Abstract
Mood disorders are serious diseases that affect a large portion of the population. There have been many hypotheses put forth over the years to explain the development of major depression (MDD), bipolar disorder (BD) and other mood disorders. These hypotheses include disruptions in monoamine transmission, HPA axis function, immune function, neurogenesis, mitochondrial dysfunction and neuropeptide signaling (to name a few). Nearly all people suffering from mood disorders have significant disruptions in circadian rhythms and the sleep/wake cycle. In fact altered sleep patterns are one of the major diagnostic criteria for these disorders. Moreover, environmental disruptions to circadian rhythms including shift work, travel across time zones, and irregular social schedules tend to precipitate or exacerbate mood-related episodes. Recent studies have found that molecular clocks are found throughout the brain and body where they participate in the regulation of most physiological processes, including those thought to be involved in mood regulation. This review will summarize recent data which implicates the circadian system as a vital regulator of a variety of systems which are thought to play a role in the development of mood disorders.
Keywords: circadian rhythms, depression, bipolar disorder, immune system, metabolism, neurogenesis
Introduction
Mood disorders are among the most prevalent and serious diseases. Furthermore, the rate of antidepressant use has almost tripled in the last 15 years and only one third of patients attain remission (1). The seemingly ever growing rates of mood disorder diagnosis might involve our modern, hectic lifestyle which includes increasing exposure to artificial light (and computer screens) at night, shift work, travel across time zones, and reduced exposure to daytime sunlight. Throughout most of human evolution, there was no electricity so people mostly stuck to a routine diurnal schedule. These environmental disruptions to circadian rhythms may be particularly deleterious for vulnerable individuals (i.e. those with a mutation in one of more circadian genes). Circadian rhythm abnormalities have been described in patients with mood disorders since the 1950s (2). These include rhythms in activity, sleep, blood pressure, hormone secretion, and monoamines (3). The Social Zeitgeber Theory of mood disorders was put forth in the late 1980s which postulates that stressful life events lead to changes in the sleep/wake schedule which then alter molecular and cellular rhythms in vulnerable individuals, leading to mood-related episodes (4). This theory is backed by a several clinical studies which find direct correlations between the severity of rhythm disruptions and mood and restoration of rhythms with treatment (2, 3). Indeed essentially all current treatments for mood disorders shift or stabilize circadian rhythms (2, 3). However the mechanisms by which circadian rhythm disruptions might lead to changes in mood has been unclear.
Multiple hypotheses have been put forth over the years to try to explain the development of depression and other mood disorders. The circadian system is best known for its role in controlling the timing of sleep and certainly chronic sleep deprivation can exacerbate mood-related problems and may play a direct role in the development of mood disorders (5, 6). However recent studies have determined that the circadian system is also intricately involved in the molecular and cellular control of a wide range of processes which are hypothesized to underlie mood disorders. Thus both genetic and environmental disruption of circadian rhythms could lead to mood-related problems via any (or all) of these potential paths.
The circadian system and mood
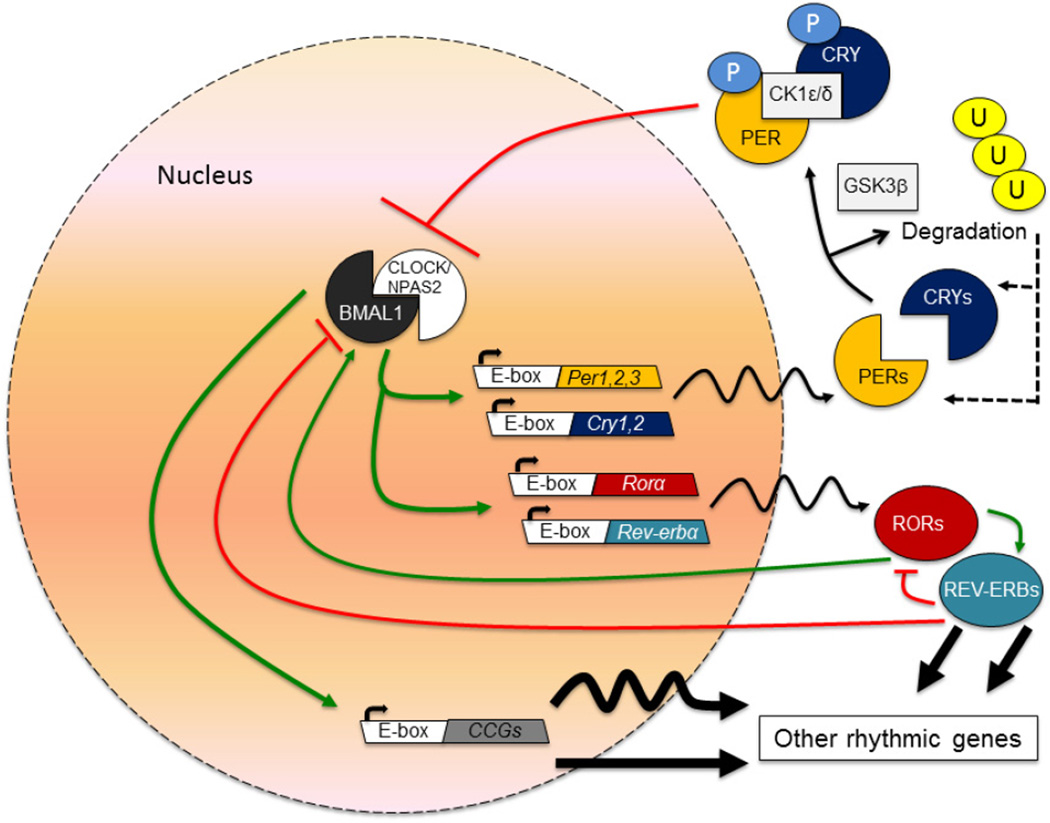
The proteins that make up the core molecular clock are shown in Figure 1 (7). The master circadian oscillator is located in the suprachiasmatic nucleus (SCN) of the hypothalamus where it receives light input via a direct retinohypothalamic tract (RHT). The SCN then sends signals via direct and indirect projections throughout the brain. It also coordinates the timing of the release of multiple peptides and hormones including melatonin which promotes sleep onset (8). Interestingly, the SCN seems to regulate the timing of rhythms in the periphery via alterations in body temperature which serve as a universal cue to entrain multiple organ systems to the light/dark cycle (9).
Figure 1.
