Abstract
Background
Several lines of evidence implicate abnormal serotonergic function in suicidal behavior and completed suicide, including low serotonin transporter binding in postmortem studies of completed suicide. We have also reported low in vivo serotonin transporter binding in major depressive disorder (MDD) during a major depressive episode using positron emission tomography with [11C]McN5652. We quantified regional brain serotonin transporter binding in vivo in depressed suicide attempters, depressed non-attempters, and healthy controls using positron emission tomography and a superior radiotracer, [11C]DASB.
Methods
51 subjects with DSM-IV current MDD, 15 of whom were past suicide attempters, and 32 healthy controls underwent PET scanning with [11C]DASB to quantify in vivo regional brain serotonin transporter binding. Metabolite-corrected arterial input functions and plasma free-fraction were acquired to improve quantification.
Results
Depressed suicide attempters had lower serotonin transporter binding in midbrain compared with depressed non-attempters (p=0.031) and controls (p=0.0093). There was no difference in serotonin transporter binding comparing all depressed subjects to healthy controls considering six a priori regions of interest simultaneously (p=0.41).
Conclusions
Low midbrain serotonin transporter binding appears to be related to the pathophysiology of suicidal behavior rather than of major depressive disorder. This is consistent with postmortem work showing low midbrain serotonin transporter binding capacity in depressed suicides, and may partially explain discrepant in vivo findings quantifying serotonin transporter in depression. Future studies should investigate midbrain serotonin transporter binding as a predictor of suicidal behavior in MDD, and determine the cause of low binding.
Keywords: serotonin transporter, depression, suicide, PET, midbrain, [11C]DASB, pathophysiology
Introduction
Abnormal serotonergic function has been associated with suicidal behavior and completed suicide. Possible explanations for this association include serotonergic effects on aggression and on decision-making (1, 2). Post-mortem studies quantifying serotonin transporter (5-HTT) protein in the brain of completed suicides have reported low Bmax (number of 5-HTT binding sites in vitro) in regions including prefrontal cortex (PFC), anterior cingulate, hippocampus, putamen, and hypothalamus, although others have reported no group differences, and one reported higher 5-HTT in frontal cortex (3, 4). One study reported low 5-HTT binding capacity (a product of receptor binding x region volume that is more analogous to PET outcome measures) in the dorsal raphe nuclei in depressed suicides (DRN) (5). Assessing the relationship between 5-HTT binding in vivo and suicidal behavior may clarify the pathophysiology of suicidal behavior, and could potentially identify a biomarker for predicting suicide risk in patients.
The serotonin (5-HT) neurotransmitter system has also been implicated in the pathophysiology of major depressive disorder (MDD). Acute tryptophan depletion provokes depressive symptoms in remitted depressed subjects and their relatives compared to healthy controls (6). Acute serotonergic challenges reveal blunted neuroendocrine responses in acutely depressed and remitted depressed subjects (7). The antidepressant efficacy of serotonergic medications in MDD is consistent with a role of 5-HT in the pathophysiology of depression (8, 9).
Many studies have examined the role of the 5-HTT specifically in the pathophysiology of MDD (10). Several, but not all, postmortem studies have found lower 5-HTT Bmax (binding density of 5-HTT in vitro) in prefrontal cortical (PFC) regions in MDD compared to controls (11). In vivo comparisons of 5-HTT binding between MDD and healthy control groups using PET and SPECT are inconsistent (10). We previously described lower 5-HTT binding in 25 antidepressant-free MDD subjects during a current major depressive episode (MDE) compared with 43 healthy controls across six regions of interest (ROIs) implicated in the pathophysiology of MDD using the radiotracer [11C]McN5652 (12). Post-hoc testing showed lower binding in midbrain and amygdala. The [11C]McN5652 radiotracer has known limitations, including poor specific-to-nonspecific binding ratio and poor quantification of cortical binding (13, 14).
[11C]DASB is a radiotracer that provides superior 5-HTT quantification compared to [11C]McN5652 (13). Other groups have used [11C]DASB to examine 5-HTT in MDD, with divergent findings. Three reports from one research group with partially overlapping subject samples found no differences in [11C]DASB binding between MDD subjects and healthy controls (15–17). One study found higher [11C]DASB binding in MDD subjects than healthy control subjects across a broad anatomic distribution (18), while two others reported lower [11C]DASB binding, one in thalamus specifically (19) and another across a broad range of cortical and subcortical regions (20). These divergent findings may be partially explained by demographic and clinical differences in in study populations, including rates of suicidal behavior, and by different PET outcome measures employed.
In addition to examining effects of diagnosis on binding, we previously examined the effect of a functional promoter polymorphism in the 5-HTT gene (SLC6A4, polymorphism: 5-HTTLPR) that regulates in vitro expression of 5-HTT (21, 22). A gene-environment interaction between the 5-HTTLPR polymorphism and the severity of stressful life events may predict the presence and severity of subsequent depression as well as the later occurrence of suicidal behavior (23–25). We found no effect of 5-HTTLPR on 5-HTT binding using [11C]McN5652 (26). In vivo findings from other studies are discordant (reviewed in (27)). We also reported an effect of early life stress on 5-HTT binding using [11C]McN5652, with low 5-HTT binding in MDD subjects reporting childhood abuse (28), but the sample size was too small to examine gene-environment interactions.
In the current study, we used [11C]DASB in the largest MDD cohort examined to date to examine the relationship between depression and suicide attempt history on SERT binding in vivo. Our primary hypotheses were that 1) MDD subjects with a history of prior suicide attempt would have low [11C]DASB binding compared to controls and MDD non-attempters in the regions identified from post-mortem studies of suicide: ventral prefrontal cortex (vPFC), anterior cingulate (ACN), and midbrain (containing DRN, which cannot be reliably delineated on MRI) and that 2) [11C]DASB binding would be low in unmedicated current MDD subjects as compared to healthy controls across the six brain regions identified in our study using [11C]McN5652. We anticipated that 5-HTTLPR genotype would not be associated with [11C]DASB binding. In exploratory analyses, we examined the effects of reported childhood abuse, and of a gene-environment interaction between 5-HTTLPR and reported childhood abuse, on [11C]DASB binding.
