Abstract
Purpose
To develop a chemical exchange saturation transfer (CEST) based technique to measure free creatine (Cr) and to validate the technique by measuring the distribution of Cr in muscle with high spatial resolution before and after exercise.
Methods
Phantom studies were performed to determine contributions from other creatine kinase metabolites to the CEST effect from Cr (CrCEST). CEST, T2, magnetization transfer ratio (MTR) and 31P magnetic resonance spectroscopy (MRS) acquisitions of the lower leg were performed before and after plantar flexion exercise on a 7T whole-body MR scanner on healthy volunteers.
Results
Phantom studies demonstrated that while Cr exhibited significant CEST effect there were no appreciable contributions from other metabolites. In healthy human subjects, following mild plantar flexion exercise, increases in CrCEST were observed, which recovered exponentially back to baseline. This technique exhibited good spatial resolution and was able to differentiate differences in muscle utilization among subjects. CrCEST results were compared with 31P MRS results showing good agreement in the Cr and PCr recovery kinetics.
Conclusion
Demonstrated a CEST based technique to measure free creatine changes in in vivo muscle. CrCEST imaging can spatially map changes in creatine concentration in muscle following mild exercise. This may serve as a tool for the diagnosis and treatment of various disorders affecting muscle.
Keywords: CEST, Creatine, Muscle, Chemical Exchange, Endogenous Contrast
INTRODUCTION
The creatine kinase (CK) reaction plays a vital role in the muscle energetics(1).
| [1] |
Adenosine triphosphate (ATP), derived from the conversion of phosphocreatine (PCr) to creatine (Cr) by the CK reaction, provides an essential chemical energy source that governs muscle contraction (2,3). While ATP represents the fundamental energy currency, PCr serves as the reservoir of cellular energy. During exercise, PCr is depleted to maintain the ATP supply leading to an increase Cr concentration.
Magnetic Resonance (MR) techniques have been used extensively for noninvasive functional investigations of exercising muscle metabolism and are becoming increasingly more important for studying muscular diseases(4). 31P Magnetic Resonance Spectroscopy (MRS) was the first and is currently the most utilized method to study oxidative metabolism of skeletal muscle(5–8). Additionally, intracellular pH can also be measured noninvasively from the chemical shift of the Pi peak(9).
The ability of 31P MRS to measure high-energy phosphate compounds as well as pH changes has made it a great tool for studying metabolic abnormalities associated with various myopathies(10–12) as well as cardiac energetics(13,14). 31P MRS has also provided key information about secondary problems of muscle metabolism associated with common conditions such as heart failure(15,16), peripheral vascular disease(17), and thyroid disease(18).
1H MRS can only measure total creatine and cannot differentiate between PCr and Cr and thus has little application towards measuring energy metabolism (19,20). While there have been a limited amount of 1H MRI studies utilizing T2 relaxation rates to study muscle energetics, the mechanism of the T2 increases in muscle exercise is still poorly understood(21–23).
Although these MR methods have been critical to our understanding of muscle energetics, they also suffer from several drawbacks. 31P MRS, like all spectroscopy techniques, suffers from poor spatial resolution as well as low sensitivity. Furthermore, neither 1H MRS nor 31P MRS can measure free Cr.
Chemical exchange saturation transfer (CEST) is new sensitivity enhancement mechanism to indirectly detect metabolite content based on exchange-related properties (24,25). In a CEST experiment, saturated magnetization from exchangeable protons on a pool of solute molecules is transferred to a much larger pool of bulk water protons. This reduction in water signal results in a solute concentration dependent contrast in CEST water images(26–28). CEST has been used to image endogenous mobile proteins and peptidesin biological tissue by selectively saturating their labile protons (29–31).
More recently, it has been shown that Cr exhibits a concentration dependent CEST effect between its amine (-NH2) and bulk water protons (32–34). Cr amine protons have an exchange site at ~1.8 ppm from water and the CEST effect from creatine is linearly proportional to the Cr concentration in the physiological pH range(34).
In this work, for the first time, we demonstrated the potential of measuring the CEST effect from Cr (CrCEST) in spatial and temporal mapping of muscle energetics. Phantom studies were performed to demonstrate minimal contributions from other creatine kinase reaction metabolites to the observed CrCEST under the same saturation parameters used in the skeletal muscle experiments in vivo. We also demonstrated that CrCEST has over three orders of magnitude higher sensitivity compared to 31P MRS. Changes in CrCEST in calf muscles of healthy human volunteers were mapped following plantar flexion exercises. Additionally, we measured changes in magnetization transfer ratio (MTR) and T2 following exercise. Finally, we showed complementary recovery kinetics of CrCEST and 31P MRS under the same exercise conditions.
MATERIALS AND METHODS
MRI Scans
All imaging experiments were performed on a 7T whole body scanner (Siemens Medical Systems, Erlangen, Germany). A 32 Channel 1H head coil was used for phantom imaging while a 28 channel 1H knee coil was utilized for Calf imaging. Localized B0 shimming was performed with second order shims using the Siemens product interactive shim program to keep reported water line widths to less than 45Hz (0.15 ppm). 31P MRS was performed with a custom built 7 cm diameter 31P transmit/receive surface coil. An MR compatible pneumatically controlled foot pedal was designed and used for plantar flexion exercise (figure 1a). The pressure applied to the pedal was controlled with a pneumatic gauge and held constant across all subjects. In CEST imaging experiments, we utilized a 500 ms long saturation pulse train consisting of a series of 99.6 ms Hanning windowed saturation pulses (with peak amplitude set to provide desired B1rms) with a 400 μs interpulse delay (99.6% duty cycle, 100 ms pulse train), followed by a single shot RF spoiled gradient echo (GRE) readout with centric phase encoding order. The excitation bandwidth of this saturation pulse train was 10 Hz for a 500 ms saturation duration with a 1% bandwidth of 40 Hz. A chemical shift selective fat saturation pulse was applied prior to image readout to reduce the signal from fat in the muscle. CEST images of the lower leg at varying saturation amplitude and duration were acquired on a healthy volunteer in order to empirically optimize muscle CEST saturation parameters within the constraints of imaging of humans at 7T. Water saturation shift reference (WASSR) images and B1 maps were collected, as described previously (35,36), for all CEST studies before and after exercise to correct for B0 and B1 inhomogeneities. WASSR images were collected from −0.5 ppm to 0.5 ppm with a step size of 0.05 ppm and saturation pulse train amplitude, B1rms of 12.4 Hz (0.3 μT) and 200 ms duration using the same sequence as used for CEST imaging and identical readout parameters. Cr amine protons have a chemical shift of 1.8 ppm downfield from water and thus CEST images were collected in a frequency shift range with respect to the water resonance of +1.5 ppm to +2.1 ppm and −1.5ppm to −2.1 ppm with a 0.3 ppm step size to calculate CrCESTasym and allow for adequate B0 inhomogeneity correction.