This core molecular clock is composed of a series of transcriptional and translational feedback loops. The basic helix-loop-helix-PAS (Period-Arnt-Single-minded)-containing transcription factors, Circadian Locomotor Output Cycles Kaput (CLOCK) and Brain and Muscle Arnt-Like protein 1 (BMAL1; also called MOP3 and ARNTL) heterodimerize and bind to E-box containing sequences in a number of genes including the three Period (Per) genes (Per1, Per2 and Per3) and the two Cryptochrome (Cry) genes (Cry1 and Cry2). Over time, the PER and CRY proteins dimerize and are shuttled back into the nucleus where CRY proteins can directly inhibit the activity of CLOCK and BMAL1 forming a negative feedback loop which cycles every twenty-four hours. In addition to this feedback loop, the CLOCK and BMAL1 proteins regulate the expression of the nuclear hormone receptors, Rev-erbα and Rora which in turn can repress or activate Bmal1 transcription, respectively, through actions at the Rev-Erb/ROR response element in the promoter. Outside of the SCN, Neuronal PAS-Domain Protein 2 (NPAS2; also known as MOP4) can heterodimerize with BMAL1 and control Per and Cry gene expression. There are several key proteins which regulate the timing of the molecular clock through phosphorylation, sumoylation, and other mechanisms. Casein kinase 1 delta and epsilon proteins (CK1δ and CK1ε) phosphorylate the PER, CRY and BMAL1 proteins altering their stability and nuclear entry. Glycogen synthase kinase 3 beta (GSK3β) also phosphorylates the PER2 protein facilitating nuclear entry, the Rev-erbα protein increasing protein stability, and the CRY2 and CLOCK proteins leading to proteasomal degradation.
To date, at least 50 human genetic studies have identified polymorphisms in several of the circadian genes which associate with various psychiatric diseases using candidate gene approaches and genome wide association studies. These results have recently been reviewed (10, 11). This argues that circadian rhythm abnormalities might be the cause of mood disruption rather than the effect of mood disruption, though likely one exacerbates the other. Moreover, a number of animal studies have implicated individual circadian genes in the regulation of mood, anxiety and reward (12). Mice with an induced mutation in the Clock gene which creates a protein with dominate-negative function (ClockΔ19) (13) have a behavioral profile which strongly resembles that of bipolar patients specifically in the manic state (14). When these mice are given the mood stabilizing drug, lithium, the majority of their behavioral responses are normalized towards those of wild type mice (14). Other circadian gene mutations lead to similar behavioral phenotypes. These include transgenic mice overexpressing GSK3β (15) and mice with a mutation in F-box protein 3 (Fbxl3, a protein that targets CRY for degradation)(16). Sirtuin 1 (SIRT1) is a histone deacetylase (HDAC) which antagonizes the transcriptional activating properties of CLOCK and BMAL1 (17). SIRT1 knock-out mice also have a decrease in anxietyrelated behavior at baseline, and lowered depression-related behavior following chronic social defeat (18). Overexpression of SIRT1 leads to the opposite effect (18). SIRT1 knock-out should lead to increased CLOCK activity yet the mutant mouse has a similar manic-like phenotype, suggesting that perhaps any circadian gene mutation will lead to the same behavioral profile. However, while Per2 knock-out mice (Per2Brdm1) also have reduced immobility in the forced swim test, (19), mice with mutations in both mPer1 and mPer2 (mPer1;mPer2ldc double mutant) have a normal locomotor response to novelty, and an increase in anxiety-related behavior which is the opposite of what is observed in the ClockΔ19 and other mutant mice (20). Thus these genes have some specificity in their function and the same phenotype is not produced with the mutation of any circadian factor. Of course some of these proteins are involved in other processes besides circadian rhythm regulation and it is possible that the mood and anxiety-related behavioral phenotypes are due to changes in other systems.
How do rhythm disruptions lead to mood disruptions?
Monoamine signaling
Antidepressant, antipsychotic and mood-stabilizing drugs all alter monoaminergic (i.e. serotonin (5-HT), dopamine (DA) and norephinephrine (NE)) function. For example, the major class of antidepressant drugs used today are selective serotonin reuptake inhibitors (SSRIs). Multiple human imaging studies have also found alterations in these systems in subjects with mood disorders (21). Animal studies have now taken advantage of optogenetics or other means of controlling neuronal activity to confirm the importance of the monoaminergic circuitry in mood, reward and anxiety-related behavior. Some results are controversial in that stimulation of dopamine neurons in the VTA can lead to both a pro-depressive and anti-depressive response in certain chronic stress models, so the exact function of these monoamines is still unclear (22, 23).
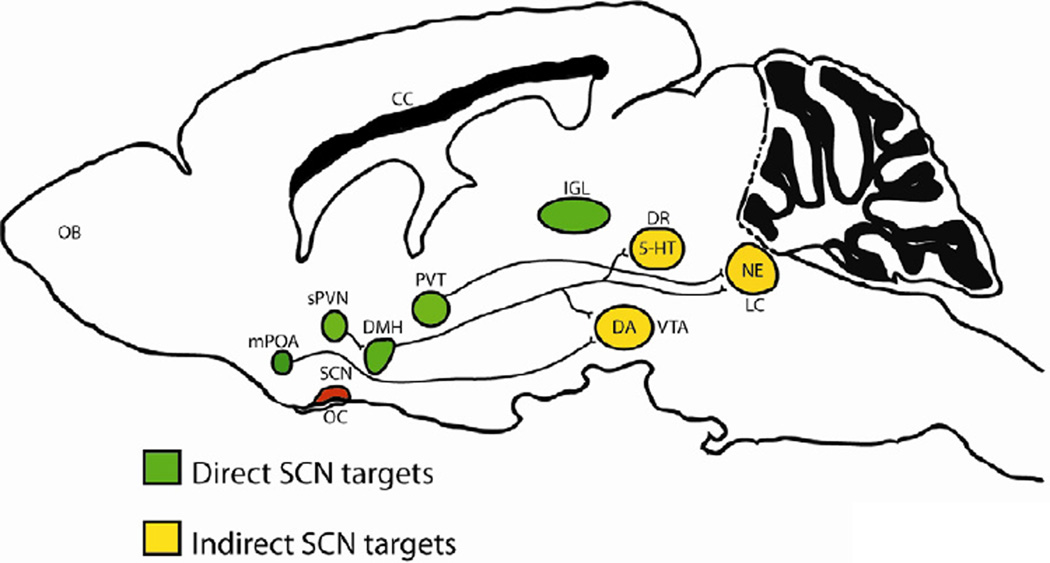
Administration of the antidepressant, agomelatine (a melatonin receptor agonist and weak 5-HT2C receptor antagonist) leads to direct (2 day) and indirect (14 day) increases in monoaminergic neuronal activity which can be blocked with the melatonergic antagonist S22153 (24). These results point to a modulatory role for melatonin in monoaminergic activity, linking the circadian and monoamine systems. Numerous studies have found that serotonin, norepinephrine and dopamine all have a circadian rhythm in their levels, release, synthesis related enzymes and receptors (3). Since these systems are keenly involved in arousal, motivation, and reward, it seems like it would be advantageous for these circuits to have a diurnal rhythm so that the motivation to find a mate or look for food are not in temporal conflict with the drive for sleep. Some of the rhythms in these circuits arise from projections from the SCN (Figure 2). All of these neuronal populations also express circadian genes which directly regulate expression of genes involved in monoamine synthesis and release. One example is monoamine oxidase A (MAOA), a gene important in monoaminergic metabolism, which is a direct transcriptional target of NPAS2, BMAL1 and PER2 in the striatum (25).