Methods
Sample
Currently depressed participants (n=51) with MDD and healthy controls (n=32) were recruited prospectively through print and online advertisements. Eligibility was assessed by psychiatric and medical history, chart review, physical examination, routine blood tests, pregnancy test, and urine toxicology. Axis I diagnoses were based on the Structured Clinical Interview for DSM-IV (SCID) (29), conducted by doctoral- or masters’-level psychologists and reviewed in a consensus conference of research psychologists and psychiatrists. Inclusion criteria for MDD subjects included: 1) current MDE; 2) 17-item Hamilton Depression Rating Scale (HDRS) ≥16 at screening; 3) age 18–65; 4) off all psychotropic and other types of drugs likely to interact with 5-HTT for a minimum of 14 days (off antipsychotics for ≥3 weeks). While this was the minimum duration according to inclusion criteria, 11 MDD subjects were antidepressant-naïve, and among the 40 MDD subjects with prior psychotropic medication exposure, the mean duration off psychotropic medication at time of scan was 122 weeks (median = 11.5 weeks, range = 14 days to 35.9 years). Short-acting benzodiazepines were allowed for distressing anxiety or insomnia up to 72 hours prior to PET scanning, but were only used by six subjects. Exclusion criteria included 1) current or past psychotic illness or bipolar disorder; anorexia nervosa or bulimia nervosa in the past year; drug or alcohol abuse within the past two months or dependence within six months; 2) first-degree family history of schizophrenia in subjects <33 years old to exclude individuals possibly presenting with the prodrome of schizophrenia (mean onset of schizophrenia = 21.4 in males and 26.8 in females (30)); 3) significant active physical illness; 4) lack of capacity to consent to study participation; 5) pregnancy or lactation among women; 6) previous head injury with loss of consciousness; 7) exposure to 3,4-methylenedioxymethamphetamine (MDMA) on more than two occasions.
For healthy controls, inclusion criteria included: 1) absence of current or past DSM-IV Axis I diagnosis, with the exception of specific phobia; 2) absence of cluster B personality diagnosis as assessed using the SCID-II (31); 3) age 18–65. Exclusion criteria included MDD exclusion criteria 3–7 above as well as: 1) past or present alcohol/substance abuse or dependence; 2) first-degree relative with history of major depression, schizophrenia, schizoaffective disorder, or suicide attempt; two or more first-degree relatives with a history of substance dependence.
The Beck Depression Inventory (32) and HDRS (33) were used to assess depression severity and functional impairment. Lifetime history of aggression was measured by the Brown Goodwin Aggression History Scale (34). The Columbia Suicide History Form was used to assess suicide attempt history (35), and the Beck Medical Lethality Scale was used to rate the degree of medical damage caused by their most lethal attempt (36). The scale scores medical damage from 0 (no injury) to 8 (fatal), with anchor points dependent on the method of attempt. In a semi-structured interview, participants were asked whether they experienced physical and/or sexual abuse over the course of their lifetime. If subjects endorsed a history of abuse, they were asked whether the abuse took place before age 15.
Genotyping
Genotyping of the triallelic 5-HTTLPR polymorphism (LA, LG, and S) was performed as previously described.(21) The triallelic genotypes were classified by their reported level of in vitro expression as follows: LA was reclassified as higher expressing L′; LG and S were classified as lower expressing S′.
Radiochemistry and input function measurement
Preparation of [11C]DASB, measurement of arterial input function, metabolites, and plasma free fraction (fP) were performed as previously described (37, 38). The chemical purity of [11C]DASB was ≥95%. Injected mass, injected dose, and fP did not differ between MDD and controls, or between MDD suicide attempters and non- attempters (Table 1).
Table 1.
[11C]DASB PET scan parameters of the sample.
| Controls (n=31) | MDD (n=51) | t, p-value | MDD Suicide Attempters (n=15) | MDD Non- Attempter (n=36) | t, p-value | |
|---|---|---|---|---|---|---|
| Injected Dose (mCi) | 16.14 ± 2.36 | 16.28 ± 2.14 | −0.27, 0.79 | 16.34 ± 1.80 | 16.25 ± 2.29 | 0.13, 0.90 |
| Injected Mass (micrograms) | 4.36 ± 2.31 | 4.49 ± 2.18 | −0.27, 0.91 | 5.07 ± 2.30 | 4.25 ± 2.11 | 1.24, 0.22 |
| Free Fraction (fP) | 0.12 ± 0.03 | 0.11 ± 0.02 | 1.59, 0.12 | 0.11 ± 0.03 | 0.11 ± 0.02 | −0.13, 0.90 |
PET Protocol
Details of the PET protocol are described elsewhere (38). Briefly, a venous catheter was used for radiotracer injection and an arterial catheter was used to obtain arterial samples for the input function. A polyurethane head holder system (Soule Medical, Tampa, FL, USA) was molded around the subject’s head for immobilization purposes. PET imaging was performed with the ECAT HR+ (Siemens/CTI, Knoxville, TN, USA). A 10-minute transmission scan was obtained prior to radiotracer injection. At the end of the transmission scan, [11C]DASB was administered intravenously as a bolus over 30 seconds (Table 1). Emission data were collected in 3D mode for 100 minutes with 19 frames of increasing duration (38).
Magnetic Resonance Imaging
Acquisition of T1-weighted MRI images for co-registration of PET images and identification of ROIs was performed as previously described using a 1.5 T Signa Advantage or a 3 T Signa HDx system (General Electric Medical Systems, Milwaukee, WI) (39).
Image Analysis
To correct for subject motion, PET frames were registered to the eighth frame using the FMRIB linear image registration tool (FLIRT), version 5.0 (FMRIB Image Analysis Group, Oxford, UK). An automated algorithm identified ROIs (midbrain, vPFC, putamen, amygdala, thalamus, hippocampus, and ACN) as well as cerebellar gray matter (CGM) on individuals’ T1-weighted MRIs (40). Each subject’s mean PET image was coregistered to their MRI using FLIRT, optimized as previously described (41). Time activity curves were generated by plotting measured activity within ROIs over the time course of the PET acquisition.
Outcome Measure Estimation
As we previously demonstrated that no brain region is devoid of specific binding with [11C]DASB (42), we used an outcome measure that does not rely on a reference region: VT/fP (where VT = volume of distribution in the region of interest). This outcome measure has been used in several studies by different groups in cases where a reference region is not available (43–47). [11C]DASB regional VT values were derived using likelihood estimation in the graphical approach (LEGA), which reduces the noise- dependent bias inherent in the graphical approach (48, 49). Brain activity was corrected for the contribution of plasma activity assuming a 5% blood volume in the regions of interest (50). For purposes of comparison to other [11C]DASB studies using different outcome measures, the following outcome measures were also estimated: BPF ((VT(ROI) − VT(REF))/fP); BPP (VT(ROI) − VT(REF)); and BPND ((VT(ROI) − VT(REF))/VT(REF)), using CGM as reference region. As there is approximately 30% specific/displaceable [11C]DASB binding in the reference region (42), VT(REF) overestimates the distribution volume of the nondisplaceable compartment (VND) (51), leading to biases in estimates of these binding potential measures (52). Results with these alternate outcome measures are described concisely in results section and are presented in greater detail in supplement 1.
