Abstract
Introduction
PLHIV have higher rates of smoking and lower motivation to quit smoking; thus to impact smoking rates, cessation interventions need to be acceptable to a wider range of PLHIV smokers as well as feasible to implement in a busy clinical setting. The purpose of this study was to evaluate the acceptability, feasibility, and effects of a Screening, Brief Intervention, and Referral for Treatment (SBIRT) model in an HIV/AIDS clinic among a sample of PLHIV.
Methods
PLHIV smokers (N = 40) were randomized at baseline, irrespective of their self-reported discrete smoking cessation motivation status, to receive either 8-weeks of combination nicotine replacement therapy (NRT) in conjunction with brief counseling (SBIRT framework) (n = 23) or usual care (n = 17). Smoking outcome measures included cigarettes smoked per day, nicotine dependence, smoking urge, and smoking withdrawal symptoms.
Results
The SBIRT intervention appeared to be acceptable and feasible, and produced medium to large reductions in cigarettes smoked per day, physical nicotine dependence, smoking urge, and smoking withdrawal symptoms, even for smokers not ready to quit within 6 months.
Conclusions
Findings provide preliminary support for the integration of an SBIRT model in an HIV/AIDS clinic setting to screen and provide active treatment to all smokers, regardless of readiness to quit smoking. Given the high prevalence and incredible health burden of continued smoking in this population, identifying brief and effective interventions that are easily translated into clinical practice represents an enormous challenge that if met, will yield significant improvements to overall patient outcomes.
1. Introduction
In the U.S., 40-70% of people living with HIV/AIDS (PLHIV) are current smokers, a striking disparity compared with a national prevalence estimate of 21% in the general population (Benard et al., 2007; Burkhalter, Springer, Chhabra, Ostroff, & Rapkin, 2005; Crothers et al., 2005; Durazzo et al., 2007; Elzi et al., 2006; Lifson et al., 2010; Nahvi & Cooperman 2009; Niaura et al., 2000; Webb, Vanable, Carey, & Blair, 2007). Medical advances in the treatment of HIV have resulted in substantial increases in life expectancy among PLHIV (Lau, Gange, & Moore, 2007; Lifson et al., 2010; Palella et al., 2006) and as a consequence PLHIV smokers are now, more than ever, at heightened risk for tobacco-related health harms. Continued smoking among PLHIV is associated with numerous adverse health outcomes, including bacterial and opportunistic infections, pneumonia, Chronic Obstructive Pulmonary Disease (COPD), emphysema, lung cancer, and increased all-cause mortality (Crothers et al., 2005; Durazzo et al., 2007; Elzi et al., 2006; Fiore et al., 2008; Lifson et al., 2010; Niaura et al., 2000; Pines, Koutsky, Buskin, 2011; Rahmanian et al., 2011).
Similarly, continued smoking among PLHIV has been linked to lower quality of life (Crothers et al., 2005; Ingersoll, Cropsey, & Heckman, 2009), increased pain (Turner et al., 2001), and diminished cognitive functioning (Turner et al., 2001). Moreover, both uncontrolled HIV infection and antiretroviral therapy may confer an elevated risk for cardiovascular disease that is further exacerbated by cigarette use (Elzi et al., 2006; Lifson et al., 2010).
Despite scientific advances contributing to longer life expectancy in PLHIV (Niaura et al., 2000), many PLHIV continue to feel that they will not live long enough to reap the health benefits associated with smoking cessation (Burkhalter et al., 2005; Mamary, Bahrs, & Martinez, 2002; Reynolds, Neidig, & Wewers, 2004). In addition, PLHIV smokers are a population who face unique barriers to smoking cessation, such as comorbid drug and alcohol use and mental illness (Bing et al., 2001), lower socioeconomic status (Diaz et al., 1994; Karon, Fleming, Steketee, & De Cock, 2001), lower quality of life (Fang, Hsiung, Yu, Chen, & Wang, 2002), inadequate social support (Gostin & Webber, 1998), and reliance on cigarettes to cope with the stress associated with their illness (Reynolds et al., 2004). In addition to patient barriers, few HIV providers have received training to treat tobacco dependence (Shuter, Bernstein, & Moadel, 2012) and less than half of HIV providers reported assessing tobacco use and dependence among their patients (Tesoriero, Gieryic, Carrascal, & Lavigne, 2010). However, 94% of HIV providers indicated that they would be willing to provide smoking cessation services, but 65% believed that patient resistance was their primary barrier to doing so. A study conducted at several HIV clinics in New England found that only 20% of PLHIV smokers were considering quitting in the near future (Niaura et al., 2000). This stands in stark contrast to the high levels motivation to quit noted in the general population of smokers, almost 50% of whom are attempting to quit smoking at any given time (Herzog & Blagg, 2007).
