Abstract
Relapse to alcohol abuse is a critical clinical issue, frequently caused by cue-induced drug craving. Therefore, disruption of the memory for the cue-alcohol association is expected to prevent relapse. It is increasingly accepted that memories become labile and erasable soon after their reactivation through retrieval, during a memory reconsolidation process that depends on protein synthesis. Here, we show that reconsolidation of alcohol-related memories triggered by the sensory properties of alcohol itself (odor and taste) activates mammalian target of rapamycin complex 1 (mTORC1) in select amygdalar and cortical regions in rats, resulting in increased levels of several synaptic proteins. Furthermore, systemic or central amygdalar (CeA) inhibition of mTORC1 during reconsolidation disrupts alcohol-cue associated memories, leading to a long-lasting suppression of relapse. Our findings provide evidence that the mTORC1 pathway and its downstream substrates play a crucial role in alcohol-related memory reconsolidation, and highlight this pathway as a therapeutic target to prevent relapse.
Keywords: Ethanol, addiction, rapamycin, reconsolidation, mTOR, memory, relapse
Alcohol abuse is a worldwide problem with concomitant medical, social and economic burdens1, for which pharmacotherapeutic approaches are limited2. Most alcoholic patients will relapse within the first year of abstinence3, highlighting relapse as a critical clinical issue. A main cause of relapse is cue-induced drug craving4, a process in which a cue previously associated with the reinforcing effects of alcohol elicits craving for alcohol itself, thereby increasing the likelihood of relapse. Thus, disruption of the memory for the cue-drug association is expected to reduce or prevent cue-induced relapse.
Current conceptions of memory processes hold that upon retrieval of the memory it is reactivated, and undergoes a process of destabilization followed by a process of reconsolidation. Following destabilization, a temporary “reconsolidation window” opens, during which the memory becomes labile and can be strengthened or attenuated5, 6, e.g., by administration of amnestic agents shortly after memory reactivation5, 7. Disruption of the reconsolidation of memories associated with drugs of abuse has been proposed as a potential strategy to decrease relapse4, 8, 9. However, while the dependence of reconsolidation on de novo protein translation is established10, 11, the specific signaling molecules and proteins that are required for drug memory reconsolidation remains largely unknown, especially for alcohol.
The mammalian target of rapamycin complex 1 (mTORC1)-mediated signaling pathway is required for the translation of a subset of dendritic proteins12, and is implicated in synaptic plasticity12, 13, as well as in memory processes12. Interestingly, mTORC1 is reported to contribute to memory processes involved in cocaine-conditioned place preference and cue-induced reinstatement14, 15, as well as to reconsolidation of fear and spatial recognition memories16-20, which raises the possibility that this pathway is involved in the reconsolidation of memories associated with drugs of abuse, including alcohol. Here, we tested whether reconsolidation of alcohol-related memories requires activation of mTORC1, and, if so, whether these memories can be disrupted by mTORC1 inhibition, resulting in prevention of relapse.
Results
Retrieval of alcohol-associated memories activates mTORC1
First, to determine whether the mTORC1 signaling pathway is activated after retrieval (reactivation) of alcohol-related memories (i.e., during memory reconsolidation), rats were trained to voluntarily consume excessive amounts of alcohol in their home cage for 7 weeks, using the intermittent access to 20% alcohol 2-bottle choice procedure21, 22. This procedure generates an average blood alcohol concentration (BAC) of ~81mg%23, which corresponds to the definition of binge drinking in humans according to the NIAAA. Rats were then trained in operant chambers for 4-5 weeks to lever press for 0.1 ml aliquots of a 20% alcohol solution in daily 30-min sessions, followed by 10 d of alcohol abstinence in the home cage. Alcohol-associated memories were then reactivated by a 5-min exposure to the behavioral context in which alcohol was received (conditioning chambers) as well as to a non-pharmacologically active alcohol prime (0.2 ml 20% alcohol) that served as a compound odor-taste cue (Suppl. Table 1). Control rats received identical training except that the reactivation stage was omitted (See Suppl. Fig 1 for schematic timeline). Thirty min after memory reactivation, mTORC1 activation was assessed by measuring the phosphorylation levels of its downstream substrates, eukaryotic translation initiation factor-4E binding protein (4E-BP) and S6 kinase (S6K), as well as S6K substrate, S624.
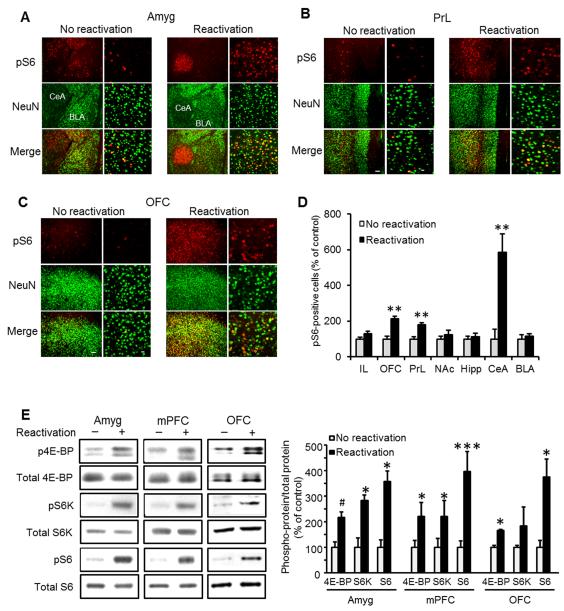
We found that memory reactivation induced mTORC1 activation, specifically in the CeA and in the prelimbic (PrL) and orbitofrontal (OFC) region of the prefrontal cortex (Fig. 1), but not in infralimbic cortex (IL), nucleus accumbens (NAc), basolateral amygdala (BLA) or dorsal hippocampus (Fig. 1 and Suppl. Fig. 1). Taken together, these data show that reactivation of an alcohol-associated memory activates the mTORC1 signaling pathway in the CeA, PrL and OFC.
Figure 1. The mTORC1 signaling pathway is activated in the central amygdala, medial prefrontal and orbitofrontal cortices following reactivation of alcohol-associated memories.
