Abstract
Purpose
To study the effects of serum and growth factors on propagation of porcine male germline stem cells (MGSCs) in vitro and develop a culture system for these stem cells.
Methods
Fresh testicular cells from neonatal piglets were obtained by mechanical dissociation and collagenase-trypsin digestion. After differential plating, non-adhering cells were cultured in media supplemented with different concentrations of serum (0, 1 %, 2 %, 5 %, 10 %). After 10 days of primary culture, the cells were maintained in media supplemented with different concentrations of growth factors (basic fibroblast growth factor and epidermal growth factor at 1, 5, 10 ng/ml). The number of MGSC-derived colonies with different sizes was determined in each treatment to assess the effects of serum concentrations and growth factors.
Results
The number of MGSC-derived colonies was significantly higher in the presence of 1 % rather than 10 % fetal bovine serum (FBS). Basic fibroblast growth factor (bFGF) at 1, 5 ng/ml and epidermal growth factor (EGF) at 5, 10 ng/ml significantly promoted colony formation. Immunocytochemistry, reverse transcriptase-polymerase chain reaction (RT-PCR) and xenotransplantation assays demonstrated the presence of functional stem cells in cultured cell population.
Conclusions
In vitro propagation of porcine MGSCs could be maintained in the presence of 1 % FBS and supplementation of growth factors for 1 month.
Keywords: Pig, Male germline stem cell, Serum, Growth factor, Culture
Introduction
Spermatogenesis is a continuous process during which spermatogonia undergoes a series of highly organized events of proliferation, differentiation, meiosis and morphogenesis to form spermatozoa throughout the adult life of a male. This process of spermatogenesis is supported by male germline stem cells (MGSCs) which are comprised of gonocytes and spermatogonial stem cells (SSCs) [12, 13]. MGSCs can spontaneously form embryonic-like stem cells in vitro [28] and thus could be applied as a model to develop strategies for regenerative therapy for humans as embryonic stem cells (ES) or induced-pluripotent stem cells (iPS). The utilization of MGSC-derived pluripotent stem cells could avoid ethical controversies regarding the use of ES cells and also preclude the necessity of exogenous transcription factors for generating iPS from somatic cells [29]. In addition, human MGSCs would contribute to treatment for chemotherapy-induced infertility and therefore benefit cancer patients [11]. Corrections of defective genes in vitro before transplantation could be applied to either cure male infertility or eliminate transmission of mutant alleles which are responsible for genetic diseases. However, MGSCs are rare in number in testes [30]. Therefore, development of strategies for maintenance and expansion of MGSCs in vitro is a prerequisite to attain the great potential of MGSCs in agriculture and medicine.
Pigs have been established as a main non-rodent model for biomedical and pharmacological research because of their anatomical and functional similarities to humans and also because of the availability of disease models. The transfer of research findings obtained in pigs to humans is much more exact compared with other animal models [31]. However, few attempts have been done to develop porcine MGSC culture in vitro. Goel et al. enriched porcine gonocytes by discontinuous Percoll density gradient and cultured the resulting gonocytes for 1 week [8]. In another report, Goel et al. demonstrated the multipotential ability of porcine MGSCs from primary culture by xenotransplantation [7]. Kuijk et al. investigated the effects of growth factors on the primary-cultured testicular cells from piglets [20]. However, they did not xenotransplant the cultured cells to recipient mouse testes to detect MGSC potential.
In this present study, we isolated the MGSCs from testes of piglets, and compared the culture of MGSCs under different concentrations of serum. Furthermore, we studied the effects of growth factors on propagation of MGSCs in vitro. In our culture system, the proliferation of MGSCs from piglets was successfully maintained for 1 month. These cultured cells would facilitate the manipulation in vitro as well as studies in porcine SSC biology.
Materials and methods
Collection of porcine testes
All animal experimental procedures were approved by the Northwest A&F University’s Institutional Animal Care and Use Committee. Testis samples were obtained from 1-week-old hybrid piglets, transported to the laboratory in Dulbecco’s phosphate-buffered saline (DPBS) within 2 h.