Statistics
To borrow strength across all ROIs and properly account for correlation among ROIs measured on the same subject, we fit linear mixed-effects models to the ROI-level VT/fP estimates with region and diagnostic group as fixed effects and subject as the random effect, and this approach was taken for all analyses involving more than one ROI. Other fixed effects considered in linear mixed-effects modeling include sex, age, antidepressant exposure, depression severity, and genetic and environmental factors. Data entered in linear mixed-effects models were first log transformed, to remedy slight skewness of VT/fP estimates (53–55), to stabilize the variance, and because our principal hypothesis of a difference between groups specifies that differences in each ROI are proportional to each ROI’s binding level. Log transformation has been used in numerous PET studies by our group and others to address these issues (12, 26, 28, 55–67). As the natural log is a monotone transformation, demonstrating a difference in log(VT/fP) is equivalent to demonstrating a difference (in the same direction) in VT/fP. Estimated VT/fP values were weighted in the model according to standard errors computed using a bootstrap algorithm taking into account errors in metabolite, plasma, and brain data (68). Analyses on single regions were performed using linear models. T-tests were performed in SPSS Statistics 19 (http://www.spss.com/software/statistics/). All other analyses were performed in R 2.10.0 (http://cran.r-project.org).
Results
Demographics
Demographic and clinical variables are presented in Table 2. Among MDD participants, 36 (70.6%) had at least one comorbid axis I diagnosis, including 8 (15.7%) with remitted alcohol or substance use disorder; 32 (62.8%) with current or past anxiety disorder; four (7.8%) with lifetime dysthymia; three (5.9%) with current ADHD; and one (2%) with remitted bulimia. Rates of these comorbidities did not differ between MDD attempters and non-attempters (remitted alcohol or substance use disorder: Fisher’s exact p = 0.41; comorbid anxiety: p = 0.13; Table 2). MDD attempters had an earlier age of onset than MDD non-attempters. While age differed between MDD subjects and controls, it did not differ between MDD attempters and MDD non-attempters (Table 2). 5-HTTLPR genotype did not differ between MDD subjects and controls, nor between MDD attempters and MDD non-attempters (Table 3).
Table 2.
Clinical and demographic characteristics of the sample.
| Controls N=31 | MDD N=51 | Control v. MDD (χ2, p-value) | MDD Suicide Attempters N=15 | MDD Non- Attempters N=36 | Attempter vs. Non-Attempter (χ2, p-value) | |
|---|---|---|---|---|---|---|
| Females | 16 (51.6%) | 28 (54.9%) | 0.08, p=0.77 | 8 (53.3%) | 20 (55.6%) | 0.021, 0.88 |
| Inpatient | - | 14 (27.5%) | - | 6 (40.0%) | 8 (22.2%) | 2.13, 0.15 |
| # with family history of MDD in 1st degree relatives | - | 25 (49.0%) | - | 5 (33.3%) | 20 (55.6%) | 2.09, 0.15 |
| Current or Past Comorbid Anxiety Disorder | - | 32 | - | 7 (46.7%) | 25 (69.4%) | 2.35, 0.13 |
| Axis 2 Comorbidity | - | 21 (37.2) | - | 6 (40.0%) | 13 (36.1%) | .069, .69 |
| Race/Ethnicity | 0.105* | 0.09* | ||||
| Asian | 6 (19.4%) | 3 (5.9%) | 0 (0%) | 3 (8.3%) | ||
| African American | 6 (19.4%) | 5 (9.8%) | 3 (20%) | 2 (5.6%) | ||
| Caucasian | 15 (48.4%) | 33 (64.7%) | 8 (53.3) | 26 (72%) | ||
| Hispanic | 2 (3.2%) | 8 (15.7%) | 2 (13.3%) | 5 (13.9%) | ||
| >1 Race | 1 (3.2%) | 2 (3.9%) | 2 (13.3%) | 0 (0%) | ||
| Unknown | 1 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Any Current Tobacco Use | 2 (6.5%) | 7 (13.7%) | 0.47* | 1 (6.7%) | 6 (16.7%) | 0.66* |
| (t, p-value) | (t, p-value) | |||||
| Age | 32.6 ± 11.3 | 40.3 ± 10.7 | −3.02, p=.004 | 38.5 ± 11.5 | 41.0 ± 10.5 | −0.76, 0.45 |
| Hamilton Depression Rating Scale (24 item) | 1.7 ± 2.4 | 24.6 ± 6.4 | −19.01, p<.001 | 26.5 ± 6.2 | 23.8 ± 6.4 | 1.40, 0.17 |
| Beck Depression Inventory | 1.3 ± 1.7 | 25.7 ± 8.8 | −15.19, p<.001 | 27.5 ± 9.2 | 24.9 ± 8.7 | 0.94, 0.35 |
| Median # of Depressive Episodes | - | 4 | - | 5 | 2.5 | −1.70, 0.088** |
| Median Length of Current Depressive Episode (weeks) *** | - | 52 | 52 | 52 | −1.15, 0.25** | |
| Age at 1st Depressive Episode | - | 19.3 ± 10.8 | - | 14.7 ± 5.8 | 21.2 ± 11.9 | −2.03, 0.05 |
| # of Suicide Attempters | - | 15 (29.4%) | - | 15 (100%) | - | - |
| Mean # of Attempts | 2.1 ± 1.6 | - | - | |||
| Maximum Lethality of Attempts**** | 2.3 ± 2.0 | - | - | |||
| Lethality of Most Recent Attempt | 1.5 ± 1.6 | - | - | |||
Fisher’s exact p-value as cells contain values too small to fulfill assumptions of χ2 test
Mann Whitney Test presented as (Z, p-value), as data are not normally distributed.
Best estimate from patient self report
From Columbia University Suicide History Form (details in methods)
Table 3.
