Abstract
Background
Ketamine is reported to have rapid antidepressant effects, however there is limited understanding of the time-course of ketamine effects beyond a single infusion. A previous report including 10 participants with treatment-resistant major depression (TRD) found that six ketamine infusions resulted in a sustained antidepressant effect. In the current report, we examined the pattern and durability of antidepressant effects of repeated ketamine infusions in larger sample, inclusive of the original.
Methods
Participants with TRD (n=24) underwent a washout of antidepressant medication followed by a series of up to six intravenous (IV) infusions of ketamine (0.5 mg/kg) administered open-label three times weekly over a 12-day period. Participants meeting response criteria were monitored for relapse for up to 83 days from the last infusion.
Results
The overall response rate at study end was 70.8%. There was a large mean decrease in Montgomery–Asberg Depression Rating Scale (MADRS) score at two hours following the first ketamine infusion (18.9±6.6, p<0.001) and this decrease was largely sustained for the duration of the infusion period. Response at study end was strongly predicted by response at four hours (94% sensitive, 71% specific). Among responders, median time to relapse following the last ketamine infusion was 18 days.
Conclusions
Ketamine was associated with a rapid antidepressant effect in TRD that was predictive of a sustained effect. Future controlled studies will be required to identify strategies to maintain an antidepressant response among patients who benefit from a course of ketamine.
Keywords: major depressive disorder, treatment-resistant depression, ketamine, antidepressant, glutamate, experimental therapeutics
INTRODUCTION
Major depressive disorder (MDD) is associated with a very high degree of morbidity and public health cost, in part due to the limited effectiveness of current antidepressant treatments (1-3). Against this background, reports of a rapid-onset antidepressant effect associated with the N-methyl-D-aspartate (NMDA) glutamate receptor antagonist ketamine have generated considerable interest among both clinicians and researchers (4-7). Notably, rapid antidepressant effects are observed even in individuals who have failed to respond to previous treatment attempts. This group of patients can be described as suffering from treatment-resistant depression (TRD) and, as a group, they suffer more severe depressive symptoms, exhibit more illness-related disability, and experience a more chronic or relapsing course of illness compared to their non-TRD counterparts (8, 9).
The large majority of clinical research involving ketamine in depression has focused on the safety and efficacy of a single low-dose (0.5 mg/kg) intravenous (IV) infusion (4-6). An initial placebo-controlled, double blind crossover study in inpatients with major depression reported a large mean reduction in depression severity (14±12 points on the 25-item Hamilton Depression Rating Scale) 72 hours following a single ketamine infusion (4). A second study using a similar design reported a rapid antidepressant effect within two hours and a 71% response rate at 24 hours (5). In total, four placebo-controlled studies of a single ketamine infusion in unipolar or bipolar depression support the rapid antidepressant effects of ketamine in mood disorders (4, 5, 10, 11).
Most individuals who respond to ketamine experience a relapse within several days or up to a week, although there is considerable variability in time to relapse following a single infusion (5, 12, 13). A paramount clinical issue, therefore, is to identify a strategy to maintain the antidepressant effects of ketamine (7, 14). To begin to address this question, our group previously reported on the safety and efficacy of up to six infusions of ketamine over a 12-day period in 10 patients with TRD (15). In that study, ketamine was found to be safe and well tolerated.
In the current study, we sought to extend the findings of our previous study (15) in order to further characterize the pattern of change in depressive symptoms and durability of response in the context of repeated ketamine infusions in a larger sample of subjects with TRD (inclusive of participants from the original report). Specifically, (1) we measured the overall proportion of response following up to six ketamine infusions; (2) determined the time-point associated with the largest change in symptom severity; (3) compared the trajectory of symptom change between study responders and non-responders; (4) estimated time to relapse among responders following cessation of ketamine and (5) investigated the effects of ketamine on individual symptoms of depression.
MATERIALS AND METHODS
Participants
Study participants were recruited from physician referrals, media advertisement or from an academic outpatient psychiatric clinic. Participants had chronic or recurrent MDD that was the primary presenting problem as assessed by a trained rater using the Structured Clinical Interview for DSM-IV (SCID) (16) and a diagnostic interview with a study psychiatrist. To be eligible, participants had to have failed to respond to at least two U.S. Food and Drug Administration (FDA)-approved antidepressant medications in the current episode according to the Antidepressant Treatment History Form (ATHF) (17). If a participant was taking antidepressant medication at the time of screening, a washout of ≥2 weeks (or 4 weeks for fluoxetine) was required prior to enrollment and participants remained free of antidepressant medication throughout the infusion period. Additional inclusion criteria included a score of ≥32 on the Inventory of Depressive Symptomatology – Clinician Rated (IDS-C) (18) at screen and baseline and a negative urine toxicology screen. Exclusion criteria included uncontrolled hypertension, any unstable medical condition, any Axis I disorder other than MDD that was judged to be the primary presenting problem, substance abuse or dependence in the 3 months prior to screen, lifetime history of psychosis, any psychotic disorder, bipolar disorder, developmental disorder or recreational use or abuse of ketamine or PCP. Physical examination, vital signs, weight, ECG, standard blood tests and urinalysis confirmed absence of unstable medical illnesses. Women of childbearing potential were required to have a negative pregnancy test prior to enrollment.