To produce significant changes in smoking rates in the PLHIV population, cessation studies will need to be both acceptable to the larger segment of PLHIV smokers who are not interested in receiving treatment as well as feasible to implement in busy HIV clinics. With the exception of one study that recruited motivated and unmotivated PLHIV smokers (Llyod-Richardson, 2009), previous studies have provided intensive treatments to PLHIV seeking treatment to quit smoking (Cummins, Trotter, Moussa, & Turham, 2005; Elzi et al. 2006; Ingersoll et al. 2009; Llyod-Richardson et al. 2009; Vidrine, Arduino, Lazev, & Gritz, 2006; Vidrine, Marks, Arduino, & Gritz, 2012; Wewers, Neidig & Kihm, 2000). However, providing cessation services, including nicotine replacement therapy, to non-treatment seeking smokers from the general population has been shown to increase quit attempts and cessation rates (Carpenter et al., 2011).
In addition, while screening individuals seeking medical care in primary care and specialty settings for cigarette use is becoming more routine (Aveyard et al., 2011; Fiore et al., 2008), provision of smoking cessation interventions are generally only provided to smokers expressing motivation to make a cessation attempt (Fiore et al., 2008). Smokers who express low motivation to make a cessation attempt at the time of their medical visit are generally provided no active intervention other than brief physician advice to quit (Fiore et al., 2008). SBIRT (Screening, Brief Intervention, and Referral for Treatment) is a public health model for the screening of substance abuse and delivery of low-intensity substance abuse treatments in the primary care setting (Babor et al., 2007). SBIRT is particularly well-suited to HIV settings, since both PLHIV, as well as providers, are focused on HIV disease management. In this context, smoking cessation treatment may not be offered until tobacco-related illness is detected or until the patient expresses interest in quitting. Because detection and treatment of cigarette use prior to the onset of smoking-related disease is critical to PLHIV smokers, the evaluation of SBIRT among PLHIV smokers warrants consideration.
The current investigation was a pilot study designed to evaluate the acceptability, feasibility, and effects of integrating the SBIRT model in a busy HIV clinic among PLHIV smokers who were randomized to either the SBIRT model or usual care (described later) for smoking cessation. The intervention chosen for this study was predicated on the notion that motivation to quit smoking varies on a moment-to-moment basis (e.g., Hughes et al., 2005; Peters & Hughes, 2009), and therefore: 1) interest in quitting when a patient is in clinic should not be required for the provision of an active smoking cessation intervention; and 2) some sort of quit aid (e.g., pharmacotherapy) should be readily available when motivation to quit is high, which is likely to occur outside of the clinic setting. Participants in the SBIRT model of the current study were therefore provided with two forms of nicotine replacement therapy for use at their own discretion and brief smoking cessation counseling irrespective of their motivational status at baseline. It was hypothesized that participants receiving the SBIRT intervention would demonstrate a decrease in cigarette consumption, nicotine dependence, urge to smoke, and smoking withdrawal symptoms relative to those receiving usual care. Furthermore, to examine motivation to quit, we evaluated the relationship between the Stages of Change algorithm (Etter & Perenger, 1999; Herzog, 2008; Herzog & Blagg, 2007; Prochaska & DiClemete, 1983; West, 2005, 2006), which categorizes smokers into discrete motivational stages, and smoking outcomes. In the transtheoretical model, individuals are believed to cycle through stages of readiness to quit including: precontemplation (not even thinking of quitting smoking), contemplation (wanting to quit but not ready to engage in a quit attempt), preparation (in anticipation of quitting, starting to make changes, e.g., telling friends and family), action (actively engaging in a quit attempt), and maintenance (quit smoking and trying to prevent relapse). Under the Stage of Change model, individuals who are further along on the model (e.g., contemplation or preparation) are believed to be more likely to successfully make the change than individuals in an earlier stage of the model (e.g., precontemplation; Prochaska & DiClemete, 1983). It was hypothesized that participants’ discrete motivational stage at the time of their clinic visit would not significantly impact their smoking behavior at follow-up time points.
2. Methods
2.1. Participant Eligibility and Recruitment
Participants were recruited through the UAB HIV Clinic, which is the largest HIV clinic in Alabama. Potential participants, self-reported smokers, were approached between July, 2011 and October, 2011 for participation. Participants who smoked at least five cigarettes per day and were engaged in HIV care at the clinic were invited to participate. Participants were excluded from participation if they had recently quit smoking, were pregnant or breastfeeding, less than 19 years of age, reported a history of allergic reaction or sensitivity to nicotine replacement, were non-English speaking, or were in too poor of health to safely use nicotine replacement.
