Abstract
Background
Biased attention to threat is found in both individuals with anxiety symptoms and children with the childhood temperament of behavioral inhibition (BI). Although perturbed fronto-amygdala function is implicated in biased attention among anxious individuals, no work has examined the neural correlates of attention biases in BI. Work in this area may clarify underlying mechanisms for anxiety in a sample at risk for internalizing disorders. We examined the relations among early childhood BI, fronto-amygdala connectivity during an attention bias task in young adulthood, and internalizing symptoms, assessed in young adulthood.
Methods
Children were assessed for BI at multiple age points from infancy through age seven. Based on a composite of observational and maternal report data, we selected 21 young adults classified as having a history of BI and 23 classified as non-BI for this study (N=44). Participants completed an event-related fMRI attention-bias task involving threat (angry faces) and neutral trials. Internalizing symptoms were assessed by self-report and diagnostic interviews.
Results
The young adults characterized in childhood with BI exhibited greater strength in threat-related connectivity than non-behaviorally inhibited young adults. Between-group differences manifested in connections between the amygdala and two frontal regions: dorsolateral prefrontal cortex and anterior insula. Amygdala-insula connectivity also interacted with childhood BI to predict young adult internalizing symptoms.
Conclusions
BI during early childhood predicts differences as young adults in threat and attention-related fronto-amygdala connectivity. Connectivity strength, in turn, moderated the relations between early BI and later psychopathology.
Keywords: Attention Bias, Temperament, Internalizing Problems, Functional Connectivity, Granger Causality, Imaging
Introduction
Behavioral inhibition (BI) is a temperament characterized by fear of novelty in infancy (1, 2), social reticence in childhood (3, 4), and internalizing difficulties in later life (5–8). However, only a subset of behaviorally inhibited children manifest psychopathology as adults (9). Unique patterns of neural connectivity may impact the relations between early childhood BI and later-emerging socio-emotional maladjustment. This study examined the neural correlates of attention bias to threat in young adults with a childhood history of BI. The study then considered the degree to which these correlates moderate the relations between childhood BI and adult internalizing symptoms.
Anxiety and depression are associated with biased orienting towards threat (10–13), which may play a causal role in the emergence of socio-emotional difficulties (14, 15). Threat bias may moderate the long-term outcomes of BI, strengthening the link between early BI and later social withdrawal (16, 17). Imaging studies have delineated the neural circuitry supporting biased orienting to threats in anxious individuals (18–20), but no imaging studies have examined attention biases in BI. Such work may help explain the interrelations among childhood BI, adult maladjustment, and the neural correlates of attention bias.
Attention orienting engages brain circuitry encompassing the amygdala and three areas of the prefrontal cortex (PFC): ventrolateral (vlPFC), insula, and dorsolateral (dlPFC) (21, 22). Individual differences in this circuitry are evident during a standard attention bias task--the dot-probe task (11). To date, four dot-probe functional magnetic resonance imaging (fMRI) studies (18, 19, 23, 24) and a fifth magneto-encephalography (MEG) study (20) have examined threat bias in adolescent anxiety disorders. One additional study examined these mechanisms in adults with post-traumatic stress disorder (PTSD) (25). Together, these studies show that anxiety is associated with perturbed activation patterns in the amygdala and PFC, although their precise nature varies with participant-related and study-design features (21, 26, 27).
Most dot-probe studies compare individual activation levels in response to presentation of angry faces, noting perturbations in the amygdala and PFC among anxious versus healthy participants. However, recent dot-probe imaging studies examined fronto-amygdala connectivity—better reflecting the networks supporting observed behavior (19). The current study extends this work by comparing the strength and directionality of connectivity in young adults initially assessed for BI as children. Specifically, we tested the hypothesis that fronto-amygdala connectivity differs in young adults with a history of early-childhood BI, relative to participants with no such history. Given prior findings (16, 17), a second analysis considered the degree to which connectivity impacts the relations between early-childhood BI and young-adult internalizing problems (28). Prior work (29–31) suggests that BI is linked to unique neural responses to both aversive and appetitive stimuli. Thus our analyses considered relations both with threats (12) and positive stimuli, to evaluate specificity of the findings for threat and extend prior work on reward responding (32).
Methods
Participants
Fifty-six young adults participated, drawn from 153 individuals initially selected at 4 months (33, 34) and behaviorally assessed for BI at ages 14 months, 24 months (33, 35), four years, and seven years (33, 36). Maternal ratings were collected at each time point (37, 38). A composite score was used to index stable BI, based on observations and maternal-report data from each time point (see supplement) (16). Higher scores reflect higher levels of BI (Full cohort sample: Mean=0.019, SD=0.60; Cronbach’s alpha=0.83).
