Abstract
The purpose of this study was to examine the association between aphasia severity and neurocognitive function, disease duration and temporoparietal atrophy in 21 individuals with the logopenic variant of primary progressive aphasia (lvPPA). We found significant correlations between aphasia severity and neurocognitive severity as well as temporoparietal atrophy; but not disease duration. Cluster analysis identified three variants of lvPPA: (1) subjects with mild aphasia and short disease duration (mild typical lvPPA); (2) subjects with mild aphasia and long disease duration (mild atypical lvPPA); and, (3) subjects with severe aphasia and relatively long disease duration (severe typical lvPPA). All three variants showed temporoparietal atrophy, with the mild atypical group showing the least atrophy despite the longest disease duration. The mild atypical group also showed mild neuropsychological impairment. The subjects with mild aphasia and neuropsychological impairment despite long disease duration may represent a slowly progressive variant of lvPPA.
Keywords: Primary progressive aphasia, Logopenic aphasia, Neurocognitive impairment, Temporoparietal atrophy, Voxel-based morphometry
1. Introduction
The logopenic variant of primary progressive aphasia (lvPPA) is a clinical phenotype distinct from other types of language impairment that occur secondary to neurodegeneration, (i.e., agrammatic PPA and the semantic variant of PPA) (Gorno-Tempini et al., 2008; Gorno-Tempini et al., 2011) and other variants of Alzheimer’s disease (early-onset typical amnestic AD and posterior cortical atrophy) (Migliaccio et al., 2009; Ridgway et al., 2012). Characteristic features of lvPPA include impaired word retrieval in spontaneous speech and naming, impaired repetition of sentences and phrases, phonologic errors in spontaneous speech, and relatively spared single word comprehension, object knowledge and motor speech (Gorno-Tempini et al., 2011).
Imaging studies of lvPPA consistently show a pattern of gray matter reduction and cortical thinning affecting primarily the left temporoparietal cortex, including the inferior parietal lobe, posterior middle and superior temporal gyri, and Brodmann area 37 which is evident at early stages of the disease process (Gorno-Tempini et al., 2008; Gorno-Tempini et al., 2004; Mesulam et al., 2009; Mesulam, Wieneke, Thompson, Rogalski, & Weintraub, 2012; Migliaccio et al., 2009; Ridgway et al., 2012; Rohrer et al., 2010; Sapolsky et al., 2010). A longitudinal study showed that as the disease progresses, there also may be varying degrees of involvement of medial parietal and temporal lobes, posterior cingulate, right temporoparietal cortex and frontal regions (Rogalski et al., 2011).
Research shows that there is an association between regions of atrophy and degree of impairment in specific language functions in individuals with PPA. For example, one study found that naming correlated with bilateral temporal lobes, sentence repetition correlated with left superior temporal volumes, sentence comprehension correlated with left dorsal middle and inferior frontal gyri, and fluency correlated with left ventral middle and inferior frontal gyri (Amici et al., 2007). Another study also found an association between left inferior frontal cortical thickness and severity of impairment on fluency. Impairment in grammar/syntax also correlated with atrophy in this region. Unlike the previous study, severity of impairment in comprehension was correlated with left temporopolar cortical thickness (Sapolsky et al., 2010). A more recent longitudinal study of a group of subjects with PPA failed to find an association between percent change in total normalized cortical volume and percent change in the WAB-AQ over a two year (Rogalski et al., 2011). Each of these studies evaluated the variants of PPA in aggregate (i.e., agrammatic, semantic, and logopenic variants were not separated out), and therefore the relationship between aphasia severity and gray matter changes unique to lvPPA is still unknown.
Many studies of lvPPA include the assessment of neuropsychological function in addition to careful characterization of language deficits. Verbally mediated tasks, especially verbal memory and tasks that tap verbal working memory, such as digit and letter span, are commonly impaired relative to normal controls (Galantucci et al., 2011; Gorno-Tempini et al., 2008; Gorno-Tempini et al., 2004; Rabinovici et al., 2008; Rohrer et al., 2010; Wicklund, Rademaker, Johnson, Weitner, & Weintraub, 2007). Both cognitive and language data suggest a core deficit in phonologic loop functions (Gorno-Tempini et al., 2008). There also are varying degrees of impairment reported in other cognitive domains such as scanning and visuomotor tracking, divided attention and cognitive flexibility (i.e., regular and modified trailmaking test), and visuospatial/visuoconstructional abilities (i.e., VOSP Cube, regular and modified Rey-O Complex Figure) (Galantucci et al., 2011; Gorno-Tempini et al., 2004; Machulda et al., 2012; Rabinovici et al., 2008; Rohrer et al., 2010; Wicklund et al., 2007; Wilson et al., 2010). Although these studies provide information on cognitive dysfunction in lvPPA, none of them specifically examine the association between aphasia severity and degree of neurocognitive impairment.