5-HTTLPR triallelic genotype distribution.
| Goldman Functional Genotype | S′S′ | L′S′ | L′L′ | Fisher’s exact p- value | S′ allelic frequency | L′ allelic frequency | Chi2 p- value |
|---|---|---|---|---|---|---|---|
| Controls (n=31) | 11 | 13 | 3 | 0.32 | 35 | 19 | 0.21 |
| MDD (n=51) | 16 | 22 | 13 | 54 | 48 | ||
| MDD Attempters (n=15) | 6 | 6 | 3 | 0.74 | 18 | 12 | 0.48 |
| MDD Non- Attempters (n=36) | 10 | 16 | 10 | 36 | 36 |
Possible Covariates
Across the six ROIs, there was no effect of sex (F=1.55, DF=1,79, p=0.22) or prior antidepressant exposure (F=0.68, DF=1,79, p=0.41) on 5-HTT binding. Because the MDD and control groups differed in age, we explored the relationship between age and binding. There was no effect of age on binding in the combined sample (F=0.02, DF=1,79, p=0.89) and no interactions were detected between age and diagnosis (F=2.13, DF=1,78, p=0.15) or age and region (F=1.60, DF=5,400, p=0.16) on binding. Nonetheless, as some studies have previously described a regional age-related decline in 5-HTT binding (15, 19, 69–77), we included age and age-by-region interaction as covariates in statistical models.
Suicide Attempt History
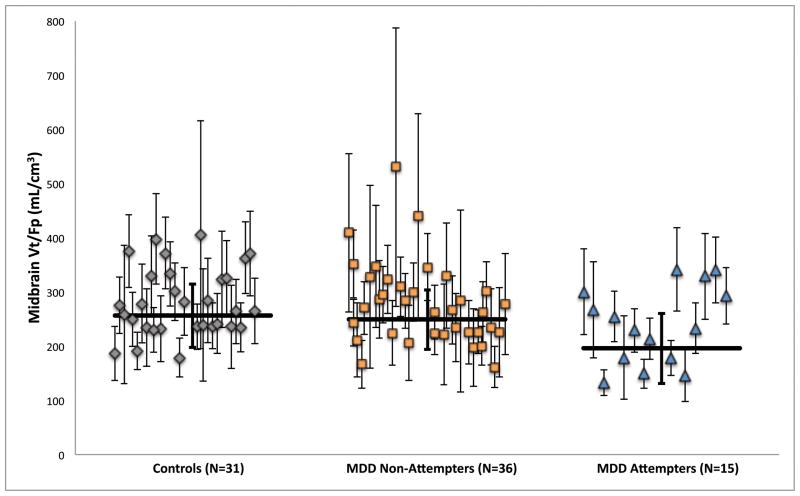
5-HTT binding differed between controls, MDD attempters, and MDD non-attempters in midbrain (Figure 1, F=3.77, DF=2,78, p=0.027), with MDD attempters having lower midbrain binding than both MDD non-attempters (F=5.88, DF=1,78, p=0.031) and controls (F=7.12, DF=1,78, p=0.0093); midbrain binding did not differ significantly between MDD non-attempters and controls (F=0.40, DF=1,78, p=0.53). Low midbrain 5-HTT binding in MDD suicide attempters compared to MDD non-attempters was significant in all analyses with alternative PET outcome measures examined (table S1 in Supplement 1; BPF: F=7.27, DF=1,78, p=0.0086; BPP: F=6.15, DF=1,78, p=0.015; BPND: F=7.51, DF=1,78, p=0.0076). 5-HTT binding did not differ as a function of suicide attempt history in the two other regions examined, vPFC and ACN (vPFC: F=0.87, DF=1,78, p=0.35; ACN: F=0.13, DF=1,78, p=0.72).
Figure 1.
Comparison of 5-HTT binding (raw VT/fP,) as a function of suicide attempt history in midbrain. Scatter plot displays each subject’s midbrain VT/fP value with its associated standard error, computed using a bootstrap algorithm that takes into account errors in metabolite, plasma, and brain data. Horizontal lines indicate weighted means for each group; thick error bars indicate the corresponding equivalent of the standard deviation of the weighted means. Depressed suicide attempters have lower 5-HTT binding than depressed non-attempters (p=0.031) and than controls (p=0.0093).
Diagnosis Effect
Considering six a priori ROIs simultaneously (dorsal putamen, amygdala, thalamus, hippocampus, midbrain, and anterior cingulate), 5-HTT binding did not differ between MDD and controls (table S2 in Supplement 1; F=0.69, DF=1,79, p=0.41). This finding was consistent for all alternative outcome measures examined (BPF: F=1.53, DF=1,79, p=0.22; BPP: F=3.49, DF=1,79, p=0.066; BPND: F=1.79, DF=1,79, p=0.19; table S2 in Supplement 1). Within the MDD group, we did not observe a relationship between depression severity assessed by the HDRS and binding across the 6 ROIs (F=0.009, DF=1,48, p=0.93).
Genetic and Environmental Effects
Considering 5-HTTLPR, we did not observe a stepwise effect of the number of L′ alleles on 5-HTT binding across the six ROIs (F=0.04, DF=1,74, p=0.84). MDD subjects reporting childhood abuse had higher binding than non-abused MDD subjects across the 6 ROIs (F=4.34, DF=1,48, p=0.043). There was no gene-environment interaction detected between number of 5-HTTLPR LA alleles and childhood abuse status on binding in MDD (F=0.65, DF=1,47, p=0.42). Including childhood abuse as a covariate did not alter the significance of the contrast of midbrain 5-HTT binding between MDD suicide attempters and non-attempters (F=6.31, DF=1,78, p=0.014).
Comment
Primary findings and comparison to existing literature
This study examined the effects of prior suicide attempt and diagnosis in the largest cohort to date of MDD subjects undergoing 5-HTT quantification using PET or SPECT. We observed lower 5-HTT binding in MDD attempters compared with both MDD non-attempters and controls in midbrain, and no differences as a function of suicide attempt history in vPFC or ACN. In addition, we found no difference in 5-HTT binding between MDD and control groups in six a priori regions. Taken together, these findings suggest that regionally specific, lower 5-HTT binding in midbrain in MDD attempters may be related to the pathophysiology of suicidal behavior, rather than of MDD. The lack of a depression effect on 5-HTT binding is consistent with a series of previous [11C]DASB studies using the outcome measure BPND (15–17), although others have reported lower (19, 20) and higher (18) [11C]DASB binding in MDD using BPP and BPND. It is notable that the current finding was replicated with all alternative PET outcome measures examined (BPF, BPP, and BPND).
Our data suggest that discrepant [11C]DASB PET findings in MDD may be at least partly due to differences in the proportion of suicide attempters in previous samples. Four of six previous [11C]DASB MDD studies did not report rates of suicide attempt history in their samples.(15–17, 19) Of the two [11C]DASB studies reporting suicide attempt status, one found lower 5-HTT binding in anteroventral striatum in MDD attempters compared with MDD non-attempters, in the same direction as our finding (18). The other study had only two attempters out of 12 MDD subjects, which did not allow direct examination of an effect of suicide attempt status on binding (20).