Potential participants were selected from the larger cohort based on childhood BI in order to reflect the span of scores and were invited to participate in the fMRI study. Individuals taking psychotropic medications or presenting with acute psychopathology in need of urgent treatment were excluded, although other psychopathology was permissible (see below). Fifty-six participants were included in the final sample. Of these, 12 did not provide useable data due to excessive movement, technical difficulties, or low task accuracy (<80% correct). Of the remaining 44 participants, 21 were behaviorally inhibited and 23 were non-BI as children.
There were no significant differences in BI scores, gender, or IQ between the included and excluded participants (p’s>0.14). Included BI and non-BI participants did not differ in gender or IQ (p’s>0.15; see Table 1). Participants were screened using the Structured Clinical Interview for DSM Disorders (SCID) (39), revealing current psychiatric diagnoses in five participants: Major Depressive Disorder (two BI; one non-BI) and anxiety (one BI and one non-BI). Removing these five individuals from the data analyses did not affect the findings; thus, they were included in the analyses.
Table 1.
Demographic characteristics and behavioral results for included and excluded participants for both the BI and non-BI groups. All calculations are reported as the mean unless otherwise noted. Standard deviations (±) are presented in parentheses.
| Included Participants | Excluded Participants | |||
|---|---|---|---|---|
| Group | BI | NON-BI | BI | NON-BI |
| Sample size | 21 | 23 | 5 | 7 |
| Gender | 12m/9f | 8m/15f | 3m/2f | 3m/4f |
| Age | 19.91 (0.86) | 20.03 (0.70) | 20.1 (0.87) | 20.1 (0.81) |
| IQ | 114.71 (8.81) | 116.10 (10.42) | 113.0 (11.69) | 109.0 (9.22) |
| BI Score | 0.61 (0.72) | −0.43 (0.24) | 0.38 (0.46) | −0.60 (0.45) |
| Internalizing Score | 8.52 (7.51) | 8.35 (5.48) | 5.80 (5.70) | 13.67 (11.59) |
| Accuracy Rate | 89.29% (7.17) | 88.80% (10.22) | 78.39% (12.93) | 81.02% (12.74) |
| Reaction Time (ms) | 766.56 (64.76) | 776.77 (84.23) | 824.10 (84.99) | 802.57 (84.19) |
| Threat Bias Scores | 13.45 (32.43) | 6.29 (30.69) | −6.23 (28.77) | 8.50 (36.64) |
| Happy Bias Scores | 2.23 (31.17) | −6.49 (39.89) | 18.21 (34.06) | −3.00 (36.23) |
Current internalizing symptoms were rated by participants using Achenbach’s Adult Self Report (ASR) (40). We focused on the broad-band internalizing scale because of the low incidence of ongoing diagnoses and previous links between BI and internalizing difficulties (41). The use of the broad-band scale also minimized Type I errors that would accrue from individual tests for the many measures of anxiety and depression that can be obtained.
The study was approved by the institutional review boards at the National Institute of Mental Health, Bethesda, MD, the University of Maryland, College Park, and George Mason University, Fairfax, VA. All participants provided informed consent prior to the study.
Dot-Probe Task
We used the same procedures as Monk et al. (18). Each trial began with a 500ms fixation point (Figure 1) followed by a face pair of the same individual (42) displaying an angry/neutral, happy/neutral, or neutral/neutral expressions (500ms). A pair of dots then appeared in one hemi-field (1100ms), and participants indicated by button-press if the dots were vertical or horizontal. All participants completed 24 practice trials outside of the scanner prior to the experiment.
Figure 1.
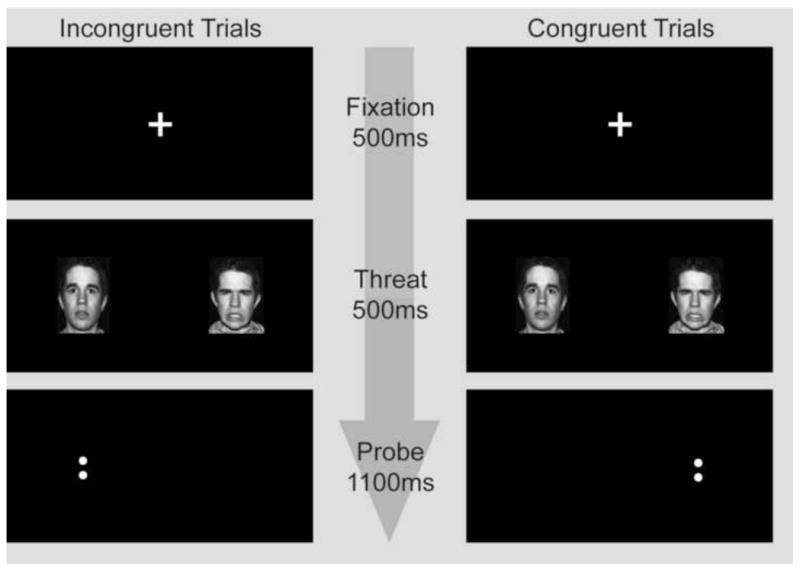
Example of visual task illustrating congruent and incongruent threat trials. The only difference between trial types is the location of the probe (dots) relative to the angry face. In congruent trials the probe appeared on the same side as the angry face (threat), for incongruent trials the probe appeared on the same side as the neutral face. Trials with happy/neutral and neutral/neutral face pairs were also shown. The same actor always appeared for the two expressions within a single trial. Here the dots are vertical; however in half of the trials the dots were horizontal.