Isolation of testicular cells from piglets
The collected testes were washed three times with DPBS. Tunica albuginea and collective tissues were removed, and testis tissues were minced into small pieces and incubated in Dulbecco’s modified eagle medium (DMEM, high glucose; Hyclone, USA) supplemented with collagenase type IV (2 mg/ml; Invitrogen, USA) and DNase I (7 mg/ml; Bio Basic, Canada) for 60 min, at 37 °C. The separate fragments of seminiferous tubule were washed three times with DPBS to remove interstitial cells and erythrocyte. To obtain single cell suspension, the resulting seminiferous tubules were incubated in 0.25 % (w/v) trypsin-EDTA (Hyclone, USA) for 5 min, at 37 °C. The dispersed testicular cells were filtered through a 40 μm mesh. The single cell suspension was pelletted by centrifugation at 600 g for 5 min, and re-suspended in DMEM (high glucose; Hyclone, USA) supplemented with 1 % (v/v) fetal bovine serum (FBS; Hyclone, USA).
In vitro culture of porcine MGSCs
To study the effects of serum, the isolated testicular cells were subjected to differential plating to remove surplus somatic cells, then seeded on 12-well multidish (Corning, USA) at a density of 1 × 105 cells/cm2 and cultured at 37 °C in a humidified atmosphere of 5 % CO2 in air. The basic culture medium was composed of DMEM (high glucose; Hyclone, USA) supplemented with 100 IU/ml penicillin (Hyclone, USA), 100 μg/ml streptomycin (Hyclone, USA), 1 % (v/v) non-essential amino acids (Sigma-Aldrich, USA), 1 % (v/v) L-glutamine (Invitrogen, USA) and 0.1 % (v/v) 2-mercaptoethanol (Invitrogen, USA). The cells were maintained in the basic culture medium supplemented with different concentrations of FBS (0, 1 %, 2 %, 5 %, 10 %; Hyclone, USA). The medium was changed every other day. After 10 days of primary culture, the number of MGSC-derived colonies in each treatment was determined.
To study the effects of growth factors, after 10 days of primary culture, MGSC-derived colonies were dissociated with 0.05 % (w/v) trypsin-EDTA (Hyclone, USA) and re-seeded on mitomycin C-treated autologous Sertoli cells at a density of 5 × 104 cells/cm2. The medium for subculture was the basic culture medium supplemented with 1 % FBS (Hyclone, USA) and different addition of growth factors-recombinant human basic fibroblast growth factor (bFGF; R&D Systems, USA) or epidermal growth factor (EGF; R&D Systems, USA) at 1, 5, 10 ng/ml. The medium was changed every other day. The number of MGSC-derived colonies in each treatment was counted after 10 days of subculture.
Based on the aforementioned observations, to develop a culture system for MGSCs, the isolated testicular cells were first maintained in the basic culture medium supplemented with 1 % FBS (Hyclone, USA) for 10 days and the MGSC-derived colonies were subcultured in medium supplemented with 1 % FBS (Hyclone, USA), 5 ng/ml bFGF (R&D Systems, USA) and 10 ng/ml EGF (R&D Systems, USA). The medium was changed every other day, and the MGSC-colonies were passaged every 7 to 10 days. Digital images were made with Olympus IX71 (Olympus, Japan) inverted fluorescence microscope camera.
MGSC-derived colony assessment
MGSC-derived colonies were assessed by the method described by Abu Elhija et al. with a minor modification [1]. Briefly, cells were designated as colonies when no less than ten cells showing a spherical configuration and a large size aggregated, and colonies were further classified into small colonies (S, 10–50 cells), medium colonies (M, 50–100 cells) and large colonies (L, >100 cells). The total number of colonies was determined in each well of multidish.
Alkaline phosphatase (AP) activity
To detect the AP activity in MGSC-derived colonies, the medium was removed and cells were fixed in 2 % paraformaldehyde (PFA) for 10 min. Next, cells were washed three times with tris-buffered saline (TBS), and stained with BCIP/NBT AP substrate (TIANGEN, China) according to instructions of the manufacturer. After 1 h of incubation in dark, cells were viewed with Olympus IX71 (Olympus, Japan) inverted fluorescence microscope camera for AP staining.