Figure 2.
The circadian system regulates multiple monoaminergic brain regions that control mood, anxiety and motivated behaviors, through local expression of clock genes as well as indirect connections originating from the master pacemaker in the suprachiasmatic nucleus (SCN). The SCN projects monosynaptically to multiple hypothalamic nuclei (in green), which subsequently communicate with regions (in yellow) that synthesize dopamine (DA), serotonin (5-HT) and norepinephrine (NE). As a result, serotonin, norepinephrine and dopamine all have a circadian rhythm in their levels, release, and synthesis-related enzymes. Abbreviations: medial preoptic area (mPOA), sub-paraventricular nucleus of the hypothalamus (sPVN), dorsomedial hypothalamus (DMH), paraventricular nucleus of the thalamus (PVT), dorsal raphe (DR), ventral tegmental area (VTA), locus coeruleus (LC), optic chiasm (OC), corpus callosum (CC), olfactory bulb (OB).
Per2Brdm1 mice have increased dopamine levels and altered neuronal activity in the striatum, which may explain some of their abnormal behavioral phenotypes in measures of mood, reward and anxiety (19). In response to chronic stress, mPer1 and mPer2 mRNA levels are altered in the NAc (20). Furthermore, selective knock-down of both mPer1 and mPer2 via RNA interference (RNAi) in the NAc is sufficient to produce an increase in anxiety-like behavior, suggesting prominent roles for the Per genes in this region (20). ClockΔ19 mutants have increased dopamine synthesis and increased dopaminergic activity (26). Moreover, they have an increase in tyrosine hydroxylase (TH) expression in the VTA (27). Chronic lithium treatment restores normal levels of VTA dopaminergic activity to the ClockΔ19 mice (26) suggesting that this activity may underlie their manic-like behavior. The regulation of TH expression and dopamine levels by CLOCK appears to be evolutionarily conserved as fruit flies (Drosophila melanogaster) which carry a mutation in the Clock gene (ClkJrk) have increased TH levels and increased dopamine synthesis (28). Many, but not all, of the manic-like phenotypes of the ClockΔ19 mice are rescued by expression of a functional CLOCK protein specifically in the VTA (14). Interestingly, knock-down of Clock expression only in the VTA of otherwise wild type mice leads to a “mixed state” where mice are less anxious and hyperactive, but have greater levels of depression-related behavior (29). This is particularly interesting given the fact that bipolar patients cycle through periods of depression, mania, and mixed states. Thus circadian genes appear to have a prominent and direct role in the VTA and likely other monoaminergic regions in the regulation of anxiety and mood-related behavior. Undoubtedly this function is key in understanding the mechanisms that connect disrupted circadian rhythms to mood disorders and should be the focus of future study. However, other systems involving the periphery and additional brain regions may also factor into these diseases.
Immune function
Alterations in the immune system are thought to underlie a number of health problems including rheumatoid arthritis, inflammatory bowel disease and asthma. Many of these disorders are associated with increased depression (30). Thus, a neuroinflammatory hypothesis has recently been proposed to explain the development of depression in general and particularly in people with immune-associated conditions. In humans and animal models, pro-inflammatory cytokines can themselves induce a syndrome resembling depression with feelings of anhedonia, psychomotor slowing and fatigue (31, 32). Pro-inflammatory cytokines in the brain lead to reduced neurogenesis, decreased synaptic plasticity, and less long-term potentiation (LTP) in the rodent hippocampus, which is similar to effects often seen with chronic stress (33). Moreover, the reduction in neurogenesis and abnormal behaviors produced in response to stress in rodents can be blocked with an inhibitor of NF-κB (34). Pro-inflammatory cytokines can also impact monoamine signaling leading to reductions in 5-HT and dopamine release, as well as altered HPA axis function in animal models (linking several systems that are discussed in this review) (33).
Circadian rhythm disruption in people and in animal models leads to an increase in proinflammatory cytokines including TNF-α, macrophage inflammatory protein 2 (MIP-2) and leukemia inhibitory factor (LIF) (35–37). In turn, TNF-α, IFN-γ, and IL-6 alter the sleep/wake cycle and circadian gene expression via nuclear factor-κB (NF-κB) signaling pathways (38). Several studies have found that a lipopolysaccharide (LPS) induced immune challenge leads to a depressive-like phenotype in rodents and it also alters circadian gene expression and SCN activity (39, 40). Interestingly, a central infusion of IL-1β causes a significant phase delay in locomotor rhythms, a state often associated with an increased risk for depression (36). Constant darkness (for 4 weeks) in rodents leads both increased depression-like behavior and elevated levels of plasma IL-6 and hippocampal Interleukin 1 receptor, type 1 (Il1-R1) (41). In addition, mice with a deletion of IL-6 (a target gene of NF-kB) do not develop depression-like behavior in constant darkness, suggesting a prominent role for NF-kB signaling and cytokine induction in the development of depression-like behavior following diurnal rhythm disruption (41). Recently, it was discovered that the CLOCK protein interacts directly with NF-κB to activate transcription at NF-κB responsive promoters (42). Furthermore, activation of NF-κB in response to immunostimuli is reduced in cells which lack the CLOCK protein (42). Interestingly, RORα inhibits NF-κB function by inducing IκB-α, a protein that antagonizes NF-κB signaling (43). Thus there appears to be complex bi-directional cross talk between the circadian and immune systems.
Thus a scenario could be proposed in which genetic or environmental disruptions of circadian rhythms lead to a pro-inflammatory response in the brain which alters monoamine signaling, SCN function, and hippocampal neuroplasticity, ultimately leading to a depression-like state. However a recent review of clinical data by Raison and Miller (2011) concluded that “The question of whether depression is an inflammatory disorder is a resounding “no”” (30). Rather, certain groups of depressed individuals have elevated inflammatory biomarkers which might indicate either a discrete depressive subtype or that even very low levels of inflammation could cause depressive-like symptoms in certain individuals through interactions with other compromised systems (30). Moreover, records from the STAR*D trial (a large multicenter study of antidepressant efficacy) found that the remission rate fell from 54% to 40% in patients taking SSRIs when they were also taking an anti-inflammatory drug, an effect that was also replicated in animal models of depression (44–46). However, a small trial (n=20) found that the addition of a cyclooxygenase-2 inhibitor (celecoxib, a drug which inhibits the production of pro-inflammatory cytokines) to an antidepressant (reboxetine a NE-transport inhibitor) produced a more robust anti-depressive effect (47). Thus the role of a pro-inflammatory response in the development of depression is still unclear and is very likely a contributing factor in certain individuals rather than a central cause of depression. Indeed, depression scores did improve significantly in patients with psoriasis and co-morbid depression following treatment with etanercept (a TNF-α inhibitor) in a phase III clinical trial (48), and anecdotal evidence supports an antidepressant effect of minocycline (a tetracycline-like antibiotic) in patients with somatoform spectrum disorders and other infections (49).