The scanner task involved 192 trials (ITI average 400ms; 200–600ms min/max) divided across 2 runs, each with 5 trial types: 1) angry/neural face pair followed by a dot pair in the same position as the angry face (congruent); 2) angry/neutral face a dot pair in the position of the neutral face (incongruent); 3) happy/neutral face pair with congruent dot presentation; 4) happy/neutral face pair with incongruent dot presentation; 5) neutral/neutral face pair with dot presentation. There were 24 trials for each condition across both runs, except for neutral/neutral trials, which were shown 48 times, providing comparisons for emotion conditions. Forty-eight blank trials of the same duration as the other five trial types were included to introduce random jitter and provide an additional baseline. For each participant, trial order was randomly determined. Emotional faces and dots were displayed an equal number of times to each hemi-field. Twelve separate actors were used and each appeared in all 5 conditions.
Task stimuli were viewed with mirrors on the head coil. Foam padding constrained head movement. A custom built two button box recorded behavioral data.
Behavioral analyses and results appear in supplement 1.
fMRI Analysis
Data Acquisition
The first 27 participants were scanned using a Signa VH/i 3 Tesla scanner (General Electric, Waukesha, WI). Due to scanner decommissioning, the final 17 participants underwent scanning on a GE Signa HDx 3 Tesla scanner. Both scanners used the same GE head coil. Analyses found no significant differences in blood oxygen level-dependent (BOLD) activity across scanners in the regions of interest for this study (0.60<p’s<0.95). Each brain volume consisted of 36 interleaved slices 2.6 mm thick acquired in the axial plane using a T2*-weighted echo-planar sequence with a repetition time (TR) of 2300ms, echo time (TE) of 25ms, and flip angle of 90. Voxel dimension was 2.5×2.5×2.6 mm. Matrix size was 96×96, and field of view (FOV) was 24 cm. To allow for signal stabilization, four acquisitions were obtained before task onset. A high resolution structural image was also acquired for each participant using a T1-weighted standardized magnetization prepared spoiled gradient recalled echo sequence: 124 1.2 mm slices, 8100ms TR, 32ms TE, 15° flip angle, 256×256 matrix, 24 cm FOV.
Preprocessing
Functional imaging data were analyzed using Analysis of Functional and Neural Images (AFNI) software (43), including slice-time correction, motion correction, and 6 mm full-width half-maximum smoothing kernel. For motion correction, we censored TRs with motion in excess of the Euclidean norm of 0.8 mm. Each participant’s EPI time series was manually placed in Talairach space and normalized by the voxel-wise temporal mean so that the effect estimates can be interpreted as percentage signal change. Only correct and within-range (150ms<RTs<1100ms) trials were included in the analyses.
Regression
Preprocessed time series data were analyzed by multiple regression in a model including six regressors of interest, six regressors for residual motion in x, y, and z planes and in the yaw, pitch, and roll dimensions, and two regressors for baseline and linear trends for each of the runs.
Regressors of interest comprised emotion type and dot pair location, modeling angry-congruent, angry-incongruent, happy-congruent, happy-incongruent, and neutral trials separately. They were created through convolving the stimulus timing with a gamma variate function that modeled a prototypical hemodynamic response (44). Idealized signal time courses were estimated based on even onset times, with blank trials providing implicit baseline. An additional regressor modeled excluded nuisance (incorrect, out-of-range, and null response) trials.
Analysis
Details of our initial activation analysis for angry, happy, and neutral faces are presented in the supplement. Briefly, bilateral amygdala activation occurred for the angry and neutral trials across the entire sample (BI and BN together). These results support our use of anatomically-delineated amygdala seeds in the PPI analysis.
PPI Analysis
This analysis delineated between-group differences in amygdala-PFC connectivity in the context of angry-versus-neutral trials using established procedures (45, 46). At the individual level, the first eigenvariate time series incorporated the anatomically-defined amygdala, as defined by the Talairach atlas, as the “seed” in two separate analyses for the right amygdala and left amygdala based on the initial fMRI group analysis. These time series were deconvolved with a presumed hemodynamic response function before a psychophysiological interaction term was created between the angry/neutral pair versus neutral/neutral pair conditions. This maps differences in amygdala-PFC connectivity across the angry, relative to neutral, dot-probe trials. Group differences were analyzed.
Post-hoc analyses extracted mean connectivity between the amygdala and voxels identified in the insula and the dlPFC. These data were then used to both decompose significant results and examine associations with concurrent internalizing symptoms. The interrelations between the variables of interest were examined in a moderated mediation model (28) (see supplement 1).
Granger Causality
Regions that differed between groups in the PPI analysis were submitted to a secondary Granger causality analysis designed to model the strength and direction of connectivity among the amygdala, dlPFC and insula--PPI maps only magnitude differences in connectivity among nodes. This analysis began by selecting as nodes the anatomically-delimited whole amygdala and the two PFC regions functionally defined from the PPI analysis. Directionality was assessed in Granger causality models, with vector autoregressive modeling that estimated lag effects by capturing the temporal and cross-regional interactions in the designated network (47). Lag effects for each condition formed the basis for inferring causality between experimental manipulation and regional activation.
Statistics were determined using a two-step process at the individual- and group-levels. At the individual level, the average time series for each participant in each condition was extracted, yielding two average time series for each ROI; these were submitted to the AFNI program 1dGC that estimated the one-TR lag path coefficients for each condition and ROI separately. At the group level, the path coefficients among the regions in the network were compared between the BI and non-BI groups. 1dGC tested group differences in the direction of the path coefficients between nodes in each condition separately, plus any possible differences between the conditions. In this analysis, data from 12 participants (4 BI; 8 non-BI) were omitted due to excessive time-period censoring.
Statistical Thresholds
For all analyses, the statistical threshold was set at the cluster-level p=0.05, FWE-corrected for multiple comparisons. This statistical threshold was accomplished with a voxel-wise p<.005 threshold, followed by cluster thresholds set through Monte Carlo simulations with 3dClustSim in AFNI.
Results
fMRI Results
Findings from the initial activation and behavioral analyses are noted in the supplement.
PPI
Analyses of between-group differences in fronto-amygdala connectivity identified two right-hemisphere clusters surpassing statistical thresholds, one in the dlPFC (x,y,z= 49,4,21; 14 voxels) and the other in the anterior insula (x,y,z=36,14,6; 14 voxels) (Figure 2). Both findings reflected significantly greater negative right fronto-amygdala connectivity in response to angry-versus-neutral contrast in BI relative to non-BI participants, with large effects (dlPFC: t(42)=−3.81, d=−1.15; insula: t(42)= −4.03, d=−1.23). Weights for the angry-versus-neutral PPI contrast values were extracted for right amygdala-insula and right amygdala-dlPFC connectivity to decompose these effects and to correlate with behavioral measures.
Figure 2.
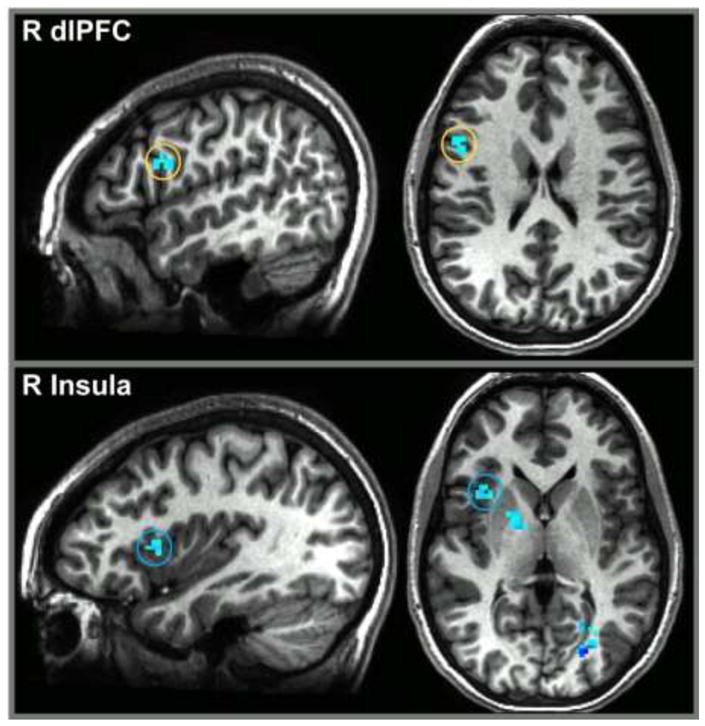
PPI activation in the BI group (vs. BN) for angry relative to neutral faces. Fronto-amygdala connectivity revealed between-group differences in the right dlPFC (top panel; x, y, z = 49, 4, 21; 14 voxels) and the right insula (bottom panel; x, y, z = 36, 14, 6; 14 voxels). Activation is shown at p = 0.005.