There are some clinical and demographic differences between the MDD attempters and non-attempters in our sample: while depression severity did not differ between attempters and non-attempters, attempters had an earlier onset of major depressive illness, consistent with previous studies (78). This raises the possibility that low midbrain 5-HTT binding among attempters is driven by specific genetic loading associated with early-onset depression (79). Moreover, attempters had a trend toward greater depression chronicity as measured by number of prior major depressive episodes. While this may be a potential confound in the interpretation of our results, we did not find a relationship between age of onset of major depressive illness and midbrain 5-HTT VT/fP (r=0.03, p=0.84).
We found no effect of suicide attempt status in vPFC or ACN. The low signal-to-noise ratio in vPFC (binding is only 14% higher in vPFC than in cerebellar gray matter) may have limited our ability to detect group differences. We did not examine the relationship between regional 5-HTT binding and suicide attempt lethality or objective medical damage, given the limited range of lethality in the current sample.
Interpretation of Findings
Low regional 5-HTT binding among MDD attempters may be due to less gene expression. Consistent with our previous findings, 5-HTT binding was not associated with 5-HTTLPR genotype in this study, but other functional promoter 5-HTT loci need to be examined. Additionally, epigenetic differences may drive differential 5-HTT binding: studies in nonhuman primates find that DNA methylation, but not 5-HTT genotype, is significantly associated with peripheral blood mononuclear cell 5-HTT mRNA expression (80). Discrepant findings reported regarding the potential association between 5-HTT binding and 5-HTTLPR genotype may also be due to biallelic vs. triallelic genotyping, different brain imaging outcome measures, as well as racial stratification differences in study populations.
An alternative explanation for low regional 5-HTT binding among suicide attempters is that it is a result of accelerated 5-HTT internalization in response to low 5-HT release. Evidence supporting a 5-HT deficiency related to suicidal behavior includes low CSF 5-HIAA associated with suicidal behavior and risk of suicide (81), postmortem studies reporting lower brainstem 5-HT or 5-HIAA in suicides (82), and lower CSF 5-HIAA in more lethal suicide attempters with MDD (83).
Serotonergic abnormalities may contribute to suicidal behavior through effects on aggressive traits, decision-making or problem solving. Measures of aggression have been correlated with several serotonergic measures, including low in vivo 5-HT1A receptor binding (84), blunted prolactin responses to serotonergic challenge with fenfluramine (85, 86), and low CSF 5-HIAA (87). However, we do not find an effect of lifetime aggression assessed via the Brown Goodwin Lifetime History of Aggression scale on 5-HTT VT/fP in vPFC (F=0.084, DF=1,77, p=0.77).
Reported Childhood Abuse
In exploratory analyses, we did not replicate our previous finding of low 5-HTT in MDD with reported childhood abuse history compared to MDD without childhood abuse history (28). Given these discrepant findings, and the report of lower HTT binding in adult monkeys with a history of maternal deprivation (88), replication is required with a larger sample, using a validated measure of childhood abuse such as the Childhood Trauma Questionnaire (89). We did not observe a gene-environment interaction between reported childhood abuse and 5-HTTLPR genotype on 5-HTT binding within the MDD sample. A definitive examination of a gene-environment interaction affecting 5-HTT binding as a mediator of depression risk would necessitate a large sample stratified across a continuous range of depression severity.
Strengths And Limitations
Strengths of this imaging study include the large sample size, favorable properties of [11C]DASB, quantitative estimation of VT/fP using a metabolite-corrected arterial input function, and careful diagnostic assessment. A limitation of this study is the lack of age matching between MDD subjects and controls. This is unlikely to have impacted the reported findings for the following reasons: 1) we did not observe an effect of age on 5-HTT binding in our sample; 2) we co-varied for age in all analyses; 3) age did not differ between MDD attempters and MDD non-attempters, who nonetheless differed in midbrain 5-HTT binding; and 4) if age-related decline in 5-HTT binding were present, it would bias our results toward lower binding in MDD subjects than controls, which we did not observe.
A longer minimum antidepressant-free interval than the two-week minimum used in the present study may be preferable, but ethical requirements prevent this approach. An alternative strategy would be to recruit drug naïve participants. Nonetheless, in this sample, the median antidepressant-free interval in those MDD subjects with prior antidepressant-exposure was 11.5 weeks. Moreover, we did not observe a difference in 5-HTT VT/fP as a function prior antidepressant exposure status, nor did mean antidepressant-free interval differ between MDD attempters and MDD non-attempters, which makes the minimum antidepressant-free interval employed in this study an unlikely explanation for reported findings.
Other clinical and demographic factors have previously been associated with 5-HTT binding, including cigarette smoking (90, 91) and anxiety (19). We did not include these as covariates in the current analysis given the large number of covariates examined, and as smoking history did not differ between groups and anxiety comorbidity did not differ between MDD attempters and non-attempters. It should be noted that while differences in midbrain VT/fP between MDD suicide attempters and non-attempters are statistically significant, there is overlap between groups in binding, and as such this measure cannot be used alone to differentiate these groups. Future studies with improved 5-HTT quantification and the combination of imaging and clinical measures may improve group differentiation.
A limitation common to most studies quantifying 5-HTT in vivo is the lack of a reference region in the brain that is devoid of 5-HTT. We chose one approach to address this issue, using the outcome measure VT/fP, thereby avoiding the error introduced when using other available outcome measures that subtract out, or subtract and then divide by, the volume of distribution measured in a reference region that actually contains specific binding. Use of VT/fP does not account for non-specific binding in the brain, and it is thus possible that low midbrain binding in MDD attempters is due to differences in non-specific binding. Other approaches to address this methodological challenge have been proposed (52). However, our results were similar when using all other outcome measures that do attempt to correct for non-specific binding in the brain using a reference region (BPF, BPP, and BPND), so this methodological issue is unlikely to be driving our reported findings.
Conclusions
5-HTT binding is low in vivo in the midbrain of depressed suicide attempters. This abnormality is consistent with postmortem findings in suicides and with a serotonergic deficit model of suicidal behavior. We are currently studying the prognostic significance of low 5-HTT binding as a predictor of7 repeated suicide attempt.
Supplementary Material
Acknowledgments
Research presented in this manuscript was supported by NIMH grants 5P50 MH62185 (Dr. Mann, principal investigator) and 2 R01 MH040695 (Dr. Mann, principal investigator).