As with other neurodegenerative conditions such as Alzheimer’s Disease and frontotemporal dementia, individuals with PPA show a decline in cognitive function over time (Wicklund et al., 2007). Over an approximate three year period, the PPA subjects (which included agrammatic, semantic and logopenic variants) showed prominent decline on language measures. Attention and verbal memory also declined though the authors clarify that the decline in verbal memory was likely influenced by the aphasia (i.e., word access problems) and rather than being representative of a true deficit in retention. No studies have specifically examined whether aphasia severity correlates with disease duration in PPA, and more specifically in lvPPA.
It remains unclear how well neuropsychological function beyond the verbal domain and neuroimaging abnormalities correlate with aphasia severity and whether they represent good biomarkers of disease progression unique to lvPPA. In addition, it is unknown whether aphasia severity correlates well with disease duration. Understanding the relationships between the clinical features of lvPPA is important for determining patient prognosis; therefore, the aim of this study was to examine the association between aphasia severity and neurocognitive function, disease duration and temporoparietal atrophy in a group of well characterized individuals with lvPPA. Because disease duration did not correlate with aphasia severity in the patients studied, we also performed a cluster analysis to determine whether there were groups of outliers that would explain this lack of correlation.
2. Methods
2.1 Subjects
We recruited 21 subjects that met our clinical criteria for lvPPA. We included only subjects who spoke English as their primary language, and who had an informant to provide an independent evaluation of functioning [and corroboration of the history of language impairment].
All subjects underwent detailed speech and language examination, neurological evaluation, neuropsychological testing and neuroimaging analysis over a span of 48-72 hours. All subjects had video and audio recordings of their entire formal speech and language assessment, as well as general conversation. Two speech-language pathologists (JRD and EAS) made the diagnosis of lvPPA prior to or during a consensus meeting, solely based on data from speech and language assessment, without any knowledge of neurological, neuropsychological or neuroimaging results. The clinical criteria used to determine a diagnosis for lvPPA were as follows: 1) presence of aphasia, 2) impaired sentence repetition and comprehension, 3) presence of anomia with evidence of relatively spared single word comprehension, 4) evidence of phonemic paraphasias, 5) slowed rate of verbal expression due to pauses for word retrieval or verbal formulation, and 6) absence of agrammatic or telegraphic verbal output. All subjects would also meet recent clinical consensus criteria for lvPPA (Gorno-Tempini et al., 2011).
Speech and language assessments included the Western Aphasia Battery (WAB), revised (Kertesz, 2007), Part A, as the primary measure of global language ability and aphasia severity, including the composite scores for Spontaneous Speech, Auditory Verbal Comprehension, Repetition, and Naming/Word Finding. The WAB aphasia quotient (AQ) was felt to be the best measure of disease severity given its comprehensive nature. All neurological assessments were performed by one Behavioral Neurologist (KAJ) and included the Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) to assess global cognitive impairment, and the Clinical Dementia Rating Scale sum of boxes (CDR-SB) (Morris, 1993) to assess functional impairment.
Subjects also underwent neuropsychological assessments. A trained psychometrist administered all neuropsychological tests. A clinical neuropsychologist (MMM) oversaw test administration, scoring accuracy and quality control. The cognitive domains assessed (in addition to the comprehensive speech and language evaluation) included (1) memory [Wechsler Memory Scale-III (WMS-III) Logical Memory I/II which assesses immediate and delayed recall of paragraph-length stories and Visual Reproduction I/II which assesses immediate and delayed recall of designs (Wechsler, 1997) and Rey Auditory Verbal Learning Test (AVLT) (Rey, 1964) which is a list learning test that includes five learning trials, an interference trial, immediate recall and delay recall trials, and recognition]; (2) executive function [Trailmaking Test B (Reitan, 1958) which is a test of scanning and visuomotor tracking, divided attention, and cognitive flexibility and Delis-Kaplan Executive Function (DKEFS) Card Sort (Delis, Kaplan, & Kramer, 2001) which is a conceptual task that evaluates problem-solving, verbal and nonverbal concept formation, and flexibility of thinking]; and, (3) visuospatial function [Rey-Osterreith Complex Figure Test ( Osterrieth, 1944) which is a measure of visual perception and constructional praxis and Visual Object and Space Perception (VOSP) cube and incomplete letters subtests (Warrington & James, 1991). The VOSP cube subtest is a block counting task. The VOSP incomplete letter subtest shows a series of large alphabet letters, one to a card, which have been randomly degraded so that only 30% of the original shape remains. The subject is asked to identify the letter.]