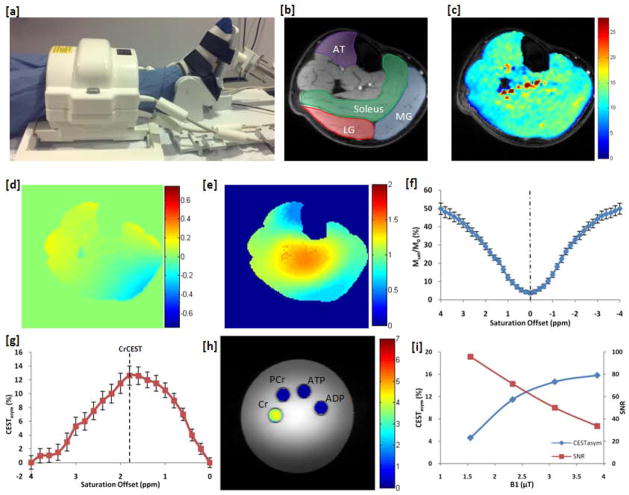
Figure 1.
CrCEST Mapping and B1 Optimization. (a) In magnet exercise setup consisting of an MR compatible pneumatically driven pedal and a 28 channel knee coil for imaging of the lower leg. (b) Anatomical image with the major muscles [lateral gastrocnemius (LG), medial gastrocnemius (MG), soleus, and anterior tibialis (AT)] of the lower leg manually segmented. (c) CrCESTasym map at 7T, (d) local static magnetic field (B0), and (e) radiofrequency field (B1) maps of the lower leg. (f,g) The z-spectra and corresponding asymmetry plots for the soleus muscle at baseline. (h) Phantom containing of nmr tubes with solutions of 10 mM Cr, 15 mM PCr, 10 mM ATP, and 10 mM ADP immersed in a beaker containing PBS. CrCEST images were acquired around 1.8 ppm with a saturation pulse train with B1rms = 123 Hz (2.9 μT) and a 500 ms duration. (i) Plot of the CESTasym as well as the signal-to-noise ratio (SNR) of the anatomical image following saturation as a function of saturation pulse B1rms for a 500 ms pulse train used to empirically optimize CEST saturation parameters.
Phantom Imaging
All phantoms were prepared in PBS and imaged at 37°C. Samples of 10 mM Cr, 15 mM PCr, 10 mM ATP, and 10 mM ADP were prepared to observe any potential contributions the Cr CEST effect. The imaging parameters were as follows: slice thickness = 4 mm, flip angle = 10°, TR = 7.1 ms, TE = 3.5 ms, field of view = 100 × 100 mm2, matrix size = 192 × 192, with one saturation pulse every 8 seconds. CEST images were collected with a B1rms of 123 Hz (2.9 μT) and 500 ms duration.
Human Studies
All Studies were conducted under an approved Institutional Review Board protocol of the University of Pennsylvania. Written informed consent from each volunteer was obtained after explaining the study protocol. CEST, T2/MTR and 31P MRS acquisitions of the calf were performed in separate exercise bouts at 7T on healthy volunteers (n=8, 5 male, 3 female, ages 19–30) with various activity levels (sedentary to active). Additionally, 1H MRS was performed on one healthy subject.
For each methodology, baseline imaging was performed for 2 minutes, followed by 2 minutes of mild plantar flexion exercise and then 8 minutes of post exercise imaging. Calf CEST imaging was performed with the same CEST saturation pulse train described for the phantoms and the following imaging parameters: slice thickness = 4 mm, flip angle = 10°, TR = 6.1 ms, TE = 2.9 ms, field of view = 130 × 130 mm2, matrix size = 128 × 128. To compute MTR maps, the same sequence with a saturation duration of 1 second (B1rms= 2.9 μT) was performed at 20 ppm along with a sequence without selective saturation. To obtain T2 maps, a T2 spin-echo prepared FLASH sequence was used with the parameters mentioned above at echo times of 2.4, 12.5, 25, 37.5, and 50 ms. Additionally, a z-spectra acquisition was performed on the lower leg of one healthy subject at baseline from −4.0 ppm to 4.0 ppm with a step size of 0.2 ppm, a B1rms of 123 Hz (2.9 μT) and a 500 ms duration. 1H MRS spectra were obtained from a region in the lateral gastrocnemius muscle using a standard point resolved spectroscopy (PRESS)(37) localization technique with the following parameters: voxel size = 10 × 20 ×10 mm3, spectral width = 3 kHz, number of points = 2048, average = 16, TE = 20 ms, and TR = 3 s. Finally, non-localized 31P MRS was performed with a 7-cm diameter surface coil positioned on the calf and the following parameters: spectral width = 5 kHz, number of points = 512, averages = 4, and TR = 3 s. In order to determine the depth of penetration of the 31P surface coil, a 2 M sodium phosphate phantom was imaged using a 31P ultra-short echo time (UTE) sequence with the following parameters: FOV =140 × 140 mm2, matrix size = 64 × 64, flip angle = 20°, TR = 50 ms, TE = 100 μs, averages = 4.