Immunocytochemistry of cultured MGSCs
Cultured MGSCs were subjected to immunocytochemistry to detect expressions of MGSC markers. Cells were fixed in 2 % PFA for 10 min, washed three times with TBS, and permeabilized with 0.1 % Triton X-100 for 10 min. In order to block non-specific binding, after washing three times with TBS, cells were incubated with 10 % donkey serum in TBS for 2 h at room temperature. The primary antibodies used for immunocytochemistry included goat anti-UCHL1 (1: 200; Santa Cruz Biotechnology, USA), goat anti-PLZF (1: 100; Santa Cruz Biotechnology, USA), rabbit anti-THY1 (1: 300; Santa Cruz Biotechnology, USA) and rabbit anti-VASA (1: 400; Abcam, UK). Cells were incubated with one of the aforementioned primary antibodies at 4 °C overnight. After overnight incubation, cells were washed three times with TBS and incubated with donkey anti-rabbit or donkey anti-goat Texas Red-conjugated secondary antibody (1: 200; Santa Cruz Biotechnology, USA) for 30 min, at 37 °C. After washing three times with TBS, cells were labeled with 4, 6-diamidino-2-phenylindole (DAPI, 1: 1000; Beyotime, China), and viewed with Olympus IX71 (Olympus, Japan) inverted fluorescence microscope camera.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of MGSCs
To detect the expression of marker genes of MGSCs, total RNA from freshly isolated MGSCs and cultured MGSC colonies was isolated by Trizol reagent (Invitrogen, USA), respectively. gDNA Eraser (RT reagent Kit With gDNA Eraser; TaKaRa, Japan) was used to eliminate contaminating genomic DNA, then RNA was reversely transcribed with RT Primer Mix and RT Enzyme Mix I (RT reagent Kit With gDNA Eraser; TaKaRa, Japan) according to instructions of the manufacturer. The amplification of resulting cDNA was carried out by conventional polymerase chain reaction (PCR), using Taq MasterMix (CWBIO, China). Specific primers for PCR and other relevant information were shown in Table 1. The PCR products were run on 2 % agarose gels, stained with ethidium bromide (EB) and visualized under ultraviolet (UV) light.
Table 1.
Specific primers used for PCR and other relevant information
| Gene | Primer sequence (5′-3′) | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|
| UCHL1 | F: CTCTATGAACTTGATGGTCGGA | 57 | 191 |
| R: ACGGGGATAAAGCGAAGG | |||
| PLZF | F: CGCCCAGGAAATCTACCC | 59 | 194 |
| R: ATGACCACATCGCACAGC | |||
| NANOG | F: ACATCCTGAACCTTAGCTACAA | 57 | 341 |
| R: AACATAGTTGTTGAGCTGGCTA | |||
| β-actin | F: TCCCTGGAGAAGAGCTACGA | 59 | 154 |
| R: CGCACTTCATGATCGAGTTG |
Xenotransplantation of porcine MGSCs into immunodeficient mouse testes
Xenotransplantation assay was performed to probe the stem cell potential of MGSCs as previously described [10, 18]. Freshly isolated germ cells and cultured colonies were transplanted into testes of recipient Balb/c nude (nu/nu) mice, respectively. The recipient mice were treated with busulfan (40 mg/kg) 1 month before transplantation to devastate endogenous spermatogenesis. The injected donor cells were pre-labeled with fluorescent dye PKH26 (PKH26 Red Fluorescent Membrane Linker Dye; Sigma-Aldrich, USA), and re-suspended in DMEM (high glucose; Hyclone, USA) for xenotransplantation. Two months after transplantation, the recipient mouse testes were examined to detect the stem cell potential of MGSCs as PKH26-labeled cell colonies emanated from the donor MGSCs.
Statistical analyses
Overall statistical analyses were carried out by SPSS v17.0 software (SPSS, USA). Multiple comparisons were conducted using one-way analysis of variance (ANOVA) followed by least-significant difference (LSD) test. Data were presented as mean ± standard error of the mean (SEM) and differences were considered significant at P < 0.05.