HPA axis regulation
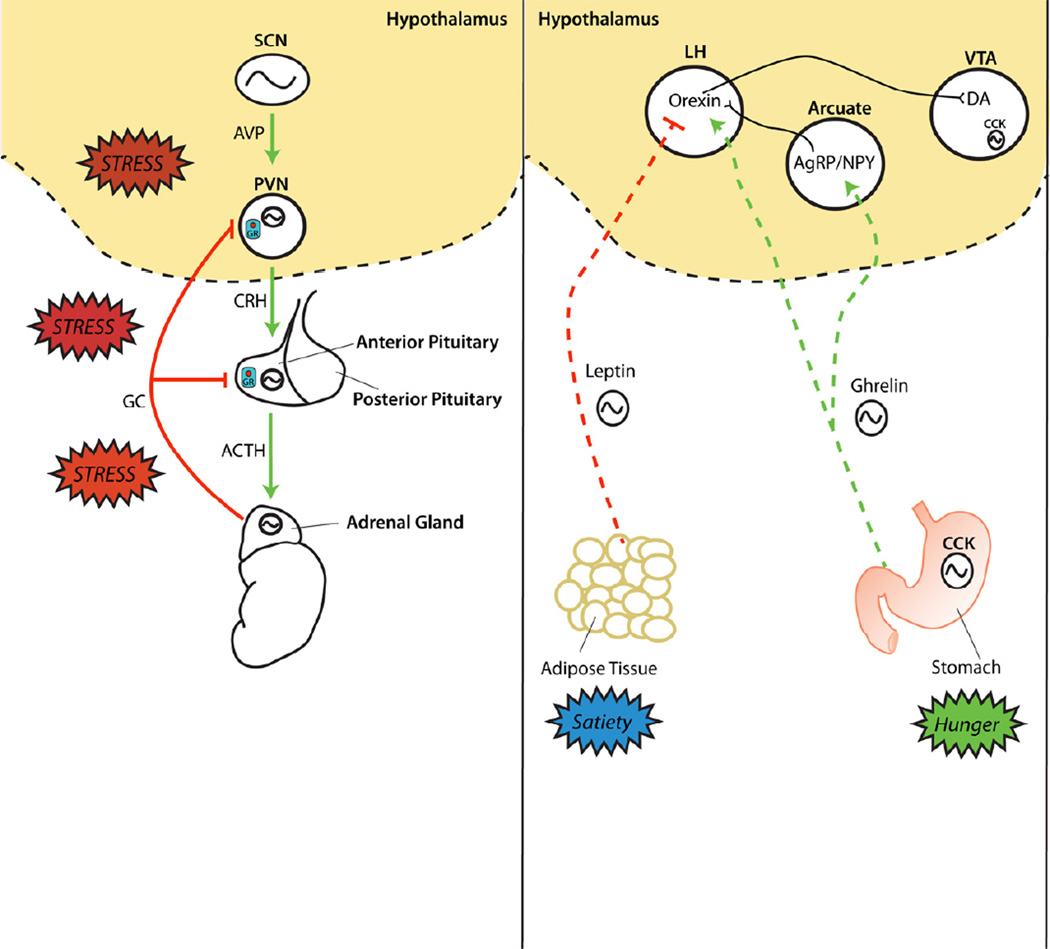
Glucocorticoids are multifunctional adrenal steroid hormones which are associated with metabolism and arousal. They are also intimately involved in the response to stress via activation of the hypothalamus-pituitary-adrenal (HPA) axis (Figure 3). Glucocorticoids have a strong circadian rhythm in expression and peak in expression just prior to the onset of awakening (8). CRY proteins also interact with the glucocorticoid receptor (GR) in a ligand-dependent fashion leading to rhythmic repression in GR activity (50). Furthermore, CLOCK directly acetylates GR leading to decreased sensitivity to glucocorticoids in the morning and increased sensitivity at night when acetylation is reversed (51).
Figure 3.
The circadian system regulates many hormones and peptides in the brain and periphery that impact mood and reward. (left panel) The circadian control of the hypothamo-pituitary-adrenal (HPA) axis originates in the SCN which projects to the paraventricular nucleus (PVN) with arginine vasopressin (AVP) synthesizing neurons, causing the release of corticotropin-releasing hormone (CRH). Subsequently, CRH stimulates the synthesis and release of adrenocorticotropin hormone (ACTH) from the anterior pituitary, which travels through the blood stream and stimulates the release of glucocorticoids (GC) from the adrenal gland. Glucocorticoids negatively feedback to multiple sites via interaction with the intracellular glucocorticoid receptor (GR) in order to maintain basal stress hormone levels within a homeostatic range. Rhythmic clock gene expression coordinates incoming hormonal signals to rhythms in local receptor expression at the level of the PVN, pituitary and adrenal gland. (right panel) The hormone leptin is expressed in adipose tissue and is a satiety signal conveying a positive energy balance to the brain. Leptin levels also fluctuate on a daily basis, as clock genes are expressed in adipose tissue, and lead to circadian rhythms in hunger and satiety. Leptin is sleep-inducing and may alter mood by inhibiting orexin neurons in the lateral hypothalamus (LH). An opposing metabolic signal, ghrelin, is released by the stomach to convey a negative energy balance to the brain by stimulating orexigenic Agouti-Related peptide (AgRP)/Neuropeptide Y (NPY)- secreting neurons in the arcuate nucleus of the hypothalamus. Additionally, ghrelin directly stimulates orexinergic neurons in the LH, which induces arousal and feeding behavior. These orexigenic neurons also project directly to dopaminergic neurons in the ventral tegmental area (VTA). Finally, cholecystokinin (CCK) is a circadian regulated peptide that is synthesized in the gut and contributes to feeding behavior, as well as the VTA, which contributes to motivated behavior and reward sensitivity.