Data Processing
All image processing and data analysis was performed using in-house written MATLAB (version 7.5, R 2007b) scripts. B0 maps were used to generate corrected CEST images (±1.8 p.p.m.) using the WASSR method(35). Similarly, B1 maps were created from two images obtained using preparation square pulses with flip angles of 30° and 60°. A B1 calibration curve for muscle was developed from calf CEST data at varying saturation amplitudes and used in conjunction with B1 maps to correct for B1 inhomogeneities(36). CEST contrast was computed by subtracting the normalized magnetization signal at the Cr proton frequency (Δω = +1.8 ppm), from the magnetization at the corresponding reference frequency symmetrically at the opposite side of the water resonance (−Δω)(38)
| [2] |
Anatomical images were manually segmented and applied to CrCESTasym maps in order to determine the CrCESTasym changes in each individual muscle group before and after exercise. Similarly, MTR maps were computed by
| [3] |
Where M0 is the magnetization without saturation and MSat is the magnetization with a saturation pulse applied at Δω = 20 ppm. T2 maps were created by fitting images acquired with various echo times to S=Soe−TE/T2 and solving for T2 on a pixel-wise basis. Eddy current corrected 1H MRS and 31P MRS Spectra were phased and baseline corrected and then fitted using nonlinear squares methods with Lorentzian or Gaussian functions.
RESULTS
In vivo CEST parameter optimization
Our experimental setup is shown in figure 1a. The anatomical image of an axial slice of the lower leg with various muscle groups segmented is shown in figure 1b. The CESTasym map overlaid on the anatomical image showed fairly uniform CrCESTasym at baseline (Fig. 1c–1e). The z-spectra and CEST asymmetry plot from the soleus muscle at baseline was broad and showed maximum CEST contrast at ~ 1.8 ppm (Fig. 1f,g). Figure 1i shows a plot of the CESTasym as well as the signal-to-noise ratio (SNR) of the anatomical image following saturation as a function of saturation pulse B1rms for a 500 ms pulse train. Increasing B1rms leads to an increase in labile proton saturation efficiency that increases the CESTasym. However, when the saturation efficiency is already high, increases in B1rms result in increased direct water saturation, which decreases the CESTasym. Increases in B1rms also decrease the SNR of images. Thus, in order to maximize CESTasym and SNR, a saturation pulse train with B1rms of 123 Hz (2.9 μT) and 500 ms duration was chosen for this study. We define the CEST effect at Δω = 1.8 ppm using those saturation parameters as CrCEST.
Potential contributions from other creatine kinase metabolites
All the major metabolites of the creatine kinase reaction have amine group protons which exchange with water protons. With these in vivo empirically optimized experimental parameters, a 4.1% (±0.2%) CrCESTasym was observed from 10mM Cr, while negligible CrCEST effects were observed from 15 mM PCr (0.3±0.1%), 10 mM ATP(0.2±0.1%), and 10 mM ADP(0.3±0.1%) (fig 1h). This is predominantly due to their unfavorable exchange rates and differences in the chemical shifts of their amine protons. At physiological pH and temperature, ATP and ADP, have much slower exchange rates (120±50 s−1) than Cr (950±100 s−1)(34). Similarly, PCr has a much slower exchange rate (140±50 s−1 at its 2.5 ppm peak and 120±50 s−1 for its 1.8 ppm peak) and thus has minor effects at 1.8 ppm with the parameters used(34).
In vivo human studies
Plantar flexion exercise led to an increase in the CrCESTasym in all subjects. Figure 2a shows CrCESTasym maps for the same subject before and after mild plantar flexion exercise with a temporal resolution of 48 seconds. The time dependence of the mean CrCESTasym for each segmented muscle group (fig 1b) is plotted in figure 2b along with error bars representing the standard deviation in CrCESTasym in each region. The principle muscles involved in plantar flexion are located in the posterior compartment of the leg and include the soleus muscle as well as the medial (MG) and lateral (LG) heads of the gastrocnemius muscles. Thus, plantar flexion exercise is expected to increase creatine levels primarily in the soleus and gastrocnemius muscles of the leg. This is exhibited in CrCESTasym maps, which show that the majority of CrCESTasym increases were localized in the posterior compartment of the leg. Whereas the CEST maps showed fairly uniform CrCESTasym at baseline, the first post-exercise map showed a 6.7%(±1.0%) and 7.2%(±0.8%) increase in CrCESTasym in the medial and lateral gastrocnemius muscles, respectively, following exercise. A 2.6%(±0.9%) increase was observed in the soleus, the other major muscle of the posterior compartment. The tibialis anterior (AT) is predominantly involved in dorsiflexion and as a result, less than a 1% increase in CrCESTasym was observed. The CrCESTasym in all the muscles was seen to recover exponentially back to baseline after roughly three time points or about 2 minutes. Both T2 and MTR maps did not show any significant differences (ΔT2< 0.5 ms, ΔMTR < 1.0%) [data not shown].
Figure 2.
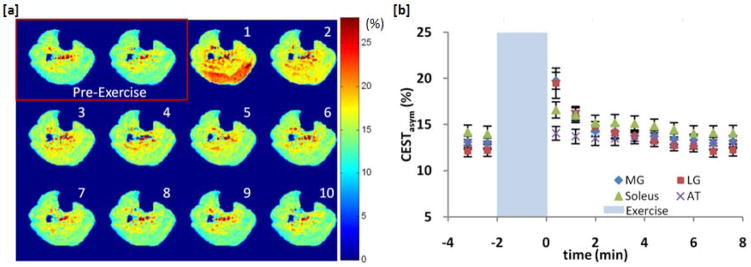
Plantar flexion exercise data for subject 1. (a) CrCESTasym maps of a human calf muscle before and every 48 seconds after (in order by number) 2 minutes of plantar flexion exercise. Color bar represents CrCESTasym in percent. (b) Plot of the average CrCESTasym as function of time in 4 different muscles of the calf segmented from anatomical images. Error bars represent the standard deviation in the CrCESTasym in each region.