Published norms were used for the WMS-III (Wechsler, 1997), VOSP (Bonello, Rapport, & Millis, 1997; Warrington & James, 1991), and DKEFS (Delis et al., 2001) subtests. Mayo Older American Normative Studies age-adjusted scaled scores were used for the AVLT and Trailmaking Test (Ivnik, Malec, Smith, Tangalos, & Petersen, 1996; Ivnik et al., 1992; Machulda et al., 2007). We converted age-adjusted scaled scores to z-scores for the WMS-III, AVLT, Trailmaking Test, DKEFS Card Sort, and Rey-Osterreith Complex Figure Test. We calculated z-scores for VOSP performances based on published norms. Domain z-scores were calculated by averaging the z-scores for each test within a domain. A global z-score was calculated by averaging the z-scores for all three cognitive domains.
Standard protocol approvals and patient consents
The Mayo Clinic Institutional Review Board approved this study. All subjects provided written informed consent before participating in any research activity.
2.2 MR Image acquisition
All subjects underwent a standardized MRI imaging protocol at 3.0 Tesla that included a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (TR/TE/T1= 2300/3/900ms; flip angle=8°; FOV=26cm; in-plane matrix=256×256; phase FOV=0.94; slice thickness=1.2 mm, in-plane resolution = 1.0 mm; bandwidth=31.25 kHz).
2.3 MRI analysis
MPRAGE images underwent pre-processing correction for gradient non-linearity (Sled, Zijdenbos, & Evans, 1998) and intensity non-uniformity (Jovicich et al., 2006). Atlas-based parcellation using SPM5 unified segmentation with custom elderly template and priors and an in-house modified version of the automatic anatomical labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) was used to calculate volumes of the left lateral temporal lobe (inferior + middle + superior temporal gyri) and inferior parietal lobe (inferior parietal lobe + supramarginal gyrus + angular gyrus) for each lvPPA subject and for a cohort of 21 age- and gender-matched controls. Left lateral temporal and inferior parietal volumes for each lvPPA subject were converted to z-scores representing difference from the controls, and were averaged to generate a temporoparietal z-score for each lvPPA subject.
Voxel-based morphometry (VBM) (Ashburner & Friston, 2000) using SPM5 was also used to assess patterns of gray matter atrophy in each cluster compared to the cohort of 21 age- and gender-matched controls. All images were normalized to a study-specific customized template and segmented using unified segmentation (Ashburner & Friston, 2005). Gray matter images were modulated and smoothed at 8mm full-width-at-half-maximum. Two-sided t tests were used to compare each lvPPA group to controls, and results were assessed after correction for multiple comparisons using the false discovery rate (FDR) correction at p<0.01.
2.4 Statistics
Statistical analyses were performed using JMP computer software (“JMP, Version 8,” 1989 - 2008). Pair-wise correlations were used to assess correlations between the WAB AQ score and the individual and global neurocognitive z-scores, temporoparietal z-score, and disease duration. Pair-wise correlation was also performed between WAB repetition sub-score and disease duration. This was performed to support the findings with the WAB AQ, since impaired repetition is one of the specific features of lvPPA. Linear regression was utilized to determine which variable/s best predicted the WAB AQ. A linear model was assessed with WAB AQ as the outcome variable and neurocognitive global z score, temporoparietal z-score, and disease duration as the predictor variables. Given that disease duration did not correlate with WAB AQ score, we performed a Hierarchical cluster analysis using Ward’s minimum variance with WAB AQ and disease duration, to determine whether there were groups of outliers that would help explain the lack of correlation. Hierarchical clustering begins with each point in its own cluster and then combines points that are closest together into a single cluster. The process is repeated until there is one cluster containing all points. Clinical and demographic features were compared across clusters using Kruskall-Wallis tests followed by Mann-Whitney U test for continuous variables and Chi-squared test for categorical variables. Statistical significance was set at p < 0.05.