A-C. Immunohistochemical staining of S6 phosphorylation 30 min after reactivation of alcohol-associated memory. Shown is dual-channel immunofluorescence images of phosphoS6 (pS6, red), NeuN (a marker for neurons, green), and overlay (yellow), in the basolateral (BLA) and central (CeA) nuclei of the amygdala (A), the prelimbic (PrL) region of the medial prefrontal cortex (B), and the orbitofrontal cortex (OFC; C). Images are representative of results from 4 rats (3-4 sections/region/rat). Scale bar, left: 100 μm; right: 20 μm. Quantification of the immunohistochemical staining of pS6-positive cells normalized by the total area in 3 slices per brain region from each rat. Data are mean ± SEM (t’s(6)>4.17; **p<0.01, n=4). D. Quantification of the immunohistochemical staining of S6 phosphorylation. Data are mean ± SEM expressed as percentage of no reactivation controls (t’s(6)>4.17; **p<0.01, n=4). IL=infralimbic cortex, OFC-orbitofrontal cortex, PrL=prelimbic cortex, NAc=nucleus accumbens, Hipp=dorsal hippocampus, CeA=central nucleus of the amygdala, BLA=basolateral amygdala E. Western blot analyses of 4E-BP, S6 kinase (S6K) and S6 phosphorylation in the amygdala (Amyg), medial prefrontal cortex (mPFC) and OFC. Immunoreactivity of 4E-BP, S6K and S6 phosphorylation was normalized to the total protein and expressed as percentage of control (no reactivation). Data are mean ± SEM, (t’s(6)>2.50; *p<0.05, **p<0.01, ***p<0.005, #p=0.08, n=4 per group).
Alcohol memory retrieval causes synaptic protein synthesis
mTORC1 controls the translation of 5′ terminal oligopyrimidine tracts (5′TOP), and all components of the mTORC1-dependent translational machinery are present at the synapse12. Thus, mTORC1 plays an essential role in local dendritic translation of mRNAs12, 25-27. For example, the translation of the synaptic proteins, Arc28, PSD-9529, 30, and the AMPA and NMDA receptor subunits, GluR130, 31 and NR131, respectively, is mTOR1 dependent. Each of these proteins plays an important role in synaptic plasticity and certain learning and memory processes32-35. Therefore, next, we tested the hypothesis that reactivation of an alcohol-associated memory increases the levels of key synaptic proteins whose translational is controlled by mTORC1.
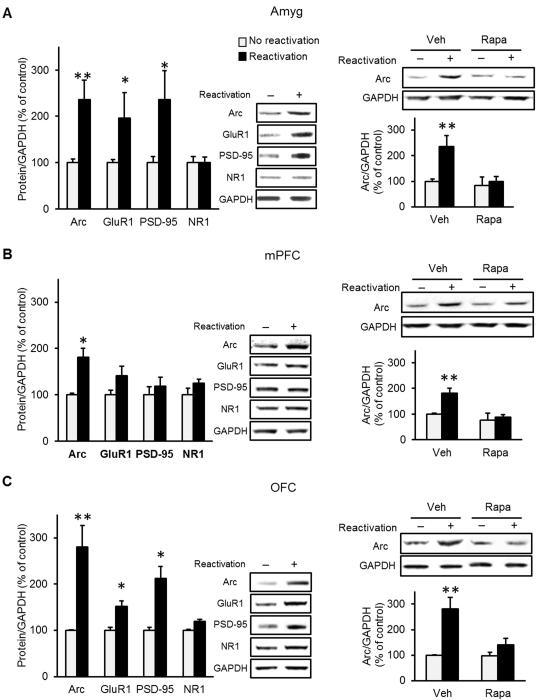
Using the same reactivation procedure described above, we found that memory reactivation increased the protein levels of Arc in the amygdala, OFC and medial prefrontal cortex (mPFC), as well as the levels of GluR1 and PSD-95 in the amygdala and OFC (Fig. 2). We further found that the increase in Arc levels induced by memory reactivation was abolished by mTORC1 inhibition in all three brain regions (Fig. 2), and that the increase in GluR1 and PSD-95 was reduced in the OFC (Suppl. Fig. 2). Taken together, these findings suggest that the consequence of mTORC1 activation during reconsolidation of alcohol-associated memories is translation of specific synaptic proteins that take part in plasticity processes.
Figure 2. Reactivation of alcohol-associated memories increases levels of synaptic proteins.
A-C. Immunoblotting of mTORC1-regulated proteins in the amygdala (Amyg; A), mPFC (B) and OFC (C), 60 min after reactivation of alcohol-associated memory. A-C (left pane). The levels of Arc, GluR1, PSD-95 and NR were determined by western blot analysis and normalized to GAPDH. A-C (right pane). The memory reactivation-induced increase in Arc immunoreactivity was blocked by rapamycin (20 mg/kg, i.p) administered immediately after memory reactivation. Data are mean ± SEM and expressed as percentage of control. (A-C, left pane: t test; t’s(6)>2.50; *p<0.05, **p<0.01, A-C, right pane, Two-way ANOVA; Reactivation X Treatment interaction, [F(1, 12)>4.90, p<0.05], post-hoc comparisons **p<0.01; n=4 per group).