Figure 3 shows CrCEST results from a different subject. Unlike the first subject, these CEST maps show that the majority of CrCESTasym increase is localized in the soleus muscle and the muscle is well segmented in the first post-exercise CEST Map. A 5.2% (±1.1%) increase in CrCESTasym was observed in the soleus muscle compared to 1.4% (±0.5%) and 1.6% (±0.5%) detected in the medial and lateral gastrocnemius muscles respectively. Similar to subject 1, the CrCESTasym in all the muscles recovered exponentially back to baseline in about 2 minutes (fig 3b). In all volunteers, no significant changes were observed in the T2 (ΔT2< 0.5 ms) and MT (ΔMTR < 1.0%) maps following exercise [data not shown]. This demonstrates that this method can detect subject dependent changes in muscle group recruitment during exercise.
Figure 3.
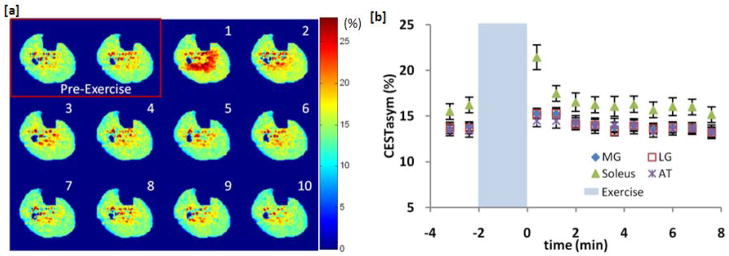
Plantar flexion exercise data for subject 2 showing variability in muscle activation between subjects. (a) CrCESTasym maps before and every 48 seconds after (in order by number) 2 minutes of plantar flexion exercise. Color bar represents CrCESTasym in percent. (b) Plot of the average CrCESTasym as function of time in 4 different muscles of the calf segmented from anatomical images. Error bars represent the standard deviation in the CrCESTasym in each region.
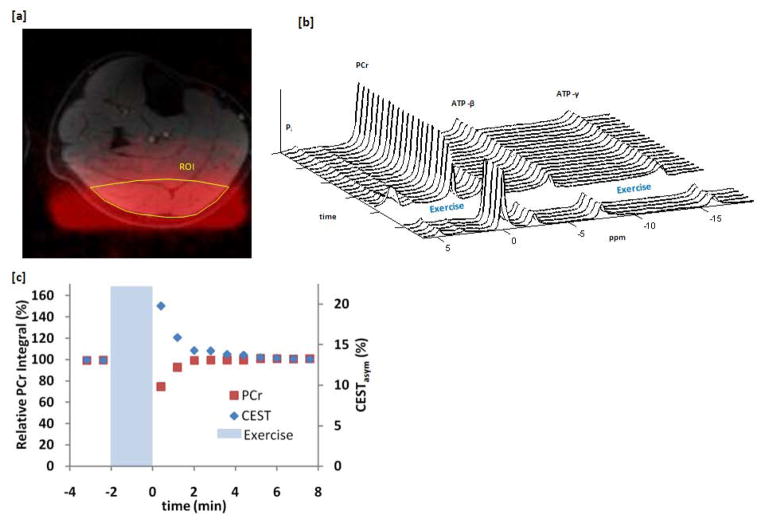
A free induction decay (fid) was used for 31P MRS acquisition in the current study, and thus the signal was unlocalized. The 31P MRS surface coil excitation profile measured using phantoms is shown in figure 4a overlaid on an anatomical image of the lower leg. The majority of the 31P MRS signal comes from the gastrocnemius muscle with minor contributions from the soleus muscles. 31P MRS spectra were acquired 2 minutes before and 8 minutes after exercise with a temporal resolution was 12 seconds per fid spectrum. A stacked plot of every alternate spectra acquired is shown for subject 1 in figure 4b. A decrease in the PCr peak area following exercise is evident, which recovers back to the pre-exercise levels in about 2 minutes. The size and area of the three ATP peaks stayed constant before and after this exercise. The Pi peak increased after exercise and then quickly decayed back to baseline. No shift or splitting of the Pi peak was observed indicating that there were negligible pH changes. Finally, when the PCr area was integrated to have an equal temporal resolution to that of CEST measurements, a very good agreement was observed in the rates at which the CrCESTasym from the region of interest pictured in fig 4a and the 31P MRS signal recovered to baseline (fig 4c).
Figure 4.
31P MRS data of the healthy human calf muscle before and after exercise. (a) Excitation profile of the 31P surface coil (red) overlaid on an anatomical image of the lower leg with corresponding segmented CrCEST region of interest outlined in yellow. (b) Stacked plot of every alternate 31P MRS spectra acquired 2 minutes before and 8 minutes after exercise. (c) Comparison between the CrCESTasym and 31P MRS signal as a function of time at an equal temporal resolution of 48 seconds.