Results
Comparison of cultures under different serum concentrations
Freshly isolated testicular cells (Fig. 1a) were first subjected to differential plating to remove surplus somatic cells. The ratio of putative male germline stem cells (UCHL1+ cells) improved from 5 % to 16 % via differential plating. To probe the effect of serum on primary culture of testicular cells from piglets, non-adhering cells were cultured in media supplemented with different concentrations of serum (0, 1 %, 2 %, 5 %, 10 %). With time, MGSC-derived colonies (Fig. 1b), which showed AP activity (Fig. 1c), formed in all treatment groups except the one without serum. Colonies were classified into three categories according to their sizes (Fig. 1d–f). There was no marked difference among groups for the number of either medium or large colonies. However, small colonies formed in the group of 10 % FBS were significantly fewer than those developed in the group of 1 % FBS, though the numbers of small colonies were not significantly different among other groups (Fig. 2). Consequently, 1 % FBS in media was used for further explorations.
Fig. 1.
Images of male germ cells from piglets. a Freshly isolated testicular cells. b MGSC-derived colonies after 10 days of culture. c Positive AP reactivity of colonies. d Small colony (10–50 cells aggregated). e Medium colony (50–100 cells aggregated). f Large colony (>100 cells aggregated). Scale bars = 50um
Fig. 2.
Comparison of colony formation under different concentrations of serum after 10 days of culture. Columns without a common superscript letter represent significant differences (P < 0.05). Data are presented as mean ± S.E.M. of 4 independent experiments
Effects of growth factors on in vitro propagation of porcine MGSCs
In order to study the effects of growth factors on in vitro propagation of porcine MGSCs, the subculturing cells were maintained in media supplemented with different concentrations of growth factors. Accordingly, the number of MGSC-derived colonies with different sizes was determined in each group after 10 days. Rather unexpectedly, no colony formation was observed at the concentration of 10 ng/ml bFGF. Compared with the control group (no addition of growth factors), more large colonies developed in treatment groups with either bFGF at 1, 5 ng/ml or EGF at 5, 10 ng/ml, and the maximum number of large colonies was shown at dose of 10 ng/ml EGF. There was no significant difference between the control group and the group with the addition of 1 ng/ml EGF in terms of the number of colonies (Fig. 3).
Fig. 3.
Comparison of colony formation under different addition of growth factors after 10 days of subculture. Columns without a common superscript letter (i.e. a, b or x, y or A–D) represent significant differences (P < 0.05). Data are presented as mean ± S.E.M. of 3 independent experiments
Immunocytochemical analysis of cultured MGSCs
Immunocytochemical staining was applied for characterization of MGSC-derived colonies with different sizes. Markers for undifferentiated spermatogonia from piglets, UCHL1, PLZF [23] and THY1 [20], were expressed in the colonies. VASA, a marker of differentiating spermatogonia [22], was also expressed in the colonies (Fig. 4). These observations indicated that the MGSC-derived colonies were mixtures of undifferentiated and differentiating spermatogonia.
Fig. 4.
Immunocytochemical staining of colonies for UCHL1, PLZF, THY1 as well as VASA after 20 days of culture. Scale bars = 50um
RT-PCR analysis of MGSCs
To detect the transcripts of markers for MGSCs, RNA was isolated from fresh testicular cells (after differential plating) and MGSC-derived colonies after 20 days of culture. RT-PCR analysis demonstrated that UCHL1, PLZF and NANOG, which characterized undifferentiated spermatogonia in pigs [6, 23], were expressed in fresh testicular cells and cultured MGSCs (Fig. 5).
Fig. 5.