Footnotes
Financial Disclosures:
Dr. Ogden and Ms. Hesselgrave report no biomedical financial interests or potential conflicts of interest. Dr. Miller has received financial compensation for psychiatric evaluations of subjects enrolled in medication studies sponsored by Pfizer and Orexigen Therapeutics, unrelated to the current manuscript. His family owns stock in Johnson & Johnson. Dr. Sullivan serves as a member of the Scientific Advisory Board of TONIX Pharmaceuticals, Inc. and has received compensation in the form of stock shares; he has served as a consultant for Ono Pharma USA, Inc.; and he has a US patent application for a use of tianeptine. None is related to the current manuscript. Dr. Mann received past unrelated grants from GSK and Novartis, and royalties for a rating scale, C-SSRS. Dr. Oquendo receives royalties for the use of the Columbia Suicide Severity Rating Scale and received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to the current manuscript. She was the recipient of a grant from Eli Lilly to support a year of the salary for the Lilly Suicide Scholar, Enrique Baca-Garcia, MD, PhD. She has received unrestricted educational grants and/or lecture fees from Astra-Zeneca, Bristol Myers Squibb, Eli Lilly, Janssen, Otsuko, Pfizer, Sanofi-Aventis, and Shire. Her family owns stock in Bristol Myers Squibb. Dr. Parsey was the recipient of grants from Pfizer, Lundbeck, Sepracor, Novartis, and General Electric, all unrelated to this manuscript. He has a US patent on voxel-based methods for assessing subjects using PET.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Courtet P, Gottesman II, Jollant F, Gould TD. The neuroscience of suicidal behaviors: what can we expect from endophenotype strategies? Transl Psychiatr. 2011:1. doi: 10.1038/tp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonda X, Fountoulakis KN, Harro J, Pompili M, Akiskal HS, Bagdy G, et al. The possible contributory role of the S allele of 5-HTTLPR in the emergence of suicidality. J Psychopharmacol. 2011;25:857–866. doi: 10.1177/0269881110376693. [DOI] [PubMed] [Google Scholar]
- 3.Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- 4.Purselle DC, Nemeroff CB. Serotonin transporter: a potential substrate in the biology of suicide. Neuropsychopharmacology. 2003;28:613–619. doi: 10.1038/sj.npp.1300092. [DOI] [PubMed] [Google Scholar]
- 5.Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 6.Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 7.Bhagwagar Z, Whale R, Cowen PJ. State and trait abnormalities in serotonin function in major depression. Br J Psychiatry. 2002;180:24–28. doi: 10.1192/bjp.180.1.24. [DOI] [PubMed] [Google Scholar]
- 8.Serrano-Blanco A, Gabarron E, Garcia-Bayo I, Soler-Vila M, Carames E, Penarrubia-Maria MT, et al. Effectiveness and cost-effectiveness of antidepressant treatment in primary health care: a six-month randomised study comparing fluoxetine to imipramine. J Affect Disord. 2006;91:153–163. doi: 10.1016/j.jad.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 10.Meyer JH. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci. 2007;32:86–102. [PMC free article] [PubMed] [Google Scholar]
- 11.Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 12.Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, et al. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006;163:52–58. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- 13.Frankle WG, Huang Y, Hwang D-R, Talbot PS, Slifstein M, Van Heertum R, et al. Comparative Evaluation of Serotonin Transporter Radioligands 11C-DASB and 11C-McN 5652 in Healthy Humans. J Nucl Med. 2004;45:682–694. [PubMed] [Google Scholar]
- 14.Szabo Z, McCann UD, Wilson AA, Scheffel U, Owonikoko T, Mathews WB, et al. Comparison of (+)-(11)C-McN5652 and (11)C-DASB as serotonin transporter radioligands under various experimental conditions. J Nucl Med. 2002;43:678–692. [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- 16.Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin Transporter Occupancy of Five Selective Serotonin Reuptake Inhibitors at Different Doses: An [(11)C]DASB Positron Emission Tomography Study. Am J Psychiatry. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- 17.Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, et al. Brain Serotonin Transporter Binding Potential Measured With Carbon 11-Labeled DASB Positron Emission Tomography: Effects of Major Depressive Episodes and Severity of Dysfunctional Attitudes. Arch Gen Psychiatry. 2004;61:1271–1279. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- 18.Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, et al. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Reimold M, Batra A, Knobel A, Smolka MN, Zimmer A, Mann K, et al. Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: a [11C]DASB PET study. Mol Psychiatry. 2008;13:606–613. 557. doi: 10.1038/sj.mp.4002149. [DOI] [PubMed] [Google Scholar]
- 20.Selvaraj S, Venkatesha Murthy N, Bhagwagar Z, Bose SK, Hinz R, Grasby PM, et al. Diminished brain 5-HT transporter binding in major depression: a positron emission tomography study with [(11)C]DASB. Psychopharmacology. 2009 doi: 10.1007/s00213-009-1660-y. [DOI] [PubMed] [Google Scholar]
- 21.Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 22.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (New York, NY. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 23.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science (New York, NY. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 24.Hung CF, Lung FW, Chen CH, O’Nions E, Hung TH, Chong MY, et al. Association between suicide attempt and a tri-allelic functional polymorphism in serotonin transporter gene promoter in Chinese patients with schizophrenia. Neuroscience letters. 2011;504:242–246. doi: 10.1016/j.neulet.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Karg K, Burmeister M, Shedden K, Sen S. The Serotonin Transporter Promoter Variant (5-HTTLPR), Stress, and Depression Meta-analysis Revisited: Evidence of Genetic Moderation. Archives of general psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang YY, et al. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- 27.Willeit M, Praschak-Rieder N. Imaging the effects of genetic polymorphisms on radioligand binding in the living human brain: A review on genetic neuroreceptor imaging of monoaminergic systems in psychiatry. NeuroImage. doi: 10.1016/j.neuroimage.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 28.Miller JM, Kinnally EL, Ogden RT, Oquendo MA, Mann JJ, Parsey RV. Reported childhood abuse is associated with low serotonin transporter binding in vivo in major depressive disorder. Synapse. 2009;63:565–573. doi: 10.1002/syn.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0) New York: Biometrics Research Dept., New York State Psychiatric Institute; 1995. [Google Scholar]