Interestingly, the degree of glucocorticoid dysregulation can be quite different depending upon the type of mood disorder (ie. MDD vs. BPD vs. PTSD) as well as the amount of early life trauma the individual has experienced (52). Moreover, hypercortisolemia is only observed in a subset of patients with depression (53). Thus the exact role of glucocorticoids in the development of mood disorders is uncertain, however, there is evidence to suggest that antidepressant treatment helps to stabilize HPA axis function via the serotonergic system (54). Therefore, it seems that restoration of the circadian rhythm of HPA axis function in individuals with mood disorders should be beneficial to mood stabilization (some evidence of this will be discussed later). Moreover, the direct regulation of GR expression and activity by circadian genes might be extremely important in mediating the response to chronic stress.
Metabolic peptides
Metabolic disorders are very highly co-morbid with mood disorders (55). Circadian rhythms in the liver, stomach, adipose tissue and gut are robust, leading to distinct twenty-four hour cycles in metabolic functions (56). Peptides involved in metabolism and feeding including ghrelin, orexin (also known as hypocretin), leptin, and cholecystokinin (CCK) all display a significant circadian rhythm in expression (57) (Figure 3). In human blood samples, leptin is increased during the night, orexin A levels are increased during the day, and ghrelin and CCK levels are increased prior to meals and during the night (58, 59). Leptin and ghrelin both signal through orexin neurons in the brain which enhance wakefulness when stimulated by ghrelin and promote sleep when inhibited by leptin (60). In fact, narcolepsy, a condition where individuals experience sudden sleep bouts, is caused by a dysfunctional orexin system, linking regulation of feeding and sleep to a small population of hypothalamic neurons (61). CCK is more widely expressed throughout the brain and gut including high levels of expression in GABAergic interneurons of the prefrontal cortex, dopaminergic neurons of the VTA, and the shell of the SCN (62–64). These peptides and others involved in feeding behaviors are arrhythmic in the ClockΔ19 mice, demonstrating that their expression is regulated by the core molecular clock (57). ClockΔ19 mice also gain weight rapidly on a high fat diet and display many features of obesity and metabolic syndrome (57).
Both Ghrelin and leptin from the periphery modulate reward-related circuits in the brain (65–67). Moreover, ghrelin (Ghr −/−) knock-out mice are more anxious after acute restraint stress and this is likely due to a problem with the normal glucocorticoid feedback in response to stress (68). Ghrelin receptor-null mice also show a greater response to chronic social defeat stress in tests of social interaction compared to wild type mice (69). Interestingly, chronic social defeat stress produces long-lasting disruptions in body temperature rhythms and in the diurnal rhythms of plasma ghrelin and leptin (70, 71). Thus proper daily rhythms in ghrelin and leptin signaling may be important in mood regulation.
Many studies have found a prominent role for CCK in the regulation of anxiety-related and mood-related behavior. Increased CCK or CCK receptor agonists are generally associated with increased anxiety (72). In turn, CCK receptor antagonists have antidepressant-like properties in acute rodent measures of behavioral despair (73). Moreover, chronic blockade of CCK receptors prevents HPA axis hyperactivity, reduction of hippocampal volume and cell proliferation and decreases sucrose intake normally evoked by repeated social defeat stress (74). CCK is co-released with dopamine in the NAc and acts to silence dopamine neurons in the VTA (63). CCK has a strong circadian rhythm in expression in the VTA and it is a direct transcriptional target of CLOCK in this region (75, 76). ClockΔ19 mice have very low levels of Cck in the VTA and increased dopaminergic activity (27). Recently we found that treatment with lithium restores CCK expression to wild type levels in the ClockΔ19 mice through changes in chromatin structure at the CCK promoter (76). Moreover, CCK knock-down in the VTA is sufficient to recapitulate many of the manic-like behaviors of the ClockΔ19 mice. Interestingly, CCK knock-down in the basolateral amygdala also has anxiolytic and antidepressant effects in mice suggesting that it is involved in modulating activity within multiple limbic regions (77).
The relationships between obesity, stress and inadequate sleep (which are all common in today’s society) have been examined in several studies and it is thought that these factors represent a vicious cycle of interacting epidemics (78). Undoubtedly, depression can be added to this cycle, at least for a subset of individuals. There is certainly a need for more studies which examine the metabolic and mood-related effects that come with therapeutic treatments which improve sleep and circadian rhythms. Moreover, the relationship between disrupted rhythms in metabolic factors and the consequences of these disruptions on brain function have yet to be fully explored.
Redox/mitochondria/apoptosis
A mitochondrial dysfunction hypothesis of psychiatric disorders (bipolar disorder in particular) has been proposed based on the findings that (1) Abnormalities in mitochondrial morphology, localization, and mtDNA sequences are found in postmortem brain samples from bipolar subjects, (2) Patients with bipolar disorder often show somatic symptoms that resemble those seen with mitochondrial diseases (3) In turn, patients with mitochondrial diseases often have comorbid mood disorders, (4) Bipolar patients display altered energy metabolism in the brain and reduced mitochondrial respiratory chain activity which resembles that seen in patients with mitochondrial disorders (79). Bipolar disorder is also associated with increased neuronal death and axon-dendritic degeneration (80). In turn, anti-apoptotic and neuroprotective proteins like BCL-2 are robustly induced by mood stabilizing drugs (79). Interestingly, mice with forebrain specific mitochondrial DNA mutations have behaviors that resemble human mania including altered circadian rhythms (81). The mitochondria supply most of the cell’s adenosine triphosphate (ATP) and are involved in controlling the redox state of the cell. Nicotinamide cofactors NADPH/NADP+ and NADH/NAD+ have recently been identified as essential partners of CLOCK, NPAS2, and SIRT1, providing a direct link between the redox state of the cell and circadian rhythms (17, 82, 83). Moreover, CLOCK/BMAL1 directly regulates the expression of nicotinamide phosphoribosyltransferase (Nampt) in a circadian fashion which then determines the availability of NAD+ in the cell over twenty-four hours (17).
Another measure of cellular metabolism is the ratio of AMP and ATP. An important sensor of the AMP/ATP ratio is adenosine monophosphate-dependent protein kinase (AMPK) which is activated upon binding to AMP (84). AMPK is activated in neurons in response to metabolic or ischemic stressors and elicits compensatory responses (85). AMPK phosphorylates CK1ε which enhances phosphorylation of PER2, again directly coupling cellular metabolism and the response to stress to the circadian system (86). Moreover, the mitochondrial biogenesis stimulator, peroxisome proliferator-activated receptor gamma coactivator 1-α (PCG-1α) directly regulates expression of BMAL1 and Rev-erbα and is necessary for circadian pacemaker function (87). The ultimate consequences of metabolic dysfunction in neurons are oxidative stress, hypoxia and apoptosis, so given the intimate connection between the circadian system and redox sensing, it seems reasonable to suspect that circadian rhythm disruption might lead to poor cellular health. In fact the apoptotic genes, Wnt10, β-catenin, Dishevelled2, and transcription factor 3 (TCF3) promoters are all bound by BMAL1, and WNT pathway signaling is attenuated when BMAL1 levels are reduced (88). The WNT pathway has been implicated in bipolar disorder through human genetic association studies, and major depression through studies of animal models (89). It is also interesting to note that many of the genes involved in apoptosis are also involved in neuroplasticity (89). Thus the response to antidepressant or mood stabilizing medications may involve these same pathways which are directly regulated by the circadian system. Indeed as depression and other mood disorders do not seem to be generally associated with severe neurodegeneration, the influence of cellular stress-related pathways are likely much more subtle and perhaps alter plasticity and dendritic complexity, rather than direct cell death (90).
Neurogenesis
The neurogenic hypothesis of depression stems from animal studies showing that chronic stress and depression-inducing behavioral models reduce hippocampal neurogenesis while antidepressants enhance neurogenesis (91). While studies have been mixed regarding the role of neurogenesis in the development of depression, most point towards a significant role for neurogenesis in the therapeutic effects of antidepressants (91). Neurogenesis varies greatly over the circadian cycle (92, 93). Moreover, there is a functional link between the expression of Per2 and the regulation of cell proliferation and cell death in the dentate gyrus (DG) (94). Chronic disruption in circadian rhythms via weekly phase shifts inhibits hippocampal neurogenesis, and the degree of reduction in neurogenesis depends upon the direction and duration of the shifts (95, 96). Rhythmic changes in corticosterone can help regulate the rhythmic expression of Per1 in the DG (92). Moreover, rhythms in cotricosterone are necessary for the proliferation of progenitor cells in the DG in response to fluoxetine (97). Flattening of the diurnal corticosterone rhythm in rats also prevents the stimulating action of L-NAME (a nitric oxide synthase, NOS, inhibitor) on progenitor cell proliferation in the DG as well as brain derived neurotrophic factor (BDNF) and trkB expression (98). BDNF and its’ receptor, TrkB have been shown in multiple studies to be important in the actions of antidepressant medications and both have a strong circadian rhythm in expression in the hippocampus (99, 100). BDNF loses its effects on cell proliferation rates in the absence of a daily rhythm in corticosterone (98). These results demonstrate that the diurnal rhythm of corticosterone regulates the stimulating action of antidepressants on neurogenesis and BDNF signaling in the DG. It will be interesting in future studies to determine the impact of circadian gene mutations on neurogenesis and TrkB signaling following antidepressant treatment.
Conclusions
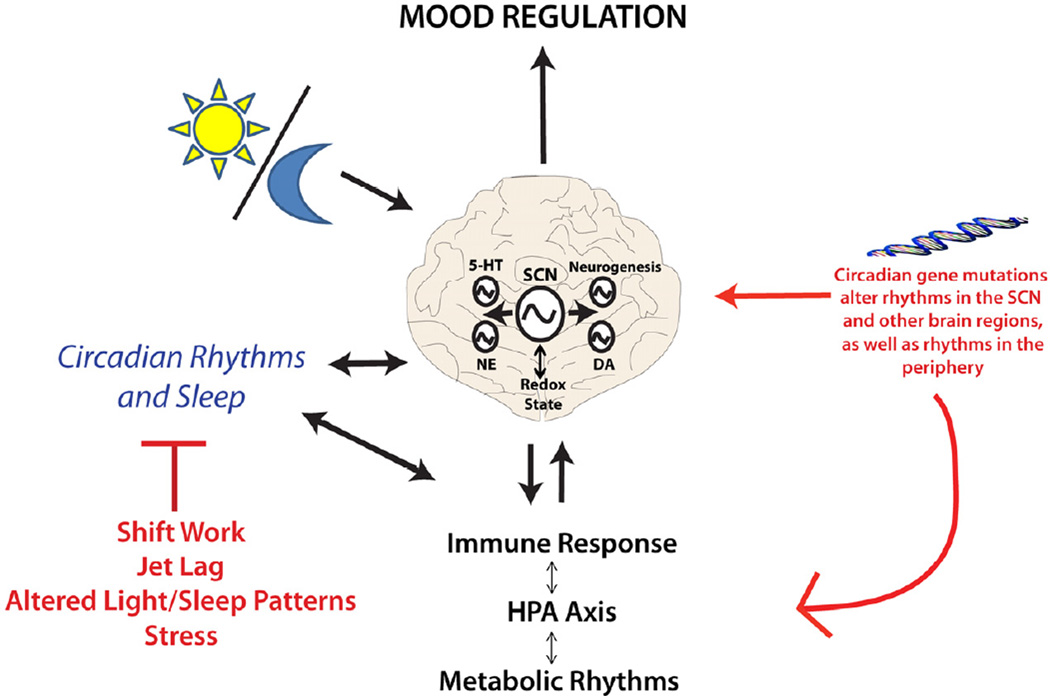
Though a great deal of work remains, our understanding of the circadian clock and how it is potentially involved in mood regulation has grown considerably over the last several years. We now know that circadian genes are not only found in the SCN, but rather they are expressed widely throughout the brain and body, including expression in mood-related centers of the brain. The circadian genes are keenly involved in the regulation of immune function, monoamine transmission, neurogenesis and metabolism (Figure 4). It is possible that circadian rhythm disruption alters mood through multiple systems. Indeed, all of these systems interact at some level. The role of sleep disruption in the pathophysiology of mood disorders is also important and should not be ignored. The circadian system is now being targeted for drug development in the hopes of treating a number of diseases including cancer, obesity, sleep disorders, and diabetes. It is likely that these types of medications could also be beneficial for the treatment of mood disorders.
Figure 4.
The circadian clock influences multiple systems and pathways which are thought to underlie mood disorders. In most cases there are reciprocal interactions which in turn regulate circadian rhythms. Circadian gene mutations might make an individual more vulnerable to mood changes and these are exacerbated by environmental deviations in the daily schedule.
Acknowledgements
I would like to thank Dr. Ryan Logan and Dr. Shibani Mukherjee for helpful comments on the manuscript and Dr. Trey Williams for work on the figures. Work from our group was funded by NIMH (MH082876) and NIDA (DA023988), as well as the McKnight Foundation and NARSAD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author declares no conflicts of interest with this manuscript.