mTORC1 inhibition disrupts alcohol memory reconsolidation
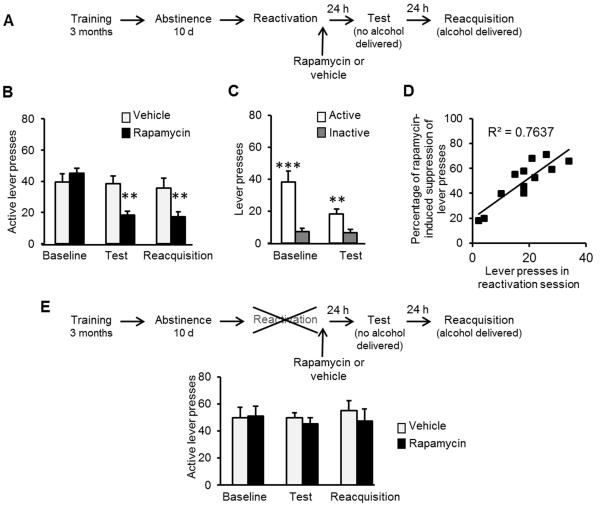
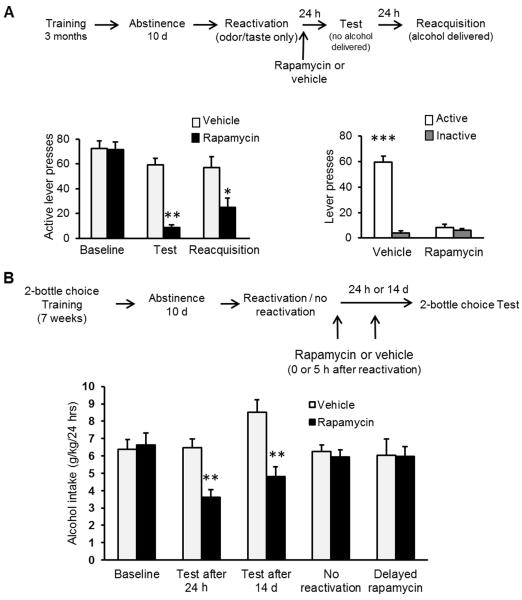
If mTORC1 is essential for reconsolidation of alcohol-associated memories, then inhibition of this pathway should disrupt this step, resulting in a subsequent reduction of relapse. To test this possibility, rats were trained to lever press for alcohol followed by a 10-d abstinence period as described above. The mTORC1 inhibitor, rapamycin (20 mg/kg, i.p), or vehicle was administered immediately following a 5-min reactivation session. Relapse to alcohol seeking and drinking was assessed using retention36 and reacquisition37 tests, 24 and 48 h after the reactivation session, respectively (Fig. 3A). We found that mTORC1 inhibition after memory reactivation suppressed alcohol seeking and consumption, 24 and 48 h later, respectively, as reflected in reduced active lever presses by rats receiving rapamycin compared to vehicle-treated rats (Fig. 3B&C). This finding indicates that mTORC1 activation is required for reconsolidation of alcohol-related memories, and that by inhibiting this pathway, the memories can be attenuated and relapse can be reduced. Furthermore, lever press number during the 5-min reactivation positively correlated with the suppressive effects of rapamycin on alcohol seeking 24 h later (Fig. 3D), suggesting that the more strongly the memory is reactivated, the more susceptible the memory becomes to mTORC1 inhibition.
Figure 3. Inhibition of mTORC1 after reactivation of alcohol-associated memories attenuates relapse measured as instrumental responding for alcohol.
A. Schematic representation of the experimental procedure. B, C & E. Data are mean ± SEM of active lever presses before abstinence (baseline), and during retention test and reacquisition stages. B. Effects of rapamycin (20 mg/kg ,i.p.) or vehicle given immediately after memory reactivation using presentation of context as well as odor-taste cue on lever presses during test and reacquisition. (Two-way ANOVA; Stage X Treatment interaction [F(2,22)=6.38, p<0.01]; post-hoc comparisons **p<0.01, n=12). C. Active and inactive lever presses during the test stage (Two-way ANOVA; Stage X Lever [F(1,22)=27.57, p<0.001]; post-hoc comparisons, active vs. inactive lever presses, **p<0.01 ***p<0.0001, n=12). D. Correlation plot of the number of lever presses during the reactivation session and the percentage of rapamycin-induced suppression in lever presses during the test (calculated as (presses in test / presses in baseline) X 100 in the rapamycin group). E. Effects of rapamycin (20 mg/kg, i.p) or vehicle, given 24 h before test without a reactivation session, on lever presses during test and reacquisition. (Two-way ANOVA; Stage X Treatment interaction [F(2,18)=0.53, p=0.59]; n=10).
Importantly, in a control experiment we found no effect when the reactivation session was omitted (i.e., rapamycin or vehicle was systemically administered 24 h before the test; Fig. 3E), showing that mTORC1 inhibition reduces relapse only if the memory is retrieved prior to the administration of rapamycin. This finding indicates that rapamycin disrupts memory reconsolidation, rather than merely the motivation to respond or consume alcohol.
Next, to test whether the effects of mTORC1 inhibition on memory reconsolidation are specific to memories associated with the reinforcing effects of alcohol, we tested the effects of post-reactivation administration of rapamycin in subjects trained to lever press for the natural reward, sucrose (2% solution), rather than alcohol. We found that lever press responding during both the retention and reacquisition tests was not different between saline- and rapamycin-treated rats (Suppl. Fig. 3), indicating that mTORC1 inhibition is effective in disrupting memories associated with alcohol, but not with other, natural reinforcers.
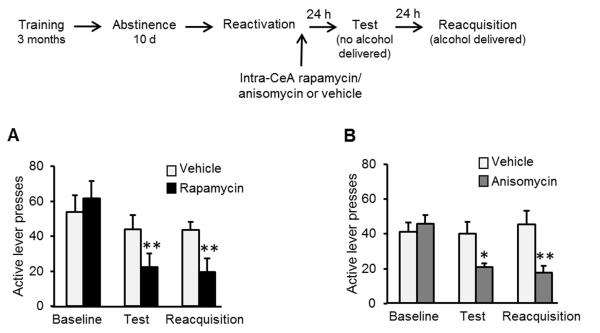
Because we saw an increase in mTORC1 activation in the CeA after reactivation (Fig. 1), we reasoned that rapamycin’s reduction of alcohol relapse was mediated, at least in part, via the inhibition of mTORC1 activity specifically within the CeA. Thus, we tested whether mTORC1 inhibition within the CeA disrupts memory reconsolidation. We found that intra-CeA infusion of rapamycin (50 μg/side; Suppl. Fig. 4) focally inhibits the mTORC1 pathway as reflected by reduced phosphorylation of S6, S6K and 4E-BP (Suppl. Fig. 5). Furthermore, as shown in Fig. 4A, infusion of rapamycin immediately after memory reactivation, suppressed relapse to alcohol seeking and consumption on subsequent days. Moreover, we found that administration of the protein synthesis inhibitor, anisomycin, into the CeA produces similar behavioral effects to those observed after intra-CeA rapamycin treatment (Fig. 4B). Together, these data suggest that mTORC1 activation within the CeA is required for reconsolidation of alcohol-associated memories, a process that is likely to be mediated via mTORC1-dependent de novo protein synthesis.
Figure 4. Infusion of rapamycin or anisomycin into the CeA after reactivation of alcohol-associated memories attenuates relapse.