Gene expressions of freshly isolated testicular cells (a) and MGSCs after 20 days of culture (b). β-actin was used as a reference marker, while “–” represented a negative control (no cDNA)
Xenotransplantation assay of porcine MGSCs
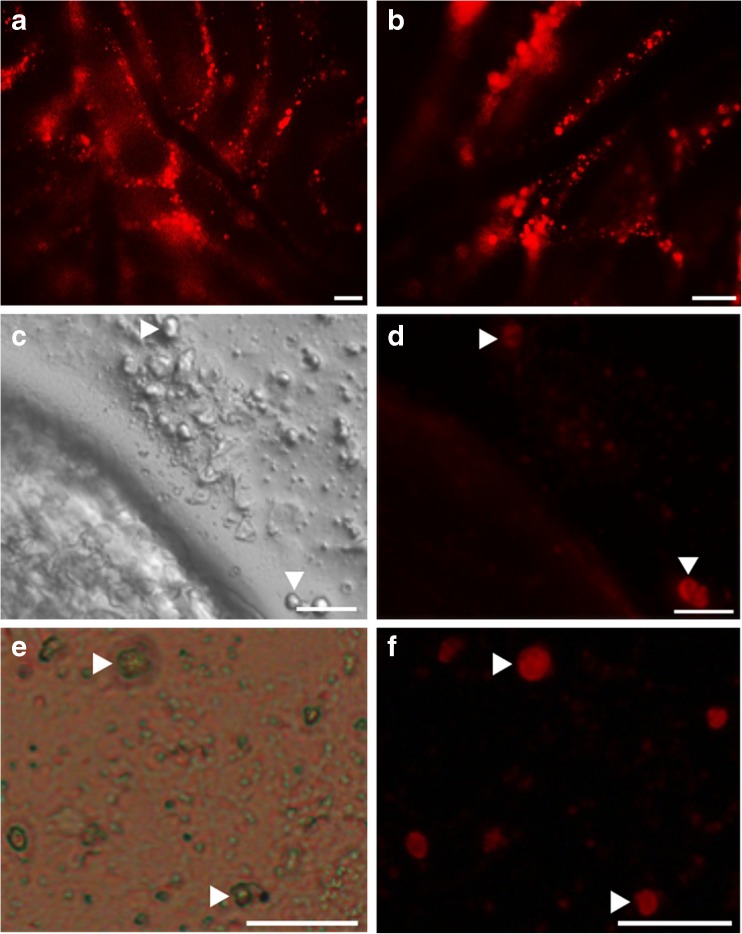
A xenotransplantation technique was performed ultimately to evaluate the stem cell potential of isolated germ cells and cultured MGSCs. To make the donor cells discernible after transplantation, cells were pre-labeled with a red fluorescent dye PKH26. Both freshly isolated cells and MGSCs after 1 month of culture colonized in the busulfan-treated recipient mouse testes 2 months after transplantation (Fig. 6), indicating that cultured cells still possessed stem cell potential. In addition, no tumor or teratoma formation was observed in any of the recipient mice.
Fig. 6.
Xenotransplantation of porcine MGSCs into recipient mouse testes. a, b Micrographs of the seminiferous tubules in recipient mouse testes in which isolated germ cells (a) and cultured MGSCs (b) colonized 2 months after transplantation. Scale bars = 100um. (d, f) Images of cultured MGSCs (red) in recipient seminiferous tubules under light (c, e) and fluorescent (d, f) microscopes. Arrowheads indicate the PKH26-labeled donor cells. Scale bars = 50um
Discussion
Male infertility has been an issue concerning human health. MGSCs are of great potential for restoration of male fertility. In addition, it is promising to apply MGSCs for generating transgenic animals, especially those with improved productivity, enormously commercial and medical value. Since neither a porcine SSC line nor a long-term culture of porcine SSCs is currently available to provide abundant cells for research, our successful attempt that maintained in vitro propagation of porcine MGSCs for 1 month would facilitate studies in porcine SSCs.
In this study, we compared the primary culture of MGSCs under different serum concentrations. Serum plays an important role in cell culture as it provides basic nutrition as well as multiple hormones and cytokines to stimulate cell proliferation, and shields cells from physical or chemical impairment. However, serum complicates culture system as it contains undefined factors and often varies from batch to batch [4, 14, 15, 24]. In addition, high concentration of serum in culture resulted in overwhelming somatic cells and abrogated proliferation of germ cells in mice and goats [3, 15]. Therefore, it is reasonable to reduce serum concentration. Our results showed that 10 % FBS indeed reduced MGSC colony formation, while a serum concentration as low as 1 % could maintain the propagation of porcine MGSCs at a comparable efficacy. In future, the specific components of serum as well as its interactions with culture microenvironment need to be further probed, and the application of Knockout Serum Replacement (KSR) could be taken into account to establish long-term cultures of porcine MGSCs.
Next, we studied the effects of growth factors on propagation of porcine MGSCs in vitro. To reduce the interference of somatic cells, the subculturing MGSCs are maintained in media supplemented with growth factors. Glial cell line-derived neurotrophic factor (GDNF) has far been demonstrated to play vital roles in SSC self-renewal and long-term cultures of SSCs across species [2, 9, 16, 17, 19, 21, 25–27, 32]. However, we observed that GDNF showed little positive effects on porcine MGSC colony formation (data not shown), which is in line with the previous report by Kuijk et al. [20]. Otherwise, little is known about the effects of other growth factors on cultured SSCs from domestic animals including pigs. In our study, the number of large colonies significantly increased in experimental groups with EGF at 5, 10 ng/ml, and when the dose of EGF increased to 10 ng/ml, the number of large colonies reached a peak. This is in agreement with the previous findings describing the positive effects of EGF on the cultured SSCs from mice, rats and pigs [15, 20, 25].
bFGF, as well as EGF, has far been used to stimulate in vitro proliferation of MGSCs from mice, rats, pigs and bulls [2, 15, 19, 20, 25]. In our study, despite the addition of bFGF at 1, 5 ng/ml significantly promoted the formation of large colonies, a devastating effect of bFGF at 10 ng/ml was observed. This is inconsistent with the previous report [20]. From our perspective, this discrepancy might be due to differences in ages, species, culture conditions and cytokine manufacturers.
Using xenotransplantation assay, the presence of functional stem cells among cultured cell population was corroborated. Only cells with stem cell potential are able to colonize on the basement membrane of recipient seminiferous tubules after transplantation [5]. In the present study, MGSCs after 1 month of culture as well as freshly isolated germ cells colonized in the recipient mouse testes 2 months after transplantation. To our knowledge, it is the first time to colonize the mouse seminiferous tubules with subcultured porcine MGSCs. Our endeavor will contribute to the establishment of a long-term culture of porcine MGSCs as well as the manipulation of stem cells in vitro.
Acknowledgments
We thank Yangling Guangming pig farm for providing porcine testis tissues. This work was supported by the National Natural Science Foundation of China (Grant No. 31072029, No. 31272439), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars from Northwest A&F University.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Footnotes
Capsule
This study showed the optimum concentrations of serum and growth factors for propagation of porcine male germline stem cells (MGSCs) in vitro and finally established a culture system for these stem cells.
References
- 1.Abu Elhija M, Lunenfeld E, Schlatt S, Huleihel M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J Androl. 2012;14:285–93. doi: 10.1038/aja.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aponte PM, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136:543–57. doi: 10.1530/REP-07-0419. [DOI] [PubMed] [Google Scholar]
- 3.Bahadorani M, Hosseini SM, Abedi P, Hajian M, Hosseini SE, Vahdati A, et al. Short-term in-vitro culture of goat enriched spermatogonial stem cells using different serum concentrations. J Assist Reprod Genet. 2012;29:39–46. doi: 10.1007/s10815-011-9687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes D, Sato G. Serum-free cell culture: a unifying approach. Cell. 1980;22:649–55. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- 5.Dores C, Alpaugh W, Dobrinski I. From in vitro culture to in vivo models to study testis development and spermatogenesis. Cell Tissue Res. 2012;349:691–702. doi: 10.1007/s00441-012-1457-x. [DOI] [PubMed] [Google Scholar]
- 6.Goel S, Fujihara M, Minami N, Yamada M, Imai H. Expression of NANOG, but not POU5F1, points to the stem cell potential of primitive germ cells in neonatal pig testis. Reproduction. 2008;135:785–95. doi: 10.1530/REP-07-0476. [DOI] [PubMed] [Google Scholar]
- 7.Goel S, Fujihara M, Tsuchiya K, Takagi Y, Minami N, Yamada M, et al. Multipotential ability of primitive germ cells from neonatal pig testis cultured in vitro. Reprod Fertil Dev. 2009;21:696–708. doi: 10.1071/RD08176. [DOI] [PubMed] [Google Scholar]