References
- 1.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 2.Wirz-Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006;21(Suppl 1):S11–S15. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- 3.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Arch Gen Psychiatry. 1988;45:948–952. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- 5.Baglioni C, Riemann D. Is chronic insomnia a precursor to major depression? Epidemiological and biological findings. Curr Psychiatry Rep. 2012;14:511–518. doi: 10.1007/s11920-012-0308-5. [DOI] [PubMed] [Google Scholar]
- 6.Manber R, Chambers AS. Insomnia and depression: a multifaceted interplay. Curr Psychiatry Rep. 2009;11:437–442. doi: 10.1007/s11920-009-0066-1. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–260. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Son GH, Chung S, Kim K. The adrenal peripheral clock: glucocorticoid and the circadian timing system. Front Neuroendocrinol. 2011;32:451–465. doi: 10.1016/j.yfrne.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etain B, Milhiet V, Bellivier F, Leboyer M. Genetics of circadian rhythms and mood spectrum disorders. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2011;21(Suppl 4):S676–S682. doi: 10.1016/j.euroneuro.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Milhiet V, Etain B, Boudebesse C, Bellivier F. Circadian biomarkers, circadian genes and bipolar disorders. J Physiol Paris. 2011;105:183–189. doi: 10.1016/j.jphysparis.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 12.McClung CA. Circadian rhythms and mood regulation: insights from pre-clinical models. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2011;21(Suppl 4):S683–S693. doi: 10.1016/j.euroneuro.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keers R, Pedroso I, Breen G, Aitchison KJ, Nolan PM, Cichon S, et al. Reduced anxiety and depression-like behaviours in the circadian period mutant mouse afterhours. PLoS One. 2012;7:e38263. doi: 10.1371/journal.pone.0038263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampp G, Albrecht U. The circadian clock and mood-related behavior. Commun Integr Biol. 2008;1:1–3. doi: 10.4161/cib.1.1.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer S, Falcon E, Kumar J, Krishnan V, Mukherjee S, Birnbaum SG, et al. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. The European journal of neuroscience. 2012 doi: 10.1111/ejn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer JH. Applying neuroimaging ligands to study major depressive disorder. Semin Nucl Med. 2008;38:287–304. doi: 10.1053/j.semnuclmed.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2012 doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2012 doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chenu F, El Mansari M, Blier P. Electrophysiological Effects of Repeated Administration of Agomelatine on the Dopamine, Norepinephrine, and Serotonin Systems in the Rat Brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockDelta19 mouse model of mania. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar S, Chen D, Sehgal A. Dopamine acts through Cryptochrome to promote acute arousal in Drosophila. Genes Dev. 2012;26:1224–1234. doi: 10.1101/gad.186338.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biological psychiatry. 2010;68:503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyre H, Baune BT. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology. 2012;37:1397–1416. doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logan RW, Sarkar DK. Circadian nature of immune function. Mol Cell Endocrinol. 2012;349:82–90. doi: 10.1016/j.mce.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 37.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bechtold DA, Gibbs JE, Loudon AS. Circadian dysfunction in disease. Trends in pharmacological sciences. 2010;31:191–198. doi: 10.1016/j.tips.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Beynon AL, Coogan AN. Diurnal, age, immune regulation of interleukin-1beta and interleukin-1 type 1 receptor in the mouse suprachiasmatic nucleus. Chronobiol Int. 2010;27:1546–1563. doi: 10.3109/07420528.2010.501927. [DOI] [PubMed] [Google Scholar]
- 41.Monje FJ, Cabatic M, Divisch I, Kim EJ, Herkner KR, Binder BR, et al. Constant darkness induces IL-6-dependent depression-like behavior through the NF-kappaB signaling pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9075–9083. doi: 10.1523/JNEUROSCI.1537-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, et al. Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2457–E2465. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delerive P, Monte D, Dubois G, Trottein F, Fruchart-Najib J, Mariani J, et al. The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response. EMBO Rep. 2001;2:42–48. doi: 10.1093/embo-reports/kve007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kronenberg S. Anti-inflammatory drugs as moderators of antidepressant effects, especially those of the selective serotonin-reuptake inhibitor class. Expert Rev Clin Pharmacol. 2011;4:575–578. doi: 10.1586/ecp.11.47. [DOI] [PubMed] [Google Scholar]
- 46.Gallagher PJ, Castro V, Fava M, Weilburg JB, Murphy SN, Gainer VS, et al. Antidepressant response in patients with major depression exposed to NSAIDs: a pharmacovigilance study. Am J Psychiatry. 2012;169:1065–1072. doi: 10.1176/appi.ajp.2012.11091325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Molecular Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 48.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 49.Pae CU, Marks DM, Han C, Patkar AA. Does minocycline have antidepressant effect? Biomed Pharmacother. 2008;62:308–311. doi: 10.1016/j.biopha.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charmandari E, Chrousos GP, Lambrou GI, Pavlaki A, Koide H, Ng SS, et al. Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PLoS One. 2011;6:e25612. doi: 10.1371/journal.pone.0025612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frodl T, O'Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids. Mood, memory, and mechanisms. Ann N Y Acad Sci. 2009;1179:19–40. doi: 10.1111/j.1749-6632.2009.04980.x. [DOI] [PubMed] [Google Scholar]
- 54.Carvalho LA, Garner BA, Dew T, Fazakerley H, Pariante CM. Antidepressants, but not antipsychotics, modulate GR function in human whole blood: an insight into molecular mechanisms. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2010;20:379–387. doi: 10.1016/j.euroneuro.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, et al. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35:1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cagampang FR, Bruce KD. The role of the circadian clock system in nutrition and metabolism. Br J Nutr. 2012;108:381–392. doi: 10.1017/S0007114512002139. [DOI] [PubMed] [Google Scholar]
- 57.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feillet CA. Food for thoughts: feeding time and hormonal secretion. J Neuroendocrinol. 2010;22:620–628. doi: 10.1111/j.1365-2826.2010.01998.x. [DOI] [PubMed] [Google Scholar]
- 59.Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121:2133–2141. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adamantidis A, de Lecea L. Sleep and metabolism: shared circuits, new connections. Trends Endocrinol Metab. 2008;19:362–370. doi: 10.1016/j.tem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Nishino S, Okuro M, Kotorii N, Anegawa E, Ishimaru Y, Matsumura M, et al. Hypocretin/orexin and narcolepsy: new basic and clinical insights. Acta Physiol (Oxf) 2010;198:209–222. doi: 10.1111/j.1748-1716.2009.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dockray GJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2012;19:8–12. doi: 10.1097/MED.0b013e32834eb77d. [DOI] [PubMed] [Google Scholar]