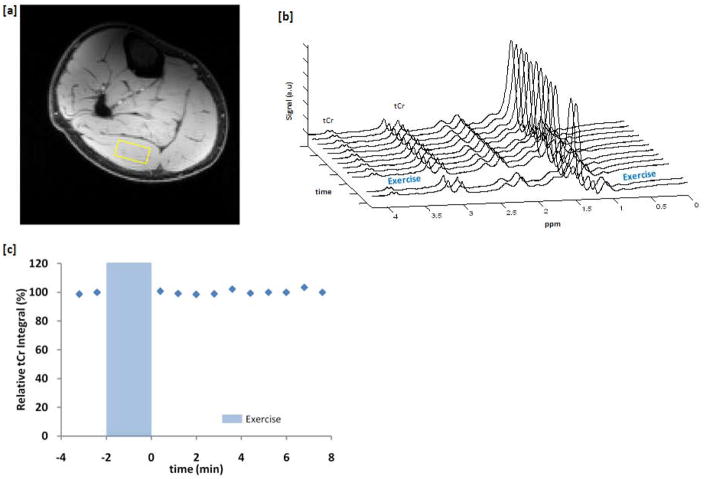
Time dependent 1H MRS provides insight into changes in total creatine(tCr), which is the combined signal from Cr and PCr. Figure 5a shows the spectroscopy voxel location overlaid on the anatomical image. Figure 5b shows the 1H MR Spectrum as a function of time before and after exercise with a temporal resolution of 48 seconds equal to that of the CEST measurements. As seen in figure 5c, the plot of the integrated tCr peak as a function of time shows no changes before or after exercise. This is due to the fact that the concentrations of Cr and PCr are tightly coupled in the creatine kinase reaction. Thus, a decrease in PCr will lead to an equivalent increase in Cr and thus the total Cr peak observed in 1H MRS will remain constant.
Figure 5.
1H MRS data of the healthy human calf muscle before and after exercise. (a) Spectroscopy voxel location overlaid on the anatomical image. (b) 1H MRS spectra 2 minutes before and 8 minutes after exercise with a temporal resolution of 48s. (c) No changes in the plot of tCr peak area as a function of time were observed.
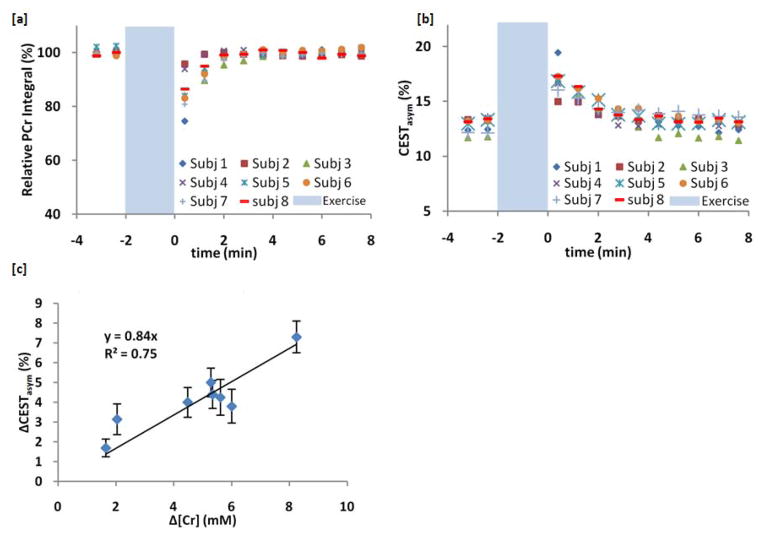
Figure 6 shows a summary of data from all 8 volunteers. The 31P MRS PCr peak integral as a function of time is shown in figure 6a. The mean CrCESTasym in the region of interest corresponding to the 31P MRS surface coil excitation profile [see figure 4a] is plotted as a function of time for each subject in figure 6b. There was some subject variability in regards to the level and involvement of each muscle group. However, the two figures show good agreement in the rate and shape at which they recover back to baseline following exercise for each subject. To compare the CESTasym and 31P MRS results, the % decrease in 31P MRS signal from baseline at the first integrated measurement following exercise was used to determine the decrease in PCr based on a 33 mM PCr baseline concentration(8). As the total Cr remains constant, the decrease in PCr concentration should equate to an equivalent increase in Cr concentration. Thus we can compare the average change in the CESTasym in the region of interest to the calculated change in Cr concentration across all subjects (fig 6c). This produces a slope of 0.84 % CrCESTasym/mM Cr with an R2 value 0.75.
Figure 6.
CrCESTasym and 31P MRS data across subjects. (a) 31P MRS PCr peak integral as a function of time before and after exercise for all subjects with a 48 second temporal resolution. (b) The average CrCESTasym as a function of time for each subject in a region of interest selected to correlate to the depth of penetration of the 31P MRS surface coil. (c) Comparison of changes in CrCESTasym Cr concentration following exercise. The % decrease in 31P MRS signal from baseline at the first integrated measurement following exercise was used to determine the decrease in PCr based on a 33 mM PCr baseline concentration8. As the total Cr remains constant, the decrease in PCr concentration was equated to an equivalent increase in Cr concentration.
DISCUSSION
The results of this work provide the first evidence that the CEST effect from Cr can be used to noninvasively measure creatine with high spatial resolution in muscle following exercise.
The CrCESTasym observed from Cr in phantom experiments was lower than what we observed in in vivo experiments. This is due to our definition of CESTasym which uses the Msat(−Δω) for normalization as it has a higher dynamic range. As the T2 of Cr phantoms is long (~800 ms), there is very little direct water saturation and as a result Msat(−Δω) is very close to M0, the magnetization without selective saturation. When M0 is used to normalize in vivo images, the CESTasym in muscle decreases by a factor of ~2. This would yield a CESTasym of 0.42% CESTasym/mM Cr, which would be in good agreement with phantom results.
Enhanced sensitivity is one of the inherent characteristics of the CEST methods. Based on the 0.42% CEST/mM Cr and muscle water proton concentration (82.5M), 1 mM Cr signal leads to 346 mM change in water. Considering that the receptivity of 31P NMR compared to that of 1H NMR is 0.066, we expect an increase in sensitivity of ~ 5240 with CEST. Thus compared to 31P NMR, CrCEST has about 3 orders of magnitude higher sensitivity.