- 8.Goel S, Sugimoto M, Minami N, Yamada M, Kume S, Imai H. Identification, isolation, and in vitro culture of porcine gonocytes. Biol Reprod. 2007;77:127–37. doi: 10.1095/biolreprod.106.056879. [DOI] [PubMed] [Google Scholar]
- 9.Hamra FK, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DL. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci U S A. 2005;102:17430–5. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod. 2002;66:21–8. doi: 10.1095/biolreprod66.1.21. [DOI] [PubMed] [Google Scholar]
- 11.Hwang K, Lamb DJ. New advances on the expansion and storage of human spermatogonial stem cells. Curr Opin Urol. 2010;20:510–4. doi: 10.1097/MOU.0b013e32833f1b71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang FX. Male germ cell transplantation: promise and problems. Reprod Fertil Dev. 2001;13:609–14. doi: 10.1071/RD01059. [DOI] [PubMed] [Google Scholar]
- 13.Jiang FX, Short RV. Male germ cell transplantation: present achievements and future prospects. Int J Dev Biol. 1998;42:1067–73. [PubMed] [Google Scholar]
- 14.Kanatsu-Shinohara M, Inoue K, Ogonuki N, Morimoto H, Ogura A, Shinohara T. Serum- and feeder-free culture of mouse germline stem cells. Biol Reprod. 2011;84:97–105. doi: 10.1095/biolreprod.110.086462. [DOI] [PubMed] [Google Scholar]
- 15.Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, et al. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–91. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 16.Kanatsu-Shinohara M, Muneto T, Lee J, Takenaka M, Chuma S, Nakatsuji N, et al. Long-term culture of male germline stem cells from hamster testes. Biol Reprod. 2008;78:611–7. doi: 10.1095/biolreprod.107.065615. [DOI] [PubMed] [Google Scholar]
- 17.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–6. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 18.Kim BG, Cho CM, Lee YA, Kim BJ, Kim KJ, Kim YH, et al. Enrichment of testicular gonocytes and genetic modification using lentiviral transduction in pigs. Biol Reprod. 2010;82:1162–9. doi: 10.1095/biolreprod.109.079558. [DOI] [PubMed] [Google Scholar]
- 19.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–94. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuijk EW, Colenbrander B, Roelen BA. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. 2009;138:721–31. doi: 10.1530/REP-09-0138. [DOI] [PubMed] [Google Scholar]
- 21.Lim JJ, Sung SY, Kim HJ, Song SH, Hong JY, Yoon TK, et al. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. 2010;43:405–17. doi: 10.1111/j.1365-2184.2010.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo J, Megee S, Dobrinski I. Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. J Cell Physiol. 2009;220:460–8. doi: 10.1002/jcp.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev. 2006;73:1531–40. doi: 10.1002/mrd.20529. [DOI] [PubMed] [Google Scholar]
- 24.Nagano MC. Techniques for culturing spermatogonial stem cells continue to improve. Biol Reprod. 2011;84:5–6. doi: 10.1095/biolreprod.110.088864. [DOI] [PubMed] [Google Scholar]
- 25.Ryu BY, Kubota H, Avarbock MR, Brinster RL. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc Natl Acad Sci U S A. 2005;102:14302–7. doi: 10.1073/pnas.0506970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305:2416–8. doi: 10.1001/jama.2011.791. [DOI] [PubMed] [Google Scholar]
- 27.Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, et al. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302:2127–34. doi: 10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- 28.Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–50. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-D. [DOI] [PubMed] [Google Scholar]
- 31.Walters EM, Wolf E, Whyte JJ, Mao J, Renner S, Nagashima H, et al. Completion of the swine genome will simplify the production of swine as a large animal biomedical model. BMC Med Genomics. 2012;5:55. doi: 10.1186/1755-8794-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Z, Falciatori I, Molyneux LA, Richardson TE, Chapman KM, Hamra FK. Spermatogonial culture medium: an effective and efficient nutrient mixture for culturing rat spermatogonial stem cells. Biol Reprod. 2009;81:77–86. doi: 10.1095/biolreprod.108.072645. [DOI] [PMC free article] [PubMed] [Google Scholar]