3. Results
Our sample consisted of 21 individuals diagnosed with lvPPA ranging from 54 to 80 years of age and disease duration ranging from one to six years. This was a relatively well-educated sample, with all individuals completing at least 12 years of education.
3.1 WAB AQ Correlations
The results of the correlation analyses are shown in Table 1. A significant positive correlation was identified between WAB AQ score and each neurocognitive domain, as well as the global neurocognitive z-score. There also was a significant association between WAB AQ and temporoparietal z-score; however, there was no association between the WAB AQ and disease duration. There was no correlation between WAB repetition sub-score and disease duration. Linear regression analysis showed that the neurocognitive global z-score best predicted WAB AQ (p=0.006), after adjusting for temporoparietal z-score (p=0.10) and disease duration (0.84); therefore, aphasia severity appears to correlate best with neuropsychological performance. The lack of correlation between disease duration and the WAB AQ was unexpected, so we ran a cluster analysis to determine whether there were groups of outliers that would help explain this lack of correlation.
Table 1.
WAB AQ correlations with neurocognitive test scores, imaging and disease duration
| Variables | Correlation coefficient & CI | P values |
|---|---|---|
| WAB AQ vs. AVLT Del + LMII + VR II Z | r = 0.6 [CI: 0.2-0.8] | 0.007 |
| WAB AQ vs. TMTB + Sorting Z | r = 0.7 [CI: 0.3-0.9] | 0.0008 |
| WAB AQ vs. Rey-O + Cube + Letters Z | r = 0.8 [CI: 0.5-0.9] | <0.0001 |
| WAB AQ vs. Global neurocognitive Z | r = 0.8 [CI: 0.5-0.9] | <0.0001 |
| WAB AQ vs. Temporoparietal Z | r = 0.6 [CI: 0.2-0.8] | 0.003 |
| WAB AQ vs. Disease Duration | r = −0.1 [CI: −0.5-0.3] | 0.53 |
| Repetition Sub-score vs. Disease Duration | r = 0.0 [CI:0.04-0.4] | 0.96 |
3.2 Cluster analysis
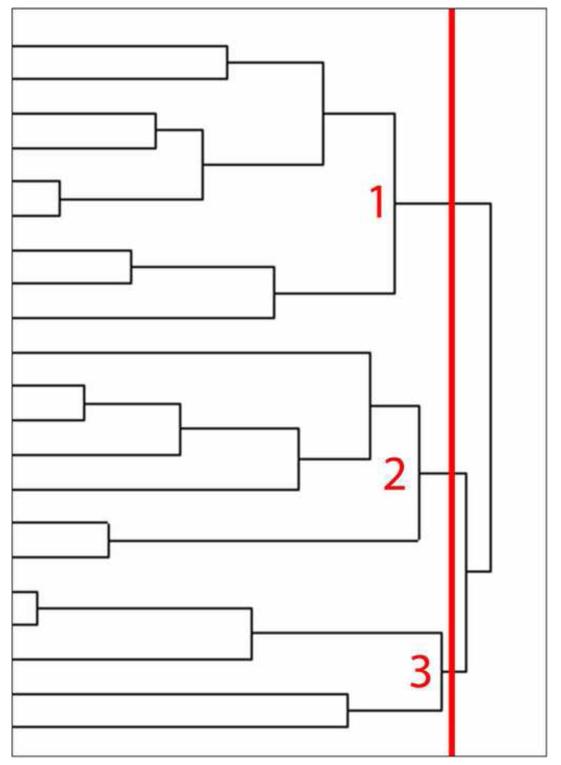
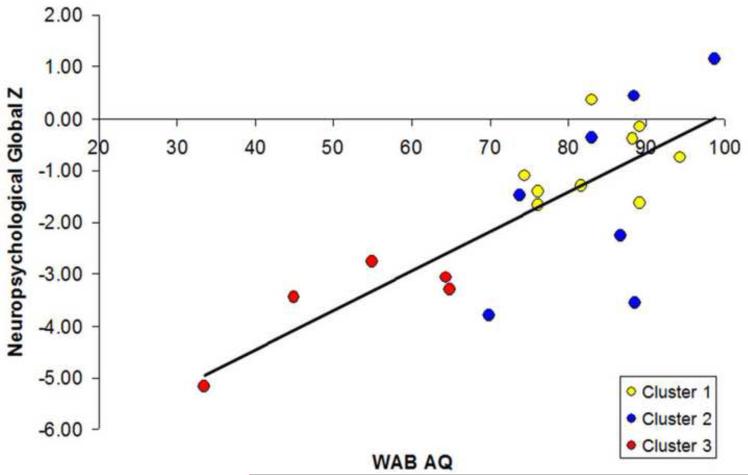
The cluster analysis identified three groups of subjects (Figure 1). The clinical and demographic features of these clusters are shown in Table 2. Cluster 1 had relatively mild aphasia based on the WAB AQ, with short disease duration (referred to as mild typical lvPPA). Cluster 2 had mild aphasia, with long disease duration (referred to as mild atypical lvPPA). Cluster 3 had severe aphasia and relatively long disease duration (referred to as severe typical lvPPA). The mild atypical lvPPA group performed similarly to the mild typical lvPPA group on the WAB AQ and all other indices of cognition (i.e., MMSE, neurocognitive domain z-scores and global z-score), but significantly better than the severe typical lvPPA group, despite having longer disease duration. The temporoparietal z-scores were also similar between the mild typical and atypical