A&B. Effects of rapamycin (A; 50 μg/side) or anisomycin (B; 62.5 μg/side) or vehicle infused into the CeA immediately after memory reactivation on lever presses during test and reacquisition. Data are mean ± SEM of active lever presses before abstinence (baseline), and during retention test and reacquisition stages. (A, rapamycin: Two-way ANOVA; Stage X Treatment interaction [F(2,14)=10.95, p<0.005]; post-hoc comparisons **p<0.01, n=8; B, anisomycin: Two-way ANOVA; Stage X Treatment interaction [F(2,11)=8.59, p<0.005]; post-hoc comparisons *p<0.5, **p<0.01, n=6-7).
Odor-taste cues evoke mTORC1-dependent reconsolidation
Alcohol is consumed orally, and therefore its odor and taste serve as potent cues for alcohol’s reinforcing effects. We predicted that disrupting the association between these cues and alcohol reinforcement would attenuate relapse driven by these potent cues, independently from specific contexts and other, more distal cues. Rats were trained and tested using the same procedure as above, except that the reactivation session was conducted in the home cage, with 10 min exposure to 2 bottles: a water bottle and an empty bottle with a tip covered with a drop of alcohol (0.2 ml, 20%) serving as an odor-taste cue, i.e., an alcohol prime. Relapse was assessed in the operant chambers as described above, 24 and 48 h later. Strikingly, mTORC1 inhibition after memory reactivation substantially reduced relapse to alcohol seeking and drinking in this procedure, as indicated by low responding at the retention and reacquisition tests as compared to vehicle-treated subjects (Fig. 5A, left panel). The complete attenuation of relapse is also apparent in the lack of difference between the number of active and inactive lever presses in rapamycin-treated rats during the retention test (Fig. 5A, right pane). These findings demonstrate that the odor and taste of alcohol are potent cues that evoke memory reconsolidation, independently of the training context, enabling a complete abolition of relapse to alcohol seeking by mTORC1 inhibition.
Figure 5. Inhibition of mTORC1 after reactivation of alcohol-associated memories in the home cage induces a potent, long-term suppression of relapse.
A. Effects of rapamycin (20 mg/kg, i.p) or vehicle, given immediately after memory reactivation using an alcohol odor-taste cue in the home cage, on active lever presses during test and reacquisition (Left pane; Two-way ANOVA; Stage X Treatment interaction [F(2,26)=14.51, p<0.0001]; post-hoc comparisons *p<0.005, **p<0.001, n=8) and on active and inactive lever presses during the test stage (Right pane; Two-way ANOVA; Stage X Lever [F(1,13)=132.27, p<0.0001]; post-hoc comparisons, active vs. inactive lever presses, ***p<0.0001, n=8). Data are mean ± SEM of lever presses. B. Effects of rapamycin (20 mg/kg ,i.p.) or vehicle, given after memory reactivation on relapse to alcohol drinking in 2-bottle choice procedure. Data are mean ± SEM of alcohol intake (g/kg/24 h) during a 24 h 2-bottle choice session, in rapamycin- or vehicle-treated rats before abstinence (baseline), 24 h after reactivation, 14 d after reactivation, in the absence of reactivation, and 24 h after reactivation with a delayed (5 h) administration of rapamycin. (Two-way ANOVA, Condition X Treatment interaction [F(4, 106)=7.12, p<0.0001], post-hoc comparisons **p<0.001, n=8-12).
To investigate the possibility that the odor-taste cue of alcohol is crucial for memory reactivation, we conducted an experiment entirely in the home cage, using the intermittent access to 20% alcohol 2-bottle choice procedure37. After 7 weeks of alcohol access, and 10 d of abstinence, alcohol-associated memories were reactivated using the alcohol odor-taste cue presented in the home cage as described above (Fig. 5B). We found that systemic administration of rapamycin immediately after memory reactivation decreased relapse to alcohol consumption 24 h later, measured as intake from the home-cage bottle (Fig. 5B). Importantly, relapse to alcohol drinking was still suppressed when the test was conducted 14 d after the reactivation session (Fig. 5B), indicating that the rapamycin-induced relapse attenuation is long-lasting. Notably, S6 phosphorylation was selectively increased in the CeA following reactivation (Suppl. Fig. 6), suggesting that activation of mTORC1 in the CeA underlies the retrieval of alcohol-related memories via the alcohol prime.
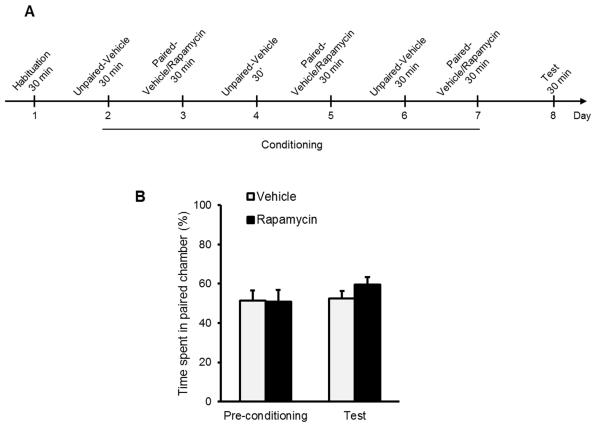
Importantly, we showed that the reduction in home-cage alcohol consumption is due to disruption of memory reconsolidation. Specifically, administration of rapamycin with the omission of the reactivation session had no effect on alcohol intake (Fig. 5B), confirming that the effects of rapamycin on alcohol consumption 24 h later requires a prior reactivation of the memory. In addition, administration of rapamycin 5 h after memory reactivation had no effect on later alcohol consumption (Fig. 5B). These results suggest that the memory lability period following its reactivation is limited to a few hours of the “reconsolidation window”, after which the memory reconsolidation process is completed, and the memories become stable4, 5 and resistant to mTORC1 inhibition. Importantly, we found that when rats were trained to consume alcohol as well as a sucrose solution, rapamycin administration after the reactivation of alcohol-associated memories had no effect on subsequent sucrose consumption, indicating that the amnestic actions of rapamycin are specific for the reactivated memories, while other memories remain intact (Suppl. Fig. 7). Finally, we show that rapamycin does not induce conditioned place aversion (Fig. 6), suggesting that the reduction in alcohol consumption we observed is unlikely to result from aversive effects of rapamycin causing either conditioned taste aversion or some other aversion-induced devaluation of the outcome.
Figure 6. Rapamycin does not induce place aversion.