- 63.Rotzinger S, Vaccarino FJ. Cholecystokinin receptor subtypes: role in the modulation of anxiety-related and reward-related behaviours in animal models. J Psychiatry Neurosci. 2003;28:171–181. [PMC free article] [PubMed] [Google Scholar]
- 64.Hannibal J, Hundahl C, Fahrenkrug J, Rehfeld JF, Friis-Hansen L. Cholecystokinin (CCK)-expressing neurons in the suprachiasmatic nucleus: innervation, light responsiveness and entrainment in CCK-deficient mice. The European journal of neuroscience. 2010;32:1006–1017. doi: 10.1111/j.1460-9568.2010.07385.x. [DOI] [PubMed] [Google Scholar]
- 65.Ogaya M, Kim J, Sasaki K. Ghrelin postsynaptically depolarizes dorsal raphe neurons in rats in vitro. Peptides. 2011;32:1606–1616. doi: 10.1016/j.peptides.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 67.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, et al. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biological psychiatry. 2012;72:457–465. doi: 10.1016/j.biopsych.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 69.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nature neuroscience. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 71.Kumar J, Chuang JC, Na ES, Kuperman A, Gillman AG, Mukherjee S, et al. Differential effects of chronic social stress and fluoxetine on meal patterns in mice. Appetite. 2013 doi: 10.1016/j.appet.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zwanzger P, Domschke K, Bradwejn J. Neuronal network of panic disorder: the role of the neuropeptide cholecystokinin. Depress Anxiety. 2012;29:762–774. doi: 10.1002/da.21919. [DOI] [PubMed] [Google Scholar]
- 73.Hernando F, Fuentes JA, Fournie-Zaluski MC, Roques BP, Ruiz-Gayo M. Antidepressant-like effects of CCK(B) receptor antagonists: involvement of the opioid system. Eur J Pharmacol. 1996;318:221–229. doi: 10.1016/s0014-2999(96)00773-x. [DOI] [PubMed] [Google Scholar]
- 74.Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel JJ. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Molecular psychiatry. 2008;13:1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- 75.Weber M, Lauterburg T, Tobler I, Burgunder JM. Circadian patterns of neurotransmitter related gene expression in motor regions of the rat brain. Neuroscience letters. 2004;358:17–20. doi: 10.1016/j.neulet.2003.12.053. [DOI] [PubMed] [Google Scholar]
- 76.Arey R, Enwright JF, III, Spencer S, Falcon E, Ozburn AR, McClung CA. An important role for Cholecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors. Molecular Psychiatry. doi: 10.1038/mp.2013.12. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Del Boca C, Lutz PE, Le Merrer J, Koebel P, Kieffer BL. Cholecystokinin knock-down in the basolateral amygdala has anxiolytic and antidepressant-like effects in mice. Neuroscience. 2012;218:185–195. doi: 10.1016/j.neuroscience.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lucassen EA, Rother KI, Cizza G. Interacting epidemics? Sleep curtailment, insulin resistance, and obesity. Ann N Y Acad Sci. 2012;1264:110–134. doi: 10.1111/j.1749-6632.2012.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, et al. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- 80.Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29:311–324. doi: 10.1016/j.ijdevneu.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T, et al. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Molecular psychiatry. 2006;11:577–593. 523. doi: 10.1038/sj.mp.4001824. [DOI] [PubMed] [Google Scholar]
- 82.Bellet MM, Orozco-Solis R, Sahar S, Eckel-Mahan K, Sassone-Corsi P. The time of metabolism: NAD+, SIRT1, and the circadian clock. Cold Spring Harb Symp Quant Biol. 2011;76:31–38. doi: 10.1101/sqb.2011.76.010520. [DOI] [PubMed] [Google Scholar]
- 83.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pimentel GD, Ropelle ER, Rocha GZ, Carvalheira JB. The role of neuronal AMPK as a mediator of nutritional regulation of food intake and energy homeostasis. Metabolism. 2012 doi: 10.1016/j.metabol.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 85.Li J, McCullough LD. Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:480–492. doi: 10.1038/jcbfm.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Um JH, Yang S, Yamazaki S, Kang H, Viollet B, Foretz M, et al. Activation of 5'-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J Biol Chem. 2007;282:20794–20798. doi: 10.1074/jbc.C700070200. [DOI] [PubMed] [Google Scholar]
- 87.Lin JD. Minireview: the PGC-1 coactivator networks: chromatin-remodeling and mitochondrial energy metabolism. Mol Endocrinol. 2009;23:2–10. doi: 10.1210/me.2008-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo B, Chatterjee S, Li L, Kim JM, Lee J, Yechoor VK, et al. The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. Faseb J. 2012;26:3453–3463. doi: 10.1096/fj.12-205781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Voleti B, Duman RS. The roles of neurotrophic factor and Wnt signaling in depression. Clin Pharmacol Ther. 2012;91:333–338. doi: 10.1038/clpt.2011.296. [DOI] [PubMed] [Google Scholar]
- 90.Hemmerle AM, Herman JP, Seroogy KB. Stress, depression and Parkinson's disease. Exp Neurol. 2012;233:79–86. doi: 10.1016/j.expneurol.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilhooley MJ, Pinnock SB, Herbert J. Rhythmic expression of per1 in the dentate gyrus is suppressed by corticosterone: implications for neurogenesis. Neuroscience letters. 2011;489:177–181. doi: 10.1016/j.neulet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 93.Tamai S, Sanada K, Fukada Y. Time-of-day-dependent enhancement of adult neurogenesis in the hippocampus. PLoS One. 2008;3:e3835. doi: 10.1371/journal.pone.0003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Borgs L, Beukelaers P, Vandenbosch R, Nguyen L, Moonen G, Maquet P, et al. Period 2 regulates neural stem/progenitor cell proliferation in the adult hippocampus. BMC Neurosci. 2009;10:30. doi: 10.1186/1471-2202-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental 'jet lag' inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One. 2010;5:e15267. doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kott J, Leach G, Yan L. Direction-dependent effects of chronic "jet-lag" on hippocampal neurogenesis. Neuroscience letters. 2012;515:177–180. doi: 10.1016/j.neulet.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 97.Huang GJ, Herbert J. Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biological psychiatry. 2006;59:619–624. doi: 10.1016/j.biopsych.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 98.Pinnock SB, Herbert J. Brain-derived neurotropic factor and neurogenesis in the adult rat dentate gyrus: interactions with corticosterone. The European journal of neuroscience. 2008;27:2493–2500. doi: 10.1111/j.1460-9568.2008.06250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schaaf MJ, Duurland R, de Kloet ER, Vreugdenhil E. Circadian variation in BDNF mRNA expression in the rat hippocampus. Brain research Molecular brain research. 2000;75:342–344. doi: 10.1016/s0169-328x(99)00314-9. [DOI] [PubMed] [Google Scholar]
- 100.Dolci C, Montaruli A, Roveda E, Barajon I, Vizzotto L, Grassi Zucconi G, et al. Circadian variations in expression of the trkB receptor in adult rat hippocampus. Brain research. 2003;994:67–72. doi: 10.1016/j.brainres.2003.09.018. [DOI] [PubMed] [Google Scholar]