- 30.Loranger AW. Sex difference in age at onset of schizophrenia. Arch Gen Psychiatry. 1984;41:157–161. doi: 10.1001/archpsyc.1984.01790130053007. [DOI] [PubMed] [Google Scholar]
- 31.First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin LS. SCID-II Personality Questionnaire. Washington, D.C: American Psychiatric Press; 1997. [Google Scholar]
- 32.Beck AT, Ward CH, Mendelson M, Mock J, Erbauh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psych. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in human correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- 35.Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: the utility and limitations of research instruments. In: First MB, editor. Standardized Evaluation in Clinical Practice. Arlington, VA: American Psychiatric Publishing; 2003. pp. 103–130. [Google Scholar]
- 36.Beck AT, Beck R, Kovacs M. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. Am J Psychiatry. 1975;132:285–287. doi: 10.1176/ajp.132.3.285. [DOI] [PubMed] [Google Scholar]
- 37.Belanger MJ, Simpson NR, Wang T, Van Heertum R, Mann JJ, Parsey RV. Biodistribution and Radiation Dosimetry of [11C]DASB in Baboons. Nuclear medicine and biology. 2004;31:1097–1102. doi: 10.1016/j.nucmedbio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Ogden RT, Ojha A, Erlandsson K, Oquendo MA, Mann JJ, Parsey RV. In vivo quantification of serotonin transporters using [(11)C]DASB and positron emission tomography in humans: modeling considerations. J Cereb Blood Flow Metab. 2007;27:205–217. doi: 10.1038/sj.jcbfm.9600329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, et al. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tisssue input functions. J Cereb Blood Flow Metab. 2000;20:1111–1133. doi: 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Milak MS, DeLorenzo C, Zanderigo F, Prabhakaran J, Kumar JSD, Majo VJ, et al. In Vivo Quantification of Human Serotonin 1A Receptor Using 11C-CUMI-101, an Agonist PET Radiotracer. J Nucl Med. 2010;51:1892–1900. doi: 10.2967/jnumed.110.076257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeLorenzo C, Klein A, Mikhno A, Gray N, Zanderigo F, Mann JJ, et al. SPIE Medical Imaging. Florida, USA: 2009. A new method for assessing PET-MRI coregistration; pp. 72592W-72592W–72598. [Google Scholar]
- 42.Parsey RV, Kent JM, Oquendo MA, Richards MC, Pratap M, Cooper TB, et al. Acute occupancy of brain serotonin transporter by sertraline as measured by [11C]DASB and positron emission tomography. Biol Psychiatry. 2006;59:821–828. doi: 10.1016/j.biopsych.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Ichise M. Neuroreceptor Imaging and Kinetic Modeling. In: Van Heertum RL, Tikofsky RS, Ichise M, editors. Functional cerebral SPECT and PET imaging. 4. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. p. 44. [Google Scholar]
- 44.Chin CL, Carr RA, Llano DA, Barret O, Xu H, Batis J, et al. Pharmacokinetic modeling and [(1)(2)(3)]5-IA-85380 single photon emission computed tomography imaging in baboons: optimization of dosing regimen for ABT-089. The Journal of pharmacology and experimental therapeutics. 2011;336:716–723. doi: 10.1124/jpet.110.173609. [DOI] [PubMed] [Google Scholar]
- 45.Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, et al. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2–18F-FA-85380. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2008;49:1628–1635. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita M, Hines CS, Zoghbi SS, Mallinger AG, Dickstein LP, Liow JS, et al. Downregulation of Brain Phosphodiesterase Type IV Measured with (11)C-(R)-Rolipram Positron Emission Tomography in Major Depressive Disorder. Biological psychiatry. 2012 doi: 10.1016/j.biopsych.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esterlis I, Cosgrove KP, Batis JC, Bois F, Stiklus SM, Perkins E, et al. Quantification of smoking-induced occupancy of beta2-nicotinic acetylcholine receptors: estimation of nondisplaceable binding. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010;51:1226–1233. doi: 10.2967/jnumed.109.072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogden RT. On estimation of kinetic parameters in graphical analysis of PET imaging data. Statistics in Medicine. 2003;22:3557–3568. doi: 10.1002/sim.1562. [DOI] [PubMed] [Google Scholar]
- 49.Parsey RV, Ogden RT, Mann JJ. Determination of Volume of Distribution using Likelihood Estimation in Graphical Analysis: Elimination of Estimation Bias. Journal of Cerebral Blood Flow and Metabolism. 2003;23:1471–1478. doi: 10.1097/01.WCB.0000099460.85708.E1. [DOI] [PubMed] [Google Scholar]
- 50.Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- 51.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 52.Turkheimer FE, Selvaraj S, Hinz R, Murthy V, Bhagwagar Z, Grasby P, et al. Quantification of ligand PET studies using a reference region with a displaceable fraction: application to occupancy studies with [lsqb]11C[rsqb]-DASB as an example. J Cereb Blood Flow Metab. 2012;32:70–80. doi: 10.1038/jcbfm.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, et al. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- 54.Rabiner EA, Wilkins MR, Turkheimer F, Gunn RN, Udo de Haes J, de Vries M, et al. 5-Hydroxytryptamine1A receptor occupancy by novel full antagonist 2-[4-[4-(7-chloro-2,3-dihydro-1,4-benzdioxyn-5-yl)-1-piperazinyl]butyl]-1,2-benzi sothiazol-3-(2H)-one-1,1-dioxide: a[11C][O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl)cyclohexanecarboxamide trihydrochloride (WAY-100635) positron emission tomography study in humans. J Pharmacol Exp Ther. 2002;301:1144–1150. doi: 10.1124/jpet.301.3.1144. [DOI] [PubMed] [Google Scholar]
- 55.Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol. 2008;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- 56.Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Parsey RV, Ojha A, Ogden RT, Erlandsson K, Kumar D, Landgrebe M, et al. Metabolite Considerations in the In Vivo Quantification of Serotonin Transporters Using 11C-DASB and PET in Humans. J Nucl Med. 2006;47:1796–1802. [PubMed] [Google Scholar]