A. Design and schedule of the rapamycin place aversion experiment: rats of the rapamycin condition were systemically administered with rapamycin (20 mg/kg) and vehicle 3 h before the 30-min conditioning paired and unpaired sessions, respectively. Rats of the vehicle condition received vehicle only. B. Place preference/aversion for rapamycin is expressed as the ratio ± SEM of the time spent in the rapamycin-paired compartment divided by time spent in paired+unpaired compartments. (Two-way ANOVA, Treatment X Conditioning interaction [F(1, 16)=1.50, p=0.24], n=9).
Discussion
Here, we demonstrate that the most behaviorally-relevant cues for relapse, the odor and taste of alcohol, are sufficient to elicit reconsolidation of alcohol-associated memories. Moreover, this process is correlated with the activation of the mTORC1 signaling pathway in the CeA and specific cortical regions. Furthermore, we show that the activation of mTORC1 leads to the translation of synaptic proteins that are important molecular contributors to memory processes32-35. Importantly, we present data that the mTORC1 inhibitor, rapamycin, disrupts the reconsolidation of these memories, resulting in a long-lasting suppression of relapse.
Interestingly, mTORC1 activation in the BLA has been implicated in reconsolidation of fearful16, 19, 38 and object recognition17 memories, however, this signaling pathway was not activated in the BLA after retrieval of alcohol-associated memories. The CeA has been implicated in behavioral responding to reward-predictive cues39-41 and in incentive42 and habit43 learning, as well as in incubation of cocaine and morphine craving44. Our findings reveal a new role for the CeA in alcohol cue memories, and suggest that this region is critical for reactivation of the association of the odor and/or taste of alcohol with its pharmacological effects. Interestingly, the CeA is additionally implicated in anxiety and stress responses45, and as such was shown to play a significant role in the development of alcohol dependence through negative reinforcement mechanisms (alleviation of anxiety)46. Rats withdrawn from alcohol in the intermittent access to 20% alcohol procedure used here show a dopamine deficiency in the NAc, which is correlated with alcohol seeking and is alleviated by alcohol intake21, implying the relevance of negative reinforcement mechanisms. It is thus plausible that retrieval of alcohol-associated memories after abstinence specifically reactivates affective (appetitive and/or aversive) aspects of memories, leading to mTORC1-dependent memory reconsolidation and synaptic protein synthesis. Inhibition of mTORC1 may disrupt these affective memories, resulting in disruption of the positive and/or negative reinforcement mechanisms that promote alcohol seeking.
Memory reactivation in the context of the alcohol self-administration chamber activated mTORC1 signaling in the PrL and OFC as well as the CeA, while only CeA activation was observed after memory reactivation in the home cage. Thus, additional associations related to the instrumental lever-press response and the contextual modulation of those associations are likely also retrieved, accounting for the activation of these cortical regions.
We note that some associations that support alcohol seeking likely remain after rapamycin treatment; when we assessed the effects of rapamycin on relapse after memory reactivation in the alcohol self-administration chamber, response levels were attenuated, but were still higher for the active lever compared to the inactive lever. In contrast, we found that relapse to alcohol seeking was completely abolished when mTORC1 was inhibited after retrieving the memory by presentation of the odor-taste cue in the home cage (i.e., outside of the alcohol-associated context), hence some instrumental associations may not be susceptible to reconsolidation disruption, accounting for the low level of responding that remained. Critically, even under conditions of reacquisition, when responding was again reinforced by alcohol, responding in the rapamycin group was still considerably lower than control levels, highlighting the potential utility of our approach for reducing relapse.
Interestingly, we found that mTORC1 inhibition after memory reactivation has no effect on relapse to consumption of a natural reward, sucrose. Specifically, sucrose intake was not altered by rapamycin administration after retrieval of sucrose-related memories or by administration of the inhibitor after reactivation of alcohol-associated memories. These findings suggest that the underlying mechanisms of memory processing are distinct for natural rewards and alcohol. This possibility is not entirely surprising, as differential effects of various manipulations on behaviors reinforced by sucrose or alcohol reward have been previously reported (e.g., 37, 47, 48).
Importantly, the alcohol-selectivity we demonstrate here has direct translational implications, potentially enabling selective interference with alcohol-related memories while leaving non-alcohol memories (e.g., natural rewards memories) intact. Furthermore, our findings that the reduction in relapse is observed even 14 days after the memory reactivation, and that this effect cannot be attributed to taste aversion or devaluation of the outcome, further highlights the translational potential of this relapse prevention approach.
Previously, we reported that alcohol exposure in pharmacologically relevant doses (2.5-6.5 g/kg, i.p. or voluntary consumption) activates the mTORC1 pathway in the NAc and that inhibition of this complex right before alcohol self-administration sessions reduces alcohol consumption49. In contrast, in the present study, we found that retrieval of alcohol-associated memories does not induce changes in mTORC1 activation in the NAc. A critical difference between the two studies is the fact that Neasta et al.49 compared alcohol-naïve to alcohol-experienced animals whereas in the present study we assessed the effects of retrieval of alcohol-associated memories per se on mTORC1 activation, in rats that all had the same exposure to alcohol and were after 10 d of abstinence. Hence, systemic administration of rapamycin has the potential to produce multiple effects on alcohol seeking behaviors by acting on multiple neural circuits: an acute effect of alcohol exposure per se, mediated by the NAc, and an effect on later relapse driven by conditioned alcohol cues mediated by the CeA. These findings as a whole indicate that multiple alcohol-induced changes in neural function are mediated by mTORC1 signaling, adding further impetus to the investigation of the mTORC1 pathway for new therapeutic approaches to treat alcohol use disorders.
Interference with the reconsolidation of memories had been proposed as a promising approach to attenuate or even erase memories, which could serve as a therapeutic strategy for several disorders associated with abnormally persistent memories, such as post-traumatic stress disorder (PTSD)50 and substance abuse and dependence4. Recently, experimental support for this approach was obtained in human heroin addicts8. The current findings suggest that disruption of reconsolidation could also be beneficial for alcohol use disorders. Virtually every behavioral experience with alcohol includes its odor and taste; thus a reconsolidation-based strategy for relapse disruption that focuses on these cues is a promising therapeutic approach. Our present results show that effective reactivation of alcohol-associated memories is achieved by a brief presentation of alcohol’s odor and taste, and that mTORC1 inhibition disrupts these memories and suppresses relapse, therefore have important translational implications for developing a novel and potent strategy to prevent relapse in alcoholism via mTORC1-mediated disruption of memory reconsolidation mechanisms.