Of the 92 individuals who were approached, 83 were eligible to participate, and 40 enrolled in the study (48%). Enrolled participants included 23 participants in the SBIRT treatment condition and 17 in the usual care comparison condition and were assigned to groups using a random numbers table. Overall, 19 (47.5%) participants were male, 23 (57.5%) were Black, and the average age was 44.5 (SD = 9.9) years old. Of the 52 individuals who did not participate, 17 (33%) reported that it was too far to drive to the clinic for extra visits, 15 (29%) initially scheduled an appointment but did not show for their baseline assessment, 11 (21%) reported no interest in participating in a research study, seven (13%) were no longer smoking, one (2%) was medically excluded by their provider, and one (2%) was incarcerated at the time of their baseline appointment. Compared to individuals who declined or were ineligible to participate, a higher number of women (52.5% versus 5.8%; p <.001) and African Americans (59.0% versus 32.7%; p =.012) enrolled in the study. There were no differences between enrolled and declined participants in age, health insurance status, substance abuse status, alcohol use, depression, anxiety, Viral Load, or CD4 count.
2.2. Procedure
As part of the Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS), ongoing computerized patient reported outcomes (PROs) that included several questions about current and past smoking behavior were collected every six months for clinical and research purposes. Potential participants who indicated current smoking on their most recent PRO assessment were initially identified through a computer search. Research staff were given a list of potential enrollees who were scheduled for upcoming routine office visits, and approached these individuals during their clinic appointment for consent and enrollment in the study. Enrolled participants completed additional baseline measures either the day of their clinic visit or during a mutually convenient return visit to the clinic. After completing baseline measures, participants were randomly assigned to either the usual care condition or the SBIRT model. The usual care condition was a no-treatment control other than standard HIV medical care, including regular HIV treatment with their provider. Participants in the usual care condition received no smoking cessation intervention from the research team and were only required to complete questionnaires on smoking characteristics, provide urine for cotinine and drug screens, and a breath sample to measure expired carbon monoxide (eCO) at time intervals that corresponded to the treatment condition (i.e., baseline, two weeks, four weeks, and eight weeks).
The SBIRT intervention was eight weeks in length and incorporated the same follow-up schedule as above (e.g., assessment at baseline, two weeks, four weeks, and eight weeks). At each of the first three visits (through Week four), participants were provided enough free nicotine replacement treatment (NRT; combination 14 mg patches and 2 mg lozenges to use together or separately) to last until their next visit. A bachelor’s level research assistant provided a one time, brief, individual 20-minute informational session about the importance of quitting or reducing smoking, techniques for cutting down, and strategies for relapse prevention. Participants were provided verbal instructions on the use of NRT, including how to use the two forms of NRT together, and told that they could use the products for a variety of purposes, including to quit smoking, reduce cigarette use, or to use in situations where they cannot smoke (e.g., public places). The research assistant and participant collaboratively problem-solved around barriers of NRT use, and discussed possible problems that may interfere with quit attempts, if the participant did decide to try and quit. In addition, all intervention participants were given a workbook which included a daily smoking diary, behavioral techniques used to quit or reduce smoking, and tips for overcoming problems scenarios (e.g., caffeine, alcohol use, living with a smoker, etc.). The workbook was modified from the treatment program used at the ACT Center at the University of Mississippi Medical Center (Payne & Crews, 2012). All participants, regardless of condition, received $20.00 for their baseline, week two, and week four appointments, and $40.00 for their week eight appointment for a potential total of $100 disbursed over eight weeks. This study was approved by the University of Alabama at Birmingham Institutional Review Board.
2.3. Measures
PROs included basic smoking information (smoking status and number of cigarettes smoked per day), clinical markers of HIV disease, adherence to antiretroviral medication, depression and anxiety scores, and substance abuse screening questions (Kitahata et al., 2008). PROs are collected through an open-source, web-based software system using touchscreen desktop computers. This method has been used with success for several years at the study site, even with patients with very limited prior computer experience, in a high volume, clinical setting (Lawrence et al, 2010; Crane et al., 2007). For the purposes of this study, the participant’s most recent PRO was combined with their baseline measures to reduce burden. PRO information provided to investigators included the participant’s basic demographics, most recent responses for alcohol use (Alcohol Use Disorders Identification Test; AUDIT-C), history of substance abuse (ASSIST), CD4 Count, Viral Load, and the Patient Health Questionnaire (PHQ-9) scales for depression (PHQ-9D) and anxiety (PHQ-A). The PHQ scales were dichotomized due to the limited number of participants and the frequency of responses. Additionally, viral load was dichotomized into detectable (>50 copies/mL) and undetectable (<50 copies/mL), in accordance with clinical use of this biomarker.
At each appointment, participants were given the Fagerström Test for Nicotine Dependence (FTND; Fagerström, 2012; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), the Questionnaire of Smoking Urges-Brief Form (QSU; Tiffany & Drobes 1991), and the Minnesota Nicotine Withdrawal Scale-Revised (MNSW-R; Hughes & Hatsukami, 1986). Participants also answered questions about their smoking history including cigarettes smoked per day (cpd), length of time smoking, and previous number of quit attempts, and completed the Stage of Change-Short Form (SOC-SF; DiClemente et al., 1991). Finally, participants exhaled into a monitor for an eCO test and provided a urine sample for cotinine and drug screening at every time point.
2.4. Data analysis
ANOVA and chi-square analyses were used to determine equivalence between groups on baseline characteristics. A repeated measures ANCOVA was then used to compare treatment conditions across cigarettes smoked per day, the FTND, the QSU, and the MNWS-R, controlling for significant covariates. As this was a pilot study designed to evaluate the acceptability and feasibility of the experimental intervention, as well as estimate its effects (i.e., rather than detect statistically significant differences between the treatment and comparison conditions), effect size was the primary outcome of interest. Eta-squared (η2) was used a measure of effect with .01, .06, and .14 representing small, medium, and large effects, respectively (Cohen, 1998). To estimate the impact of discrete motivational status on the effects of treatment condition, a baseline question from the SOC-SF was dichotomized into intention to quit within 30 days or 6 months or more (which included one person who reported no intention to quit smoking). This process yielded four groups (SBIRT group/30 days; SBIRT group/6 months; usual care/30 days; usual care/6 months) that were compared on smoking outcomes. One person from the usual care group was dropped due to incomplete data on most measures. Five treatment completers had missing data points on one or more of these scales. We imputed these missing data points via average of the scale on the previous and subsequent weeks. Eleven individuals did not complete all sessions. We conducted an intent-to-treat analysis and carried forward their last values to be able use the total sample of 39 individuals. However, 4 individuals did not have baseline measures of anxiety, which was a significant covariate, and were dropped. Thus, the retained sample consisted of 21 SBIRT and 14 usual care participants. We also analyzed the data with only completers (28 total; 14 in each group) and found a similar pattern of results. All analyses were conducted using IBM SPSS Statistics 20.
3. Results
3.1 Baseline comparisons between SBIRT versus usual care
There were no differences between the SBIRT and usual care groups in terms of age, gender, race, viral load, CD4 count, health insurance status, age of first cigarette, age of smoking daily, smoking dependence, number of cigarettes smoked daily, or depressive symptoms. However, the SBIRT group was more likely to report anxiety symptoms (38.1% versus 7.1%, p=.040) as well as history of substance abuse (68.8% versus 39.1%, p= .038). These variables were entered into the repeated-measures ANOVA as covariates.
3.2 Comparison between treatment completers and noncompleters
Individuals who completed all sessions (treatment completers; N=28; 14 in each condition) and noncompleters (N=12) were comparable on most demographic and other baseline characteristics with the exception that treatment completers were more likely to be women (64.3% versus 25.0%; p =.023), Black (71.4% versus 27.3%; p =.012), and smoke fewer cigarettes per day at enrollment (means = 14.6 vs. 25.5, p = .004).
3.3 Smoking outcomes
Panel A (Figures 1 - 4) illustrate the effects of treatment condition on smoking outcomes. As indicated in the figures, the SBIRT group reported: fewer cigarettes smoked per day (η2 = .07; p = .13); lower physical nicotine dependence (η2 = .18, p = .01) and a greater rate of decreasing physical dependence across time (η2 = .14, p =.005); lower smoking urge (η2 = .16, p = .01) and a greater rate of decreasing smoking urge across time (η2 = .11, p =.02) compared to the usual care group. Rate of change in cigarettes smoked per day did not appear to differ between the SBIRT and usual care conditions (η2 = .007, p = .71); however withdrawal symptoms (η2 = .07, p = .13) and withdrawal symptoms across time (η2 = .02, p = .51) both demonstrated small to medium effect size differences between the two groups.
Figure 1.
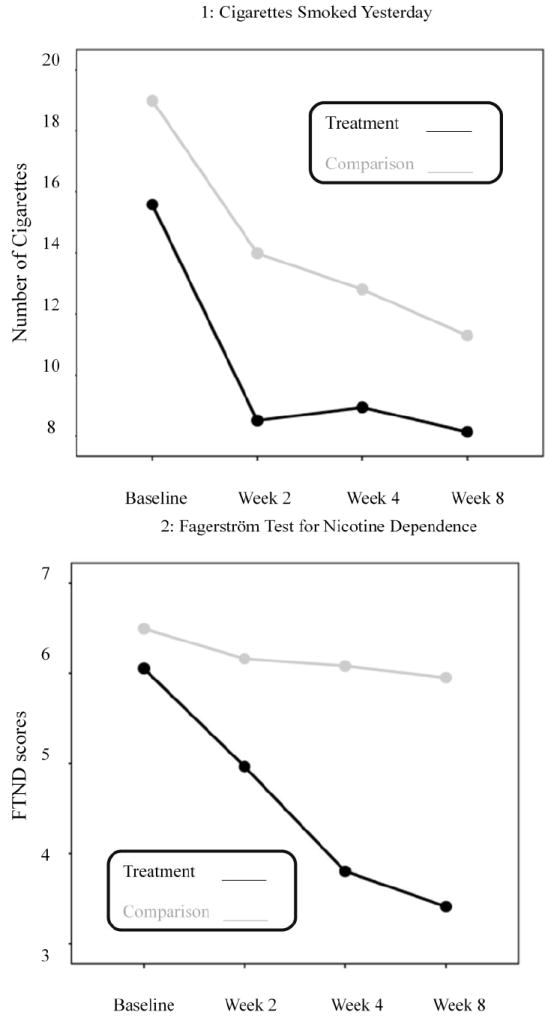
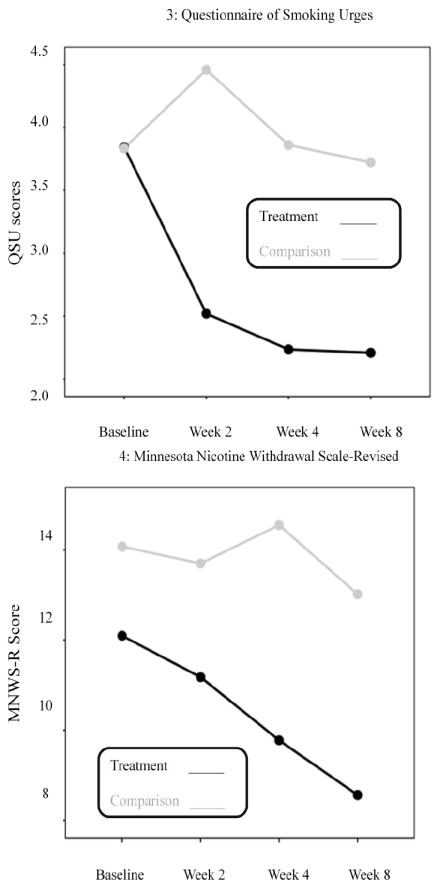
Panel A: Treatment versus Usual Care on Measures of Smoking
Note: Scale scores are labeled along the Y axis while the weeks are shown across the x axis.
3.4 Discrete motivational stage and smoking outcomes
Discrete motivation status had a minimal effect on SBIRT condition for cigarettes smoked per day across time (η2 = .02, p = .95). However, a medium effect size was shown for level of nicotine dependence across time (η2 =.16, p = .09), with individuals in both SBIRT arms decreasing their level of nicotine dependence, regardless of their readiness to quit compared to the usual care group. Similarly, a medium effect size was shown for smoking urges (η2 = .15, p = .13), with both SBIRT groups, regardless of readiness to quit, reporting less smoking urges. Finally, a medium effect size is seen for groups across time for withdrawal symptoms (η2 = .06, p = .78), with participants who received SBIRT who did want to quit for at least 6 months or more demonstrating the greatest reductions in nicotine withdrawal.
4. Discussion
The current research was a pilot study designed to evaluate the feasibility, acceptability, and effects of an SBIRT intervention, including the provision of combination NRT regardless of readiness to quit smoking, among PLHIV smokers engaged in HIV medical care. The SBIRT treatment appeared both feasible and acceptable as evidenced by rapid recruitment (3 participants per week representing 48% of eligible participants) and high retention of participants (70% completed all sessions). This rate of recruitment is higher than in most clinical trials for smoking cessation. For example, in an examination of clinical enrollment in a clinical trial for smoking cessation, Dahm et al., (2009) found only 33% of screened individuals were eligible for enrollment and, of eligible participants, only 37% were subsequently enrolled in a standard clinical trial for smoking cessation. Our eligibility rate of over 90% and subsequent enrollment of almost 50% of eligible participants exceeds most clinical trials, suggesting that this approach is both acceptable and feasible to implement widely in primary care.
In addition to high acceptability and feasibility, we observed important clinical changes resulting from this intervention. Specifically, participants in the SBIRT intervention demonstrated lower levels of cigarette use, physical nicotine dependence, urges to smoke, and withdrawal symptoms. Interesting, while the self-reported rate of cpd declined for both SBIRT and usual care groups across time, FTND, QSU, and MNWS all remained elevated across time for the usual care group. Thus, the overall pattern of results suggests moderate to strong effects of the SBIRT condition relative to the usual care condition. Moreover, study findings are also consistent with other studies that have also found support for the clinical utility of NRT in PLHIV (Cummins et al., 2005; Elzi et al., 2006; Wewers et al., 2000).
The initial evidence from this pilot indicates that provision of an active treatment, regardless of the participant’s self-reported discrete smoking cessation motivational status at baseline, was effective in bringing about reported changes to smoking behaviors including decreased cigarette use, dependence, craving, and withdrawal. These findings confirm more recent evidence suggesting that active interventions can be provided to all individuals regardless of their stage of change (Carpenter, Alberg, Gray, & Saladin, 2010; West et al.2005; 2006). Indeed, a recent meta-analysis of the efficacy of stage-based interventions demonstrated little benefit of stage-based interventions over non-stage-based treatments (Cahill, Lancaster, & Green, 2010).
The particular treatment approach used in the current study was unique and carries important implications for implementation in routine clinical practice settings. Unlike many existing interventions (e.g., Elzi et al., 2006), the SBIRT model of treatment of the current study did not distribute NRT in the context of extended counseling, which may boost its transportability into busy clinical care settings. For instance, NRT can be provided with minimal behavioral instruction, with more detailed tips for quitting provided in accompanying literature. This SBIRT model of treatment could significantly extend treatment to all smokers, regardless of self-reported motivation to make behavioral changes, which would be a similar treatment model applied to other chronic diseases (e.g., hypertension, diabetes). Using SBIRT in this setting to identify and treat smokers was not time intensive, costly, or dependent on the skills of a highly trained tobacco treatment specialist. If these pilot data are successfully replicated in a larger clinical trial, any healthcare provider (physician, nurse, social worker, intake coordinator) or non-healthcare provider (e.g., bachelor’s level assistant, as was the case in this study) could implement the intervention in a few minutes, especially since select NRT products are generally well-tolerated, safe, and available without a prescription.
While the preliminary results of this study suggest that this approach was acceptable, feasible, and efficacious, it is important to recognize the limitations inherent in the current design. First, consistent with a pilot design, the sample size was small. As a result, our study was not powered on clinical outcomes of more direct interest such as quit attempts and cessation. While we acknowledge this as a limitation, we also believe it is always necessary to first demonstrate the feasibility of an approach before expending the vast amount resources typically required for a cessation study (Carpenter et al., 2010). Moreover, while we did not focus exclusively on quit attempts and cessation, we did use well-established proxy measures of the latter outcomes (Vangeli, Stapleton, Smit, Borland, & West, 2011). Nonetheless, future randomized controlled trials employing larger samples and longer follow-up periods will be necessary to examine the efficacy of this intervention on quit attempts and ultimate cessation. Second, while we assumed that allowing participants to use NRT for whatever function they desired (e.g., to quit, cope with smoking restrictions) would result in recruiting a more representative sample across the motivational spectrum, we cannot definitively conclude that individuals who elected to not enroll in this study would have been as likely to engage in use of these products for cessation, reduction, or to use in smoking-restricted areas. However, based on the routine clinical measures collected in clinic, those who elected to not enroll in the trial were similar to participants on key measures of substance use, mental health, and HIV clinical markers although other important differences that we did not measure may have been present. Finally, while we did track provision of NRT, we did not closely monitor how or for what purpose participants elected to use these products. However, whether the person used the in a way to promote cessation was not the primary message provided to participants and is in some ways, irrelevant. While a future study will monitor the how the products were used, the results of this study demonstrated that provision of these products, regardless of how they were used, still brought about desired effects.
In sum, the prevalence of cigarette use among PLHIV is alarmingly high and linked with a number of severe health problems. With continued improvements in HIV treatment and care, PLHIV smokers will benefit more than ever from smoking reduction and/or abstinence. At present, however, there is a lack of well-controlled studies to examine the impact of smoking cessation treatments among PLHIV. While further research is needed, the preliminary results of the current study suggest that use of an SBIRT framework for providing a brief informational session plus NRT in HIV clinics, regardless of readiness to change smoking behavior, is a promising approach. If proven efficacious, this relatively simple model has strong potential for widespread dissemination that could substantively advance improved health and clinical outcomes for this patient population.
Highlights.
-
*
People living with HIV/AIDS report a lower motivation to quit smoking.
-
*
Providing NRT to unmotivated smokers has been shown to be effective.
-
*
This pilot study provided NRT to unmotivated smokers living with HIV/AIDS.
-
*
This pilot study shows the feasibility of an SBIRT model in a busy HIV/AIDS clinic.
Acknowledgments
Funding.
Dr. Cropsey was supported by grant R01CA141663 and the funding from this study was provided by the Centers for AIDS Research (CFAR)
Dr. Hendricks was supported by grant R34DA031936
Dr. Carpenter was supported by grant K23DA020482
Dr. Mugavero has received consulting fees (advisory board) from Bristol-Myers Squibb, Gilead Sciences and Merck Foundation, and grant support (to UAB) from Bristol-Myers Squibb, Pfizer, Inc, Tibotec Therapeutics, and Definicare, LLC.
Footnotes
Declaration of Interests
None of the authors has any conflicts of interest or competing interests to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Babor TB, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, Brief Intervention, and Referral to Treatment (SBIRT): toward a public health approach to the management of substance abuse. Substance Abuse. 2007;28(3):7–30. doi: 10.1300/J465v28n03_03. [DOI] [PubMed] [Google Scholar]
- 2.Benard A, Bonnet F, Tessier JF, Fossoux H, Dupon M, Mercie P, et al. Tobacco addiction and HIV infection: toward the implementation of cessation programs. AIDS Patient Care STDS. 2007;21(2):458–468. doi: 10.1089/apc.2006.0142. [DOI] [PubMed] [Google Scholar]
- 3.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry. 2001;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 4.Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, Rapkin BD. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine and Tobacco Research. 2005;7(4):511–522. doi: 10.1080/1462220050018606. [DOI] [PubMed] [Google Scholar]
- 5.Cahill K, Lancaster T, Green N. Stage-based interventions for smoking cessation. Cochrane Database of Systamatic Reviwes. 2010;(11) doi: 10.1002/14651858.CD004492. CD004492. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter MJ, Alberg AJ, Gray KM, Saladin ME. Motivating the unmotivated for health behavior change: A randomized trial of cessation induction for smokers. Clinical Trials. 2010;7:157–166. doi: 10.1177/1740774510361533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Archives of Internal Medicine. 2011;171(21):1901–1907. doi: 10.1001/archinternmed.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV positive veterans. Journal of General Internal Medicine. 2005;20(12):1142–1145. doi: 10.1111/j.1525-1497.2005.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummins D, Trotter G, Moussa M, Turham G. Smoking cessation for clients who are HIV positive. Nursing Standard. 2005;20(12):41–47. doi: 10.7748/ns2005.11.20.12.41.c4016. [DOI] [PubMed] [Google Scholar]
- 10.Dahm JL, Cook E, Baugh K, Wileyto EP, Pinto A, Leone F, Halbert CH, Schnoll RA. Predictors of enrollment in a smoking cessation clinical trial after eligibility screening. Journal of the National Medical Association. 2009;101:450–5. doi: 10.1016/s0027-9684(15)30931-7. [DOI] [PubMed] [Google Scholar]
- 11.Diaz T, Chu SY, Buehler JW, Boyd D, Checko PJ, Conti L, et al. Socioeconomic differences among people with AIDS: results from a Multistate Surveillance Project. American Journal of Preventative Medicine. 1994;10(4):217–222. [PubMed] [Google Scholar]
- 12.Elzi L, Spoerl D, Voggensperger J, Nicca D, Simcock M, Bucher HC, et al. A smoking cessation programme in HIV-infected individuals: a pilot study. Antiviral Therapy. 2006;11:787–795. [PubMed] [Google Scholar]
- 13.Fang CT, Hsiung PC, Yu CF, Chen MY, Wang JD. Validation of the World Health Organization quality of life instrument in patients with HIV infection. Quality of Life Research. 2002;11(8):753–762. doi: 10.1023/A:1020870402019. [DOI] [PubMed] [Google Scholar]
- 14.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Clinical Practice Guideline. Rockville, MD: US Public Health Service; 2008. Treating tobacco use and dependence: 2008 Update. [Google Scholar]
- 15.Gostin LO, Webber DW. The AIDS Litigation Project: HIV/AIDS in the courts in the 1990s, Part 2. AIDS & Public Policy Journal. 1998;13(1):3–19. [PubMed] [Google Scholar]
- 16.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 17.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 18.Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine replacement intervention for HIV positive smokers. AIDS and Behavior. 2009;13(3):545–554. doi: 10.1007/s10461-007-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karon JM, Fleming PL, Steketee RW, De Cock KM. HIV in the United States at the turn of the century: an epidemic in transition. American Journal of Public Health. 2001;91(7):1060–1068. doi: 10.2105/ajph.91.7.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. International Journal of Epidemiology. 2008;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. Journal of Acquired Immune Deficiency Syndromes. 2007;44(2):179–187. doi: 10.1097/01.qai.0000247229.68246.c5. [DOI] [PubMed] [Google Scholar]
- 22.Lifson AR, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, Read TR. Smoking-related health risks among persons with HIV in the strategies for management of antiretroviral therapy clinical trial. American Journal of Public Health. 2010;100(10):1896–1903. doi: 10.2105/AJPH.2009.188664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd-Richardson EE, Stanton CA, Papandonatos GD, Shadel WG, Stein M, Tashima K, et al. Motivation and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction. 2009;104:1891–1900. doi: 10.1111/j.1360-0443.2009.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamary EM, Bahrs D, Martinez S. Cigarette smoking and the desire to quit among individuals living with HIV. AIDS Patient Care and STDS. 2002;16(1):39–42. doi: 10.1089/108729102753429389. [DOI] [PubMed] [Google Scholar]
- 25.Nahvi S, Cooperman NA. Review: the need for smoking cessation among HIV-positive smokers. AIDS Education and Prevention. 2009;21:14–27. doi: 10.1521/aeap.2009.21.3_supp.14. doi: 0.1521/aeap.2009.21.3_supp.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clinical Infectious Diseases. 2000;31:808–812. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- 27.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. Journal of Acquired Immune Deficiency Syndromes. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 28.Payne TJ, Crews KM. Brief treatment of the tobacco dependent patient: A training program for healthcare providers. University of Mississippi Medical Center printing office 2012 [Google Scholar]
- 29.Pines H, Koutsky L, Buskin S. Cigarette smoking and mortality among HIV-infected individuals in Seattle, Washington (1996-2008) AIDS and Behavior. 2011;15(1):243–251. doi: 10.1007/s10461-010-9682-3. [DOI] [PubMed] [Google Scholar]
- 30.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. Journal of Consulting and Clinical Psycholology. 1983;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 31.Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A, Diaz P. Cigarette smoking in the HIV-infected population. Proceedings of the American Thoracic Society. 2011;8(3):313–319. doi: 10.1513/pats.201009-058WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds NR, Neidig JL, Wewers ME. Illness representation and smoking behavior: a focus group study of HIV-positive men. Journal of the Association of Nurses in AIDS Care. 2004;15(4):37–47. doi: 10.1177/1055329003261969. doi: org/10.1177/1055329003261969. [DOI] [PubMed] [Google Scholar]
- 33.Shuter J, Bernstein SL, Moadel AB. Cigarette smoking behaviors and beliefs in persons living with HIV/AIDS. American Journal of Health Behaviors. 2012;36(1):75–85. doi: 10.5993/ajhb.36.1.8. http://dx.doi.org/10.5993/AJHB.36.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: Prevalence, frequency, and opportunities for cessation. AIDS and Behavior. 2010;14(4):824–835. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 35.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86(11):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 36.Turner J, Page-Shafer K, Chin DP, Osmond D, Mossar M, Markstein L, et al. Adverse impact of cigarette smoking on dimensions of health-related quality of life in persons with HIV infection. AIDS Patient Care STDS. 2001;15(12):615–624. doi: 10.1089/108729101753354617. [DOI] [PubMed] [Google Scholar]
- 36.Vangeli E, Stapleton J, Smit ES, Borland R, West R. Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addiction. 2011;106(12):2110–2121. doi: 10.1111/j.1360-0443.2011.03565.x. [DOI] [PubMed] [Google Scholar]
- 37.Vidrine DJ, Arduino RC, Lazev AB, Gritz ER. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS. 2006;20:253–260. doi: 10.1097/01.aids.0000198094.23691.58. [DOI] [PubMed] [Google Scholar]
- 38.Vidrine DJ, Marks RM, Arduino RC, Gritz ER. Efficacy of cell phone-delivered smoking cessation counseling for persons living with HIV/AIDS: 3-month outcomes. Nicotine and Tobacco Research. 2012;14(1):106–110. doi: 10.1093/ntr/ntr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webb MS, Vanable PA, Carey MP, Blair DC. Cigarette smoking among HIV+ men and women: examining health, substance use, and psychosocial correlates across the smoking spectrum. Journal of Behavioral Medicine. 2007;30(5):371–383. doi: 10.1007/s10865-007-9112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: Proposal for a common standard. Addiction. 2005;100:299–303. doi: 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- 41.Wewers ME, Neidig JL, Kihm KE. The feasibility of a nurse-managed, peer-led tobacco cessation intervention among HIV-positive smokers. Journal of the Association of Nurses in AIDS Care. 2000;11(6):37–44. doi: 10.1016/S1055-3290(06)60353-1. doi:org/10.1016/S1055-3290(06)60353. [DOI] [PubMed] [Google Scholar]