The Mount Sinai School of Medicine (MSSM) Institutional Review Board approved the study and written informed consent was obtained from all subjects prior to participation. The study is registered at http://ClinicalTrials.gov (NCT00548964).
Study Procedures and Rating Instruments
The study consisted of two phases. In phase I, participants received up to six IV infusions of ketamine (0.5 mg/kg) on a Monday-Wednesday-Friday schedule over a 12-day period. In phase II, participants who met response criteria following the last dose of ketamine in phase I were followed until relapse or for the maximum follow up time of 83 days, whichever came first. Response in phase I was defined as a ≥ 50% improvement in depressive symptoms as measured by the Montgomery–Asberg Depression Rating Scale (MADRS) (19). Relapse in phase II was defined as <50% improvement in MADRS score at that visit compared to baseline for two consecutive visits.
Results from the first ten participants enrolled in the current study were previously reported in part and the methods for the current report are very similar to what was described therein (15). Briefly, participants were admitted to the Mount Sinai Clinical Research Unit (CRU) on the morning of the first infusion for a 24-hour stay to optimize safety and monitoring. All subsequent infusions occurred as an outpatient procedure at the CRU. An anesthesiologist (A.M.P.) administered racemic ketamine hydrochloride (Bedford Laboratories, USA) diluted in normal saline over 40 min by IV infusion pump with standard telemetry monitoring. In the first cohort, participants were exited from the study if they failed to achieve response following the first infusion (this occurred in one case among the n=10) (15). The protocol was subsequently changed to allow participants to remain in the study regardless of response status following the first infusion (n=14) in order to measure the effect of repeated ketamine infusions among initial non-responders.
The primary outcome for phase I was change in depressive symptoms measured by the MADRS over the 12-day infusion period. Depression severity was measured in the morning prior to the first ketamine infusion (-60 min) and then at +120 min, +240 min and 24 hours. Depression severity was measured at -60 min and +240 min for each subsequent infusion day. Responder status following the sixth infusion, or the last observation for non-completers, was used to determine overall phase I responder status. During phase II, responders were followed twice weekly for four weeks, then every other week for eight weeks or until relapse, which ever came sooner. All but two participants remained free of antidepressant medication for the duration of the follow up period. Three of the 17 responders were enrolled in a separate relapse prevention study of venlafaxine extended-release (ER) up to 300 mg daily during the follow up period (two were randomized to medication, one was randomized to placebo).
Acute dissociative and psychotomimetic effects of ketamine were measured prior to the start of each infusion, during or immediately upon completion of each infusion (+40 min) and then +240 min post-infusion. Psychotomimetic effects were measured using the four-item positive symptom subscale of the Brief Psychiatric Rating Scale (BPRS; scale range 4-28) (20); dissociative effects were measured using the Clinician-Administered Dissociative States Scale (CADSS, scale range 0-92) (21); manic-like mood elevation was measured using the mood item of the Young Mania Rating Scale (YMRS, scale range 0-4) (22); a feeling of being “high” was measured using a Visual Analog Scale (VAS, range 0-10). General side effects were measured using the Systematic Assessment For Treatment Emergent Effects Self-Report Inventory (SAFTEE) (23) administered in the morning prior to each infusion and then immediately upon completion of the infusion (+40 min) and at +240 min.
Guidelines established for clinically significant changes in vital signs during the ketamine infusions were as follows: systolic or diastolic BP > 180/100 or > 20% increase above pre-infusion reading or tachycardia > 110 beats per minute. The infusion was discontinued in the event that significant changes in vital signs occurred that did not respond to medication intervention (see Supplementary Material for details).
Statistical Analysis
Baseline characteristics were compared between responders and non-responders using the Mann-Whitney U test for continuous variables and the chi-square test for categorical variables. Changes between two time-points for continuous variables were tested using paired t-tests and associations between continuous variables were quantified using the Spearman correlation coefficient. Random effects models were used to summarize and quantify changes in the MADRS score and its component items over time, and to compare temporal differences between eventual responders and non-responders. Splines were used to determine differences in the pattern of response over time among all patients and to identify the time at which there was no additional improvement in depressive symptoms. Additionally, the relationship between response status at 2, 4, and 24 hours post baseline and end of study was summarized using sensitivity, specificity, positive and negative predictive values. Time to relapse for patients who met response criteria at the end of phase I was estimated using the Kaplan-Meier method. Analyses were performed using IBM SPSS Statistics version 19 and SAS version 9.2.
