
β-TC-6 cells, isolated pancreatic β-cells, and pancreatic sections from frozen and paraffin-embedded tissues were stained for immunofluorescence microscopy as previously described 12 and analyzed on a Zeiss Axioskop 2 plus fluorescent microscope (Carl Zeiss Werk, Gottingen, Germany) mounted with an AxioCam MRc 5 camera. Images were taken with the aid of the Axiovision (Rel 4.5) software (Carl Zeiss Imaging System). Cells were also scanned with an LSM 510 laser scanning confocal microscope (Zeiss, Heidelberg, Germany).
Apoptosis
Apoptosis was assessed either by Western blotting for cleaved caspase-3 or cleaved caspase-3 with immunofluorescence microscopy 12, 13.
Proliferation
Cell proliferation in cultured cells and pancreatic tissue was assessed by immunofluorescence microscopy 13 using an anti-Ki67 polyclonal antibody 14.
Western blotting
Western blotting were performed as previously described12. A semiquantitative measurement of band density was performed using Scion Image for Windows software (Scion Corp, MD, U.S.A.).
Immunohistochemistry
Pancreata were frozen in Tissue-Tek O. C. T. compound (Sakura Finetek, Torrance, CA, U.S.A.) or fixed in 10% formalin solution and embedded in paraffin using standard techniques. Frozen sections were fixed in 4% paraformaldehyde and paraffin sections processed according to suggested protocols (Cell Signaling Technologies). Staining was performed according to standard protocols using the Histomouse-SP (AEC broad spectrum) kit (Zymed).
Assessment of β-cell mass and size
Four 5 µm pancreas sections (separated by 200 µm)/ per mouse were used to assess β-cell mass as previously described 3. Images were taken after insulin staining, and islet size, islet number, β-cell size and the total areas of the sections determined using the Image-Pro Plus 5.0 software (Media Cybernetics, Silver Spring, MD, U.S.A.). The percentage of β-cell area in each pancreas was then determined.
Assessment of insulin content
Pancreata were homogenized using acid-alcohol as previously described 15 and insulin levels measured by radioimmunoassay at Linco Diagnostic Services (St. Louis, MO, U.S.A.).
Glucose tolerance test and estimation of insulin sensitivity
Age-matched WT and GDNF-tg mice were fasted for 6 h and baseline blood glucose levels measured with the aid of an Accu-Check Advantage blood glucose meter (Roche, Mannheim, Germany) using blood collected from the tail vein. To tests for glucose tolerance, the mice were injected intraperitoneally with 2 mg glucose/g body weight in sterile PBS and blood glucose levels measured 30, 60 and 120 min after injection 3. For the insulin sensitivity test, fasted mice were injected intraperitoneally with 0.75 U/Kg human rapid insulin (Eli Lilly Co, Indianapolis, IN, USA), and blood glucose measured 15, 30, 60, 90, and 120 min after injection16.
Assessment of insulin and c-peptide secretion
For in vivo insulin secretion, 6-h fasted mice were injected with 3 mg glucose/Kg body weight and plasma insulin measured 0 and 2.5 min post-injection using a rat/mouse insulin ELISA kit (Linco). For in vitro insulin secretion analysis, isolated islets were cultured overnight in Ham's F10 medium, and 40 hand picked islets cultured per well for 2 h in Kreb's Ringer bicarbonate buffer containing 1.67 mM glucose followed by 1 h in buffer containing 1.67 mM or 20 mM glucose. The culture media were collected, the islets lysed in acid-alcohol, and insulin concentrations measured by insulin ELISA (Linco).Values are expressed as percent of islet content relative to basal secretion.
Resistance to induction of diabetes
WT and GDNF-tg littermates were injected intraperitoneally with 75 mg/Kg streptozotocin followed by another 75 mg/Kg streptozotocin after 12 h and their blood glucose levels measured once daily to monitor onset of hyperglycemia. Hyperglycemia was defined as post-prandial blood glucose greater than 145 mg/dL. At the end of the experiments, the mice were sacrificed, pancreata embedded in paraffin, sectioned and stained for insulin and islet images obtained. The outlines of islets were marked, the images thresholded based on insulin staining intensity, and threshold area relative to total islet area calculated with the aid of the MetaMorph Offline version 7.0r3 software (Molecular Devices Corp., Downingtown, PA, U.S.A.).
To assess apoptosis, pancreas sections from STZ-treated mice were made and apoptosis assessed by cleaved caspase-3 immunofluorescence microscopy with TUNEL staining12.
Statistical Analysis
All statistical analyses were conducted using the GraphPad Prism software version 3.00 for Windows (GraphPad Software, San Diego CA, U.S.A). Data were tested for normality and subjected to t-tests or One-way ANOVA with Tukey post test.
Results
Ret and GFRα1 receptors are expressed in β-TC-6 cells and mouse pancreatic β-cells
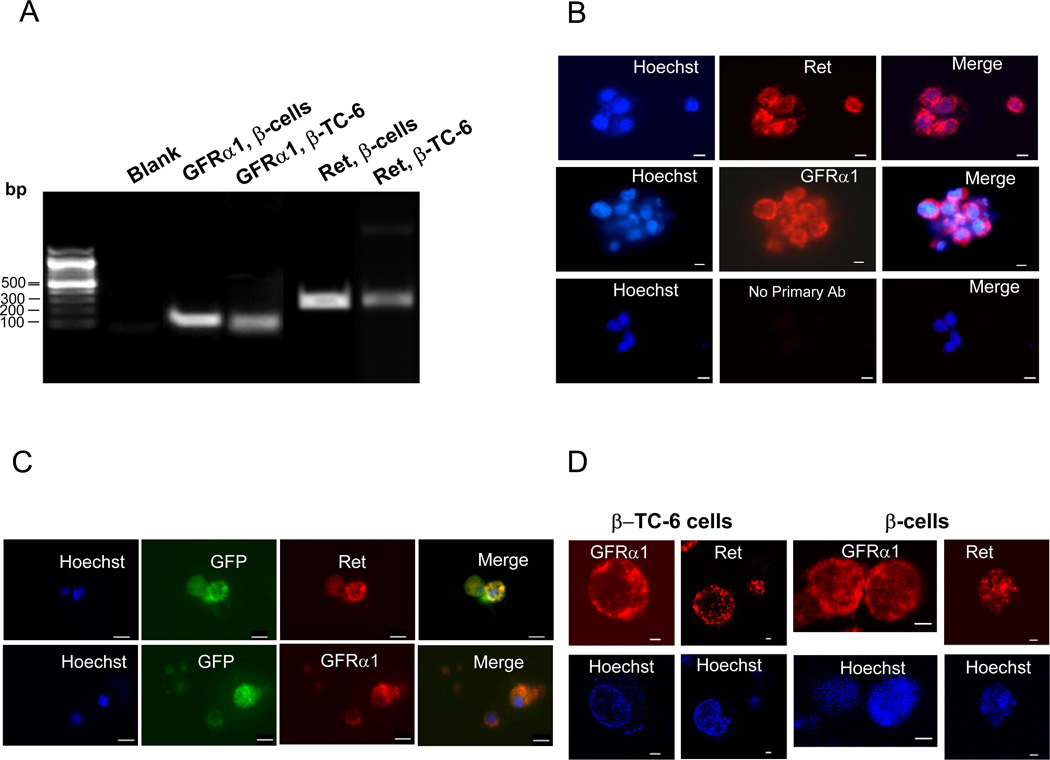
To understand the role of GDNF in β-cell growth and survival, we first analyzed by RT-PCR the expression of its receptors in cells of the insulin-secreting mouse pancreatic β-TC-6 cell line and mouse pancreatic β-cells. As seen in Fig. 1A, both cell types express significant amounts of GFRα1 and Ret receptor mRNA. The expression of the receptors was further analyzed by immunofluorescence microscopy using receptor-specific antibodies. High expression of both receptors was observed in cultured β-TC-6 cells and isolated mouse pancreatic β-cells (Fig. 1B and C). The localization of the receptors on the surface of the cells was confirmed by laser confocal microscopy (Fig. 1D).