Specifically, BI participants exhibited greater differences in connectivity, while the non-BI group did not show significant connectivity. For the BI group, this pattern resulted from positive connectivity to neutral faces (dlPFC: mean=5.38±7.81; insula: mean=5.71±13.25) and negative connectivity to angry faces (dlPFC: mean= −1.13±6.01; insula: mean= −1.94±8.37). Among non-BI adolescents connectivity to angry (dlPFC: mean=0.83±7.28; insula: mean=3.34±12.89) and neutral (dlPFC: mean= −1.51±7.81; insula: mean= −1.47±10.18) faces were non-significant in both ROIs. This pattern generated the significantly greater negative contrast weight in the angry-versus-neutral condition for the BI (dlPFC: mean= −3.81±4.88; insula: mean= −4.45±5.08) relative to non-BI group (dlPFC: mean=1.54±4.41; insula: mean=3.25±7.27), explaining the opposite connectivity signs seen between the two groups.
Reinforcing the categorical group analysis, fully-continuous individual BI scores across the full sample were correlated with the extracted coefficients for both amygdala-dlPFC (r=−0.43, p=0.003) and amygdala-insula (r=−0.49, p=0.001) connectivity in the angry-versus-neutral contrast (Figure S1 in the Supplement). Self-reported internalizing in adulthood also correlated with amygdala-dlPFC (r=−0.32, p=0.04) but not amygdala-insula (r=−0.25, p=0.12) connectivity. The correlation between BI and self-reported internalizing problems was not significant (r=0.11, p=0.49).
For the happy-neutral analyses, no between-group difference in connectivity was found above our statistical thresholds in the main ROIs. However, an area of the posterior frontal cortex (x,y,z=29, −26,46, t=5.53) survived a whole-brain-corrected threshold. In contrast to findings for threat trials, this difference reflected greater connectivity in the BN versus BI group.
Granger Causality
Granger causality analyses extended results from PPI by modeling the strength and direction of connectivity among amygdala, dlPFC, and insula nodes. Significant group differences were found for the strength of the connection for the dlPFC-to-insula path coefficients, for both the angry (+0.24; p<.05) and neutral (+0.20; p<.05) trials. These differences reflected a significant, positively-weighted dlPFC-to-insula path in the BI group, both for angry (+0.33, p<.001) and neutral (+0.30; p<.001) trials, with no significant path coefficients in the non-BI group.
Moderated Mediation Model
Finally, exploratory moderated-mediation models examined the relations among early temperament, connectivity, and adult self-reported internalizing problems (Table 2; Figures S2 & S3 in the Supplement).
Table 2.
Predicting internalizing symptoms in young adulthood using measures of early temperament (BI composite) and neural connectivity (amygdala-dlPFC and amygdala-insula) in young adulthood. The table presents the path coefficients (standard errors) and t-values for the separate moderated mediation models.
| BI-PPI | PPI-INT | BI-INT | BI X PPI-INT | |||||
|---|---|---|---|---|---|---|---|---|
| a | b | c′ | ab | |||||
|
| ||||||||
| β (SE) | t | β (SE) | t | β (SE) | t | β (SE) | t | |
| Amygdala- dlPFC | −2.99 (1.05) | −2.84** | −0.40 (0.20) | −2.01* | −2.38 (1.91) | −1.25 | −0.46 (0.27) | −1.73+ |
|
| ||||||||
| Amygdala- Insula | −5.11 (1.32) | −3.88** | −0.27 (0.16) | −1.67+ | −1.84 (1.74) | −1.05 | −0.40 (0.20) | −2.03* |
p<0.01,
p<0.05,
p<0.10
BI-Behavioral Inhibition
PPI-Connectivity measure
INT-Internalizing Raw Score from ASR
BI X PPI-Interaction between BI and PPI
For amygdala-insula connectivity, the direct path between early BI and connectivity was significant (t=−3.88, p<0.001), while the connectivity-internalizing (t=−1.67, p=0.10) and the BI-Internalizing (t=−1.05, p=0.30) paths were non-significant. However, the interaction between BI and insula connectivity significantly predicted internalizing symptoms (t=−2.03, p=0.05), reflecting stronger relation in the BI than non-BI group.
For amygdala-dlPFC connectivity, the direct path between BI and connectivity was significant (t=−2.84, p=0.007), as was the connectivity-internalizing path (t=−2.01, p=0.05) but not the BI-Internalizing path (t=−1.25, p=0.22). Thus, a mediation relation was not supported. Although resembling the pattern with BI and insula connectivity, the BI-dlPFC connectivity interaction was not significant (t=−1.73, p=0.09).