Methods
Reagents
The following antibodies were purchased from Cell Signaling Technology (Danvers, MA): NMDAR1 (NR1; no. 4204), phospho-S6 Ribosomal Protein S235/236 (pS6; no. 2211), total S6 (no. 2217), phospho-S6 kinase Thr389 (pS6K; no. 9234), phospho-4E-BP Thr37/46 (p4E-BP; no. 2855), total S6K (no. 2708), and total 4E-BP2 (no. 2845; 4E-BP2 is the main 4E-BP isoform in the brain1, 2). Antibodies against GAPDH (sc-25778), Arc (sc-17839) and PSD-95 (sc-32290) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). GluR1 antibody (no. 06-306) was purchased from Upstate (Billerica, MA). Mouse monoclonal anti-neuronal nuclei (NeuN) and nitrocellulose membrane were purchased from Millipore (Billerica, MA). EDTA-free Complete Mini Protease Inhibitors Mixture was purchased from Roche (11873580001, Indianapolis, IN). Phosphatase Inhibitors Mixtures 1 and 2, DMSO and anisomycin were purchased from Sigma-Aldrich (St. Louis, MO). Alexa Fluor 594 donkey anti-rabbit and Alexa Fluor 488 donkey anti-mouse, BCA Protein Assay kit was purchased from Pierce, NuPAGE Bis-Tris precasted gels were purchased from Invitrogen (Carlsbad, CA). Enhanced Chemiluminescence Plus was purchased from GE Healthcare (Buckinghamshire, UK) and BioMax MR Film was purchased from Kodak (Rochester, NY). Alcohol was purchased from Gold Shield Chemical. Rapamycin (R-5000) was purchased from LC Laboratories (Woburn, MA). Isoflurane was purchased from Baxter Health Care (Deerfield, IL).
Animals
Male Long–Evans rats (Harlan; 270-300 g at the beginning of training) were housed under a 12 h light/dark cycle (lights on at 7:00 a.m.) with food and water available ad libitum. All animal procedures in this report were approved by the Gallo Center Institutional Animal Care and Use Committee and were conducted in agreement with the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996.
Preparation of solutions
Alcohol solution was prepared from ethyl alcohol absolute anhydrous (190 proof) diluted to 20% alcohol (vol/vol) in tap water. Rapamycin and anisomycin were dissolved in 100% DMSO.
Western-blot analysis
Western blot analysis was conducted as previously described3. Briefly, brain regions were chosen according to the immunohistochemistry results (Fig. 1A-C). The amygdala, mPFC and OFC were rapidly dissected, and immediately homogenized in a RadioImmuno Precipitation Assay (RIPA) buffer containing (in mM): 25 Tris-HCl pH 7.6, 150 NaCl, 1 EDTA, 1% (vol/vol) NP-40, 0.5% (weight/vol) sodium deoxycholate, 0.1% (weight/vol) SDS and protease and phosphatase inhibitors. Protein concentration was determined using BCATM assay, and an equal amount of samples (40μg) was denatured with Laemmli buffer, boiled for 10 min and resolved on a 4-12% SDS-PAGE, and transferred to a nitrocellulose membrane. Membranes were blocked for 1 h with 5% (weight/vol) non-fat milk in Tris Buffer Saline/0.1% (vol/vol) Tween 20 (TBS-T) and then incubated overnight at 4°C with the appropriate antibody. After extensive washing with TBS-T, bound primary antibodies were detected with HRP-conjugated secondary antibody and visualized by ECL plus. Membranes were then striped for 30 min at 50°C in buffer containing 100 mM 2-Mercaptoethanol, 2% (weight/vol) SDS, 62.5 mM Tris-HCl pH 6.7, followed by extensive washing in TBS-T before re-blocking and re-probing with the appropriate total antibody. The optical density of the relevant immunoreactive band was quantified using NIH ImageJ 1.63 program. The optical density values of the phospho-protein signal was normalized to the signal of the total protein in the same sample. The optical density values of Arc, GluR1, PSD-95 and NR1 were normalized to the level of GAPDH. Results were expressed as a percentage of the control.
Immunohistochemistry
Immunofluorescent staining was conducted as previously described4. Briefly, Free-floating paraformaldehyde-fixed 50-μm thick sections were incubated with 50% ethanol for 20 min to permeablize the tissue, rinsed in PBS, then blocked with 10% normal donkey serum in PBS for 30 min, and then incubated for 48 h at 4°C on an orbital shaker with a mixture of primary antibodies: anti-pS6, and anti-neuronal nuclei (NeuN). Sections were then rinsed with PBS, and incubated in 2% normal donkey serum for 10 min and incubated for 12 hours with secondary antibodies: Alexa Fluor 594 donkey anti-rabbit, Alexa Fluor 488 donkey anti-mouse. After staining, sections were rinsed in PBS, mounted on gelatin-subbed slides and coverslipped using Vectashield mounting medium (Vector Labs, Burlingame, CA). Images were acquired using Zeiss LSM 510 META laser confocal microscope (Zeiss, Thornwood, NY) with using factory recommended settings. Quantification was done by counting the number of pS6-positive cells and normalizing by area. All counts were performed blind with respect to treatment groups.
Intermittent-access to 20% alcohol in the 2-bottle choice drinking procedure
Intermittent-access to alcohol was performed as previously described4, 5. Briefly, animals were given 24 h of concurrent access to one bottle of 20% vol/vol alcohol in tap water and another bottle of water, starting at 11:00a.m. on Monday, Wednesday, and Friday, with 24 or 48 h of alcohol-deprivation periods in between the alcohol-drinking sessions. The placement (left or right) of each solution was alternated between each session to control for side preference. The water and alcohol bottles were weighed after 24 h of access.
Operant alcohol self-administration after history of high voluntary alcohol consumption
The operant training began after rats achieved a stable baseline of alcohol consumption following 7 weeks training in the intermittent access to 20% alcohol 2-bottle choice drinking procedure as described above, when rats maintained a stable baseline of alcohol consumption of 5.5-6 g/kg/24 h. Rats were then trained to self-administer an alcohol solution in the operant self-administration chambers (Med Associates, Georgia, VT), as previously described5, leading to a stable baseline of operant performance to obtain the delivery of 0.1 ml of a 20% alcohol solution under a fixed ratio 3 (FR3) schedule, during 30 min sessions, 5 days per week.