RESULTS
Twenty-four participants received at least one ketamine infusion. Twenty-two participants received at least two infusions and 21 participants received all six scheduled ketamine infusions. Among the three participants who did not receive the full schedule of ketamine infusions: one was exited following one infusion due to non-response as per protocol for the first cohort (see Methods); one experienced hemodynamic elevation during the first infusion resulting in study exit as per protocol (see Methods above and Hemodynamic Changes below); one withdrew consent following three infusions due to perceived lack of response and desire for standard treatment.
Baseline Characteristics
Demographic and clinical characteristics of the study sample are presented in Table 1. Participants were aged 48.1±13.0 years with an age of onset of MDD of 22.8±13.6 years and had failed to respond to 6.1±3.3 antidepressant treatment trials and 2.3±2.3 augmentation trials in the current major depressive episode.
Table 1.
Demographic and Clinical Characteristics of Total Study Sample and Responder/Non-Responder Subgroups
| Total Sample | Responder Subgroup | Non-Responder Subgroup | P Value | |
|---|---|---|---|---|
| No. of Participants (%) | 24 (100%) | 17 (71%) | 7 (29%) | - |
| Gender (M/F) | 15/9 | 10/7 | 5/2 | 0.56 |
| Age at Enrollment (yrs) | 48.1 ± 13.0 | 48.9 ± 11.8 | 46.1 ± 16.5 | 0.73 |
| Education (yrs) | 16.0 ± 2.5 | 15.2 ± 2.7 | 17.1 ± 1.0 | 0.016 |
| Age at Onset of MDD (yrs) | 22.8 ± 13.6 | 26.4 ± 14.2 | 14.0 ± 6.8 | 0.039 |
| Length of Current Episode (yrs) | 18.1 ± 16.8 | 17.0 ± 16 | 20.6 ± 19.4 | 0.74 |
| No. of Lifetime Episodes | 1.8 ± 1.1 | 1.7 ± 0.9 | 2.0 ± 1.5 | 0.89 |
| First Degree Relative with Mood Disorder (n, %) | 14 (58.3%) | 10 (58.8%) | 4 (57.1%) | 0.94 |
| No. of Failed Antidepressants (Current Episode)a | 6.1 ± 3.3 | 6.1 ± 3.6 | 6.0 ± 2.7 | 0.70 |
| No. of Failed Antidepressant Augmentations (Current Episode)a | 2.3 ± 2.3 | 2.4 ± 2.2 | 2.0 ± 2.5 | 0.65 |
| Lifetime History of ECT (n, %) | 4 (16.7%) | 2 (11.8%) | 2 (28.6%) | 0.32 |
| Lifetime History of Suicide Attempt (n, %) | 3 (12.5%) | 1 (5.9%) | 2 (28.6%) | 0.13 |
| Past Substance Use Disorder (n, %) | 8 (33.3%) | 5 (29.4%) | 3 (42.9%) | 0.53 |
| Current Anxiety Disorder (n, %) | 6 (25%) | 4 (23.5%) | 2 (28.6%) | 0.80 |
| Current Pain Disorder (n, %) | 3 (12.5%) | 2 (11.8%) | 1 (14.3%) | 0.87 |
| Baseline MADRS Score | 31.8 ± 6.1 | 31.6 ± 6.3 | 32.1 ± 6.2 | 0.63 |
| Baseline IDS-C Score | 44.0 ± 9.8 | 45.1 ± 10.5 | 41.6 ± 8.1 | 0.46 |
P values result from Mann-Whitney U or Chi-Square tests comparing study phase I responder and non-responder subgroups. Statistical significance was defined at the .05 level, two-tailed. ECT, electroconvulsive therapy; IDS-C, Inventory of Depressive Symptomatology – Clinician Rated; MADRS, Montgomery–Asberg Depression Rating Scale; MDD, major depressive disorder.
Only includes failed trials with a score ≥3 according to the Antidepressant Treatment History Form (ATHF)
Phase I: Time-Course of Antidepressant Effects of Ketamine
The overall response rate at study end was 70.8% (17 of 24 participants). Within two hours of the first dose of ketamine, there was a large and statistically significant mean improvement in MADRS score from baseline to two hours across the full study sample: 18.9±6.6 (decrease from 31.8 to 12.9, p<0.001) (Figure 1).
Figure 1. Change in Depression Severity Following Repeated Ketamine Infusions in Treatment-Resistant Major Depression.