Discussion
Behaviorally inhibited children are at risk for internalizing difficulties in adolescence and young adulthood. The current study suggests for the first time that dynamic neural patterns in threat processing may support these documented developmental relations. For two frontal regions (dlPFC and anterior insula), childhood BI was associated with negative fronto-amygdala connectivity, evident across trials containing threat faces compared to neutral faces. In addition, connectivity patterns moderated the relations between childhood BI and adult internalizing symptoms. These relations suggest that negative fronto-amygdala functional connectivity places individuals with a history of BI uniquely at risk. Our analyses with happy faces suggest that this pattern is specific to threat processing. As such, previously observed perturbations in reward processing may not extend to attention biases (29).
Most research on the neural correlates of anxiety has quantified individual differences in risk based on measures of behavior acquired contemporaneously with measures of brain function (21, 23). The current study, however, examines young adults classified based on the degree to which they manifested the temperament of BI as young children. Brain function was examined more than 10 years after the last assessment of temperament. Our findings suggest that early-life temperament exhibits a unique relation with brain function that endures into adulthood, even after the initial behavioral or phenotypic markers are no longer evident (48). Moreover, these long term associations shed light on factors that shape adaptive functioning in adulthood. The current study builds on accruing evidence of the long-term imprint of childhood temperament on amygdala (49) and striatal (29) circuitry as well as on the central role of attention in socioemotional development (50). Our findings in this relatively healthy sample echo prior research with clinically anxious participants noting prefrontal dysfunction, including the insula and dlPFC (18, 19, 23–25). Therefore, these data suggest underlying mechanisms of risk that may inform our understanding of the neural underpinnings of anxiety.
Prior fMRI studies using the dot-probe task differ in important respects from the current study. Those studies compared frontal function in groups differing on concurrent levels of anxiety, to the extent where overt psychopathology was manifest, and found differences in mean levels of activation during threat trials. Rather than direct-group differences in activation across standard condition-based contrasts, the current study found differences in fronto-amygdala connectivity as a function of early BI. In particular, we found greater negative connectivity for both the amygdala-dlPFC and amygdala-insula circuits among young adults with a history of BI, in line with one previous study of adolescent GAD (19). This pattern suggests that there may be an altered inhibitory response among individuals with a history of BI in brain regions supporting the regulation of negative affect.
Of note, the current study also examined the direction of functional connections that manifest during the dot-probe task. We found a stronger input from the dlPFC to the insula in BI relative to non-BI participants. The insula possesses rich anatomical connections with both the amygdala and the dlPFC; the latter two are less strongly connected. Thus, these findings suggest that frontal regions might uniquely modulate between-group differences in amygdala function through connections from the dlPFC to the insula. The Granger causality method thus captured individual differences in the delayed effects of activation as the PFC works to modulate initial reactivity.
The available longitudinal data allow the current study to delineate relations among early-childhood temperament, brain function, and internalizing symptoms in young adulthood. Prior work in this and other samples found associations between early-childhood BI, internalizing difficulties, and adolescent anxiety (9, 51). Supporting these relations, behavioral attention biases during the dot-probe task to threat linked early BI to subsequent social withdrawal (16, 17). Here, our exploratory analysis examined whether the neural correlates of the task display a similar relation. A mediation model was only partially supported. While amygdala-dlPFC connectivity was significantly associated with both BI and internalizing symptoms, BI and symptom levels did not correlate in this relatively small sample. Rather, the data suggested that amygdala-insula connectivity moderates the link between early BI and later socioemotional difficulties, consistent with prior research noting moderation across various measures of information processing (52–54). Although statistical significance was only evident for amygdala-insula connectivity, the direction of effects was the same for amygdala-dlPFC connectivity.
No evidence emerged for temperament-related differences in amygdala function (see supplement 1); this was not unexpected. Individual differences in amygdala function are sensitive to relatively subtle variations in task parameters. Prior studies finding enhanced amygdala activation in youth characterized in childhood with BI (49) used tasks on which anxiety disorder patients also exhibit amygdala hyper-activation (46). The current dot-probe paradigm we employed 500msec threat-cue exposures. Monk et al. (18) found no differences in amygdala activation between clinically anxious and healthy adolescents with the same protocol.
The current study has some limitations. The use of two scanners was an unavoidable limitation, although analyses revealed no evidence that this influenced findings. Moreover, by introducing variability, this limitation is more likely to produce Type II than Type I errors. Most importantly, the current study was based on a small sample, with low rates of ongoing psychopathology. Thus, we were not able to compare participants with and without psychopathology who also were with or without a history of BI to examine the degree to which fronto-amygdala connectivity might moderate risk among individuals characterized in childhood with BI.
The current findings set the stage for future work in which longitudinal brain imaging studies might assess at-risk individuals. Given the pattern of findings in the current study, this approach may powerfully predict outcome among behaviorally inhibited individuals. Recent work (19, 55) suggests that our noted pattern of activation and connectivity may vary with the length of exposure to threat (e.g., increased amygdala response to masked faces). We do not know if this shift in neural functioning to rapid presentation is similarly associated with variations in observed patterns of socioemotional functioning. An examination in progress will help elucidate these questions.
