Abstract
Purpose
To highlight emerging techniques aimed at improving oocyte cryopreservation.
Methods
Review of available and relevant literature through Pubmed and Medline searches.
Results
Oocyte cryopreservation is an increasingly common procedure utilized for assisted reproduction and may benefit several patient populations. Therefore, improving efficiency is paramount in realizing the tremendous promise of this approach. However, in addition to numerous studies looking to improve oocyte cryopreservation efficacy via examination of variables involved with protocol methodology, such as type/concentration of cryoprotectant (CPA), type of storage device, or cooling/warming rates, there are more novel approaches for improvement. These alternate approaches include utilizing different the stages of oocytes, examining alteration of basal media and buffer composition, optimizing CPA exchange protocols and device loading through use of automated technology, as well as examination/manipulation of oocyte cellular composition to improve cryotolerance. Finally, elucidating more accurate or insightful indicators of “success” is crucial for continued improvement of oocyte cryopreservation.
Conclusion
Oocyte cryopreservation has improved dramatically in recent years and is receiving widespread clinical use. Novel approaches to further improve success, as well as improved methods to assess this success will aid in continued improvement.
Keywords: Oocyte, Vitrification, Cryopreservation, Meiotic stages, Microfluidics, Composition, Membrane, Buffers
Introduction
Oocyte cryopreservation is an increasingly common procedure utilized for assisted reproduction and holds tremendous promise for several patient populations. Oocyte storage permits fertility preservation for a variety of individuals, including those experiencing fertility threatening disease or treatments, those who wish to delay reproduction, as well as those who may be unable to preserve preimplantation embryos for social, medical, ethical, or legal reasons. Furthermore, oocyte cryopreservation provides logistical alternatives for cycle management, permits disease testing, and facilitates emerging services such as donor egg banks.
Not surprisingly, increasing amounts of research have focused on improving oocyte cryopreservation efficiency. However, unique aspects of the oocyte make it a notoriously difficult cell to cryopreserve. For example, the volume-to-surface ratio of the oocyte is greater than other cells, thus complicating the dehydration process [5, 73, 74, 100]. Also, membrane characteristics and permeability differences appear to make the oocyte more sensitive to chilling injury [73, 97]. It is well known that oocyte cryopreservation induces premature cortical granule release causing zona hardening that interferes with fertilization, necessitating use of ICSI [20, 35, 38, 40, 64–66, 69, 92]. Additionally, due to the high rates of oocyte derived human aneuploidy, alterations to the oocyte meiotic spindle in response to low temperatures from cryopreservation [36, 56, 86] are concerning with regard to potential impact on chromosome remodeling. Thus, while successful methods of embryo cryopreservation may be a valid starting point in exploring methods for oocyte cryopreservation, these unique oocyte qualities need to be considered when adopting or altering methodology to improve outcomes.
Though controlled-rate or slow-rate freezing of oocytes can be successful [9–11], vitrification has emerged as the apparent preferred approach that yields exceptionally high survival rates and successful live births [19, 82]. Numerous review articles have summarized the principles behind both slow-rate cooling and vitrification of cells, including oocytes [9, 44, 58, 74, 81, 82]. These reviews tend to extensively cover traditional approaches aimed at optimizing these two cryopreservation approaches, which include alterations in solution equilibration temperature, use and optimization of numerous penetrating and non-penetrating CPAs and their combinations, as well as application of various containers that can impact cooling and warming rates. These approaches have resulted in oocyte survival rates of ~90 %, and assisted reproductive outcomes approaching those of fresh oocyte cycles.
However, in addition to these widely examined improvement methods, there may be more novel approaches that could further improve oocyte cryopreservation efficiency. This review focuses on these emerging techniques and presents the preliminary data for methodologies that include altering the stage of meiotic stage of oocyte cryopreserved, altering media and buffer composition to alleviate pH stress, optimizing CPA exchange protocols and device loading through use of automated technology, as well as examining/manipulating oocyte cellular composition to improve cell selection and/or cryotolerance.
Oocyte stages for cryopreservation
Traditionally, metaphase II (MII) oocytes are selected for cryopreservation. However, as mentioned, temperature sensitivity of the meiotic spindle at this stage may be of concern in regard to fidelity of chromosome remodeling and oocyte derived embryonic aneuploidy. To review, the quiescent oocyte at prophase of meiosis I, also known as a germinal vesicle intact oocyte (GV-intact), resumes meiosis, undergoes dissolution of the nuclear membrane, then progresses to MI, anaphase I, telophase I and re-arrests at MII until fertilization occurs. During this time, the meiotic spindle is first dispersed with a pole located near the oocyte periphery. As meiosis resumes and the GV dissolves, the MI spindle forms the familiar bipolar structure as it gathers on the metaphase plate. The spindle then facilitates chromosomal separation and segregation as the cell progresses to MII.