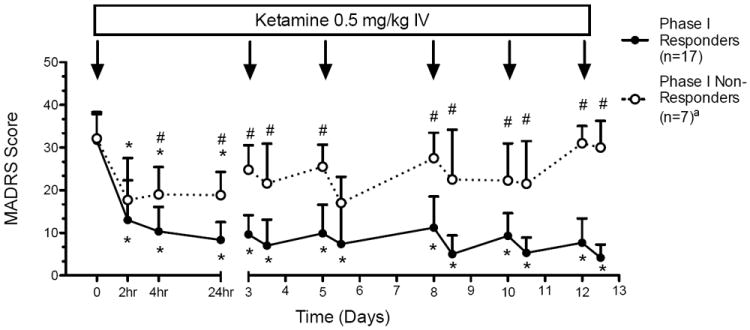
Figure depicts change in depression severity as measured by the Montgomery–Asberg Depression Rating Scale (MADRS) (mean±SD) over a 12-day period during which ketamine (0.5 mg/kg) was administered IV on a Monday-Wednesday-Friday schedule, corresponding to study days 0, 3, 5, 8, 10 and 12. Trajectories of depression severity are plotted for phase I responder and non-responder subgroups, defined using the final observed MADRS score. Depression severity was initially measured at baseline prior to the first ketamine infusion and then at 2 h, 4 h and 24 h while participants were inpatient. Subsequent infusions occurred on an outpatient basis and depression severity was measured in the morning prior to each infusion and then at 4 hours.
*MADRS score significantly decreased at given time point compared to baseline, p<0.05.
#MADRS score significantly different at given time point between responder and non-responder subgroups.
aThree participants in the non-responder group did not receive all six ketamine infusions.
The large magnitude of the two-hour response was generally maintained over the infusion period as estimated by a random effects model: average daily decrease in MADRS score of 0.128±0.17 (p=0.45). Phase I responders continued to improve slightly but significantly following the initial two-hour improvement (average daily decrease in MADRS score of 0.35±0.10, p=0.004), while phase I non-responders tended to worsen over time (average daily increase in MADRS score of 0.78±0.40, p=0.096).
The separation of responders from non-responders was clearly evident by 4 hours (MADRS score 10.35±5.74 vs. 19.0±6.46, p=0.013) and was large at 24 hours (8.35±4.2 vs. 18.8±5.5, p=0.002) (Figure 1). Ninety-four percent of study responders had responded by four hours (i.e., sensitivity was 94%), as did 29% of non-responders (i.e., specificity was 71%); with positive and negative predictive values of 0.89 and 0.83 respectively (Table 2). The relative risk of overall study non-response for two-hour non-responders was 4.0, 95% CI (1.23, 12.99).
Table 2.
Diagnostic Test Statistics Considering the Validity of Early Antidepressant Response Predicting End of Study Response During Repeated Ketamine Infusions in Treatment-Resistant Major Depression
| Hours | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 2 | 0.65 | 0.57 | 0.79 | 0.40 |
| 4 | 0.94 | 0.71 | 0.89 | 0.83 |
| 24 | 0.88 | 0.71 | 0.88 | 0.71 |
Table reports statistics of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) considering response status at the 2h, 4h and 24h time points following the first dose of ketamine as a diagnostic test for predicting subsequent phase I response. See text for details.
Phase I: Effect of Ketamine on Individual Symptoms of Depression
Within two hours of the first dose of ketamine, there was a significant reduction in each individual MADRS item score compared to baseline across the full study sample (p<0.01; with the exception of the appetite and sleep items what were not examined at the two-hour time-point; Figure 2). The non-responder subgroup manifested significant reductions in reported sadness, inner tension, pessimistic thoughts and suicidal thoughts but not the other items (p<0.05, Figure 2). The largest difference in magnitude between the phase I responders and non-responders at two hours was change in lassitude (Cohen’s d = 1.34). The observed difference between decrease in apparent sadness and concentration difficulty between the responder and non-responder subgroups was also large (Cohen’s d = 0.88 and 0.96, respectively).
Figure 2. Change in Individual Depressive Symptoms Two Hours Following the First Infusion of Ketamine.