- 58.Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, et al. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 2010;68:170–178. doi: 10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, Hu XZ, et al. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry. 2007;64:201–208. doi: 10.1001/archpsyc.64.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31:1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan GM, Oquendo MA, Simpson N, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin1A receptor binding in major depression is related to psychic and somatic anxiety. Biol Psychiatry. 2005;58:947–954. doi: 10.1016/j.biopsych.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ, et al. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology. 2009;34:2275–2284. doi: 10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milak MS, Severance AJ, Prabhakaran J, Kumar JS, Majo VJ, Ogden RT, et al. In vivo serotonin-sensitive binding of [11C]CUMI-101: a serotonin 1A receptor agonist positron emission tomography radiotracer. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:243–249. doi: 10.1038/jcbfm.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogden RT, Zanderigo F, Choy S, Mann JJ, Parsey RV. Simultaneous estimation of input functions: an empirical study. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:816–826. doi: 10.1038/jcbfm.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan GM, Ogden RT, Oquendo MA, Kumar JS, Simpson N, Huang YY, et al. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biological Psychiatry. 2009;66:223–230. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller JM, Oquendo MA, Ogden RT, Mann JJ, Parsey RV. Serotonin transporter binding as a possible predictor of one-year remission in major depressive disorder. J Psychiatr Res. 2008;42:1137–1144. doi: 10.1016/j.jpsychires.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Milak MS, Ogden RT, Vinocur DN, Van Heertum R, Cooper TB, Mann JJ, et al. Effects of Tryptophan Depletion on the Binding of [11C] DASB to the Serotonin Transporter in Baboons: Response to Acute Serotonin Defic. Biol Psychiatry. 2005;57:102–106. doi: 10.1016/j.biopsych.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 68.Ogden RT, Tarpey T. Estimation in regression models with externally estimated parameters. Biostatistics (Oxford, England) 2006;7:115–129. doi: 10.1093/biostatistics/kxi044. [DOI] [PubMed] [Google Scholar]
- 69.Ichimiya T, Suhara T, Sudo Y, Okubo Y, Nakayama K, Nankai M, et al. Serotonin transporter binding in patients with mood disorders: a PET study with [11C](+)McN5652. Biol Psychiatry. 2002;51:715–722. doi: 10.1016/s0006-3223(01)01351-8. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto M, Suhara T, Okubo Y, Ichimiya T, Sudo Y, Inoue M, et al. Age-related decline of serotonin transporters in living human brain of healthy males. Life Sci. 2002;71:751–757. doi: 10.1016/s0024-3205(02)01745-9. [DOI] [PubMed] [Google Scholar]
- 71.Newberg AB, Amsterdam JD, Wintering N, Ploessl K, Swanson RL, Shults J, et al. 123I-ADAM Binding to Serotonin Transporters in Patients with Major Depression and Healthy Controls: A Preliminary Study. J Nucl Med. 2005;46:973–977. [PubMed] [Google Scholar]
- 72.van Dyck CH, Malison RT, Seibyl JP, Laruelle M, Klumpp H, Zoghbi SS, et al. Age-related decline in central serotonin transporter availability with [(123)I]beta-CIT SPECT. Neurobiol Aging. 2000;21:497–501. doi: 10.1016/s0197-4580(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 73.Hesse S, Barthel H, Murai T, Muller U, Muller D, Seese A, et al. Is correction for age necessary in neuroimaging studies of the central serotonin transporter? Eur J Nucl Med Mol Imaging. 2003 doi: 10.1007/s00259-002-1044-6. [DOI] [PubMed] [Google Scholar]
- 74.Jacobsen LK, Staley JK, Malison RT, Zoghbi SS, Seibyl JP, Kosten TR, et al. Elevated central serotonin transporter binding availability in acutely abstinent cocaine-dependent patients. Am J Psychiatry. 2000;157:1134–1140. doi: 10.1176/appi.ajp.157.7.1134. [DOI] [PubMed] [Google Scholar]
- 75.Kuikka JT, Tammela L, Bergstrom KA, Karhunen L, Uusitupa M, Tiihonen J. Effects of ageing on serotonin transporters in healthy females. Eur J Nucl Med. 2001;28:911–913. doi: 10.1007/s002590100540. [DOI] [PubMed] [Google Scholar]
- 76.Cannon DM, Ichise M, Fromm SJ, Nugent AC, Rollis D, Gandhi SK, et al. Serotonin transporter binding in bipolar disorder assessed using [11C]DASB and positron emission tomography. Biol Psychiatry. 2006;60:207–217. doi: 10.1016/j.biopsych.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 77.Pirker W, Asenbaum S, Hauk M, Kandlhofer S, Tauscher J, Willeit M, et al. Imaging serotonin and dopamine transporters with 123I-beta-CIT SPECT: binding kinetics and effects of normal aging. J Nucl Med. 2000;41:36–44. [PubMed] [Google Scholar]
- 78.Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, et al. Effect of age at onset on the course of major depressive disorder. The American journal of psychiatry. 2007;164:1539–1546. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]
- 79.Neuman RJ, Geller B, Rice JP, Todd RD. Increased prevalence and earlier onset of mood disorders among relatives of prepubertal versus adult probands. J Am Acad Child Adolesc Psychiatr. 1997;36:466–473. doi: 10.1097/00004583-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 80.Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, et al. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes, Brain and Behavior. 2010;9:575–582. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Asberg M, Traskman L, Thoren P. 5-HIAA in the cerebrospinal fluid. A biochemical suicide predictor? Arch Gen Psychiatry. 1976;33:1193–1197. doi: 10.1001/archpsyc.1976.01770100055005. [DOI] [PubMed] [Google Scholar]
- 82.Kamali M, Oquendo MA, Mann JJ. Understanding the neurobiology of suicidal behavior. Depress Anxiety. 2001;14:164–176. doi: 10.1002/da.1062. [DOI] [PubMed] [Google Scholar]
- 83.Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biological Psychiatry. 1997;41:162–171. doi: 10.1016/s0006-3223(96)00217-x. [DOI] [PubMed] [Google Scholar]
- 84.Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT(1A) receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954:173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- 85.Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF. Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology. 1998;19:287–299. doi: 10.1016/S0893-133X(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 86.New AS, Trestman RF, Mitropoulou V, Goodman M, Koenigsberg HH, Silverman J, et al. Low prolactin response to fenfluramine in impulsive aggression. J Psychiatr Res. 2004;38:223–230. doi: 10.1016/j.jpsychires.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Stanley B, Molcho A, Stanley M, Winchel R, Gameroff MJ, Parsons B, et al. Association of aggressive behavior with altered serotonergic function in patients who are not suicidal. Am J Psychiatry. 2000;157:609–614. doi: 10.1176/appi.ajp.157.4.609. [DOI] [PubMed] [Google Scholar]
- 88.Ichise M, Vines D, Gura T, Anderson G, Suomi S, Higley J, et al. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. Journal of Neuroscience. 2006;26:4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bernstein DP, Fink L. Childhood trauma questionnaire: A retrospective self-report. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- 90.Ruhe HG, Booij J, Reitsma JB, Schene AH. Serotonin transporter binding with [123I]beta-CIT SPECT in major depressive disorder versus controls: effect of season and gender. Eur J Nucl Med Mol Imaging. 2009;36:841–849. doi: 10.1007/s00259-008-1057-x. [DOI] [PubMed] [Google Scholar]
- 91.Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, et al. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–284. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.