Prolonged strenuous exercise can lead to glycogen breakdown to provide substrates for glycolysis which through the combined production of lactate and ATP hydrolysis lowers pH (39,40). This decrease in pH will decrease the exchange rate between Cr amine protons and water protons which will lead to a lower CEST contrast. As the performed exercise was mild and aerobic, most of the energy was obtained from the oxidative TCA cycle and lactate levels and pH remained the same(41). This was validated with 31P MRS, which showed no shifts in the Pi peak. Finally, a decrease in pH, had it occurred, would lead to a decrease in CrCESTasym, and thus would underestimate the increase in Cr post exercise. Furthermore, in previous work, glycogen has been shown in vitro and in liver in vivo to exhibit a CEST effect around 1.0 ppm from exchange of its hydroxyl (-OH) protons with bulk water(42). However, as no pH changes were observed in 31P MRS, we assume that there is no glycogen contribution to the observed CrCEST changes in the mild exercise employed in this study. Hence, glycogen is not expected to contribute to the observed changes in CrCESTasym following plantar flexion exercise employed in this study.
Changes in pH provide both an opportunity and a confounding effect for CrCEST. It is well known that the Cr concentration returns to baseline faster than the pH(43,44). During strenuous exercise when the pH becomes acidic (<7), the CrCESTasym will decrease below baseline when the Cr concentration has recovered and then slowly increase back to the baseline CrCESTasym as the pH normalizes. This decrease below the baseline CrCESTasym may provide information about pH changes in the muscle. Further studies will be needed to characterize the effects of pH on the CrCEST effect of exercised muscle.
Magnetization transfer, which is caused by the bound pool of water from rigid macromolecules, also may have an effect on the CEST contrast. However, as we did not observe any changes on the calculated MTR maps before and after exercise, we do not expect any MTR contamination of the measured increase in CrCESTasym following exercise in this study.
Phantom experiments showed that ATP, ADP, and PCr do not contribute to the CrCEST effect at 1.8 ppm under the saturation parameters used in this study. 31P MRS also showed no change in ATP following exercise. Since ADP concentration is too small (< 1mM) to be reliably measured, its contribution to CrCEST is negligible. Finally, there is a decrease in PCr concentration following exercise which would have decreased the observed CrCESTasym had PCr exhibited an observable CEST effect at 1.8 ppm under these parameters. Also, any changes in pH, had they occurred would reduce the already slow exchange rates of PCr, ATP and ADP amine protons, and further reduce their contribution to the CrCEST effect. Amide protons, which are present in the calf muscle and have been used with CEST imaging, have a chemical shift of 3.5 ppm and slow exchange rate (< 50 s−1)(30,45). However, a z-spectra from muscle acquired with the saturation parameters used in this study showed less than a 1 % CESTasym at Δω = 3.5 ppm (Fig. 1g). As a minimal CEST effect is seen at 3.5 ppm with these parameters, amide protons contributions to the CrCEST effect at 1.8 ppm are expected to be small. Since there was no change in pH, T2, or MTR and the CEST effects from other creatine kinase reaction metabolites were negligible, it can be concluded that the observed changes in CrCESTasym following plantar flexion exercise presented in this study are predominantly due to changes in muscle Cr concentration.
It is also necessary to point out several potential limitations of the study. Since, a dual tuned 31P/1H 7T coil was not available, it was not possible to obtain a localized 31P spectrum of the exact slice acquired with CEST imaging or localize it to a specific muscle. Thus, the majority of the phosphorus 31P MRS signal originated from the gastrocnemius muscles from a large area of the calf. As a result, comparisons made between CrCESTasym and 31P MRS are estimates as the spectral data is obtained from a larger volume of the leg. Nonetheless, there was good agreement in the rates and shape at which the CrCESTasym and 31P MRS signal recovered to baseline across all subjects. Subjects with smaller increases in CrCESTasym post exercise similarly had smaller decreases in their PCr peak. Furthermore, in subjects where CrCESTasym changes were localized mainly in the soleus muscles with smaller changes observed in the gastrocnemius muscles, smaller decreases were observed in the 31P MRS measured PCr peak levels post exercise.
Also, CEST, T2, MTR and 31P MRS data were not collected concurrently and as a result there may have been differences in muscle changes between exercise bouts. However, since subjects were healthy volunteers and exercise was mild, these variations are expected to be small. Finally, the temporal resolution used for CrCEST mapping in this work is too long to adequately characterize creatine kinase kinetics for clinical application. However, the feasibility of measuring Cr concentration changes is demonstrated. There is potential for improving the temporal resolution by decreasing the shot TR and resolution. Work by many groups is also being conducted on methods to reduce CEST scan times while maintaining adequate B0 correction. These advances will allow this technique to be clinically applicable.
In summary, it is feasible to use CEST imaging to measure changes in Cr concentration in muscle following plantar flexion exercise. The observed increases in CrCESTasym following exercise were due mainly to increases in Cr concentration in this study. CrCEST exhibited a sensitivity enhancement of over 3 orders of magnitude compared to 31P MRS. Further, CrCESTasym maps showed good spatial resolution and were able to differentiate varying muscle usage in different subjects. There was good agreement between the recovery kinetics of 31P MRS and CrCESTasym following exercise. While the experiments demonstrated in this work were performed at 7T, the favorable exchange rate of Cr amine protons would allow for these studies to be performed at 3T as well. This may require further work to optimize saturation parameters.
Future studies using this approach may provide new insights into muscle energetics and can serve as a tool for the diagnosis and treatment of muscle disorders. In addition, this technique has the potential to provide key information about primary disorders of muscle metabolism as well as the secondary complications of muscle metabolism associated with common conditions such as heart failure, renal failure, and peripheral vascular disease. In addition to studies of skeletal muscle, CrCEST may also have applications in studies of cardiac energetics as well as functional studies of the brain.