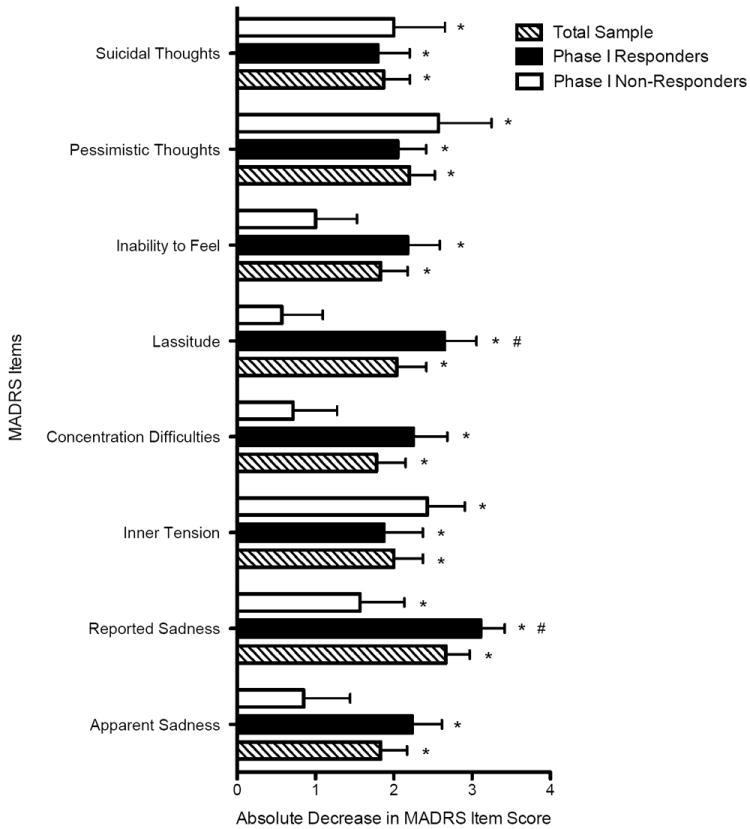
Figure depicts absolute decrease in individual items of the Montgomery–Asberg Depression Rating Scale (MADRS) from baseline to two hours following the first infusion of ketamine for the total sample, phase I responders and non-responders. The score of each item of the MADRS ranges from 0 (no symptom) to 6 (maximally severe symptom). Bars represent means±SEM.
*Item score significantly decreased at two-hour time point compared to baseline, p<0.05.
Phase II: Risk of Relapse Following Response to Ketamine
The 17 phase I responders were followed for up to 83 days in order to estimate time to relapse (Figure 3). The median time to relapse was 18 days and the 24th and 75th percentiles were 11 and 27 days, respectively. Four participants did not relapse and the estimated risk of remaining relapse free for up to 83 days is 0.25±0.11. Fourteen individuals received no psychotropic medication during the follow up period while three individuals participated in a placebo-controlled study of venlafaxine ER for relapse prevention following ketamine (see Methods). The relapse experience of the three participants receiving psychotropic medication was similar to the other responders: of the two participants randomized to venlafaxine ER, one relapsed at day 20 and one was a responder at day 83. The participant randomized to placebo post ketamine was also relapse-free at day 83.
Figure 3. Risk of Relapse Among Responders to Repeated Ketamine Infusions in Treatment-Resistant Major Depression.
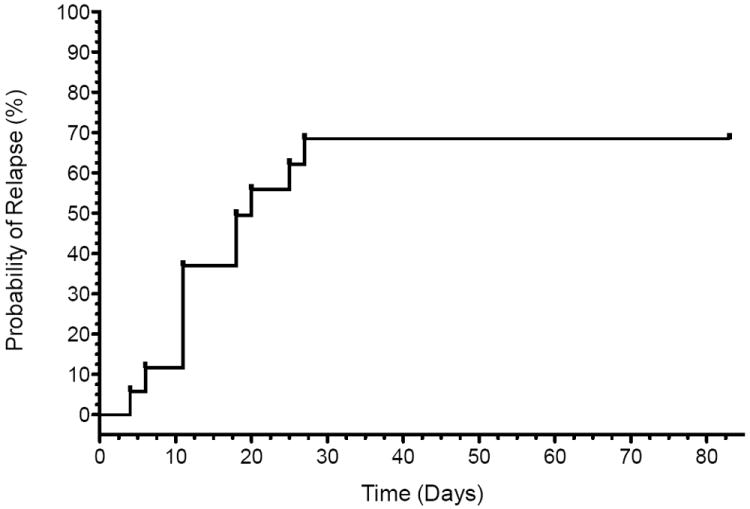
Figure depicts survival analysis conducted in 17 phase I responders following six infusions of ketamine (maximum follow up time = 83 days). Relapse in phase II was defined as <50% improvement in MADRS score at that visit compared to baseline for two consecutive visits. The number at risk at each time point is indicated along the bottom of the graph.
Acute Dissociative and Psychotomimetic Effects Associated with Ketamine
Ketamine was associated with a small but significant increase in psychotomimetic symptoms as measured by the BPRS (increase from a mean of 4.0±0.1 pre-infusion to 4.5±0.9 at the peak of the infusion, p=0.013). The BPRS score returned to a mean of 4.0 by +240 min post-infusion. Ketamine resulted in a mild, significant increase in dissociative symptoms as measured by the CADSS (increase from a mean of 0.3±0.5 pre-infusion to 7.8±12.0 at the peak of the infusion, p=0.001), which returned to baseline by +240 min post-infusion. A similar pattern was observed for elevated mood as measured by the YMRS-1 (p=0.002) or the VAS High (p<0.001). There was no trend towards increasing dissociative or psychotomimetic effects over the course of the trial. See Supplementary Table 1 for details.
There was no difference in dissociative, psychotomimetic or high feeling between responders and non-responders or any correlation between change in MADRS score and change in any of the acute measures across the infusion period.
General Side Effects
General side effects were measured in the morning prior to each infusion and then immediately upon completion of the infusion (+40 min), at +120 min and at +240 min (Supplementary Table 2). The most commonly reported side effects during the four-hour period following each infusion included feeling strange or unreal (58.3%), abnormal sensations (54.2%), blurred vision (50.0%) and feeling drowsy or sleepy (45.8%). These side effects were largely not reported at the morning pre-infusion assessments for infusions 2-6, suggesting the transient nature of the side effects. Notably, only four participants (16.7%) reported that any side effect impaired functioning at any time during the study.
Hemodynamic Changes
Sixteen participants (67%) did not experience any clinically significant change in vital signs during any of the ketamine infusions. Eight participants (33%) experienced elevated BP and/or heart rate according to pre-defined study criteria at least once during the series of infusions (see Methods section and Supplemental Material). One participant experienced elevated BP during the first infusion that did not respond satisfactorily to administration of anti-hypertensive medication, resulting in discontinuation of the infusion and study exit (maximum BP: 180/115). That participant’s blood pressure stabilized shortly after discontinuation of the ketamine infusion. No serious adverse events occurred during the study.
DISCUSSION
Herein we report the results of the largest study conducted to date on the antidepressant effects of repeated ketamine infusions in TRD. The major findings of this study are that (1) the antidepressant effect of ketamine is evident very early in the course of treatment, (2) ketamine exerts a broad-spectrum effect on individual symptoms of depression, and (3) rapid response to the first infusion is highly predictive of a sustained response to subsequent infusions.
An initial infusion of ketamine was associated with a large antidepressant effect (MADRS score decreased 18.9±6.6 from baseline to two hours) and this effect was generally maintained throughout the course of up to five additional infusions. The effect of ketamine was observed across nearly the full spectrum of depressive symptoms in the total study sample. Of particular note, suicidal ideation (SI) rapidly decreased across the total study sample, even among study non-responders. While preliminary, this result suggests that ketamine may exert a unique anti-SI effect even in the absence of a full response and is consistent with previous reports highlighting the potential anti-SI effects of ketamine in depressed populations (10, 24, 25).
We found that antidepressant response very early in the course of treatment with ketamine strongly predicted subsequent antidepressant response. Specifically, response status at 4 hours was 94% sensitive and 71% specific for predicting response status at the end of phase I. Although preliminary, these findings suggest that patients who will benefit from a course of repeated ketamine infusions will manifest a rapid improvement in depression that is then maintained over the course of treatment. Conversely, lack of a rapid response is a poor prognostic indicator for improvement following additional ketamine infusions. These data are in contrast to the time-course of response to standard antidepressants. For example, in the first step of the STAR*D study, 56% of participants who responded at some point during a 12-week trial of the serotonin selective reuptake inhibitor citalopram did so only at or after 8 weeks of treatment (26). Other groups, however, have reported that improvements within a few weeks of initiating standard antidepressant treatment are predictive of a later stable response (27, 28). A more definitive conclusion regarding the validity of early response to ketamine predicting a more durable response must await the results of future controlled studies.
Following the final ketamine infusion, the observed median time to relapse among responders was 18 days and there was considerable inter-subject variability (range 4 to > 83 days). Characterizing the durability of antidepressant response following ketamine is a critical issue in determining the potential clinical utility of ketamine as a treatment for TRD. Initial reports suggested a duration of response of several days or up to one week following a single ketamine infusion (5). A recent study of a single ketamine infusion in bipolar depression found a time to relapse of just two days using the Kaplan-Meier method (11). The findings of the current study may therefore suggest that repeated infusions yield a more durable antidepressant response compared to a single infusion, even after the infusions are discontinued. Interestingly, in a pervious placebo-controlled study of riluzole for relapse prevention following a single administration of ketamine we observed a mean time-to-relapse of 22 and 24.4 days for placebo or riluzole, respectively (6). This difference was not significant in part due to the unexpectedly long time-to-relapse of the placebo group (6). A second study of riluzole for relapse prevention following ketamine reported a time-to-relapse of 9.8 and 17.2 days for placebo or riluzole, respectively (12). Taken together, the data from the current study provide preliminary evidence for an enhanced durability of response following repeated ketamine infusions but also highlights the need to identify effective relapse prevention strategies for patients who respond to ketamine.
Future studies testing relapse prevention strategies following response to ketamine may be guided by hypothesized mechanistic synergy. While the riluzole for relapse prevention strategy was based on potential synergy between ketamine and riluzole involving modulation of glutamate signaling, the recent identification of additional signaling pathways implicated in the antidepressant action of ketamine suggests new targets for synergy (7, 29-31). In particular, the finding that inhibition of glycogen synthase kinase-3 (GSK-3) is obligatory for the antidepressant effect of ketamine in mice (31) suggests lithium – a well-known inhibitor of GSK-3 – as a potential pharmacotherapeutic strategy following ketamine.
Regarding side effects observed in this study, dissociative and psychotomimetic changes associated with ketamine were only present acutely (during and immediately post infusions) and were generally mild and well tolerated. We observed an expected increase in dissociative symptoms during administration of ketamine that returned to baseline within four hours of the start of the infusion. At no time did any participant evidence clinically significant psychotomimetic effects resulting from ketamine (e.g. paranoid, delusions, hallucinations). Other adverse effects were generally mild and no individual discontinued study participation due to side effects. Importantly, there was no evidence of increasing severity of these effects over the 12-day infusion period. There was no correlation between acute dissociative or psychotomimetic effects of ketamine and antidepressant treatment response.
Overall, our results suggest that repeated ketamine infusions may be a viable treatment strategy in the future for patients suffering from TRD. A strategy involving repeated ketamine infusions is currently being investigated as treatment for chronic pain disorders in ambulatory patients that may provide a model for ketamine treatment in TRD in the future (32). Concerns persist, however, regarding the safety and feasibility of prolonged treatment with ketamine and the optimal number of repeated treatments for safety and efficacy purposes. More preclinical and clinical research will be required before this treatment strategy can be recommended (33, 34). Chief among our concerns are a series of early preclinical studies showing that repeated administrations of very high doses of ketamine or other NMDA receptor antagonists may be neurotoxic in rodents (35, 36). Neuroimaging studies in human populations suggest that prolonged abuse of ketamine as an illicit drug may result in deleterious brain changes (37, 38), although these studies have been cross-sectional in nature and are confounded by significant comorbid substance abuse beyond ketamine. Research investigating the role of ketamine in TRD must balance concerns regarding potential toxicity against the unmet need for rapidly acting, more effective treatments for patients suffering from enormous morbidity and disability.
Our study has several limitations. Most notably, the open-label design limits the interpretation of efficacy. Specifically, it is not known to what extent the observed decrease in depression severity would have occurred even under placebo conditions. However, there are currently at least four placebo-controlled studies of ketamine in TRD or bipolar depression showing that ketamine results in a rapid antidepressant effect superior to placebo (4, 5, 10, 11). Therefore, the current study was not designed to test the antidepressant effect of ketamine per se, but rather to investigate the pattern for response to repeated administrations of ketamine over time. The second significant limitation is the modest sample size of 24 that limits the interpretations that can be drawn and the generalizability of the sample to the broader population of patients with TRD. Despite the limited sample size, however, the current report represents the largest prospective study of repeated ketamine administrations in TRD conducted to date. Notwithstanding the important limitations, we believe that the current report contributes significantly to the small but growing literature on the clinical impact of ketamine in patients with TRD.
Supplementary Material
Acknowledgments
The project described was supported in part by grant UL1RR029887 from the National Center for Research Resources, National Institutes of Health and by the Brain and Behavior Research Fund (NARSAD Distinguished Investigator Award to Dr. Charney; Young Investigator Award to Dr. Murrough). Dr. Murrough is supported by a Career Award (K23MH094707) from the National Institute Of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Mental Health or the National Institutes of Health.
We wish to thank Jazmin Moral, Drs. Adriana Feder, Kyle Lapidus, Brian Iacoviello and Laili Soleimani for their assistant in participant screening and study procedures. We wish to thank Dr. David Reich, Chair of Anesthesiology, for his continuing support of this research. We wish to thank the nursing staff at the Mount Sinai Clinical Research Unit for their support and their care of study participants. Most importantly, we wish to thank all of the patients and their families for participating in this study.
Dr. Charney, Dean of Mount Sinai School of Medicine, has been named as an inventor on a use-patent of ketamine for the treatment of depression. If ketamine were shown to be effective in the treatment of depression and received approval from the Food and Drug Administration for this indication, Dr. Charney and Mount Sinai School of Medicine could benefit financially. Within the past two years, Dr. Murrough completed a research fellowship at Mount Sinai School of Medicine that was supported in part by an educational grant from AstraZeneca to Mount Sinai. Dr. Iosifescu in the last two years has been a consultant for CNS Response, Inc. Lifetime, Dr. Iosifescu has received research support from Aspect Medical Systems, Forest Laboratories and Janssen Pharmaceutica, he has been a consultant for Forest Laboratories, Gerson Lehrman Group and Pfizer, Inc and he has been a speaker for Eli Lilly & Co., Forest Laboratories, Pfizer, Inc and Reed-Elsevier. Dr. Mathew has received research funding or salary support over the last two years from the Banner Family Fund, Brain and Behavior Fund, The Brown Foundation, Inc., Bristol-Myers Squibb, Department of Veterans Affairs, Evotec, Johnson and Johnson and the National Institute of Mental Health (5R01MH81870). He has received consulting fees or honoraria from Allergan, AstraZeneca, Cephalon, Corcept, Roche, Takeda, and has received medication from Sanofi-Aventis for a NIH sponsored study. Dr. Mathew has been named as an inventor on a use patent of ketamine for the treatment of depression. Dr. Mathew has relinquished his claim to any royalties and will not benefit financially if ketamine were approved for this use.
Continuation Ketamine in Major Depression, NCT00548964, http://clinicaltrials.gov/ct2/show/NCT00548964
Footnotes
DISCLOSURE:
Drs. Perez, Parides and aan het Rot and Ms. Pillemer, Stern and Collins reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, et al. Combining Medications to Enhance Depression Outcomes (CO-MED): Acute and Long-Term Outcomes of a Single-Blind Randomized Study. Am J Psychiatry. 2011;168:689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 4.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 5.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 6.Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murrough JW. Ketamine as a Novel Antidepressant: From Synapse to Behavior. Clin Pharmacol Ther. 2011;91:303–309. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathew SJ. Treatment-resistant depression: recent developments and future directions. Depress Anxiety. 2008;25:989–992. doi: 10.1002/da.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shelton RC, Osuntokun O, Heinloth AN, Corya SA. Therapeutic options for treatment-resistant depression. CNS Drugs. 2010;24:131–161. doi: 10.2165/11530280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Diazgranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of Ketamine’s Antidepressant Efficacy in Bipolar Depression: A Randomized Controlled Add-On Trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of Improvement in Depressive Symptoms to a Single Intravenous Infusion of Ketamine vs Add-on Riluzole: Results from a 4-Week, Double-Blind, Placebo-Controlled Study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 14.Murrough JW, Charney DS. Cracking the moody brain: lifting the mood with ketamine. Nat Med. 2010;16:1384–1385. doi: 10.1038/nm1210-1384. [DOI] [PubMed] [Google Scholar]
- 15.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 16.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis Disorders (SCID) New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 17.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- 18.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 20.Overall JE, Gorham DR, Shawver JR. Basic dimensions of change in the symptomatology of chronic schizophrenics. J Abnorm Soc Psychol. 1961;63:597–602. doi: 10.1037/h0039893. [DOI] [PubMed] [Google Scholar]
- 21.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 22.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 23.Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- 24.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 27.Szegedi A, Müller MJ, Anghelescu I, Klawe C, Kohnen R, Benkert O. Early improvement under mirtazapine and paroxetine predicts later stable response and remission with high sensitivity in patients with major depression. J Clin Psychiatry. 2003;64:413–420. doi: 10.4088/jcp.v64n0410. [DOI] [PubMed] [Google Scholar]
- 28.Henkel V, Seemuller F, Obermeier M, Adli M, Bauer M, Mundt C, et al. Does early improvement triggered by antidepressants predict response/remission? Analysis of data from a naturalistic study on a large sample of inpatients with major depression. J Affect Disord. 2009;115:439–449. doi: 10.1016/j.jad.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: A double-blind placebo controlled study. Pain. 2009;147:107–115. doi: 10.1016/j.pain.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Wolff K, Winstock AR. Ketamine: from medicine to misuse. CNS Drugs. 2006;20:199–218. doi: 10.2165/00023210-200620030-00003. [DOI] [PubMed] [Google Scholar]
- 34.Perry EB, Jr, Cramer JA, Cho HS, Petrakis IL, Karper LP, Genovese A, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 2007;192:253–260. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- 35.Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 36.Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- 37.Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, et al. Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain. 2010;133:2115–2122. doi: 10.1093/brain/awq131. [DOI] [PubMed] [Google Scholar]
- 38.Liao Y, Tang J, Corlett PR, Wang X, Yang M, Chen H, et al. Reduced dorsal prefrontal gray matter after chronic ketamine use. Biol Psychiatry. 2011;69:42–48. doi: 10.1016/j.biopsych.2010.08.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