groups, but were significantly higher (i.e., showing less atrophy) than the severe typical group. Figure 2 shows a scatterplot of the association between the WAB AQ and neurocognitive global z-score as a function of cluster membership, with marked overlap observed between Cluster 1 and 2 despite the significant difference between the groups in disease duration. There were no differences between the clusters in age at onset or education. After excluding the atypical group (Cluster 2) there was a strong correlation between WAB AQ and disease duration (r = −0.8, [CI −0.9, −0.4] p = 0.001).
Figure 1.
Dendrogram created by cluster analysis. The distance along the x-axis represents a measure of similarity between subjects, such that the closer the distance the greater the similarity between the subjects. The vertical red line illustrates the cut-point that divides the cohort of 21 lvPPA subjects into three clusters. Cluster 1 consisted of subjects with relatively mild aphasia and short disease duration (mild typical lvPPA). Cluster 2 consisted of subjects with relatively mild aphasia despite having long disease duration (mild atypical lvPPA). Cluster 3 consisted of subjects with severe aphasia and long disease duration (severe typical lvPPA).
Table 2.
Language, neurocognitive test scores and imaging data across clusters. Data shown as median (range); p values calculated using Kruskall-Wallis followed by Mann-Whitney U test for continuous variables and Chi-squared test for categorical variables (ND = Not Done)
| Clusters | P values | ||||||
|---|---|---|---|---|---|---|---|
| 1 “Mild typical lvPPA” |
2 “Mild atypical lvPPA” |
3 “Severe typical lvPPA” |
All | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |
| N | 9 | 7 | 5 | - | - | - | - |
| Gender, female | 44% | 57% | 80% | 0.44 | ND | ND | ND |
| Age at onset | 65 (54-72) | 64 (42-80) | 64 (47-70) | 0.63 | ND | ND | ND |
| Education | 16 (12-20) | 15 (12-20) | 14 (12-16) | 0.26 | ND | ND | ND |
| MMSE | 25 (24-29) | 26 (15-30) | 14 (10-21) | 0.005 | 0.96 | 0.002 | 0.007 |
| Disease duration | 2.0 (1.0, 3.0) |
5.0 (4.0, 6.0) |
3.5 (3.0, 5.0) |
0.0003 | 0.0006 | 0.005 | 0.02 |
| WAB AQ | 83.0 (74.4, 94.4) |
86.7 (69.9, 98.8) |
55.0 (33.5, 64.8) |
0.004 | 0.92 | 0.003 | 0.005 |
| AVLT Del+LM II Z+VRII Z |
−1.6 (−2.1, 0.3) |
−1.2 (−2.1, 1.7) |
−2.3 (−2.5, −2.1) |
0.003 | 0.27 | 0.003 | 0.004 |
| TMTB + Sorting Z |
−1.3 (−2.9, 0.2) |
−1.8 (−3.0, 1.3) |
−3.0 (−3.2, −2.7) |
0.02 | 0.67 | 0.009 | 0.02 |
| Rey-O +Cube + Inc. Letters Z |
−0.2 (−6.1, 0.6) |
−0.5 (−6.9, 0.6) |
−4.9 (−10.3, −3.3) |
0.03 | 0.71 | 0.01 | 0.03 |
| Global Z | −1.1 (−3.6, 0.4) |
−1.3 (−3.8, 1.1) |
−3.3 (−5.2, −2.8) |
0.03 | 0.71 | 0.01 | 0.03 |
| Temporoparietal Z |
−2.4 (−3.7, 1.8) |
−2.2 (−2.8, 0.3) |
−3.2 (−4.1, −3.1) |
0.008 | 0.15 | 0.03 | 0.005 |
Figure 2.
Scatterplot of WAB AQ versus neuropsychological global Z color coded by cluster.
We also examined neuropsychological test performances for each cluster to determine whether any test(s) influenced the cluster analysis. For subjects younger than the MOANS normative sample (n = 2), the lowest age grouping was used to derive standard scores. Subjects were assigned a score of 1 if they attempted the task but were unable to complete it. Subjects were assigned a 0 if they did not comprehend task instructions. Table 3 provides a summary of scores from which we calculated z-scores. Similar to the z-scores, Clusters 1 and 2 performed comparably across all neurocognitive measures whereas Cluster 3 consistently performed the most poorly. It does not appear that any test within each domain skewed domain z-scores.
Table 3.
Non-transformed neurocognitive test scores across clusters.For subjects younger than the MOANS normative sample (n = 2), the lowest age grouping wasused to derive standard scores. Subjects were assigned a score of 1 if they attempted the task but were unable to complete it. Subjects were assigned a 0 if they did not comprehend task instructions.
| AVLT Del* |
LMII* | VRII* | TMTB* | Sorting* | Rey-O* | Cube** | Incompl Letters** |
|
|---|---|---|---|---|---|---|---|---|
| Cluster 1 | 5.44 (3.04) |
5.22 (2.90) |
8.22 (1.98) |
5.11 (4.34) |
6.33 (3.27) |
6.88 (4.31) |
18.55 (2.12) |
7.55 (3.43) |
| Cluster 2 | 7.71 (5.21) |
7.57 (4.85) |
9.14 (5.89) |
5.28 (5.90) |
7.57 (4.03) |
6.85 (4.29) |
16.28 (7.22) |
8.0 (3.26) |
| Cluster 3 | 4.8 (1.92) |
1.25 (0.5) |
4.4 (0.54) |
1.00 (0.0) |
1.4 (1.51) |
2.6 (1.34) |
10.8 (6.87) |
0.8 (1.78) |
Mean (standard deviation) of age-adjusted scaled scores based on published means (mean = 10 ± 3).
Mean (standard deviation) of raw scores (VOSP Cube Total = 20; Incomplete Letters Total = 10).
3.3 VBM Results
Voxel-based morphometry maps demonstrate varying degrees of atrophy involving left temporoparietal cortex in all three clusters (Figure 3). Cluster 1 (mild typical lvPPA) has marked volume loss in left lateral temporal lobe with extension into the inferior parietal lobe, and some mild involvement of medial parietal lobe and right temporal lobe. Cluster 2 (mild atypical lvPPA) shows a less severe pattern of loss, involving left posterior lateral temporal lobe with some scattered areas in inferior parietal lobe. Cluster 3 (severe typical lvPPA) shows extensive loss in bilateral temporoparietal regions (left > right), with extension into occipital and frontal lobes.
Figure 3.
Patterns of gray matter volume loss in each cluster compared to a cohort of 21 age and gender matched controls.
4. Discussion
We investigated the association between aphasia severity and neurocognitive function (beyond the language domain), disease duration, and temporoparietal atrophy in a prospectively recruited group of individuals presenting with lvPPA. Our main findings are: (1) aphasia severity strongly correlates with neurocognitive function and left temporoparietal atrophy, (2) aphasia severity did not correlate with disease duration, and (3) there appeared to be an atypical variant of lvPPA with mild aphasia despite long disease duration.
In addition to the strong correlation between aphasia severity and global neurocognitive function, we found a strong correlation between aphasia severity and each neurocognitive domain, i.e., memory, visuospatial and executive function, suggesting that multiple domains of neuropsychological function are progressively affected in lvPPA in spite of the fact that language decline is the initial symptom and the predominant or only functional complaint at clinical presentation. This is likely due in part to the fact that performances on most neuropsychological measures, including those that do not require a verbal response, are mediated to some degree by language function. As the disease progresses, however, brain regions are affected that mediate other aspects of cognition (i.e., medial temporal lobe atrophy causes memory impairment, parietal atrophy causes visuospatial impairment, and frontal atrophy causes executive impairment). This is supported by the VBM results that showed more severe atrophy of temporal, parietal and frontal regions in those with more severe symptoms. We also found a strong correlation between aphasia severity and temporoparietal atrophy. We chose a temporoparietal region of interest for our analyses because several independent groups of investigators show that this is the region most commonly involved in lvPPA and is affected at the early to mid-stage of the disease (Gorno-Tempini et al., 2004; Rogalski et al., 2011). The high correlation between aphasia severity and the temporoparietal z-score demonstrates that temporoparietal atrophy is a good biomarker of aphasia severity in lvPPA.
The lack of correlation between aphasia severity and disease duration was unexpected; particularly in light of the most common course of cortical neurodegenerative disorders in which the severity of cognitive impairment typically worsens the longer symptoms are present. The cluster analysis, however, demonstrated that one of the reasons we did not observe a correlation between aphasia severity and disease duration is that there is an atypical variant of lvPPA with mild aphasia yet long disease duration. This likely represents a slowly progressive variant of lvPPA that is distinct from the other two groups. In fact after excluding the atypical group there was very good correlation between WAB AQ and disease duration. This atypical variant also had only mild neuropsychological impairment and the least temporoparietal atrophy (relative to controls), despite having the longest median disease duration of all three groups. Atrophy in the atypical lvPPA group was relatively limited to posterior regions of the left lateral temporal lobe, and was strikingly less severe than the degree of atrophy observed in the severe typical lvPPA group whose median disease duration was shorter than the atypical group.
Slowly progressive variants of frontotemporal dementia have also been described, with symptoms sometimes not progressing beyond mild levels of severity (Hornberger, Shelley, Kipps, Piguet, & Hodges, 2009; Khan et al., 2012). It is possible that those with a slowly progressive variant of lvPPA may have a better prognosis and, by virtue of their mild aphasia and cognitive impairment, have a longer window of opportunity for the successful implementation of compensatory strategies. It would be extremely valuable for clinicians to be able to identify this subset of patients when they present clinically. The presence of mild aphasia in the context of relatively long symptom duration, i.e., greater than 4 years based on patients in this study, should alert clinicians to the possibility of an atypical variant of lvPPA.
Currently there is no way to determine if patients who present with mild symptoms of short duration will follow a relatively mild course or rapidly deteriorate; hence, a key question is whether the subjects in our mild typical lvPPA group will evolve into mild atypical lvPPA or severe typical lvPPA. The severe typical lvPPA group did show more widespread and severe atrophy than the mild typical lvPPA group, and showed worse cognitive performance and longer disease duration, suggesting that it may reflect a later stage of the disease such that Cluster 1 and Cluster 3 may be a continuum of progression of the same disease; however, it is still possible that some of the mild typical lvPPA subjects will remain mildly affected even after longer disease duration. There were no clear differences between the two mild groups on WAB AQ subtest performances or in their degree of overlap on this instrument with the severe group. There were also no distinguishing features in the neuropsychological test performances or degree of atrophy in the temporoparietal region of interest between the two mild groups. Longitudinal follow-up is needed to clarify the trajectory of those with both mild typical and atypical lvPPA, and will provide an opportunity to identify measures of language or neuropsychological function that might have predictive value relative to clinical progression and underlying pathology.
BRLN_12_250_Highlights.
We found an association between aphasia and neurocognitive severity in lvPPA.
We found an association between aphasia severity and temporoparietal atrophy in lvPPA.
We did not find an association between in aphasia severity and disease duration in lvPPA.
We describe an atypical variant of lvPPA with mild clinical features despite long disease duration.
Acknowledgments
This study was funded by NIH grant R01 DC010367 from the National Institute of Deafness and Other Communication Disorders. The authors would like to thank Sarah Papenfuss for performing the neuropsychometric testing and organizing the subject’s test schedules.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amici S, Ogar J, Brambati S, Miller B, Neuhaus J, Dronkers N, et al. Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cognitive and Behavioral Neurology. 2007;20(4):203–211. doi: 10.1097/WNN.0b013e31815e6265. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bonello P, Rapport L, Millis S. Psychometric properties of the Viusal Object and Space Perception Battery in normal older adults. The Clinical Neuropsychologist. 1997;11(4):436–442. [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function System (DKEFS): Examiner’s manual.) The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Folstein M, Folstein S, McHugh P. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011;134(10):3011–3029. doi: 10.1093/brain/awr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71(16):1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M, Shelley BP, Kipps CM, Piguet O, Hodges JR. Can progressive and non-progressive behavioural variant frontotemporal dementia be distinguished at presentation? Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(6):591–593. doi: 10.1136/jnnp.2008.163873. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos E, Petersen RC. Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, Stroop, TMT, and JLO. The Clinical Neuropsychologist. 1996;10(3):262–278. [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos E, Petersen RC, Kokmen E, et al. Mayo’s Older Americans Normative Studies: Updated AVLT norms for ages 56 to 97. The Clinical Neuropsychologist. 1992;6((Supplement)):83–104. [Google Scholar]
- JMP . Version 8 SAS Institute Inc; Cary, NC: 1989 - 2008. [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Wester Aphasia Battery (Revised)) Psych Corp; San Antonio, TX: 2007. [Google Scholar]
- Khan BK, Yokoyama JS, Takada LT, Sha SJ, Rutherford NJ, Fong JC, et al. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. Journal of Neurology, Neurosurgery & Psychiatry. 2012;83(4):358–364. doi: 10.1136/jnnp-2011-301883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda M, Ivnik R, Smith G, Ferman T, Boeve B, Knopman D, et al. Mayo’s Older Americans Normative Studies: Visual Form Discrimination and copy trial of the Rey-Osterrieth Complex Figure. Journal of Clinical and Experimental Neuropsychology. 2007;29(4):377–384. doi: 10.1080/13803390600726803. [DOI] [PubMed] [Google Scholar]
- Machulda M, Whitwell J, Dean P, Micklewright J, Duffy J, Strand E, et al. Neuropsychological Correlates of Parietal Atrophy in Logopenic Progressive Aphasia (P02.029) Neurology. 2012;78(Meeting Abstracts 1):P02–029. [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Archives of Neurology. 2009;66:1545–1551. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012 doi: 10.1093/brain/aws080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio R, Agosta F, Rascovsky K, Karydas A, Bonasera S, Rabinovici GD, et al. Clinical syndromes associated with posterior atrophy. Neurology. 2009;73(19):1571–1578. doi: 10.1212/WNL.0b013e3181c0d427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Osterrieth P. Le test de copie d’une figure complex: Coontribution a l’etude de la perception et de la memoire. Archives de Psychologie. 1944;30:286–356. [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Aβ amyloid and glucose metabolism in three variants of primary progressive aphasia. Annals of Neurology. 2008;64(4):388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual & Motor Skills. 1958;8:271–276. [Google Scholar]
- Rey A. L’examen clinique en psychologie) Presses Universitaires de France; Paris: 1964. [Google Scholar]
- Ridgway GR, Lehmann M, Barnes J, Rohrer JD, Warren JD, Crutch SJ, et al. Early-onset Alzheimer disease clinical variants. Neurology. 2012;79:80–84. doi: 10.1212/WNL.0b013e31825dce28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam M-M. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76(21):1804–1810. doi: 10.1212/WNL.0b013e31821ccd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, et al. Progressive logopenic/phonological aphasia: Erosion of the language network. NeuroImage. 2010;49(1):984–993. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky D, Bakkour A, Negreira A, Nalipinski P, Weintraub S, Mesulam M-M, et al. Cortical neuroanatomic correlates of symptom severity in primary progressive aphasia. Neurology. 2010;75(4):358–366. doi: 10.1212/WNL.0b013e3181ea15e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Warrington E, James M. The Visual Object and Space Perception Battery) Thames Valley Test Company; Bury St. Edmunds, Suffolk, England: 1991. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-III. Psychological Corporation; New York: 1997. [Google Scholar]
- Wicklund AH, Rademaker A, Johnson N, Weitner BB, Weintraub S. Rate of cognitive change measured by neuropsychologic test performance in three distinct dementia syndromes. Alzheimer Disease and Associated Disorders. 2007;21(4):S70–S78. doi: 10.1097/WAD.0b013e31815bf8a5. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133(7):2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]