Acknowledgments
We gratefully acknowledge V. Kassey and A. Bonner for their help in designing and building the MR compatible exercise setup. We thank D. Reddy for his help with human studies. This work was supported by a NIBIB supported resources center grants P41EB015893, P41EB015893S1 and a training grant T32EB009384.
References
- 1.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation.3. The steady state. J Biol Chem. 1955;217(1):409–427. [PubMed] [Google Scholar]
- 2.Cain DF, Davies RE. Breakdown of adenosine triphosphate during a single contraction of working muscle. Biochem Biophys Res Commun. 1962;8(5):361–366. doi: 10.1016/0006-291x(62)90008-6. [DOI] [PubMed] [Google Scholar]
- 3.Davies RE. A molecular theory of muscle contraction - calcium-dependent contractions with hydrogen bond formation plus atp-dependent extensions of part of myosin-actin cross-bridges. Nature. 1963;199(489):1068–1074. doi: 10.1038/1991068a0. [DOI] [PubMed] [Google Scholar]
- 4.Bendahan D, Giannesini B, Cozzone PJ. Functional investigations of exercising muscle: a noninvasive magnetic resonance spectroscopy-magnetic resonance imaging approach. Cell Mol Life Sci. 2004;61(9):1001–1015. doi: 10.1007/s00018-004-3345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoult DI, Busby SJW, Gadian DG, Radda GK, Richards RE, Seeley PJ. Observation of tissue metabolites using P-31 nuclear magnetic-resonance. Nature. 1974;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- 6.Dawson MJ, Gadian DG, Wilkie DR. Muscular fatigue investigated by phosphorus nuclear magnetic-resonance. Nature. 1978;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- 7.Ingwall JS. Phosphorus nuclear magnetic-resonance spectroscopy of cardiac and skeletal-muscles. Am J Phys. 1982;242(5):H729–H744. doi: 10.1152/ajpheart.1982.242.5.H729. [DOI] [PubMed] [Google Scholar]
- 8.Kemp GJ, Meyerspeer M, Moser E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by P-31 MRS: a quantitative review. NMR Biomed. 2007;20(6):555–565. doi: 10.1002/nbm.1192. [DOI] [PubMed] [Google Scholar]
- 9.Burt CT, Glonek T, Barany M. Analysis of phosphate metabolites, intracellular ph, and state of adenosine-triphosphate in intact muscle by phosphorus nuclear magnetic-resonance. J Biol Chem. 1976;251(9):2584–2591. [PubMed] [Google Scholar]
- 10.Argov Z, Bank WJ. Phosphorus magnetic-resonance spectroscopy (P-31 MRS) in neuromuscular disorders. Ann Neurol. 1991;30(1):90–97. doi: 10.1002/ana.410300116. [DOI] [PubMed] [Google Scholar]
- 11.Argov Z, Lofberg M, Arnold DL. Insights into muscle diseases gained by phosphorus magnetic resonance spectroscopy. Muscle Nerve. 2000;23(9):1316–1334. doi: 10.1002/1097-4598(200009)23:9<1316::aid-mus2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Tarnopolsky MA, Parise G. Direct measurement of high-energy phosphate compounds in patients with neuromuscular disease. Muscle Nerve. 1999;22(9):1228–1233. doi: 10.1002/(sici)1097-4598(199909)22:9<1228::aid-mus9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, Mader H, Kromer EP, Riegger GAJ, Lackner K, Ertl G. P-31 magnetic-resonance spectroscopy in dilated cardiomyopathy and coronary-artery disease - altered cardiac high-energy phosphate-metabolism in heart-failure. Circulation. 1992;86(6):1810–1818. doi: 10.1161/01.cir.86.6.1810. [DOI] [PubMed] [Google Scholar]
- 14.Yabe T, Mitsunami K, Inubushi T, Kinoshita M. Quantitative measurements of cardiac phosphorus metabolites in coronary-artery disease by P-31 magnetic-resonance spectroscopy. Circulation. 1995;92(1):15–23. doi: 10.1161/01.cir.92.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Massie BM, Conway M, Rajagopalan B, Yonge R, Frostick S, Ledingham J, Sleight P, Radda G. Skeletal-muscle metabolism during exercise under ischemic conditions in congestive heart-failure - evidence for abnormalities unrelated to blood-flow. Circulation. 1988;78(2):320–326. doi: 10.1161/01.cir.78.2.320. [DOI] [PubMed] [Google Scholar]
- 16.Mancini DM, Coyle E, Coggan A, Beltz J, Ferraro N, Montain S, Wilson JR. Contribution of intrinsic skeletal-muscle changes to P-31 NMR skeletal-muscle metabolic abnormalities in patients with chronic heart-failure. Circulation. 1989;80(5):1338–1346. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- 17.Kemp GJ, Hands LJ, Ramaswami G, Taylor DJ, Nicolaides A, Amato A, Radda GK. Calf muscle mitochondrial and glycogenolytic atp synthesis in patients with claudication due to peripheral vascular-disease analyzed using P-31 magnetic-resonance spectroscopy. Clin Sci. 1995;89(6):581–590. doi: 10.1042/cs0890581. [DOI] [PubMed] [Google Scholar]
- 18.Argov Z, Renshaw PF, Boden B, Winokur A, Bank WJ. Effects of thyroid-hormones on skeletal-muscle bioenergetics – in vivo P-31 magnetic-resonance spectroscopy study of humans and rats. J Clin Invest. 1988;81(6):1695–1701. doi: 10.1172/JCI113508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bottomley PA, Lee YH, Weiss RG. Total creatine in muscle: Imaging and quantification with proton MR spectroscopy. Radiology. 1997;204(2):403–410. doi: 10.1148/radiology.204.2.9240527. [DOI] [PubMed] [Google Scholar]
- 20.Rico-Sanz J, Thomas EL, Jenkinson G, Mierisova S, Iles R, Bell JD. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by H-1-MRS. J Appl Physiol. 1999;87(6):2068–2072. doi: 10.1152/jappl.1999.87.6.2068. [DOI] [PubMed] [Google Scholar]