Importantly, the meiotic spindle is a dynamic conglomerate of tubulin and associated proteins that are in a constant state of flux as microtubules/proteins depolymerize and re-polymerize. Decreases in temperature appear to shift this dynamic equilibrium so that at least a portion of these spindle components depolymerize, though it should be mentioned that increasing temperature permits apparent normal re-polymerization [37, 56]. Importantly, as mentioned, not all stages of oocytes have distinctly formed/organized meiotic spindles and, interestingly, not all stages of oocytes appear to have the same level of sensitivity to thermal effects on the meiotic spindle. For example, it was shown that telophase I mouse oocytes maintained more spindle stability when cooled to 4 °C for 10 min compared to both MI and MII oocytes, as indicated by polarized light microscopy [36].
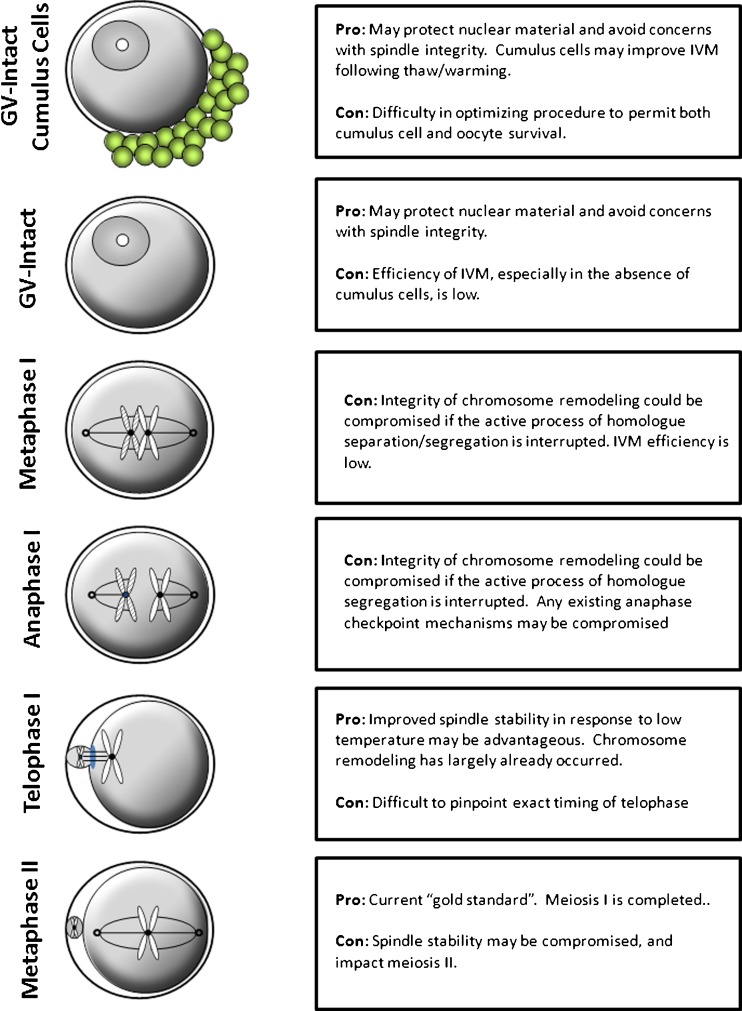
Certainly, spindle stability has previously been examined as a method to improve developmental competence following cryopreservation. Several studies have examined use of pharmacologic compounds, such as Taxol, to manipulate the stability of the meiotic spindle in the hopes of improving cryotolerance [31, 62, 63, 79]. However, the use of pharmacologic compounds may not be clinically feasible. Regardless, if a specific meiotic stage of oocyte displays greater/less spindle integrity, this lays the basic groundwork and promotes the question of whether one stage of oocyte meiotic progression may be better suited to withstand the thermal stresses or other insults conveyed by the cryopreservation process [12] (Fig. 1).
Fig. 1.
Cryopreservation of oocytes at different meiotic stages may offer more treatment options and potentially improve survival/outcomes due to unique characteristics of cells at the various stages. Each cell stage has positive and negative properties that may make it appealing for cryopreservation
GV-intact oocytes lack a distinct meiotic spindle, and genetic material is confined within the nuclear envelope. Thus, cryopreservation at this stage may provide a means of mitigating damage from cryopreservation and of improving oocyte survival. However, early studies indicated difficulty with preserving human GV-intact oocytes, as evidenced by low rates of success [89, 90]. Current survival rates of vitrified GV oocytes after warming are in the range of 55 to 75 % [2, 18]. In at least one study, vitrification of GV oocytes had no apparent impact on meiosis resumption as these oocytes achieved full nuclear maturation up to metaphase II post-warming and maintained normal methylation of genes such as H19DMR and KvDMR1 [2]. As an additional benefit, GV-oocytes can be obtained from unstimulated or minimally stimulated cycles, which may protect the patient from detrimental conditions associated with hormone stimulation, and is less expensive. Of course, efficient in vitro oocyte maturation (IVM) is also a pre-requisite for GV-oocyte cryopreservation and carries with it concerns of its own. Cryopreserved oocytes mature less efficiently than their fresh counterparts [15, 28]. Furthermore, presence of cumulus cells is a factor to consider, as their presence improves oocyte maturation, however they may not survive cryopreservation efficiently in protocols optimized for oocyte survival [87]. Currently, only one live birth has been reported following cryopreservation of human GV-oocytes that were subsequently matured in vitro before ICSI [93]. Thus, at this time, efficiency of GV-intact cryopreservation is still inferior to MII oocytes
Alternatively, whether cryopreservation of telophase oocytes, due to their apparent improved spindle stability at low temperature, may permit superior survival rates or outcomes compared to traditional MII oocytes remains unknown. Interestingly, it was reported that slow rate freezing of oocytes within 2 h of retrieval resulted in higher embryo quality, pregnancy and implantation rates compared to oocytes frozen after 2 h [68]. Because oocytes are retrieved at a time prior to ovulation, their final maturation is completed in vitro. Telophase oocytes do display a polar body. Thus, it is feasible that a portion of these oocytes frozen <2 h after retrieval were still at the telophase I stage. Very little information exists as to how metaphase I or anaphase I oocytes tolerate cryopreservation. It should also be mentioned that fertilization following thawing of cryopreserved oocytes would need to be optimized to ensure proper maturational status and fertilization [22, 45]. This would be easier than maturation of cryopreserved GV-intact oocytes, as specialized IVM media would not likely be required.
Media
Much of the attention on media used for cryopreservation focuses on the type, concentration and exposure timing of cryoprotectants (CPAs). CPAs function as their name implies, guarding against cryo-induced cellular damage, including mechanical damages from ice crystal formation. Several CPAs exist, both permeating and non-permeating, and have been reviewed elsewhere [88], and a thorough examination of each compound is beyond the scope of this review. However, it is clear that not all CPAs function equally well with the delicate oocyte. This likely stems from a balance between the CPA’s permeability in conjunction with the size and unique permeability of the oocyte membrane, as well as the CPA’s toxicity. As a result, some CPAs have received more widespread use than others, and this certainly remains an area for future improvement
Use of novel macromolecules and synthetic polymers also hold potential for improving oocyte cryopreservation. Studies examining use of ficoll [16, 23], fetuin [42] and hyaluronan [54] have all shown promise in improving cryopreservation outcomes, including that of oocytes. Use of these compounds and novel methods for their cellular loading, like microinjection, have been discussed elsewhere [88].
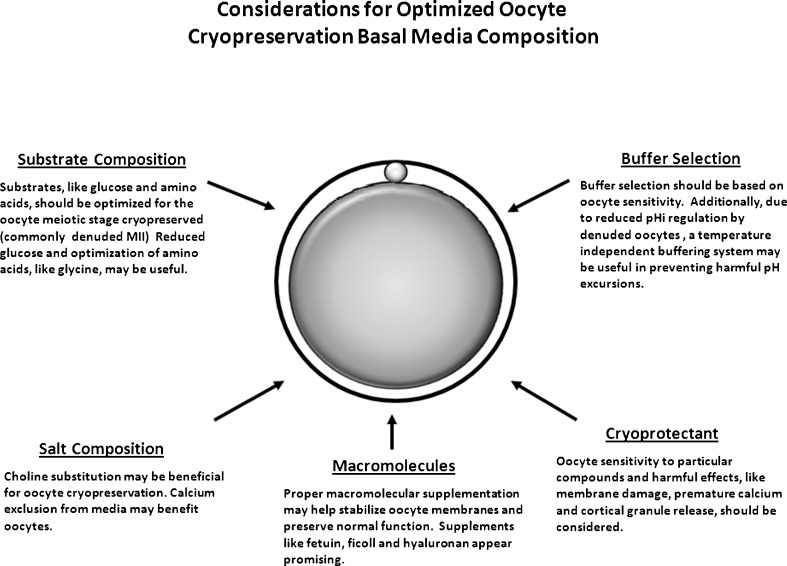
A less well-studied aspect of improving oocyte cryopreservation includes consideration of the basal medium utilized (Fig. 2). For example, many media utilize a standard HEPES or MOPS handling base, which are often formulated for various stage embryos and include elevated levels of glucose. Denuded oocytes do not have the same metabolic requirements as embryos and levels of glucose or other substrates should likely be adjusted. Other unique oocyte physiologic requirements, such as amino acid complement, should also be considered. The oocyte has unique methods of regulating cell volume [6, 102], and this is a critical consideration due to the extreme osmolality changes experienced during exposure to and removal from solutions used in cryopreservation. Due to the role of amino acids as osmolytes, the correct amino acids should also be included. For example, glycine is a potential osmolyte and elevated levels have been found beneficial for oocyte vitrification, safeguarding mitochondrial function [98].
Fig. 2.
Use of oocyte specific cryoprotectants, aimed at addressing oocyte membrane permeability characteristics and other unique physiologic properties, is critical in optimizing outcomes. Similarly, consideration to oocyte requirements should be given selecting the basal media formulation, including substrate and salt composition. Though use of a single basal media and cryopreservation system for preimplantation embryos of various stages and oocytes may be convenient, conditions should be optimized based on the physiology of the oocyte
In addition to energy substrate requirements, which should likely be optimized based on oocyte physiology, rather than using basal medium formulation for various stages of preimplantation embryos, consideration should be given to other components of the basal media. Lest we forget, base media can also impact the toxic effects of CPAs [49]. For example, choline replacement of sodium chloride improved oocyte slow-rate cooling [84, 85]. Additionally, due to the role of calcium oscillations in oocyte development and function, including a role if cortical granule exocytosis, and the potential for cryopreservation to elicit or interfere with these oscillations, depletion of calcium from oocyte cryopreservation media may be beneficial and prudent [57]. Using a Ca+2 free media, it was reported that vitrified mouse oocytes had less zona hardening and improved fertilization and embryo development.
In a similar fashion, other basal media alterations may be important. Specifically, basal media contain zwitterionic buffers to stabilize pH and prevent detrimental perturbations in hydrogen ion concentration that could compromise oocyte function. It is known that improper pH can impact embryo development, metabolism and fetal development [53, 83, 99]. It can also disrupt organization of the cytoskeleton and mitochondria [70, 83]. Similar impacts may be apparent in the oocyte as well. This likelihood is increased when one considers the fact that the denuded mature oocyte, the stage most commonly cryopreserved, lacks robust mechanisms to regulate internal pH [29, 30] and is thus heavily reliant upon stability external pH of the media. Additionally, vitrification can result in a temporary lapse in membrane bound regulatory mechanisms aimed at regulating internal pH within the embryo [53]. Thus, vitrification likely further compromises what little ability the denuded mature oocyte has to regulate its internal pH. Therefore, maintenance of a stable and appropriate external pH during oocyte cryopreservation is especially important.
Because different media and their buffers respond differently to the freeze process, some leading to large perturbations in pH [96], this could be problematic for slow-rate protocols, which may expose cells to pH perturbations over time. For example, use of a phosphate buffered medium appears to become acidic when cooled, while buffers such as HEPES or MOPS result in alkalinization as evidenced by the colormetric pH indictor phenol red [96]. Formulation of a temperature independent buffered medium though combination of buffers may be beneficial in stabilizing pH during the cooling process. Indeed, such an approach was utilized for cooling of somatic cells [80] and similar approach may be useful for oocytes. Alternatively, specific buffers may convey other beneficial effects independent of their pH stabilizing ability. Sperm cryosurvival and membrane damage is differentially impacted by different buffers [14, 21, 33, 39, 96]. The same may hold true for oocytes. For example, it was reported that MOPS was superior to HEPES in vitrifying human embryos, though the rationale for this is unclear and other variables existed [24]. Additionally, TES may be a beneficial buffer for cryopreservation due to structural similarity to some cryoprotectants [47].
Therefore, selection of specific buffers based on oocyte sensitivity, exploration of novel buffer combinations to mitigate pH changes and resulting oocyte stress, as well as inclusion of these buffers in a basal medium formulated on the metabolic needs of the oocytes may help further refine and improve current oocyte cryopreservation methodologies.
Optimized CPA exposure and loading
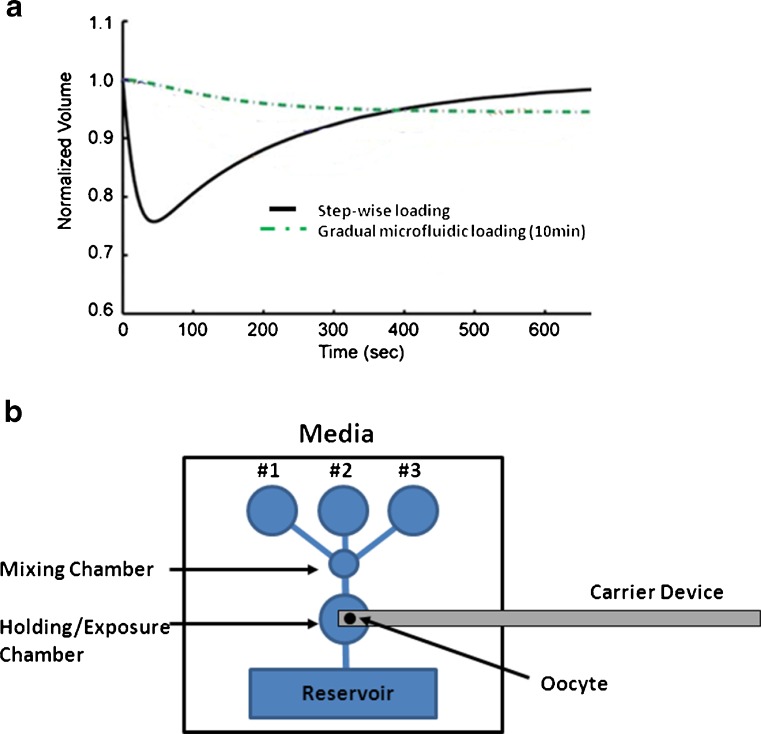
Perhaps as important as CPAs, the conditions of CPA exposure can impact cell function and survival; with factors like concentration and time of exposure as crucial variables [96]. This is especially true for vitrification approaches, which utilized high cryoprotectant concentrations. It thought that optimizing CPA exchange procedures might help reduce sub-lethal oocyte damage (Fig. 3).
Fig. 3.
Optimized loading of cryoprotectants (CPA) may improve oocyte cryosurvival by mitigating stresses due to rapid media exchange and resulting osmolality shifts. a A microfluidic device has been shown to be able gradually load CPA into mouse oocytes and prevent rapid volume excursions normally experience with current manual methods of moving oocytes between separate media (0 mM PROH to 1.5 mM PROH; adapted from [41]). Additionally, this approach may reduce stress due to physical movement of the oocyte. b Schematic of a theoretical device to automate and optimize oocyte CPA mixing and loading by flowing media over the oocyte. Incorporation of a carrier device into the CPA loading chamber may alleviate variability and reduce errors with device loading. Following exposure, the loading device can be removed and immediately cooled/vitrified. A similar gradual mixing device may be useful during thawing/warming to optimize CPA unloading
Importantly, practical issues exist with current CPA exposure regimes that limit preciseness and repeatability. Most protocols give broad time ranges for cell exposure. Also, discrete manual dilution steps are required to make to CPA exposure feasible. However, while logistically important for the embryologist, these approaches may not ideal for the cell. Thus, it is clear that currently, laboratories lack the tools needed to perform CPA exchange precisely at the cellular level.
Although the three-step CPA exchange concept is now broadly accepted for oocyte vitrification, the exact procedure differs from lab to lab contributing to the variability in cryopreservation success rates. More fundamentally, there is a problem that there are more potential combinations of CPAs and CPA exchange procedures that could be utilized than can be efficiently and reliably tested for by trial and error experiments alone. The approach of using specialized bioengineered devices to design vitrification CPA exchange protocols, where manual dilutions do not limit efficacy, might help alleviate both the osmotic shock (mechanical effects) and CPA toxicity (chemical effects). A more gradual, rather than sudden stepwise increases, in CPA concentration is known to increase viability [51]. There is a practical and capability limit, however, in terms of how many exposure steps and in what timing the CPA exchange can be performed using the current gold standard: manual pipetting. Furthermore, the transfer of oocytes from solution to solution for each step involves rapid pipetting that can introduce mechanical stress [51].
To address these current limitations with manual approaches, microfluidic devices have been designed where, rather than moving the oocyte from one CPA solution to another by pipetting, the oocyte is kept stationary with automated CPA solutions flow and exchange over the cell. This permits continuous and gradual cellular exposure, rather than discrete step increases in CPA concentrations. Preliminary reports indicate controlled CPA exposure with a microfluidic platform reduced oocyte size change compared to traditional approaches, which may be important for cell viability [41] (Fig. 3a). Similar approaches have been used with embryos [61].
Another potential improvement evident via automated cryoprotectant exposure is standardization of oocyte cryo-device loading. Indeed, a limitation that has hindered rapid and widespread acceptance of vitrification approaches has centered on ease-of-use issues of vitrification devices. There are numerous commercially available and in-house devices to contain oocytes for low temperature storage following vitrification [17]. These utilize very small volumes of solution to permit rapid cooling/vitrification and avoid damaging ice crystal formation. While both open and close containers can be used successfully, the combination of the low volume and rapid time need needed to load devices to prevent toxicity from CPA exposure can be problematic, especially compared to 0.25 cc straws used with slow-rate approaches. A novel platform regulating solution exposure to oocytes, that also integrates a freezing device, could simply bypass the current step-wise procedure and eliminate errors with manual dilutions and movement of cells through various media and loading of complicated devices (Fig. 3b).
Oocyte composition
While several attempts to improve oocyte cryopreservation have focused on technical approaches or methodology involving CPA exposure regimes, cooling/warming rates or vessels to store oocytes, the inherent cell composition is a crucial variable to consider. Components of the cell that control membrane fluidity and/or regulate water/CPA exchange will impact dynamics of the freezing/vitrification process and influence mechanical stresses experienced by the cell (Table 1).
Table 1.
Oocyte composition modifications and approaches used that may improve cryotolerance
| Composition modification | Approach | Cell type/Species | Reference |
|---|---|---|---|
| Cytoplasmic lipid reduction | Micromanipulation/mechanical removal | Oocyte/Porcine | [7, 67] |
| Media adjustment/pharmacologic manipulation | Embryo/Bovine | ||
| Membrane fatty acid composition alteration | Liposomes | Oocyte, Sperm/Bovine | [4, 19, 101] |
| Maturation medium supplementation | |||
| Membrane cholesterol content manipulation | Cyclodextran loading | Oocyte/Bovine | [43, 76] |
| Membrane aquaporin content | cRNA microinjection | Oocyte/Mouse | [97] |
Indeed, attempts at modifying bovine oocyte plasma membrane composition by altering the polyunsaturated fatty acid composition with liposomes to resist injury following chilling to ~16 °C or 0 °C have been attempted and appear to improve viability as determined by increased blastocyst formation [4, 101]. Similarly, maturation of bovine oocytes with medium supplemented with high levels of non-esterified fatty acids could negatively impact cryotolerance, possibly through membrane composition alterations, though this remains to be proven [78]. Conversely, increasing cholesterol content of sperm or oocyte membranes with cholesterol loaded cyclodextran also appears to improve cryotolerance [43, 76]. These factors likely convey their effects through alterations in membrane fluidity and changes in resistance to mechanical stressors.
Additional membrane compositional changes that may benefit oocytes during cryopreservation include those that regulate water permeability. Increasing mouse oocyte membrane aquaporin 3 expression via injection of cRNA appeared to increase water permeability and to helped improve cryotolerance, as measured by improved embryo development following vitrification/warming [97].
Cytoplasmic lipid content is another aspect of cell composition that influences cell cryotolerance. High cytoplasmic lipid content reduces survival of bovine and porcine embryos following vitrification and culture of embryos in the presence of compounds that reduce cytoplasmic lipid content improves cryotolerance [1, 71]. In theory, the importance of lipid content for cryotolerance may also hold true for oocytes and culture approaches may be adapted to manipulate oocyte lipid content to improve success. In support of this theory, mechanical removal of cytoplasmic lipid from GV-intact porcine oocytes improved cryosurvival following vitrification [67].
If cytoplasmic lipid content is inversely proportional to cryosurvival, then lipid content, or lack thereof, may be an important factor in the criteria for selection of oocytes that will best survive cryopreservation. Unfortunately, lipid quantification has been limited by its techniques and has yet to be a common assay integrated into the clinical setting. Current approaches for cell lipid quantification include fluorescence labeling [44, 58, 81], thin layer and gas chromatography [31, 37, 62, 63] and electron microscopy [12, 32, 79]. However, these destructive techniques are unable to be used towards selections of candidate oocyte for eventual cryopreservation. Therefore, if methods of non-invasive lipid quantification can be developed and integrated into a clinical setting, this may prove useful in selecting candidate oocytes for cryopreservation and improving strategies to improve management of patient cycles. Indeed, non-invasive quantification of oocyte lipid is being explored to facilitate this endeavor. Coherent anti-raman stokes microscopy (CARSM) has been utilized to quantify lipid content of oocytes from species with known difference, including mouse, human and pig [46].
Methods of viability assessment
Despite success with oocyte vitrification, there is still much work to be completed in terms of suppressing oocyte sub-lethal damage. For example, although the intent of CPAs is to prevent damage during cooling/freezing, some of these compounds can themselves result in cellular damage. In oocytes, assessment of damage from CPAs and cooling has tended to focus on ultrastructural modifications of the meiotic spindle, actin cytoskeleton, chromosomal arrangement and other organelle distribution. Other studies have examined functional endpoints by through assessment of fertilization and embryo development. Unfortunately, adverse effects may not be so overt. The exact mechanisms by which the toxicity of cryoprotective agents arise are still being identified. Examples of damage induced by improper cell exposure to CPAs range from removal or fusing of cellular membranes to impaired enzyme activity, altered organelle structure and function, as well as perturbed protein interactions [3, 26, 27]. Detrimental effects of CPA exposure on genotoxicity, even without cryopreservation, have additionally been demonstrated [8]. Even sub-lethal genotoxicity such as DNA fragmentation [60] can still cause catastrophic outcomes [25, 94]
Thus, as important as novel methods to improve oocyte cryopreservation are, determination of novel methods of endpoint viability assessment may also be useful in improving protocols (Table 2). These include molecular and biochemical markers that can be used to assess the efficacy of experimental cryopreservation approaches. Furthermore, if one wishes to apply viability assessment to selection of oocytes post thawing, then development of noninvasive techniques are also required. There are many of novel assessment techniques postulated for assessing efficacy of cryopreservation approaches, including quantification of intracellular calcium, metabolomics, proteomics, cellular imaging, assessment of hydrogen bonding and analysis of epigenetic modification.
Table 2.
Methods of oocyte viability assessment. Several invasive assessment methods exist that may be used to further improve experimental cryopreservation and thawing protocols. However, especially useful are methods that permit non-invasive assessment of oocyte viability following warming/thawing to better aid in selection of high quality oocytes and resulting embryos for subsequent clinical use
| Target | Assessment | Reference |
|---|---|---|
| Pyruvate uptake | Decreased pyruvate uptake can be used as a proxy for cryo-induced stress on the oocyte. If analytic methods are sensitive enough and turn-around times appropriate, cells could be cultured individually to aid in selection of embryos for transfer that came from higher quality oocytes. | [52] |
| LDH appearance | Lactate dehydrogenase (LDH) appearance in the media surrounding the oocyte can be used to indirectly assess cryo-induced membrane damage. | [13] |
| Meiotic spindle stability/formation | Polscope imaging allows rapid visualization of the meiotic spindle of living and unfixed MII oocyte without compromising viability. Those oocytes displaying an intact meiotic spindle may be more suitable for use. | [50, 95] |
Quantification of intracellular calcium (Cai) is one method to examine endpoint viability in the context of oocyte cryopreservation. Cai is responsible for initiating cortical granule release after sperm penetration, and therefore its analysis can be essential to the diagnosis of cellular trauma [48]. Cai oscillations can be quantified using fluorescent calcium-indicators, such as Indo-1, and resultant changes can be quantified using CCD or photomultiplier tube-based detection systems. While not likely useful for clinical selection purposes, such an approach may be useful in experimental optimization protocols. There are several reports of cryo-induced zona hardening [57, 59] secondary to Cai-induced cortical granule release, and analysis of Cai has revealed that exposure to conventional permeating cryoprotectants, such as propanediol, ethylene glycol and DMSO, all independently result in an increase in Cai, which in turn has the potential to initiate oocyte activation, culminating in zona hardening [34]. Thus, use of less harmful cryoprotectants and/or concentrations to minimize calicium release may be useful. However, utilization of intracytoplasmic sperm injection (ICSI) can bypass some of these detrimental effects on subsequent fertilization.
Another method that may be useful in not only optimizing protocols, but also as a selection tools, may be through examination of the oocyte metabolome. In a study by Lane & Gardner, non-invasive measurement of pyruvate uptake of vitrified oocytes was found to be lower than fresh oocytes, however, greater than slow-rate frozen oocytes, suggesting that pyruvate uptake can be used as a proxy for cryo-induced stress on the oocyte [52]. Similar studies have indicated that by measuring the appearance of lactate dehydrogenase (LDH) in the media surrounding the oocyte, it is possible to indirectly assess cryo-induced membrane damage [13]. Importantly, to be clinically useful, these approaches would have to be able to be performed quickly, and with the sensivity to detect levels from individually cultured oocytes if used a means of viability assessment/selection.
With recent developments in mass spectrometry, it is now possible to assess specific protein expression patterns, or the proteomes, that reflect different biologic states [72, 77]. By using surface enhanced laser desorption/ionization time-of-flight mass spectrometry it is possible to determine the proteome of small groups of oocytes [55]. The advantage of this approach is that is possible to obtain through protein chips, a comprehensive profile of proteins in gametes under different conditions, such as those frozen with different CPAs. This has been used to show major alterations with oocytes cryopreserved with slow rate freezing techniques compared to vitrification [34]. Thus, step-wise experimental approaches aimed at producing oocytes with similar proteome profiles following cryopreservation, more similar to those of fresh oocytes, may be a means of improving various protocols.
Perhaps most practically at this point, advances in imaging technology allow for morphologic assessment of the meiotic spindle and cytoskeleton without requiring cell fixation. With the advent of a polarized light microscopy, it is now possible to image the meiotic spindle of the living and unfixed MII oocyte without compromising viability [50, 95]. This allows for almost continual monitoring of the spindle during cryopreservation and could further elucidate cellular changes related to different CPAs or cryopreservation/warming protocols and perhaps identify cells that will or have “survived” the process most effectively. Interestingly, various other non-invasive imaging approaches exist that have been shown to be compatible with embryo viability and may facilitate assessment of more novel endpoints to assess oocyte viability post-warming/thaw.
Finally, epigenetic modifications can be utilized to monitor changes secondary to cryopreservation. DNA methylation and histone modifications are two epigenetic modifications that alter the functional state of chromatin and activate or repress gene activation. The epigenetic mechanism whereby one copy of a gene is silenced, leading to parental-specific expression, is known as genomic imprinting. A study by Trapphoff et al. demonstrated that ultra-rapid vitrification of mouse preantral follicles followed by long-term IVM to GV oocytes led to Snrpn LOM (1/50 strands) but not Igf2r LOM (0/15 strands) or H19 GOM (0/58 strands) in pooled vitrified oocyte [91]. In a second study, mice produced following whole ovary cryopreservation maintained normal H19 and Kcnq1ot1 methylation ratios. Unfortunately, one limitation of this study is that averaging methylation levels from three tissues from 5 to 36 mice may have obscured imprinting defects in individual mice [75]. In humans, vitrification of GV oocytes followed by short-term IVM resulted in H19 GOM in MII oocyte pools (5/29 strands), although this was not significantly different from IVM-only MII oocytes (3/34 strands) [2]. Analysis of 17 human vitrified-IVM MII oocytes showed KCNQ1OT1 LOM (1/28 strands), which again was not significantly different 20 IVM-only MII oocytes (2/37 strands) [2]. Overall, these studies as a whole suggest that freezing does not impart greater risk than IVM alone. However, little insight is given regarding possible impact of specific steps of the vitrification process on the epigenetic status of the oocytes. Additionally, practical application of this approach for oocyte selection is likely limited. Future studies using similar epigentic endpoint measurements, focusing on optimizing oocyte cryopreservation protocols may prove insightful.
Conclusion
It is apparent that oocyte cryopreservation is a valuable tool in the arsenal of ART and improving efficiency carries substantial reward. Though there may lie potential for improvement by cryopreserving oocytes at earlier meiotic stages, the most widespread approach currently used includes MII oocytes. Thus, it is likely best to focus on means to improve methodology to optimize outcomes using this meiotic stage. This may include use of novel CPAs and macromolecules, and certainly should entail use of basal media formulated for the specific needs of the oocyte, perhaps even modifying their composition or membrane structure to increase cryotolerance. Additionally, through the use of emerging technology, the current approach of manually moving oocytes through discrete steps of CPAs and loading onto/into cumbersome storage devices can be avoided. Rather, in the foreseeable future, select oocytes of the correct meiotic stage or with the proper cellular/membrane composition to better survive the stresses of cryopreservation can be chosen for cryopreservation. These oocytes can be loaded into novel chambers designed to optimize CPA exposure and also serve as loading devices to streamline procedures and reduce variability. Certainly oocyte cryopreservation has a rapidly expanding place in ART and every attempt should be made to facilitate its continued improvement.
Footnotes
Capsule Novel strategies to improve oocyte cryopreservation may permit greater efficacy and increased application.
References
- 1.Abe H, Yamashita S, Satoh T, Hoshi H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol Reprod Dev. 2002;61:57–66. doi: 10.1002/mrd.1131. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khtib M, Perret A, Khoueiry R, et al. Vitrification at the germinal vesicle stage does not affect the methylation profile of H19 and KCNQ1OT1 imprinting centers in human oocytes subsequently matured in vitro. Fertil Steril. 2011;95:1955–60. doi: 10.1016/j.fertnstert.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Anchordoguy TJ, Rudolph AS, Carpenter JF, Crowe JH. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology. 1987;24:324–31. doi: 10.1016/0011-2240(87)90036-8. [DOI] [PubMed] [Google Scholar]
- 4.Arav A, Pearl M, Zeron Y. Does membrane lipid profile explain chilling sensitivity and membrane lipid phase transition of spermatozoa and oocytes? Cryo-lett. 2000;21:179–86. [PubMed] [Google Scholar]
- 5.Arav A, Zeron Y, Leslie SB, et al. Phase transition temperature and chilling sensitivity of bovine oocytes. Cryobiology. 1996;33:589–99. doi: 10.1006/cryo.1996.0062. [DOI] [PubMed] [Google Scholar]
- 6.Baltz JM, Tartia AP. Cell volume regulation in oocytes and early embryos: connecting physiology to successful culture media. Hum Reprod Update. 2010;16:166–76. doi: 10.1093/humupd/dmp045. [DOI] [PubMed] [Google Scholar]
- 7.Barceló-Fimbres M, Seidel GE. Effects of either glucose or fructose and metabolic regulators on bovine embryo development and lipid accumulation in vitro. Mol Reprod Dev. 2007;74:1406–18. doi: 10.1002/mrd.20700. [DOI] [PubMed] [Google Scholar]
- 8.Berthelot-Ricou A, Perrin J, Di Giorgio C, et al. Assessment of 1,2-propanediol (PrOH) genotoxicity on mouse oocytes by comet assay. Fertil Steril. 2011;96:1002–7. doi: 10.1016/j.fertnstert.2011.07.1106. [DOI] [PubMed] [Google Scholar]
- 9.Boldt J. Current results with slow freezing and vitrification of the human oocyte. Reprod Biomed Online. 2011;23:314–22. doi: 10.1016/j.rbmo.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Borini A, Bianchi V, Bonu MA, et al. Evidence-based clinical outcome of oocyte slow cooling. Reprod Biomed Online. 2007;15:175–81. doi: 10.1016/S1472-6483(10)60706-7. [DOI] [PubMed] [Google Scholar]
- 11.Borini A, Coticchio G. The efficacy and safety of human oocyte cryopreservation by slow cooling. