Abstract
Objective
To assess the performance of a tandem mass spectrometry technology in a newborn screening laboratory to simultaneously measure α-galactosidase, acid-α-glucosidase, and α-L-iduronidase for the detection of infants at risk to develop Fabry, Pompe, or Mucopolysaccharidosis-I (MPS-I) diseases.
Study design
Enzyme activity was assayed from a 3.2-mm punch from 100,000+ anonymous newborn blood spots. Punches with low enzyme activity were further evaluated by nucleotide sequence analysis of the responsible gene. Confirmation of affected infants was dependent on identification of mutations compatible with diminished enzyme activity.
Results
The technology for simultaneously measuring multiple enzyme activities by tandem mass spectrometry was successful. The confirmation of diagnosis for Fabry, Pompe, or MPS-I, by DNA sequencing estimated the prevalence of Fabry disease at 1/7,800 males (95% CI: 1/17,800 - 1/3,600); Pompe disease at 1/27,800 newborns (95% CI: 1/90,000 – 1/10,200); and MPS-I at 1/35,500 newborns (95% CI: 1/143,000 – 1/11,100). These estimates of prevalence are two to four times greater than the prevalence estimated by clinical diagnosis. The combined prevalence for the three disorders was 1/7,700 newborns (95% CI: 1/13,500 –1/4,500).
Conclusions
Tandem mass spectrometry for the simultaneous assay of multiple lysosomal enzymes can be successfully introduced into a routine newborn screening laboratory. The technology has a positive predictive value equal to, or better, than methods currently used for the detection of non-lysosomal disorders. Using newborn blood spots, the combined prevalence of Fabry, Pompe, and MPS-I is estimated at 1/7,700 newborns based on low-enzyme activity and confirmation by mutation analysis.
More than 50 lysosomal storage diseases (LSDs), with an estimated clinical prevalence of 1/7,000 to 1/9,000 in European populations, have been described (1,2). LSD symptoms and severity vary, from central nervous system degeneration in infancy in severe Krabbe disease or Mucopolysaccharidosis-I, to minor somatic complications in Gaucher disease, type 1. Each of the disorders, however, has a wide spectrum of clinical presentations depending on the effect mutations have on the residual enzyme activity. Even with the same mutation, differences in factors controlling gene expression and the environmental milieu will influence disease manifestation. Because of the variable clinical presentation and non-specific symptoms of the LSDs, there is often delay in clinical recognition and diagnosis. This delay in diagnosis may postpone the timely initiation of therapy for the prevention of serious central nervous system or somatic involvement (3).
Since the recognition by Chamoles et al (4,5) that lysosomal enzymes retain activity in dried blood spots on filter paper, our group has been designing novel substrates that can be used in diagnostic and newborn screening laboratories for the detection of LSD patients (6). We have designed substrates and internal standards for the quantitative measurement of lysosomal enzyme activities by tandem mass spectrometry (MS/MS). These include methods for the detection of Gaucher (acid β-glucosidase), Fabry (α-galactosidase A), Pompe (acid α-glucosidase), MPS-I (α-Liduronidase), MPS-II (α-L-iduronide-2-sulfatase), Niemann-Pick diseases types A and B (acid sphingomyelinase), MPS-IVA (galactose-6-sulfate sulfatase), MPS-VI (N-acetylgalactosamine-4-sulfatase), and Krabbe (galactocerebrosidase) diseases (7–13).
Many of these enzyme activity assays can be “multiplexed” by incubating samples with a cocktail containing substrates and internal standards in a common buffer, measuring the products by MS/MS (14–18), and calculating enzyme activities. To evaluate the robustness of the MS/MS method, we tested a multiplexed assay for Fabry disease, Pompe disease, and MPS-I in the Washington State Newborn Screening Laboratory on over 100,000 anonymous newborn dried blood spots. These three disorders were selected on the belief that they would be clinically acceptable to the newborn screening community for early detection and intervention.
Methods
The screening of ~110,000 newborns was performed in the Washington State Newborn Screening Laboratory. Institutional Review Board approval was obtained from the Washington State Department of Health with the stipulation that the screening be done anonymously after all required newborn screens had been performed. Punches were obtained from blood spots that had been stored at 18°C for 8–10 months from collection.
Retention of 80% of initial enzyme activity was validated under these conditions. Two 3.2-mm punches were collected for each anonymous newborn screening sample. The first punch was used to determine enzyme activity. The second punch was used for sequence analysis for samples with enzyme activities in the affected range. MS/MS was used for quantitating enzyme activity for α-galactosidase A (GLA), acid αglucosidase (GAA), and α-L-iduronidase (IDUA) as reported by Duffey et al. (14).
Cocktails were prepared from vials containing substrate and internal standard from the Centers for Disease Control and Prevention, Atlanta, Georgia (CDC) (19).
A 3.2-mm punch from each newborn dried blood spots was placed into a well of a 96-deep-well plate, and 30 μL of ammonium formate buffer (0.1 M, pH 4.4) containing 0.48 mM IDUA substrate (IDUA-S), 3.1 μM IDUA internal standard (IDUA-IS), 0.2 mM GAA substrate (GAA-S), 2.0 μM GAA internal standard (GAA-IS), 0.6 mM GLA substrate (GLA-S), 1.2 μM GLA internal standard (GLA-IS) and 8 μM acarbose (SIGMA-ALDRICH) was added. In addition to the newborn dried blood spots, each 96-deep-well plate contained six wells with a blank filter paper punch and two wells each of the 3.2-mm punches from quality control dried blood spots from the CDC (15). The blanks and QC samples were in the first and last columns of each plate. All pipetting was performed using a Rainen Liquidator 96-tip pipetter. The plates were sealed with aluminum plate sealing film (VWR) and incubated at 37° C for 16 hours (overnight) in an orbital, shaking incubator at 225 RPMs.
In the morning, the plates were quenched by the addition of 100 μL of ammonium acetate buffer (0.1 M, pH 5.5), and 400 μL ethyl acetate was added to the wells and mixed by aspirating the liquid 20–40 times using the Rainen Liquidator. The plates were covered with foil, centrifuged for 5 minutes at 3,000 g, and 200 μL of the top ethyl acetate layer was transferred to a new 96-shallow-well plate. The ethyl acetate was evaporated in a stream of air with an SPE Dry 96 Dual Argonaut sample concentrator system,(Biotage) with a flow rate of 40–80 psi of air and heating <35 C (typically <30 minutes), and the residue was re-suspended in 100 μL of the mass spectrometry mobile phase (80% acetonitrile, 20% water, 0.2% formic acid). The plates were placed on an orbital shaker for five minutes, covered with aluminum foil, and placed into the injector tray for MS/MS analysis.
A Waters Acquity TQD Ultra Performance tandem quadrupole MS/MS was used for analysis. Ten μL of each 100 μL sample was injected using a Waters 2777C sample manager via flow injection. The injection fluid was 80/20 acetonitrile/water with 0.2% formic acid at 0.10 ml/min for 1.10 minutes and then 0.5 ml/min for 0.40 minutes. The enzyme activity was calculated from the abundance ratio of product to internal standard with subtraction of the average of the six blanks. Enzyme activity was reported in μmol/hr/L of blood, assuming each punch contained 3.2 μL of blood.
For samples with enzyme activities in the affected range, genotype was determined by analysis of the nucleotide sequence of the appropriate gene. Genomic DNA was isolated from the duplicate 3.2-mm punch using a QIAamp DNA micro kit (Qiagen, Carlsbad, CA, USA). The Vector NTI Advance 11 computer program from Invitrogen (Carlsbad, CA, USA) was used to develop primers for amplification. Exons and the flanking intron boundaries of the GAA and GLA genes were amplified by PCR using Tsg DNA Plus Polymerase (D102, Lamda Biotech, St. Louis, MO, USA), following the supplier’s instruction. The exons and the flanking introns of IDUA were amplified using the GC-RICH PCR system (#12140306001, Roche Applied Science, Mannheim, Germany) following the supplier’s instructions. The PCR amplicons were sequenced using a 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Unless otherwise noted, primers used for PCR and sequencing reactions were identical. The Mutation Surveyor DNA Variant Analysis software from SoftGenetics (State College, PA, USA) was used to confirm sequencing genotypes. GenBank entries NM_000152.3 (GAA), NM_000169.2 (GLA), and NM_000203.3 (IDUA) were used as references for coding regions, whereby nucleotide A of the translation initiation codon ATG constituted numbering +1 of the cDNA sequences. We followed the standard naming convention of the Human Genome Variation Society such that methionine encoded by the translation mutation codon was designated as position 1 in amino acid numbering.
Dried blood spots used for quality control (QC) were obtained from the CDC (19). Four different samples were obtained: 1) unprocessed cord blood (high QC), 2) 50% cord blood in leukocyte-reduced base pool (medium QC), 3) 5% cord blood in leukocyte-reduced base pool (low QC), and 4) leukocyte-reduced base pool (blank). Two 3.2-mm punches from each quality control blood spot were included in each 96-well assay plate. A continuous 95% Confidence Interval (CI) was calculated for each QC sample and any plate where the QC sample was outside the 95% CI was reason to repeat or exclude the plate from the final analysis. The interday coefficient of variation (CV) for each control sample was typically less than 10% (Table I).
Table I.
Calculated coefficient of variation for interday enzyme variation based on quality control samples supplied by the CDC (13).
| QC Samples | GLA | GAA | IDUA | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| meanx | std dev | CV | meanx | std dev | CV | meanx | std dev | CV | |
| low | 1.24 | 0.12 | 9.73 | 1.58 | 0.20 | 12.42 | 1.41 | 0.13 | 9.52 |
| med | 9.18 | 0.76 | 8.23 | 10.07 | 1.00 | 9.94 | 3.75 | 0.32 | 8.44 |
| high | 18.01 | 1.36 | 7.53 | 15.47 | 1.54 | 9.94 | 6.23 | 0.51 | 8.21 |
activity is reported as μmole/hr/L blood
Results
Over 100,000 newborn blood spots were assayed for the enzymes αgalactosidase (GLA), α-glucosidase (GAA), and α-L-iduronidase (IDUA). Based on experience during method development, arbitrary enzyme activity cutoffs of ≤ 19% of the daily mean value for GLA, ≤15% of the daily mean value for GAA, and ≤32% of the daily mean value for IDUA were selected for further analysis by DNA sequencing.
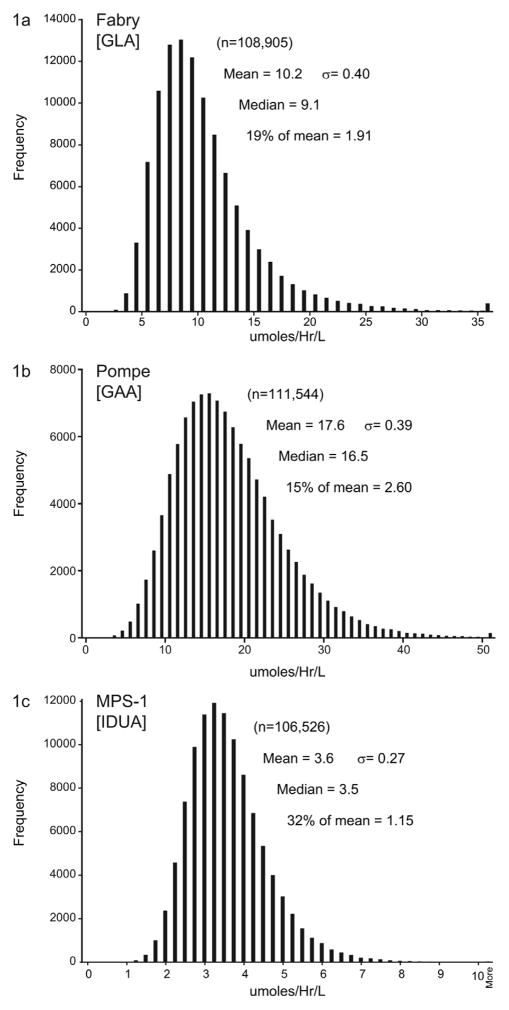
GLA activity was measured in 108,905 samples (Figure, A). Enzyme activity was distributed as a log-normal curve; and mean activity was 10.2 μmol/hr/L blood. This activity is similar to that using a MS/MS method to quantitate GLA enzyme activity reported by Dajnoki (20). Based on our prior studies of activity in blood spots from males affected with Fabry disease (14), a cut-off of 1.91 μmol/hr/L blood was used (≤ 19% of the mean value). Sixteen samples were at or below 1.91 μmol/hr/L blood. Seven samples had nucleotide changes within the GLA gene consistent with Fabry disease (Table II). Consistent with the X-linked inheritance of the disease, all were males. Samples from three female and six male infants had enzyme activities between 1.91 and 1.12 μmol/hr/L but no detectable mutations in the GLA gene. Based on this data, the estimated prevalence of Fabry disease in a U.S. population of mixed ethnic background of approximately 54,800 male infants was 1/7,800 (95% CI: 1/17,800 - 1/3,600). The positive predictive value for any sample, male or female, was 0.43 (95% CI: 0.21–0.70); and the false positive rate was estimated at 1/12,100 (95% CI: 1/6,300 – 1/23,300).
Figure.
Distribution curves for the enzyme activity of GLA, GAA, and IDUA obtained from newborn dried blood spots.
Table II.
Summary of mutation data for those blood spots identified with low enzyme activity. Identified mutations were used to imply the clinical phenotype.
| Enzyme Activity (μmol/hr/L blood) | cDNA | Amino acid | |
|---|---|---|---|
| Fabry Disease (n=7):
| |||
| 1.90 | c.660G>A | p.Arg220Gln | |
|
| |||
| 1.66 | c.1001G>A | p.Ser334Asn | |
|
| |||
| 1.34 | c.70T>A | p.Trp24Arg | |
|
| |||
| 1.28 | c.427C->A | p.Ala143Thr | |
|
| |||
| 1.21 | c.758T>C | p.Ila253Thr | |
|
| |||
| 0.89 | c.938A>G | p.Asp313Gly | |
|
| |||
| 0.58 | c.644A>G | p.Asn215Ser | |
|
| |||
| Unaffected with low GLA activity (n=9): | |||
|
| |||
| 1.91 – 1.12 | wt/n= 6 | ||
| wt/wt n=3 | |||
|
| |||
| Pompe Disease (n=4): | |||
|
| |||
| 2.53 | IVS1-13t>g | ||
| IVS1-13t>g | |||
|
| |||
| 1.70 | c.365T>A | p.Met122Lys | |
| c.1925T>A | p.Val642Glu | ||
|
| |||
| 1.57 | IVS1-13t>g | ||
| IVS1-13t>g | |||
|
| |||
| 1.43 | IVS1-13t>g | ||
| c.1-17C>T | p.Ser(-6)Phe | ||
|
| |||
| Unaffected with low GAA activity (n=13): | |||
|
| |||
| 2.45 - 2.20 | carrier/wt | n=4 | |
|
| |||
| 2.38 – 2.20 | carrier/pseudo def | n=3 | |
|
| |||
| 2.44 – 1.49 | pseudo def/wt | n=6 | |
|
| |||
| MPS-I (n=3): | |||
|
| |||
| 1.15 | c.246C>G | p.His82Gln* | |
| c.246C>G | p.His82Gln | ||
|
| |||
| 1.06 | c.355G>T | p.Asp119Tyr | |
| c.250G>A | p.Gly84Ser | ||
|
| |||
| 1.05 | c.208C>T | p.Gln70X | |
| c.208C>T | p.Gln70X | ||
|
| |||
| Unaffected with low IDUA activity (n=6): | |||
|
| |||
| 1.15 – 0.87 | carrier/wt | n=1 | |
|
| |||
| poor punch | n=2 | ||
|
| |||
| wt/wt | n=3 | ||
may be a low-activity polymorphism.
GAA activity was measured in 111,544 samples. Enzyme activity was distributed as a log-normal curve; and mean activity was 17.6 μmol/hr/L (Figure, B). A cutoff of ≤ 2.60 μmol/hr/L (≤ 15% of mean) was used. Seventeen samples had enzyme activities at or below 2.60 μmol/hr/L blood. Four samples were confirmed to have nucleotide changes consistent with possibly developing Pompe disease (Table II). Four samples had a single nucleotide change on one allele (carrier of Pompe), three were identified as carriers with an additional pseudodeficiency allele, and 6 samples were heterozygotes for a pseudodeficiency allele only. An estimate of the prevalence of infants with mutations who may eventually develop clinical symptoms of Pompe disease was 1/27,800 (95% CI: 1/90,900 – 1/10,200). The positive predictive value was 0.24 (95% CI: 0.08 – 0.50) and the false positive rate was 1/8,600 (95% CI: 1/5,000 – 1/14,800).
IDUA activity was measured in 106,526 samples (Figure, C). The mean activity was 3.6 μmol/hr/L. The variance in IDUA activity was less than that for GLA or GAA, but also had a log-normal distribution. A cutoff of ≤ 1.15 μmol/hr/L (≤ 32% of mean) was used. Three samples were confirmed to have nucleotide changes consistent with MPS-I (Table II). One sample was identified as a carrier of a common mutation for MPS-1; three samples had no identified nucleotide change; and two had low activity attributed to a poor punch from the newborn card. The estimated prevalence of MPS-I from this study was 1/35,700 (95% CI: 1/143,000 – 1/11,100). The positive predictive value was 0.33 (95% CI: 0.08 – 0.65), and the false positive rate was 1/17,750 (95% CI: 1/7,250 – 1/31,900).
The empirically set cutoffs, 19% of the mean for GLA, 15% of the mean for GAA, and 32% of the mean for IDUA, were further examined by finding distribution functions that would fit the experimental enzyme activities. All three distributions could be fitted with a log-normal probability density function according to formula,
where X is the activity relative to the experimental distribution median and σ is the standard deviation obtained by least squares fits. For GLA, the log-normal probability density function predicted six counts at or below 19% of the mean (1.91 μmol/hr/L). Likewise, the GAA distribution function predicted six counts at or below the 15% cutoff (2.60 μmol/hr/L). The IDUA distribution function predicted 13 counts at or below the 32% cutoff (1.15 μmol/hr/L). Thus, the experiment-based cutoffs are consistent with the statistical behavior of the dataset for each of the enzymes. Integration of the distribution curves from zero to the cutoff limits gives the predicted (theoretical) false positive rates which are 100 × 6/108,905 = 0.006% for GLA, 100 × 6/111,544 = 0.005% for GAA, and 100 × 13/106,526 = 0.012% for IDUA.
Discussion
Our goal was to answer three major questions with this study: (1) Can the multiplex MS/MS method for the detection of LSDs be performed as a routine procedure in a newborn screening laboratory?; (2) What is the estimated prevalence of each of the disorders?; and (3) What is the positive predictive value of the MS/MS method?
The multiplexed assay for Fabry, Pompe, and MPS-I was successfully integrated into the routine workflow of the Washington State Newborn Screening Laboratory. It required a dedicated MS/MS, nitrogen source, the availability of a 96-tip manual pipetter, and a dedicated technician. The existing software in the screening lab for data collection (Neometrix) and analysis (Quanlynx) was satisfactory.
Consistent with prior reports, the detection of LSDs by newborn screening indicates a higher prevalence in the population than the prevalence estimates by clinical diagnosis. This increase in prevalence is primarily due to recognition of later-onset forms of the diseases. We detected 1/7,800 newborn males to have low enzyme activity and nucleotide changes consistent with Fabry disease. We did not detect any female heterozygotes. We believe this was due to our cutoff of <19% of the population mean. This cutoff was based on data from affected males. This prevalence is considerably more frequent than the estimate of 1/40,000 based on clinical data (1). Spada et al (21) screened 37,164 newborn males in Northern Italy for Fabry disease using a fluorometric enzyme assay and identified ~1/3,100 as affected males. Three of the six detected males carried the p.A143T mutation that is associated with a later-onset form of the disease. In Austria, Mechtler et al (22) screened 34,736 infants by MS/MS and detected a prevalence of 1/3,859 births (~1/1,630 males) with Fabry disease. Similar to the population in Italy, p.A143T was present in three of the six males identified, suggesting a founder effect for this mutation in the Northern Italy/Austrian region of Europe. Screening for Fabry disease has also been performed in Asia using a fluorometric enzyme assay (23). In Taiwan, 1/1,250 males were identified to have Fabry disease, with many having the genotype IVS4+919G>A. This mutation is common in the Asian population and is stated to have a late-onset phenotype in males, primarily with involvement of heart and kidney (24).
We detected four infants with Pompe disease, for an estimated prevalence of 1/27,800. Based on mutation data, we believe each of the four affected infants will have a late-onset phenotype. This value is twice as common as the estimate of 1/40,000 cited for the prevalence of both infantile and late-onset phenotypes of Pompe disease in the U.S. population (25). In Taiwan, a screening program using a fluorometric assay for GAA identified five affected infants in a population of 132,538, giving a prevalence of 1/41,000 (26). A complicating factor for screening for Pompe disease in the Asian population is the presence of a pseudodeficiency allele (p.G576S; E689K) that increased the false positive rate (27). The five infants detected by screening and confirmed by clinical evaluation to have Pompe disease in Taiwan were placed on enzyme replacement therapy prior to the development of severe symptoms, and are reported to have normal cardiac function, growth, and age-appropriate developmental milestones (26).
The prevalence of 1/35,500 for MPS-1 identified by newborn screening is threefold greater than the prevalence estimated from clinical studies (1, 2). Early diagnosis of MPS-I patients with the severe (Hurler) phenotype is needed to gain maximum benefit from hematopoietic stem cell transplant. For those affected with the Hurler phenotype, the greatest benefit in intellectual outcome is achieved if the procedure can be performed at less than 2.5 years of age (28). The attenuated phenotype of MPS-I can benefit from enzyme replacement therapy to minimize the joint, organ, skin, and facial changes characteristic of the disorder (29).
In this study, the overall prevalence of disease identification for the three LSDs is 1 per 7,200 newborns. Approximately 2/3 of the identified infants will have disease manifestations outside of early childhood. This raises the ethical question as to whether the early recognition of children at risk for significant disease after infancy is the appropriate venue for screening in the newborn period (30). However, a significant number of patients with later-onset forms of LSDs have significant disease burden early in childhood and go mis- and un-diagnosed for years.
The introduction of MS/MS into the newborn screening laboratory by Millington et al and Chace et al (31, 32) has revolutionized the ability to detect many genetic disorders prior to their symptomatic presentation and has prevented significant morbidity and mortality. The expansion of the use of MSMS for the early detection of infants affected with various LSDs should prove to be just as clinically valuable as the detection of amino acid and organic acid disorders.
The compelling advantage of the MS/MS technology for the assay of enzyme activity is its ability to quantitate multiple reaction products from a single incubation and a single injection. High throughput fluorometric methods that measure enzyme activity and immunocapture methods that can quantitate residual enzyme protein are also being evaluated for newborn screening for lysosomal diseases. Data from their adaptation in newborn screening programs is not yet available. Although we have demonstrated the ability to simultaneously measure three enzymes from a single 3.2-mm dried blood spots, we anticipate expanding this technology to include nine or more assays for the detection of additional LSDs from a single 3.2-mm dried blood spots (33).
Acknowledgments
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (HHSN267200603429C/ADB-N01-KD-6-3429), the National Institute of Diabetes and Digestive and Kidney Diseases (5R01 DK067859-13), the National Institutes of Health (to C.R.S.), and Genzyme Corporation (to C.R.S.). J.K. is Vice President of Scientific Affairs for Genzyme Corporation and was involved in initial discussions and design of the project and has submitted editorial recommendations.
We are grateful for the technical cooperation of Santosh Shaunak, Tim Davis, Gary Resler, Aaron Boyce, Arun Singh, Ben Peprah, Jessica Daiker, Juli Terao, Luis Loyola, Michelle Collins, Saan Saelee, and Stan Kosciow, within the Washington State Newborn Screening Laboratory.
Footnotes
The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–54. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 2.Poorthius BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, et al. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105:151–6. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 3.D’Aco K, Underhill L, Rangachari L, Arn P, Cox GF, Giugliani R, et al. Diagnosis and treatment trends in mucopolysaccharidosis I: findings from the MPS I Registry. Eur J Pediatr. 2012;171:911–9. doi: 10.1007/s00431-011-1644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamoles NA, Blanco M, Gaggioli D. Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin Chim Acta. 2001;308:195–6. doi: 10.1016/s0009-8981(01)00478-8. [DOI] [PubMed] [Google Scholar]
- 5.Chamoles NA, Blanco MB, Gaggioli D, Casentini C. Hurler-like phenotype: enzymatic diagnosis in dried blood spots on filter paper. Clin Chem. 2001;47:2098–102. [PubMed] [Google Scholar]
- 6.Gelb MH, Turecek F, Scott CR, Chamoles NA. Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders. J Inherit Metab Dis. 2006;29:397–404. doi: 10.1007/s10545-006-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Turecek F, et al. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004;50:1785–96. doi: 10.1373/clinchem.2004.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Brockmann K, Turecek F, Scott CR, Gelb MH. Tandem mass spectrometry for the direct assay of enzymes in dried blood spots: application to newborn screening for Krabbe disease. Clin Chem. 2004;50:638–40. doi: 10.1373/clinchem.2003.028381. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Turecek F, Scott CR, Gelb MH. Quantification of cellular acid sphingomyelinase and galactocerebroside β-galactosidase activities by electrospray ionization mass spectrometry. Clin Chem. 2001;47:874–81. [PubMed] [Google Scholar]
- 10.Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis I. Clin Chem. 2008;54:2067–70. doi: 10.1373/clinchem.2008.115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffey TA, Khaliq T, Scott CR, Turecek F, Gelb MH. Design and synthesis of substrates for newborn screening of Maroteaux-Lamy and Morquio A syndromes. Bioorg Med Chem Lett. 2010;20:5994–6. doi: 10.1016/j.bmcl.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffey TA, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) Anal Chem. 2010;82:9587–91. doi: 10.1021/ac102090v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfe BJ, Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis II (Hunter syndrome) Anal Chem. 2011;83:1152–6. doi: 10.1021/ac102777s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffey TA, Bellamy G, Elliott S, Fox AC, Glass M, Turecek F, et al. A tandem mass spectrometry triplex assay for the detection of Fabry, Pompe, and mucopolysaccharidosis-I (Hurler) Clin Chem. 2010;56:1854–61. doi: 10.1373/clinchem.2010.152009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spacil Z, Elliott S, Reeber SL, Gelb MH, Scott CR, Turecek F. Comparative triplex tandem mass spectrometry assays of lysosomal enzyme activities in dried blood spots using fast liquid chromatography: application to newborn screening of Pompe, Fabry, and Hurler diseases. Anal Chem. 2011;83:4822–8. doi: 10.1021/ac200417u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang XK, Elbin CS, Chuang WL, Cooper SK, Marashio CA, Beauregard C, et al. Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin Chem. 2008;54:1725–8. doi: 10.1373/clinchem.2008.104711. [DOI] [PubMed] [Google Scholar]
- 17.Orsini JJ, Martin MM, Showers AL, Bodamer OA, Zhang XK, Gelb MH, et al. Lysosomal storage disorder 4+1 multiplex assay for newborn screening using tandem mass spectrometry: application to a small-scale population study for five lysosomal storage disorders. Clin Chim Acta. 2012;413:1270–3. doi: 10.1016/j.cca.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasper DC, Herman J, De Jesus VR, Mechtler TP, Metz TF, Shushan B. The application of multiplexed, multi-dimensional ultra-high-performance liquid chromatography/tandem mass spectrometry to the high-throughput screening of lysosomal storage disorders in newborn dried bloodspots. Rapid Commun Mass Spectrom. 2010;24:986–94. doi: 10.1002/rcm.4496. [DOI] [PubMed] [Google Scholar]
- 19.De Jesus VR, Zhang XK, Keutzer J, Bodamer OA, Mühl A, Orsini JJ, et al. Development and evaluation of quality control dried blood spot materials in newborn screening for lysosomal storage disorders. Clin Chem. 2009;55:158–64. doi: 10.1373/clinchem.2008.111864. [DOI] [PubMed] [Google Scholar]
- 20.Dajnoki A, Fekete G, Keutzer J, Orsini JJ, De Jesus VR, Chien YH, et al. Newborn screening for Fabry disease by measuring GLA activity using tandem mass spectrometry. Clin Chim Acta. 2010;411:1428–31. doi: 10.1016/j.cca.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, et al. High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79:31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mechtler TP, Stary S, Metz TF, De Jesus VR, Greber-Platzer S, Pollak A, et al. Neonatal screening for lysosomal storage disorders: feasibility and incidence from a nationwide study in Austria. Lancet. 2012;379:335–41. doi: 10.1016/S0140-6736(11)61266-X. [DOI] [PubMed] [Google Scholar]
- 23.Chien YH, Lee NC, Chiang SC, Desnick RJ, Hwu WL. Fabry disease: incidence of the common later-onset α-galactosidase A IVS4+919G->A mutation in Taiwanese newborns—superiority of DNA-based to enzyme-based newborn screening for common mutations. Mol Med. 2012;18:780–4. doi: 10.2119/molmed.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin HY, Huang CH, Yu HC, Chong KW, Hsu JH, Lee PC, et al. Enzyme assay and clinical assessment in subjects with a Chinese hotspot late-onset Fabry mutation (IVS4 + 919G->A) J Inherit Metab Dis. 2010;33:619–24. doi: 10.1007/s10545-010-9166-7. [DOI] [PubMed] [Google Scholar]
- 25.Hirschhorn R, Reuser AJJ. Glycogen storage disease type II: acid α-glucosidase (acid maltase) deficiency. In: Scriver CR, Beaudet AL, Sly WS, et al., editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3389–420. [Google Scholar]
- 26.Chien YH, Lee NC, Thurberg BL, Chiang SC, Zhang XK, Keutzer J, et al. Pompe disease in infants: improving the prognosis by newborn screening and early treatment. Pediatrics. 2009;124:e1116–25. doi: 10.1542/peds.2008-3667. [DOI] [PubMed] [Google Scholar]
- 27.Labrousse P, Chien YH, Pomponio RJ, Keutzer J, Lee NC, Akmaev VR, et al. Genetic heterozygosity and pseudodeficiency in the Pompe disease newborn screening pilot program. Mol Genet Metab. 2010;99:379–83. doi: 10.1016/j.ymgme.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 28.de Ru MH, Boelens JJ, Das AM, Jones SA, van der Lee JH, Mahlaoui N, et al. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. [Accessed October 22, 2012];Orphanet J Rare Dis. 2011 55 doi: 10.1186/1750-1172-6-55. Available at: http://www.ojrd.com/content/6/1/55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabrielli O, Clarke LA, Bruni S, Coppa GV. Enzyme-replacement therapy in a 5-month-old boy with attenuated presymptomatic MPS I: 5-year follow-up. Pediatrics. 2010;125:e183–7. doi: 10.1542/peds.2009-1728. [DOI] [PubMed] [Google Scholar]
- 30.Ross LF. Newborn screening for lysosomal storage diseases: an ethical and policy analysis. J Inherit Metab Dis. 2012;35:627–34. doi: 10.1007/s10545-011-9435-0. [DOI] [PubMed] [Google Scholar]
- 31.Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis. 1990;13:321–4. doi: 10.1007/BF01799385. [DOI] [PubMed] [Google Scholar]
- 32.Chace DH, Millington DS, Terada N, Kahler SG, Roe CR, Hofman LF. Rapid diagnosis of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal blood spots by tandem mass spectrometry. Clin Chem. 1993;39:66–71. [PubMed] [Google Scholar]
- 33.Spacil Z, Haribabu T, Scott CR, Turecek F, Gelb MH. High throughput assay of nine lysosomal enzymes for newborn screening. Clin Chem. 2012 doi: 10.1373/clinchem.2012.189936. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]