- 21.Fisher MJ, Meyer RA, Adams GR, Foley JM, Potchen EJ. Direct relationship between proton t2 and exercise intensity in skeletal-muscle Mr images. Invest Radiol. 1990;25(5):480–485. doi: 10.1097/00004424-199005000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Weidman ER, Charles HC, Negrovilar R, Sullivan MJ, Macfall JR. Muscle-activity localization with P-31 spectroscopy and calculated T2-weighted h-1 images. Invest Radiol. 1991;26(4):309–316. doi: 10.1097/00004424-199104000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Adams GR, Duvoisin MR, Dudley GA. Magnetic-resonance-imaging and electromyography as indexes of muscle function. J Appl Physiol. 1992;73(4):1578–1589. doi: 10.1152/jappl.1992.73.4.1578. [DOI] [PubMed] [Google Scholar]
- 24.WOLFF S, BALABAN R. NMR imaging of labile proton-exchange. J Magn Reson. 1990;86:164–169. [Google Scholar]
- 25.Guivel-Scharen V, Sinnwell T, Wolff S, Balaban R. Detection of proton chemical exchange between metabolites and water in biological tissues. J Magn Reson. 1998;133:36–45. doi: 10.1006/jmre.1998.1440. [DOI] [PubMed] [Google Scholar]
- 26.Ward K, Aletras A, Balaban R. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143:79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 27.Ward K, Balaban R. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST) Magn Reson Med. 2000;44:799–802. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Wilson D, Sun P, Klaus J, van Zijl P. Quantitative description of proton exchange processes between water and endogenous and exogenous agents for WEX, CEST, and APT experiments. Magn Reson Med. 2004;51:945–952. doi: 10.1002/mrm.20048. [DOI] [PubMed] [Google Scholar]
- 29.Jones C, Schlosser M, van Zijl P, Pomper M, Golay X, Zhou J. Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med. 2006;56:585–592. doi: 10.1002/mrm.20989. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, van Zijl P. Chemical exchange saturation transfer imaging and spectroscopy. Prog in Nuc Magn Reson Spectrosc. 2006;48:109–136. [Google Scholar]
- 31.Cai K, Haris M, Singh A, Kogan F, Greenberg J, Hariharan H, Detre J, Reddy R. Magnetic resonance imaging of glutamate. Nat Med. 2012;18(2):302–306. doi: 10.1038/nm.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun P, Benner T, Kumar A, Sorensen A. Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magn Reson Med. 2008;60(4):834–841. doi: 10.1002/mrm.21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A, Haris M, Cai K, Hariharan H, Reddy R. Chemical Exchange Transfer Imaging of Creatine. Proceedings of the 19th Annual Meating of ISMRM; Montreal, Canada. 2011. p. 2767. [Google Scholar]
- 34.Haris M, Nanga RPR, Singh A, Cai K, Kogan F, Hariharan H, Reddy R. Exchange rates of creatine kinase metabolites: feasibility of imaging creatine by chemical exchange saturation transfer MRI. NMR Biomed. 2012;25:1305–1309. doi: 10.1002/nbm.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim M, Gillen J, Landman B, Zhou J, van Zijl P. Water Saturation Shift Referencing (WASSR) for Chemical Exchange Saturation Transfer (CEST) Experiments. Magn Reson Med. 2009;61:1441–1450. doi: 10.1002/mrm.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh A, Cai K, Haris M, Hariharan H, Reddy R. On B1 inhomogeneity correction of in vivo human brain glutamate chemical exchange saturation transfer contrast at 7T. Magn Reson Med. 2012 doi: 10.1002/mrm.24290. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bottomley PA. Spatial localization in NMR-spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu G, Gilad AA, Bulte JWM, van Zijl PCM, McMahon MT. High-throughput screening of chemical exchange saturation transfer MR contrast agents. Contrast Media Mol Imaging. 2010;5(3):162–170. doi: 10.1002/cmmi.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iotti S, Lodi R, Frassineti C, Zaniol P, Barbiroli B. In-vivo assessment of mitochondrial functionality in human gastrocnemius-muscle by P-31 MRS - the role of pH in the evaluation of phosphocreatine and inorganic-phosphate recoveries from exercise. NMR Biomed. 1993;6(4):248–253. doi: 10.1002/nbm.1940060404. [DOI] [PubMed] [Google Scholar]
- 40.Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):502–516. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]
- 41.Rossiter HB, Ward SA, Howe FA, Kowalchuk JM, Griffiths JR, Whipp BJ. Dynamics of intramuscular P-31-MRS P-i peak splitting and the slow components of PCr and O-2 uptake during exercise. J Appl Physiol. 2002;93(6):2059–2069. doi: 10.1152/japplphysiol.00446.2002. [DOI] [PubMed] [Google Scholar]
- 42.van Zijl PCM, Jones CK, Ren J, Malloy CR, Sherry AD. MR1 detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST) Proc Natl Acad Sci USA. 2007;104:4359–4364. doi: 10.1073/pnas.0700281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial-function invivo in human skeletal-muscle by means of P-31 NMR. Magn Reson Med. 1984;1(3):307–315. doi: 10.1002/mrm.1910010303. [DOI] [PubMed] [Google Scholar]
- 44.Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle samples obtained after dynamic exercise. Pflugers Arch. 1976;367(2) doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- 45.Zhou J, Lal B, Wilson D, Laterra J, van Zijl P. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003;50:1120–1126. doi: 10.1002/mrm.10651. [DOI] [PubMed] [Google Scholar]