Operant-based memory reconsolidation procedure
Pre-training
Rats were trained in operant chambers to self-administer a 20% alcohol solution, as described above, or for a 2% sucrose solution, as previously described6. After 4-5 weeks of training in FR3, when a stable response and alcohol consumption levels were obtained, rats were subjected to 10 d of abstinence from alcohol or sucrose in their home-cage.
Memory reactivation
After completing 10 d of abstinence rats were re-exposed to the alcohol- or sucrose-associated context and odor-taste cues. Specifically, rats were confined to the behavioral chamber for 5 min with the levers presented, and a non-pharmacologically-active alcohol prime (0.2 ml 20% alcohol) or sucrose prime (0.2 ml sucrose 2% solution) was delivered immediately at the beginning of the session, serving as an odor-taste cue. Alcohol/sucrose was not delivered following lever presses in the remainder of the session. Control (‘no reactivation’) rats were handled but were not presented with the context/cues. In experiments where rapamycin (dissolved in DMSO) or vehicle was administered, the injection was given immediately after the reactivation session.
Test
Relapse to alcohol or sucrose seeking was assessed in a retention test stage, taking place 24 h after the reactivation session. Rats were placed in the operant chambers for a 30-min session similarly to the self-administration training sessions, except that no alcohol/sucrose was delivered following either lever presses. In addition, an alcohol/sucrose prime was non-contingently delivered at the beginning of the session, as in the reactivation session.
Reacquisition
Relapse to alcohol or sucrose consumption was assessed in a reacquisition stage, taking place 24 h after the test session (i.e., 48 h after the reactivation session). This session was identical to the test stage, except that alcohol/sucrose was delivered following lever presses (at FR3) as in the pre-training sessions. See Figures for a schematic timeline of experiments.
Non-operant memory reconsolidation in a 2-bottle choice procedure
Pre-training
Rats were first trained for 7 weeks to voluntarily consume high levels of alcohol in their home-cage, as described above. After obtaining a stable baseline alcohol consumption level (5.5-6.5 g/kg/24 h; Figure 4), rats were subjected to 10 d of abstinence from alcohol in their home-cage.
Memory reactivation
After completing 10 d of abstinence rats were re-exposed to the alcohol-associated odor-taste cues. Specifically, the ad lib water bottle was taken out, and rats were presented for 10 min with two bottles in a similar manner to their 2-bottle choice experience, however one bottle contained water, whereas the other bottle was empty, with a 0.2 ml drop of alcohol applied on the tip to serve as an odor-taste cue. Control rats (‘no reactivation’) were presented with 2 water bottles. In experiments where rapamycin or vehicle was administered, the injection was given immediately, or 5 h, after the reactivation session, as indicated.
Alcohol intake test in 2-bottle choice
Relapse to alcohol drinking was assessed by measuring alcohol and water intake in a 24-h 2-bottle choice drinking session.
Relapse to sucrose consumption test in 2-bottle
After 7 weeks of training in the intermittent access to 20% alcohol in 2-bottle choice, rats had access to a bottle containing sucrose solution (0.5% w/v), as well as to a bottle of water, for 3 weeks. Sucrose and water intake were monitored daily. Following 10 d of access only to water (abstinence period), the alcohol-associated memory was reactivated in the home-cage as described above, and rapamycin (20 mg/kg, i.p.) or vehicle was given immediately after memory reactivation. The next day, sucrose intake was tested in a 24 h 2-bottle choice (sucrose and water) drinking session.
Surgery and microinfusion of rapamycin
Rats were anesthetized continuously with isoflurane. Guide cannulae (26 gauge; Plastics One) were aimed dorsal to the central nucleus of the amygdala (CeA; 2.50 mm posterior to bregma, 4.1 mm mediolateral, 7.4 mm ventral to the skull surface), according to the Paxinos and Watson rat brain atlas. The coordinates for the CeA were chosen based on the immunoreactivity of pS6 following reactivation of alcohol memories (Fig. 1). Microinjections began when self-administration responding retuned to pre-surgery baseline. Immediately after the memory reactivation session, rapamycin (50 μg/0.5 μl per side) or vehicle, or anisomycin (62.5 μg/0.5 μl per side) or vehicle was infused over 1 min into the CeA of gently restrained rats via injection cannulae extending 0.5 mm beyond the guide cannula tip. Injection cannulae were left in place for an additional 2 min. The dose of rapamycin was based on previous studies7, and on pilot studies demonstrating that at this does, rapamycin infusion into the CeA inhibits the mTORC1 pathway (Suppl. Fig 5). The dose of anisomycin was based on previous reconsolidation studies were the inhibitor was infused into the amygdala8.
Conditioned place preference (CPP) apparatus and procedure
Apparatus
Animals were trained in identical 3-chamber CPP boxes (Med Associates, Georgia, VT) consisting of a small gray middle chamber (12 × 21× 21 cm) joined to 2 larger side chambers (28 × 21 ×21 cm) that differ in color, lighting, and floor texture. Total time spent in each chamber as well as horizontal locomotor activity was automatically recorded by infrared beam breaks.
Place conditioning to Rapamycin
The place conditioning procedure was conducted as we previously described5 and is illustrated in Fig. 6. Briefly, Rats were allowed to explore the entire apparatus for 30 min for habituation, and to obtain the baseline measurements (day 1, pre-conditioning session). The next day, the conditioning training started with one conditioning trial per day during 6 days (days 2 to 7). Rapamycin (20 mg/kg) or vehicle was systemically administered 3 h before confinement of the animals for 30 min in the paired side chamber (days 3, 5 and 7). All animals were administered with vehicle before confinement in the unpaired side chamber (days 2, 4 and 6). On day 8, animals were allowed to explore the entire apparatus for 30 min (post-conditioning test session) as during habituation, and preference was scored by dividing the time spent in the paired compartment by the total time spent in the unpaired+paired compartment during this session (preference ratio). Three conditioning sessions were chosen as 3 to 4 sessions are generally used to obtain robust placed preference or aversion to rewarding substances, including drugs of abuse, in rodents9.