Finally, recent work suggests that attention biases to threat may play a causal role in the emergence of internalizing difficulties (56). Indeed, attention-retraining techniques might alter long-term risk for anxiety, potentially through effects on the PFC (15, 18, 24, 57, 58). A number of open questions remain as it is not clear if effects are reliant on specific training paradigm, are transferrable across contexts, or will impact risk for disorder, as opposed to current symptomatology. Importantly, the neural mechanisms underlying attention-training are, at the moment, unclear (12). The current data suggest that assessments should focus on shifts in fronto-amygdala connectivity. Current work taking advantage of this unique sample may address these open translational questions.
Supplementary Material
Acknowledgments
Funding was provided by the Intramural Research Program of NIH, grants from NIH to KPE (MH073569 and MH094633) and NAF (MH074454; R37HD17899), as well as from the NARSAD Foundation (Distinguished Investigator Award) to NAF. We would like to thank the participants and families for their continued involvement in our study.
Footnotes
Previous Presentation
This work has been presented at the Society for Neuroscience Annual Meeting, Washington, DC, November 2011
Financial Disclosures
BPB and KM report consultancy work for GSK in relation to research into obesity, which is unrelated to this project. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, et al. Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology. 1997;30:127–40. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Marshall PJ, Reeb BC, Fox NA. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Developmental Science. 2009;12:568–82. doi: 10.1111/j.1467-7687.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox NA, Schmidt LA, Henderson HA. Developmental psychophysiology: Conceptual and methodological perspectives. In: Cacioppo John T, LGT, Berntson Gary G., editors. Handbook of psychophysiology. 2. Cambridge: Cambridge University Press; 2000. pp. 665–86. [Google Scholar]
- 4.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–62. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 5.Chronis-Tuscano A, Degnan K, Pine D, Perez-Edgar K, Henderson H, Diaz Y, et al. Stable behavioral inhibition during infancy and early childhood predicts the development of anxiety disorders in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:928–35. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gladstone GL, Parker GB, Mitchell PB, Wilhelm KA, Malhi GS. Relationship between self-reported childhood behavioral inhibition and lifetime anxiety disorders in a clinical sample. Depression and Anxiety. 2005;22:103–13. doi: 10.1002/da.20082. [DOI] [PubMed] [Google Scholar]
- 7.Hayward C, Killen J, Kraemer H, Taylor C. Linking self-reported childhood behavioral inhibition to adolescent social phobia. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:1308–16. doi: 10.1097/00004583-199812000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–15. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–46. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- 10.Romens SE, Pollack SD. Emotion regulation predicts attetnion bias in maltreated children at-risk for depression. Journal of Child Psychology & Psychiatry. 2012;53:120–7. doi: 10.1111/j.1469-7610.2011.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg M, van IJzendoorn M. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Fox NA, Pine DS. Temperament and the emergence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:125–8. doi: 10.1016/j.jaac.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kujawa AJ, Torpey D, Kim J, Hajcak G, Rose S, Gotlib IH, et al. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. Journal of Abnormal Child Psychology. 2011;39:125–35. doi: 10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pine DS, Helfinstein SM, Bar-Haim Y, Nelson EE, Fox NA. Challenges in developing novel treatments for childhood disorders: Lessons from research on anxiety. Neuropsychopharmacology. 2009;34:213–28. doi: 10.1038/npp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, et al. Attention Bias Modification Treatment: A meta-analysis towards the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68:982–90. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, Fox NA. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10:349–57. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, et al. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology. 2011;39:885–95. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attention bias in responsive to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163:1091–7. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 19.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65:568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Britton JC, Bar-Haim Y, Carver FW, Holroyd T, Norcross MA, Detloff A, et al. Isolating neural components of threat bias in pediatric anxiety. Journal of Child Psychology & Psychiatry. 2012;53:678–86. doi: 10.1111/j.1469-7610.2011.02503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12(1):92–8. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- 22.Hooker CI, Knight RT. The role of lateral orbitofrontal cortex in the inhibitory control of emotion. In: Zald DH, Rauch, editors. The Orbitofrontal Cortex. New York: Oxford University Press; 2006. [Google Scholar]