Figure 1. Analysis of GFRa1 and Ret receptors expression.
(A) RT-PCR analysis of GFRα1 and Ret receptor mRNA expression in the β-TC-6 cell line and mouse pancreatic β-cells. (B) Immunofluorescence staining for GFRα1 and Ret receptors in β-TC-6 cells. Scale bar, 10 µm. Cell nuclei are labeled with the nuclear stain Hoechst (blue) (C) Immunofluorescence staining for GFRα1 and Ret receptors on pancreatic β-cells isolated from MIP-GFP mice. Scale bar, 10 µm. (D) Laser confocal microscopy images of β-TC-6 cells and mouse pancreatic β-cells stained with anti-GFRα1 and Ret antibodies. Scale bar: 2 µm.
GDNF promotes β–cell survival and proliferation in vitro
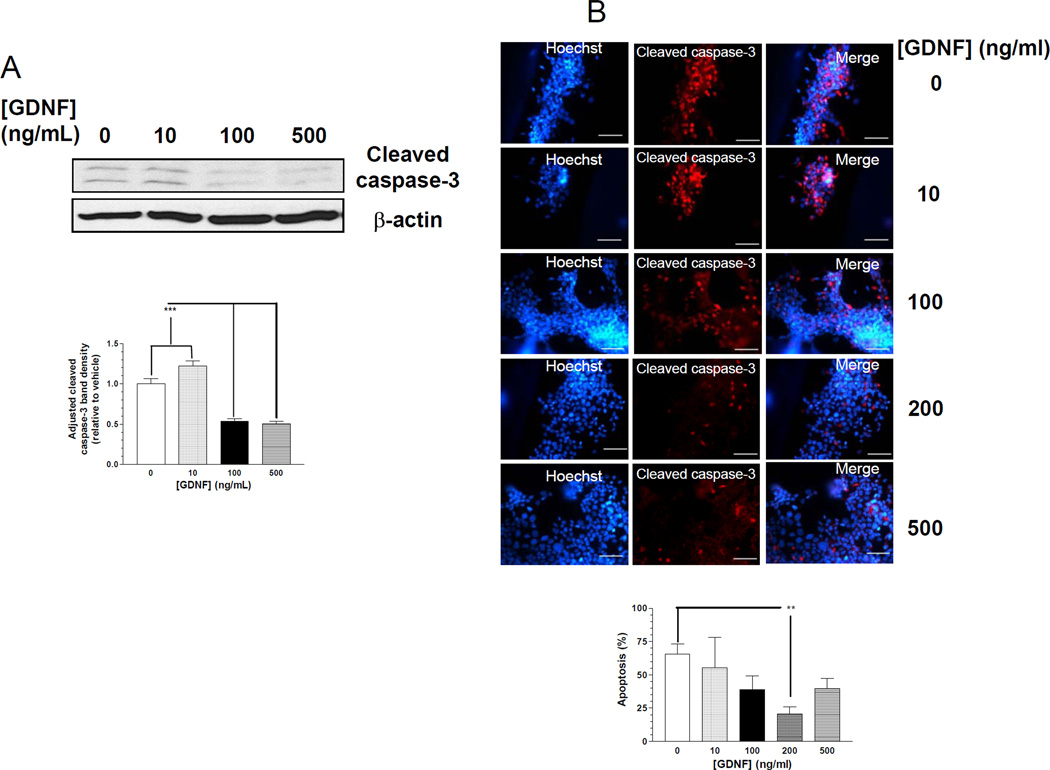
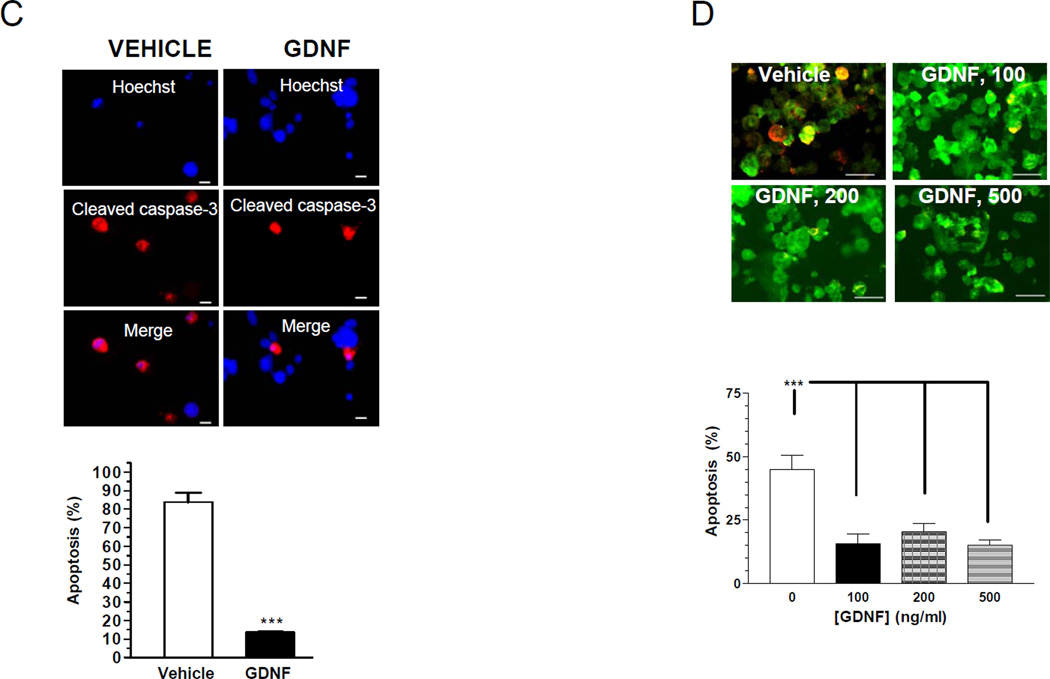
GDNF is known to be a trophic factor for neurons, but its role in β-cell growth and survival is not known. We thus tested if GDNF (10–500 ng/ml) could prevent apoptosis and promote proliferation of primary β-cells and β-TC-6 cells. Apoptosis was assessed by blotting for cleaved caspase-3 and cleaved caspase-3 immunocytochemistry. GDNF treatment for 72h suppressed caspase-3 cleavage in β-TC-6 cells in a dose-dependent fashion (Fig. 2A and B). To further investigate the ability of GDNF to promote β-cell survival, we assessed the ability of GDNF to block the effects of thapsigargin, a pro-apoptotic stimulus for β-cells. GDNF significantly reduced thapsigargin-induced apoptosis in β-TC-6 cells compared to vehicle treated cells (Fig. 2C). Similarly, isolated mouse pancreatic β-cells from MIP-GFP mice revealed a significant reduction in apoptosis when cultured for 48h in the presence of GDNF (100–500 ng/ml) compared to vehicle (Fig. 2D).
Figure 2. GDNF promotes β-cell survival.
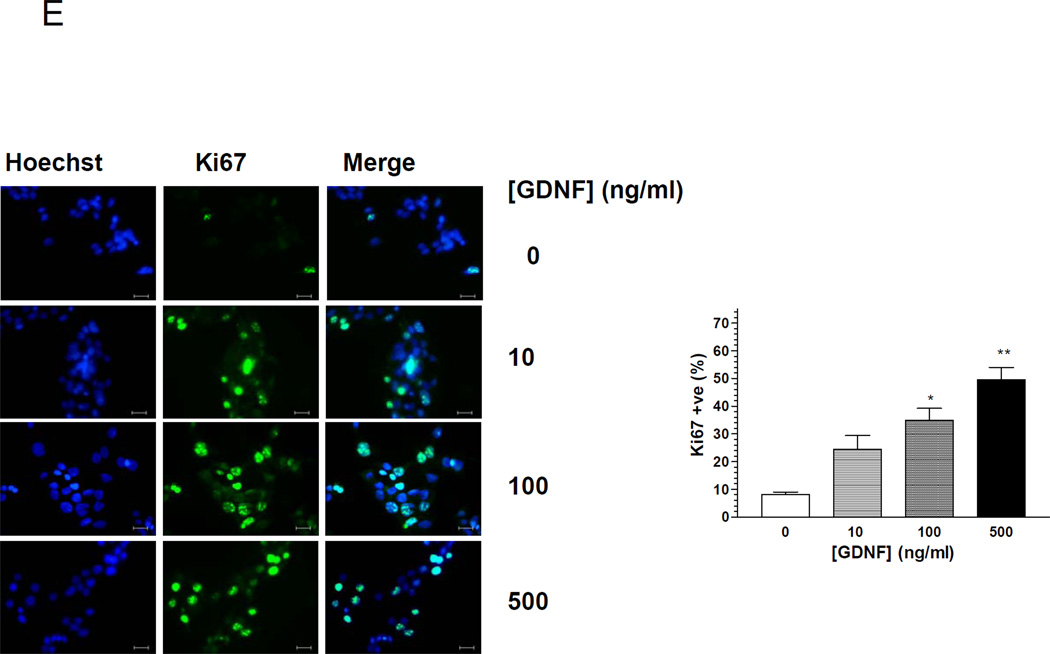
(A) Western blot analysis of cleaved caspase-3 in β-TC-6 cells cultured for 72 h in the presence of vehicle or GDNF, and histogram comparison of the relative band densities. Plotted are means ± SE. (B) Immunofluorescence staining for cleaved caspase-3 (red) and Hoechst (nuclear, blue) in β-TC-6 cells exposed for 48h to vehicle or GDNF, and histogram showing rate of apoptosis (C) Immunofluorescence staining for cleaved caspase-3 (red) and Hoechst nuclear staining (blue) in β-TC-6 cells exposed for 24 h to thapsigargin (0.1 µM) in the presence of vehicle or GDNF. Scale bar, 20 µm. Histogram shows the rates of apoptosis. (D) Representative images showing cleaved caspase-3 staining (yellow) in isolated MIP-GFP pancreatic β-cells (green) cultured for 24 h in the presence of vehicle or GDNF (100–500 ng/ml). Scale bar: 10 µm. Histogram shows rate of apoptosis (mean ± SE, ***, P<0.001). Data are representative of four independent experiments. (E) Assessment of proliferation by fluorescent staining for Ki67 (green) in β-TC-6 cells cultured for 24 h in serum-free medium with or without GDNF. Cell nuclei are counterstained with DAPI. Scale bar, 20 µm. Histogram shows the percentages of Ki67 positive cells (mean ± SE *, P<0.05; **, P<0.01). Data are representative of four independent experiments.
We next assessed if GDNF could promote the proliferation of β-cells in vitro. β-TC-6 cells were cultured in serum-free medium for 48 h followed by 24 h in the presence or absence of GDNF and assessed for proliferation by staining for the proliferation marker Ki6717. GDNF increased the number of Ki67 positive cells in a dose dependent fashion (Fig. 2E). Taken together these data suggest an important survival and proliferation role for GDNF in β-cells in vitro.
GDNF Signals through the PI-3-kinase/Akt pathway to promote β- cell survival
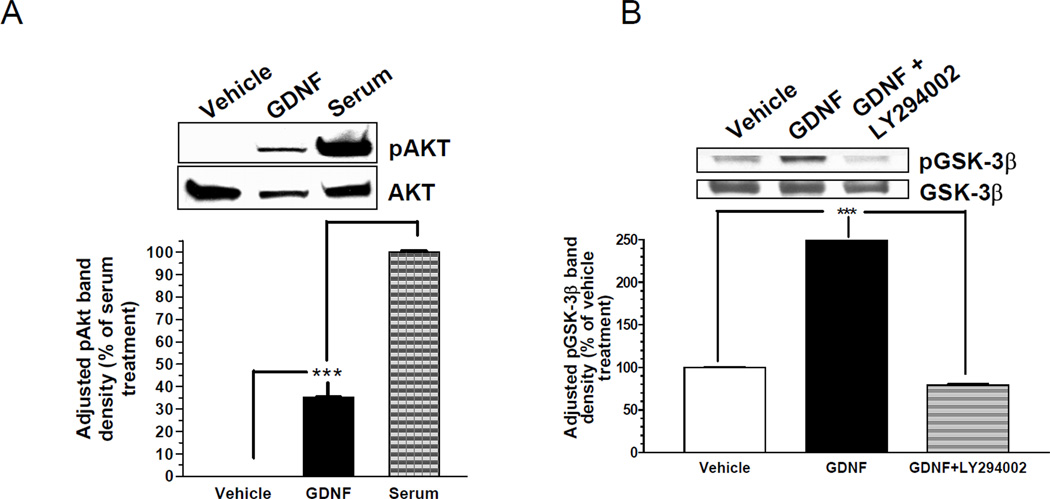
Since GDNF signals through the PI-3-kinase/Akt pathway in neurons to promote cell survival, we examined the possible activation of this pathway in β-cells by GDNF. β-TC-6 cells pre-cultured in serum-free medium for 48 h were stimulated with vehicle only (no GDNF), GDNF (100ng/ml) or serum (15%) for 30 min, and analyzed by Western blotting for Akt phosphorylated at ser 473. Significant amounts of phospho-Akt were detected in cells cultured in the presence of GDNF, but absent in cells cultured in vehicle only (P<0.001; Fig. 3A). We also investigated the ability of GDNF to stimulate the phosphorylation of glycogen synthase kinase-3β (GSK3β), a downstream target of Akt, in these cells. More than 2.7 fold more phospho-GSK3β (ser9) was detected by Western blotting in β-TC-6 cells cultured for 30 min with GDNF than in cells cultured in vehicle only (P<0.001; Fig. 3B). This increase was lost when the cells were cultured with GDNF in the presence of the PI3-kinase inhibitor LY294002. These data, thus, demonstrate that GDNF activates the PI-3-K/Akt signaling pathway in β-cells in vitro.
Figure 3. GDNF stimulates the phosphorylation of Akt and its downstream target GSK3β in β-TC-6 cells.
(A) Western blot analysis of Akt phosphorylation (ser473) in β-TC-6 cells treated with vehicle, GDNF (100ng/ml) or fetal bovine serum for 30 min. Total Akt was assessed to control for protein loading. Histogram shows relative phospho-Akt (pAkt) band densities (mean ± SE) adjusted for protein loading and expressed as a percentage of that of cells treated with serum. (B) Western blot analysis of GSK3β phosphorylation (ser9) and total GSK3β in β-TC-6 cells treated for 30 min with vehicle, GDNF (100 ng/ml) or GDNF plus the PI3K inhibitor LY294002 (50 µM). Histogram shows relative phospho-GSK3β band densities adjusted for protein loading to total GSK3β staining and expressed as a percentage of that of cells treated with vehicle (mean ± S.E, ***, P<0.001). Data are representative of four independent experiments.
Mice overexpressing GDNF in pancreatic glia have higher β-cell mass and β-cell proliferation
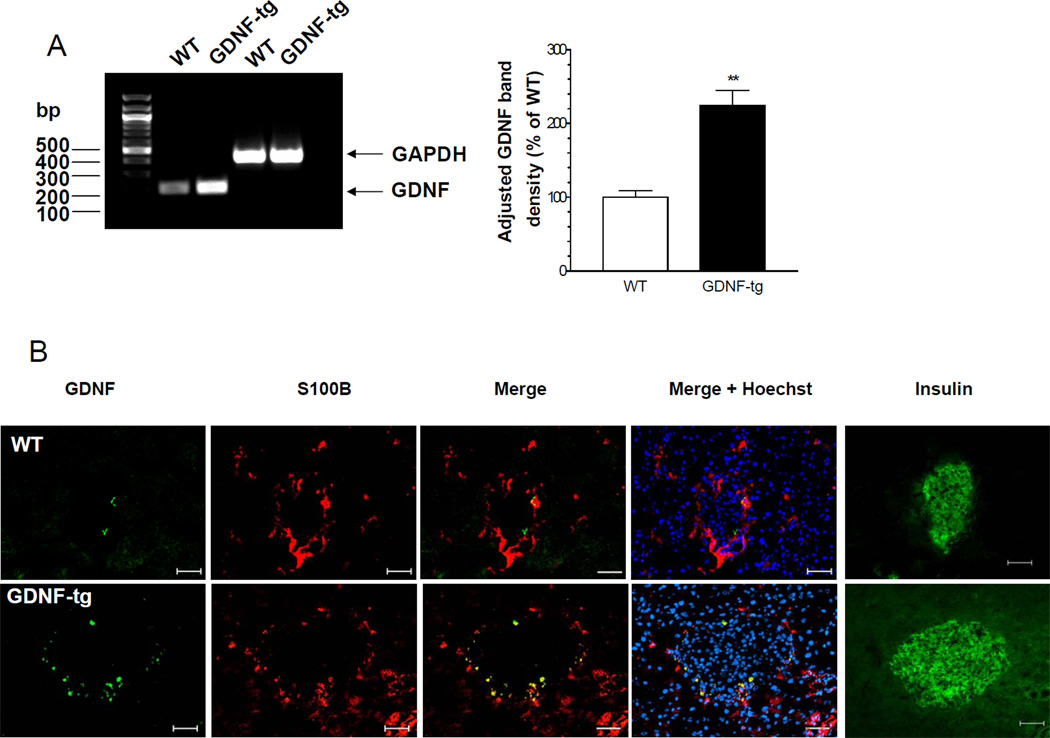
Having demonstrated the effects of GDNF in vitro we next examined the effects of GDNF in vivo using a GDNF transgenic (GDNF-tg) mouse in which the overexpression of GDNF has been demonstrated in astrocytes in the brain and spinal cord 10 and glia in the peripheral nervous system. Experiments were performed on GDNF-tg mice and their WT littermates. Both male and female GDNF-tg mice have approximately 20% lower body weight compared to their WT littermates (weight in gms at 8 Weeks, WT-M: 35.1 ± 1.4, GDNF-tg M: 30.8 ± 0.7 P<0.05, WT-F: 29.5 ± 0.8, GDNF-tg F: 23.7 ±1.2, P<0.01). Despite their lower body weights, GDNF-tg mice have normal eye opening, fur growth, and weaning and reproductive capacity, similar to WT mice. GDNF-tg mice have a slight tremor at birth that disappears by 2–3 Weeks of age. To confirm the overexpression of GDNF in the pancreas of GDNF-tg mice, we compared by RT-PCR the levels of GDNF mRNA in pancreas from WT and GDNF-tg mice. GDNF-tg mice had more than 2.2 fold more GDNF message than WT mice (P<0.01; Fig. 4A). To identify the specific areas of the pancreas where GDNF is expressed, sections of pancreas from both WT and GDNF-tg mice were immunofluorescently stained for GDNF. As seen in Fig. 4B, GDNF expression was localized to glial cells identified by the glial specific marker, S-100β, and was enhanced in GDNF-tg mice compared to WT mice.
Figure 4. GDNF-tg mice have increased GDNF expression in the islets.
(A) RT-PCR analysis of GDNF mRNA expression in pancreata from WT and GDNF-tg mice. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression shows equal mRNA loading. Histogram shows relative GDNF PCR band densities. (**, P<0.01). (B) Immunofluorescent staining of pancreas sections from WT and GDNF-tg mice showing co-localization of GDNF (green) with S100β (red). Cell nuclei are stained with Hoechst (blue). Insulin staining in the same islet from an adjacent section is shown (green). Scale bar 20 µm.
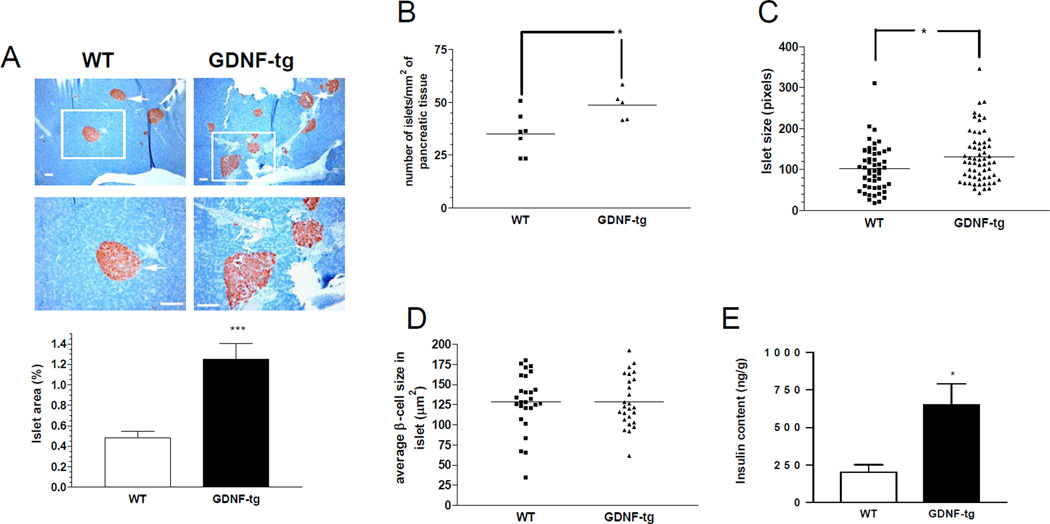
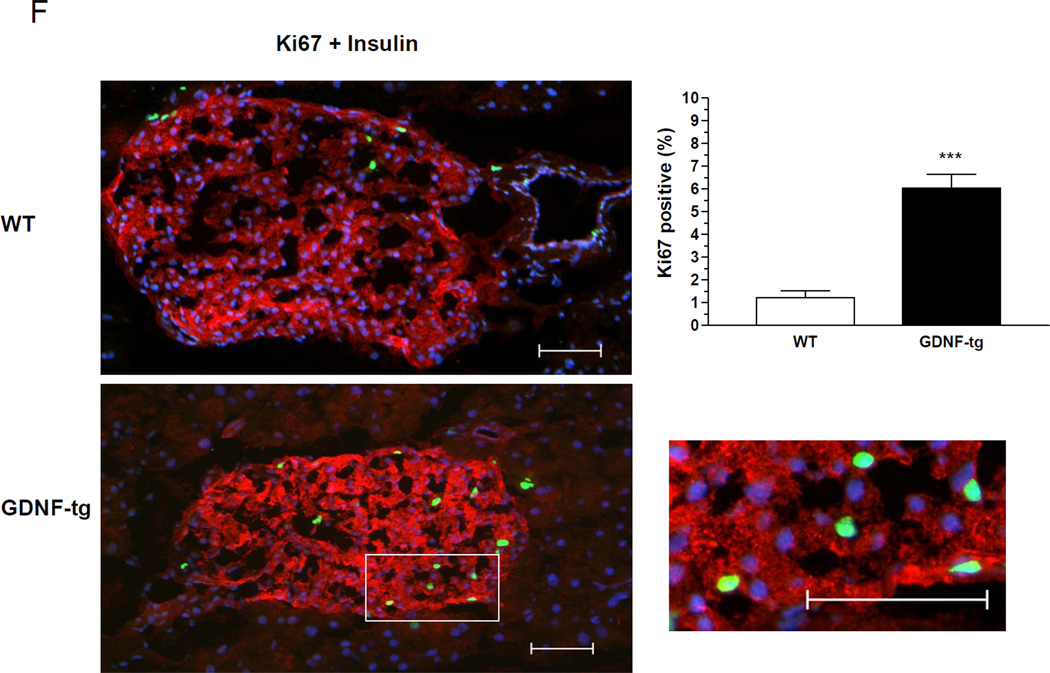
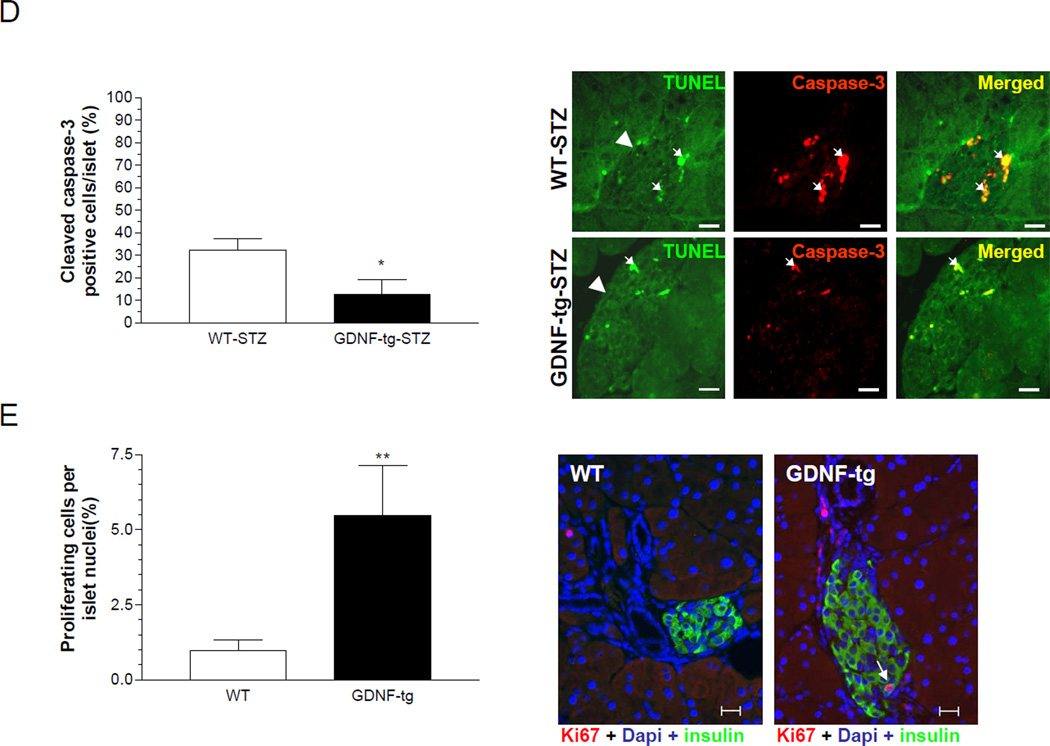
To assess the effects of increased GDNF expression in the pancreas, successive sections taken 200 µm apart from pancreata from 8 week-old WT and GDNF-tg mice were stained for insulin. Examination of these sections revealed significantly more islets in GDNF-tg mice than their WT littermates (Fig. 5A). A more detailed morphometric analysis to assess β-cell mass showed a 2.5 fold increase in β-cell area/pancreas in transgenic mice compared to WT mice (P<0.001; Fig. 5A). The number of islets per unit pancreatic tissue was higher in GDNF-tg mice compared to WT mice (P<0.05; Fig. 5B). The size of individual islets was higher in GDNF-tg mice compared to WT mice (P<0.05; Fig. 5C). However, individual β-cell size appeared similar (P >0.05; n=26, Fig. 5D) and no change in the islet architecture was noted, with a similar distribution of β and α cells. Assessment of pancreatic insulin content also revealed a 3.2 fold higher insulin content in GDNF-tg mice than in WT mice (P<0.05; Fig. 5E). To understand the processes accounting for the increased β-cell mass in GDNF-tg mice, WT and GDNF-tg mice were stained for insulin and Ki67 to assess β-cell proliferation (Fig. 5F). A significantly higher number of Ki67+/insulin+ cells were observed in islets of GDNF-tg mice compared to WT mice. (P<0.0001, n = 3).
Figure 5. GDNF transgenic mice have higher β-cell mass and increased proliferation compared to WT mice.
(A) Pancreatic sections from WT and GDNF-tg mice stained for insulin (red) and counterstained with hematoxylin (blue). Images in the lower panel are higher magnifications of the islets in the upper panel. Arrows show representative islets. Scale bar: 100 µm. Histogram shows comparison of β-cell mass (expressed as percentage insulin stained area/pancreatic area) between WT and GDNF-tg mice (means ± SE, ***, P<0.001). (B) Number of islets per unit pancreatic tissue area in WT and GDNF-tg mice. (C) Islet size of WT and GDNF-tg mice. (D) Comparison of β-cell sizes between WT and GDNF-tg mice (n=26, P>0.05). (E) Total insulin content of pancreata from WT and GDNF-tg mice (male and female, n= 6, *P<0.05). (F) Pancreatic sections from WT and GDNF-tg mice immunostained for Ki67 (green), insulin (red) and DAPI nuclear stain (blue) to show the presence of proliferating β-cells within islets. Higher magnification image of boxed area is shown. Scale bar; 20 µm. Histogram shows a plot of percentage of Ki67+ β-cells (mean ± SE, ***, P<0.001).
Functional evidence of improved glucose homeostasis in GDNF transgenic mice
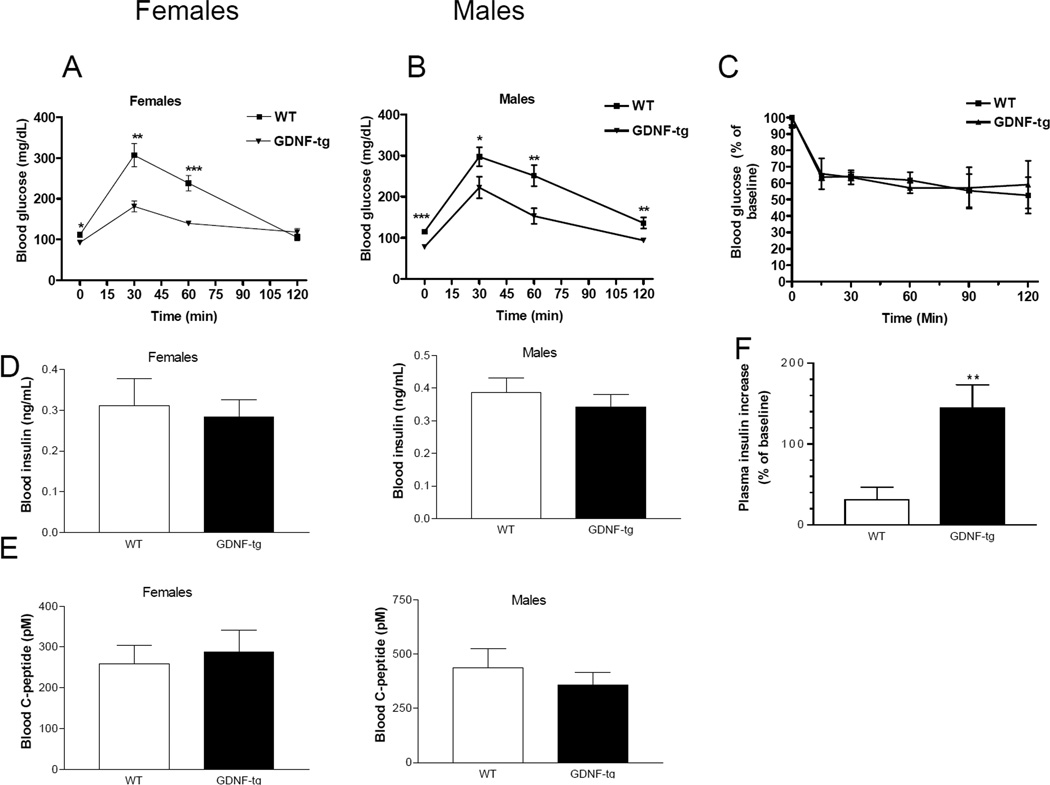
To assess the functional effect of increased β-cell mass on glucose homeostasis, glucose tolerance tests were conducted in male and female WT and GDNF-tg mice. Fasting blood glucose levels of GDNF-tg mice of both sexes were significantly (P<0.05) lower than those of WT mice (Fig. 6A, B). Following intraperitoneal glucose administration, blood glucose levels remained significantly lower in GDNF-tg mice than in WT mice at all time points except at the 120 min time point in females (Fig. 6A, B). Intraperitoneal glucose tolerance curves were compared in weight matched WT and GDNF-tg mice and the impairment in glucose tolerance persisted (blood glucose 30 min post injection of glucose (mg/dL) WT: 252 ± 34, GDNF-tg, 175 ± 12, P<0.01, WT weight (gms): 33.73 ± 0.9, GDNF-tg weight (gms): 32.94 ± 0.36, n=5 p>0.05. We also assessed if there was a difference in insulin sensitivity between WT and GDNF-tg mice using an intraperitoneal insulin sensitivity test, and found no difference (Fig. 6C). To investigate the factors contributing to the improved glucose tolerance, c-peptide and insulin levels were assessed in plasma samples collected following 6 hr of fasting. GDNF-tg mice had similar plasma c-peptide and insulin levels to WT mice (P>0.05; Fig. 6 D, E). We then assessed glucose-stimulated insulin release in vivo. We found that 2.5 minutes after intraperitoneal glucose administration, plasma insulin increase relative to baseline was 144.7 ± 28.75 % in GDNF-tg mice compared to 30.85 ± 15.52 % in WT mice (Fig. 6F, p<0.01). These data suggest that the increased β-cell mass and associated increased insulin release in GDNF-tg mice results in improved glucose tolerance. We also tested glucose-stimulated insulin secretion in vitro in islets isolated from WT and GDNF-tg mice. Glucose-stimulated insulin secretion was assessed in response to 20mM glucose compared to baseline 1.67 mM glucose. We found no difference in in vitro insulin release in islets isolated from WT vs. GDNF-tg mice (% insulin release, WT: 109.2 ± 27.68; GDNF-tg : 89.3 ± 52.61, n=4, P>0.05).
Figure 6. GDNF transgenic mice have improved glucose tolerance.
Eight Week-old mice were fasted for 6 hour, injected intraperitoneally with glucose (2 mg/g body weight), tail blood samples taken at indicated time points, and blood glucose levels measured. Data represents plasma glucose levels of (A) female and (B) male WT and GDNF-tg mice (mean ± SE, *** P<0.001; **, P<0.01; *, P<0.05; females, n = 6 in each group; males n = 8 in each group). (C) Insulin sensitivity tests. Blood glucose levels (mean ± SE) expressed as a percentage of baseline at noted time points after insulin injection are shown (n=4). (D) Fasting (mean ± SE) plasma c-peptide and (E) insulin levels of WT and GDNF-tg mice (male and female, n = 8). (F) Glucose-induced insulin release at (t=2.5 min) in WT and GDNF-tg mice, ** P<0. 01, n=6 in each group.
GDNF transgenic are resistant to the induction of diabetes mellitus
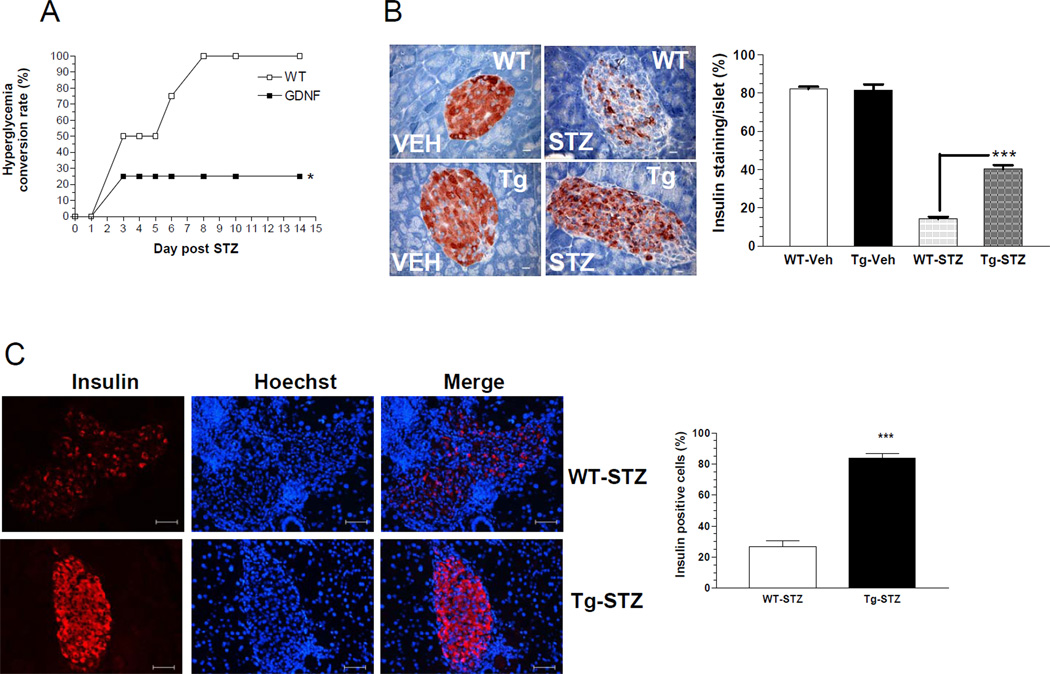
Administration of multiple low doses of streptozotocin (STZ) produces diabetes by the selective loss of β-cells18. The rate of conversion to hyperglycemia following a low dose STZ protocol was used to assess diabetes susceptibility. While WT mice developed diabetes as early as day 4 post STZ injection, GDNF-tg mice remained normoglycemic. At day 14 post STZ injection the number of hyperglycemic mice was significantly higher among WT mice than GDNF-tg mice (Fig. 7A). The effect of STZ on β-cell mass was assessed by insulin staining. STZ-treatment resulted in over 2.8 fold (P<0.001) reduction in insulin staining in WT mice than in GDNF-tg mice, which would suggest a resistance to STZ-induced destruction of β-cells in GDNF-tg mice (Fig. 7B, C). To determine the mechanisms involved in insulin loss following STZ administration, β-cells apoptosis was assessed by a combination of TUNEL staining19 with cleaved caspase-3 immunocytochemistry. GDNF-tg mice showed 4 fold less β-cell apoptosis than WT mice twelve days after injection with STZ as evidenced by the smaller number of TUNEL and cleaved caspase-3 positive β-cells in these mice (P<0.05; Fig. 7D). GDNF-tg mice also had enhanced proliferation compared to WT mice (Figure 7E). Since WT mice received more STZ compared to GDNF-tg mice due to their higher body weights, the studies were repeated in weight-matched male WT and GDNF-tg mice. The effect of STZ on induction of hyperglycemia was independent of weight as we found a difference in STZ-induced hyperglycemia in weight matched WT compared to GDNF-tg mice (blood glucose (mg/dL) post STZ, WT: 265 ± 39, GDNF-tg: 179 ± 31, P<0.05; weight (gms): WT: 33.93 ± 1.38, GDNF-tg: 31.79 ± 0.37, n=3 in each group P=0.21).
Figure 7. GDNF transgenic mice are more resistant to STZ-induced diabetes than WT mice.
(A) Diabetes conversion rates in WT and GDNF-tg mice after two injections of streptozotocin (75mg/Kg). (B) Representative images showing residual insulin staining in pancreatic islets in pancreas sections from GDNF-tg and WT mice 6 days after STZ or vehicle injection, and plot of islet residual insulin staining (expressed as percentage of total islet area for each group, mean ± SE, *** P<0.001). Scale bar, 20 µm. (C) Representative pancreas sections of STZ injected WT and GDNF-tg mice stained for insulin (red) and Hoechst (blue) 14 days after STZ-injection. Histogram shows average insulin positive cells in WT and GDNF-tg mice. (D) Representative images showing both cleaved caspase-3 (red) and TUNEL (green) positive cells within islets in pancreas sections from STZ-treated WT and GDNF-tg mice. Arrows show cleaved caspase-3 and TUNEL positive cells. Arrowheads point at islets. Scale bar, 20µm. (E) Assessment of proliferation in islets from STZ-treated WT and GDNF-tg mice. Histogram shows average Ki67+ cells. Representative images showing both Ki67 (red) and insulin (green) positive cells within islets in pancreas sections from STZ-treated WT and GDNF-tg mice. Arrow shows Ki67+/insulin+ cell. Nuclei are stained with DAPI (blue).
Discussion
The role of the enteric nervous system in influencing β-cell survival or mass has not been studied. In the current series of experiments we document the presence of GDNF receptors on β-cells, and demonstrate that GDNF can influence β-cell mass by promoting survival and increasing proliferation. Similar to its effect on neurons, GDNF stimulates the PI-3-kinase pathway in β-cells.
We demonstrated GDNF effects on β-cells in vivo using the GFAP-GDNF transgenic mouse that overexpresses GDNF in glial cells. In addition to exhibiting increased rates of β-cell proliferation and β-cell mass these mice demonstrated significantly lower fasting glucose levels and an enhanced response to an intraperitoneal glucose load. They demonstrated no difference in insulin resistance. Consistent with this finding, fasting plasma insulin levels were comparable in both mice. However, GDNF-tg mice demonstrated higher insulin release in response to intraperitoneal glucose administration compared to WT mice, which explains the improved glucose tolerance seen in these mice. The ratio of plasma insulin to blood glucose was higher in the GDNF-tg mice compared to WT mice. This is similar to what was observed in transgenic mice that overexpress placental lactogen in β-cells that also have increased β-cell mass20. The capacity of GDNF to diminish chemically induced β-cell destruction was also demonstrated in vivo. Intraperitoneal STZ injection induced less β-cell loss in GDNF-tg mice than in WT mice. This β-cell sparing effect was reflected in the maintenance of normal blood glucose levels in GDNF-tg mice injected with STZ in contrast to STZ-injected WT mice. The results of this study demonstrate novel GDNF effects that may prove useful in sustaining β-cell mass, or promoting survival.
Tissue-specific overexpression of Akt in mice increases β-cell mass3. Our in vitro results demonstrate that GDNF can activate the PI-3-kinase/Akt pathway. This may be one of the mechanisms through which GDNF enhances β-cell mass. GDNF is expressed in pancreatic enteric glia. The close proximity of these ganglia to β-cells suggests a paracrine effect of GDNF on β-cells. Further studies are necessary to examine the mechanism by which GDNF overexpression in enteric glia activates its receptors on β-cells, and to evaluate the contribution of potential indirect effects. Our studies demonstrate a three-fold increase in β-cell mass in GDNF-tg mice compared to WT mice. This degree of enhancement is similar to the three-fold increase in β-cell mass observed in other growth factor overexpression studies, including PTH-RP and placental lactogen20, 21. It is important to note that the effects of GDNF on β-cells is not a systemic effect as serum GDNF levels have been measured in GDNF-tg mice and are no different from littermate controls10.
Islet β-cell mass is influenced by changes in proliferation, apoptosis and neogenesis1. In our study we found that GDNF can reduce thapsigargin-induced apoptosis in β-cells in vitro as well as streptozotocin-induced apoptosis in vivo. GDNF induces phosphorylation of Akt and its downstream target GSK3β. Other downstream targets of Akt may be involved in this survival pathway and will be examined in future studies. GDNF has been shown to increase NPY expressing neurons. One of the possible mechanisms GDNF could influence β-cell mass could be through NPY13. Future studies will examine this possibility.
A recent study that traced the genetic lineage of newly forming adult β-cells in mice concluded that pre-existing β-cells, and not adult stem cells, are the major source of β-cell neogenesis in adults following pancreatectomy22. Several other studies, however, report the presence of a pool, in both rodents and humans, of endocrine and non-endocrine pancreatic precursors that differentiate into cells of the β-cell lineage6, 23. Future studies will examine the possibility that GDNF induces β-cell neogenesis and the involvement of notch signaling24.
The protection from STZ-induced β-cell loss in the transgenic model suggests a potential mechanism for the prevention of diabetes. We found that GDNF-tg mice have lower rates of STZ-induced β-cell destruction. Aside from a reduced apoptotic rate, other potential reasons for the increased β-cell mass in the STZ-treated transgenic mice include a greater initial β-cell mass or an enhanced ability to rapidly replenish β-cells by neogenesis or replication. Cross-breeding of GDNF-tg mice with genetic models of β-cell failure may help elucidate potential mechanisms underlying the resistance to the development of diabetes.
In summary, the current study demonstrates a novel mechanism of a neurotrophic factor expressed in the enteric nervous system that clearly influences β-cell mass. The enteric nervous system can affect both functional and structural effects of β-cells by increasing cellular proliferation, reducing apoptosis, and improving glycemic control in vivo. The current study provides new therapeutic targets for improving β-cell mass.
Acknowledgments
This work is supported by NIH grants KO8 DK067045 (S. Srinivasan), DK 062092, DK 075391 (F.A), DK06411 (S.V. Sitaraman), Digestive Diseases Research Center grant (DK064399) and a grant from the Juvenile Diabetes Research foundation (S. Mwangi, 3-2006-787). The authors would like to acknowledge Dr. Mark Rigby, Emory Transplantation Center for help with the insulin staining; and Dr. Bindu Chandrasekharan and Irene Joseph for their help with the experiments. We would also like to thank Dr. Mauricio Rocca, Edillson Torres and Jianguo Xu, Division of Pulmonary Diseases for help with histological tissue processing
Abbreviations
- GDNF
Glial Cell line-Derived Neurotrophic Factor
- WT
wild-type
- tg
transgenic
- STZ
streptozotocin
- GFAP
Glial Fibrillary Acidic Protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonner-Weir S. Life and death of the pancreatic beta cells. Trends Endocrinol Metab. 2000;11:375–378. doi: 10.1016/s1043-2760(00)00305-2. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen JH, Galsgaard ED, Moldrup A, Friedrichsen BN, Billestrup N, Hansen JA, Lee YC, Carlsson C. Regulation of beta-cell mass by hormones and growth factors. Diabetes. 2001;50(Suppl 1):S25–S29. doi: 10.2337/diabetes.50.2007.s25. [DOI] [PubMed] [Google Scholar]
- 3.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001;108:1631–1638. doi: 10.1172/JCI13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 5.Pierucci D, Cicconi S, Bonini P, Ferrelli F, Pastore D, Matteucci C, Marselli L, Marchetti P, Ris F, Halban P, Oberholzer J, Federici M, Cozzolino F, Lauro R, Borboni P, Marlier LN. NGF-withdrawal induces apoptosis in pancreatic beta cells in vitro. Diabetologia. 2001;44:1281–1295. doi: 10.1007/s001250100650. [DOI] [PubMed] [Google Scholar]
- 6.Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 7.Heuckeroth RO, Lampe PA, Johnson EM, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev Biol. 1998;200:116–129. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- 8.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 9.Teitelman G, Guz Y, Ivkovic S, Ehrlich M. Islet injury induces neurotrophin expression in pancreatic cells and reactive gliosis of peri-islet Schwann cells. J Neurobiol. 1998;34:304–318. doi: 10.1002/(sici)1097-4695(199803)34:4<304::aid-neu2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Z, Alam S, Oppenheim RW, Prevette DM, Evenson A, Parsadanian A. Overexpression of glial cell line-derived neurotrophic factor in the CNS rescues motoneurons from programmed cell death and promotes their long-term survival following axotomy. Exp Neurol. 2004;190:356–372. doi: 10.1016/j.expneurol.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Hara M, Wang X, Kawamura T, Bindokas VP, Dizon RF, Alcoser SY, Magnuson MA, Bell GI. Transgenic mice with green fluorescent protein-labeled pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- 12.Mwangi S, Anitha M, Fu H, Sitaraman SV, Srinivasan S. Glial cell line-derived neurotrophic factor-mediated enteric neuronal survival involves glycogen synthase kinase-3beta phosphorylation and coupling with 14-3-3. Neuroscience. 2006;143:241–251. doi: 10.1016/j.neuroscience.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 13.Anitha M, Chandrasekharan B, Salgado JR, Grouzmann E, Mwangi S, Sitaraman SV, Srinivasan S. Glial-derived neurotrophic factor modulates enteric neuronal survival and proliferation through neuropeptide y. Gastroenterology. 2006;131:1164–1178. doi: 10.1053/j.gastro.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 15.Montana E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest. 1993;91:780–787. doi: 10.1172/JCI116297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauro D, Kido Y, Castle AL, Zarnowski MJ, Hayashi H, Ebina Y, Accili D. Impaired glucose tolerance in mice with a targeted impairment of insulin action in muscle and adipose tissue. Nat Genet. 1998;20:294–298. doi: 10.1038/3112. [DOI] [PubMed] [Google Scholar]
- 17.Akerblom B, Anneren C, Welsh M. A role of FRK in regulation of embryonal pancreatic beta cell formation. Mol Cell Endocrinol. 2007;270:73–78. doi: 10.1016/j.mce.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien BA, Harmon BV, Cameron DP, Allan DJ. Beta-cell apoptosis is responsible for the development of IDDM in the multiple low-dose streptozotocin model. J Pathol. 1996;178:176–181. doi: 10.1002/(SICI)1096-9896(199602)178:2<176::AID-PATH433>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan S, Stevens M, Wiley JW. Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes. 2000;49:1932–1938. doi: 10.2337/diabetes.49.11.1932. [DOI] [PubMed] [Google Scholar]
- 20.Vasavada RC, Garcia-Ocana A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem. 2000;275:15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- 21.Porter SE, Sorenson RL, Dann P, Garcia-Ocana A, Stewart AF, Vasavada RC. Progressive pancreatic islet hyperplasia in the islet-targeted, parathyroid hormone-related protein-overexpressing mouse. Endocrinology. 1998;139:3743–3751. doi: 10.1210/endo.139.9.6212. [DOI] [PubMed] [Google Scholar]
- 22.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 23.Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, Monosov EZ, Barcova M, Mercola M, Levine F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med. 2006;12:310–316. doi: 10.1038/nm1367. [DOI] [PubMed] [Google Scholar]
- 24.Darville MI, Eizirik DL. Notch signaling: a mediator of beta-cell de-differentiation in diabetes? Biochem Biophys Res Commun. 2006;339:1063–1068. doi: 10.1016/j.bbrc.2005.11.111. [DOI] [PubMed] [Google Scholar]