Histology
Locations of cannulae were verified in 50-μm coronal sections of paraformaldehyde-fixed tissue stained with thionin. Only data from subjects with cannulae located within the CeA were included in the analysis (Suppl. Fig 4).
Statistical analysis
Data from western blot and immunohistochemistry were analyzed using unpaired t tests. Operant self-administration, alcohol consumption and place preference data were analyzed using two-way ANOVA with repeated measures. Fisher LSD post-hoc analysis was used where indicated. Correlation was analyzed by linear regression, and the effect size (R2 value) was calculated. No statistical test was run to determine sample size a priori. The sample sizes we chose are similar to those used in previous publications.
Supplementary Material
Acknowledgements
Supported by NIH/NIAAA Grant P50 AA017072 (DR and PHJ) and funds from the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (DR and PHJ). We thank Dr. Sebastien Carnicella for critical review of this manuscript.
Footnotes
Author contribution SB, JN, PHJ, and DR designed the research; SB, FL, QVY, SBH, JN and VK performed the research; SB, FL, SBH, QVY, JN, and DR analyzed data; and SB, PHJ, and DR wrote the paper.
Competing financial interests The authors declare no competing financial interests.
References
- 1.World Health Organization . WHO global status report on alcohol 2004. World Health Organization; Geneva: 2004. [Google Scholar]
- 2.Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Current psychiatry reports. 2011;13:398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milton A. Drink, drugs and disruption: memory manipulation for the treatment of addiction. Curr Opin Neurobiol. doi: 10.1016/j.conb.2012.11.008. (in press) [DOI] [PubMed] [Google Scholar]
- 5.Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 6.Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue YX, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milton AL, et al. Antagonism at NMDA receptors, but not beta-adrenergic receptors, disrupts the reconsolidation of pavlovian conditioned approach and instrumental transfer for ethanol-associated conditioned stimuli. Psychopharmacology (Berl) 2012;219:751–761. doi: 10.1007/s00213-011-2399-9. [DOI] [PubMed] [Google Scholar]
- 10.Miller CA, Sweatt JD. Amnesia or retrieval deficit? Implications of a molecular approach to the question of reconsolidation. Learn Mem. 2006;13:498–505. doi: 10.1101/lm.304606. [DOI] [PubMed] [Google Scholar]
- 11.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 12.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoica L, et al. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci U S A. 2011;108:3791–3796. doi: 10.1073/pnas.1014715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, et al. Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2010;30:12632–12641. doi: 10.1523/JNEUROSCI.1264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey J, Ma D, Szumlinski KK. Rapamycin attenuates the expression of cocaine-induced place preference and behavioral sensitization. Addiction biology. 2012;17:248–258. doi: 10.1111/j.1369-1600.2010.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jobim PF, et al. Inhibition of mTOR by rapamycin in the amygdala or hippocampus impairs formation and reconsolidation of inhibitory avoidance memory. Neurobiol Learn Mem. 2012;97:105–112. doi: 10.1016/j.nlm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Jobim PF, et al. Impairment of object recognition memory by rapamycin inhibition of mTOR in the amygdala or hippocampus around the time of learning or reactivation. Behav Brain Res. 2012;228:151–158. doi: 10.1016/j.bbr.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem. 2008;90:28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glover EM, Ressler KJ, Davis M. Differing effects of systemically administered rapamycin on consolidation and reconsolidation of context vs. cued fear memories. Learn Mem. 2010;17:577–581. doi: 10.1101/lm.1908310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gafford GM, Parsons RG, Helmstetter FJ. Consolidation and reconsolidation of contextual fear memory requires mammalian target of rapamycin-dependent translation in the dorsal hippocampus. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barak S, Carnicella S, Yowell QV, Ron D. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J Neurosci. 2011;31:9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barak S, Ahmadiantehrani S, Kharazia V, Ron D. Positive autoregulation of GDNF levels in the ventral tegmental area mediates long-lasting inhibition of excessive alcohol consumption. Transl Psychiatry. 2011;1:e60. doi: 10.1038/tp.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang DO, Martin KC, Zukin RS. Spatially restricting gene expression by local translation at synapses. Trends Neurosci. 2010;33:173–182. doi: 10.1016/j.tins.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu-Yesucevitz L, et al. Local RNA translation at the synapse and in disease. J Neurosci. 2011;31:16086–16093. doi: 10.1523/JNEUROSCI.4105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takei N, et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. The Journal of biological chemistry. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- 30.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Xu W. PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr Opin Neurobiol. 2011;21:306–312. doi: 10.1016/j.conb.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korb E, Finkbeiner S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 2011;34:591–598. doi: 10.1016/j.tins.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plath N, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, et al. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everitt BJ, Cardinal RN, Hall J, Parkinson J, Robbins T. Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction. In: Aggleton JP, editor. The amygdala: a functional analysis. 2000. pp. 353–390. [Google Scholar]
- 40.Calu DJ, Roesch MR, Haney RZ, Holland PC, Schoenbaum G. Neural correlates of variations in event processing during learning in central nucleus of amygdala. Neuron. 2010;68:991–1001. doi: 10.1016/j.neuron.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purgert RJ, Wheeler DS, McDannald MA, Holland PC. Role of amygdala central nucleus in aversive learning produced by shock or by unexpected omission of food. J Neurosci. 2012;32:2461–2472. doi: 10.1523/JNEUROSCI.5090-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci. 2009;29:6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lingawi NW, Balleine BW. Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J Neurosci. 2012;32:1073–1081. doi: 10.1523/JNEUROSCI.4806-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koob GF. Theoretical Frameworks and Mechanistic Aspects of Alcohol Addiction: Alcohol Addiction as a Reward Deficit Disorder. Current topics in behavioral neurosciences. 2011 doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hopf FW, et al. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron. 2010;65:682–694. doi: 10.1016/j.neuron.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Besheer J, et al. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
References for Methods section
- 1.Banko JL, et al. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puighermanal E, et al. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- 3.Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barak S, Ahmadiantehrani S, Kharazia V, Ron D. Positive autoregulation of GDNF levels in the ventral tegmental area mediates long-lasting inhibition of excessive alcohol consumption. Transl Psychiatry. 2011;1:e60. doi: 10.1038/tp.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barak S, Carnicella S, Yowell QV, Ron D. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J Neurosci. 2011;31:9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, et al. Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2010;30:12632–12641. doi: 10.1523/JNEUROSCI.1264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.