- 23.Telzer EH, Mogg K, Bradley BP, Mai X, Ernst M, Pine DS, et al. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biological Psychology. 2008;79:216–22. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maslowsky MA, Mogg K, Bradley BP, McClure-Tone E, Ernst M, Pine DS, et al. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. Journal of Child and Adolescent Psychopharmacology. 2010;20:105–11. doi: 10.1089/cap.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fani N, Tone EB, Phifer J, Norrholm SD, Bradley BP, Ressler KJ, et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychological Medicine. 2012;42:533–43. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etkin A, Prater K, Hoeft F, Menon V, Schatzberg MA. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry. 2010;167:545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etkin A, Schatzberg MA. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. American Journal of Psychiatry. 2011;168:968–78. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 28.Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- 29.Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26(24):6399–405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helfinstein SM, Benson B, Pérez-Edgar K, Bar-Haim Y, Detloff A, Pine DS, et al. Striatal responses to negative monetary outcomes differ between behaviorally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49:479–85. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, et al. Neural correlates of reward processing in adolescents with a history inhibited temperament. Psychological Science. 2009;20:1009–18. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helfinstein SM, Fox NA, Pine DS. Approach-withdrawal and the role of the striatum in the temperament of behavioral inibition. Developmental Psychology. 2012;48:815–26. doi: 10.1037/a0026402. [DOI] [PubMed] [Google Scholar]
- 33.Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- 34.Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, et al. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1771–84. [PubMed] [Google Scholar]
- 35.Kagan J, Reznick JS, Gibbons J. Inhibited and uninhibited types of children. Child Development. 1989;60(4):838–45. [PubMed] [Google Scholar]
- 36.Rubin KH. The Play Observation Scale (POS) University of Waterloo; 1989. [Google Scholar]
- 37.Goldsmith HH. Studying temperament via construction of the toddler behavior assessment questionnaire. Child Development. 1996;67:218–35. [PubMed] [Google Scholar]
- 38.Rowe DC, Plomin R. Temperament in early childhood. Journal of Personality Assessment. 1977;41:150–6. doi: 10.1207/s15327752jpa4102_5. [DOI] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- 40.Achenbach TM. Manual for the young adult self-report and young adult behavior checklist. Burlington, VT: University of Vermont; 1997. [Google Scholar]
- 41.Williams LR, Degnan KA, Pérez-Edgar K, Henderson HA, Rubin KH, Pine DS, et al. Impact of behavioral inhibition and parenting style on internalizing and externalizing problems from early childhood through adolescence. Journal of Abnormal Child Psychology. 2009;37:1063–75. doi: 10.1007/s10802-009-9331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tottenham N, Tanaka J, Leon A, McCarry T, Nurse M, Hare T, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 44.Cohen M. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 45.Klumpp H, Ho SS, Taylor SF, Phan KL, Abelson JL, Liberson I. Trait anxiety modulates anterior cingulate activation to threat interference. Depression & Anxiety. 2011;28:194–201. doi: 10.1002/da.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:109–16. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 47.Chen G, Glen DR, Saad ZS, Hamilton JP, Thomason ME, Gotlib IH, et al. Vector autoregression, structural equation modeling, and their synthesis in neuroimaging data analysis. Computers in Biology and Medicine. 2011;41:1142–55. doi: 10.1016/j.compbiomed.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz CE, Rauch SL. Temperament and its implications for neuroimaging of anxiety disorders. CNS Spectrums. 2004;9(4):284–91. doi: 10.1017/s1092852900009226. [DOI] [PubMed] [Google Scholar]
- 49.Pérez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35:1538–46. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Edgar K, McDermott JM, Korelitz K, Degnan KA, Curby TW, Pine DS, et al. Patterns of sustained attention in infancy shape the developmental trajectory of social behavior from toddlerhood through adolescence. Developmental Psychology. 2010;46:1723–30. doi: 10.1037/a0021064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pérez-Edgar K, Fox NA. Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics of North America. 2005;14:681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 52.McDermott JM, Pérez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox N. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–8. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reeb-Sutherland BC, Helfinstein SM, Degnan KA, Pérez-Edgar K, Henderson HA, Lissek S, et al. Startle modulation in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:610–7. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reeb-Sutherland BC, Vanderwert RE, Degnan KA, Marshall PJ, Pérez-Edgar K, Chronis-Tuscano A, et al. Attention to novelty in behaviorally inhibited adolescents moderates risk for anxiety. Journal of Child Psychology and Psychiatry. 2009;50:1365–72. doi: 10.1111/j.1469-7610.2009.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miskovic V, Schmidt LA. Early information processing biases in social anxiety. Cognition & Emotion. 2012;26:176–85. doi: 10.1080/02699931.2011.565037. [DOI] [PubMed] [Google Scholar]
- 56.Shechner T, Britton JC, Pérez-Edgar K, Bar-Haim Y, Ernst M, Fox NA, et al. Attention biases, anxiety, and development: Toward or away from threats or rewards? Depression & Anxiety. 2012;29:282–94. doi: 10.1002/da.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eldar S, Ricon T, Bar-Haim Y. Plasticity in attention: Implications for stress response in children. Behaviour Research & Therapy. 2008;46:450–61. doi: 10.1016/j.brat.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Eldar S, Apter A, Lotan D, Pérez-Edgar K, Naim R, Fox NA, et al. Attention bias modification in clinically anxious children: A randomized control trial. American Journal of Psychiatry. 2012;169:213–22. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


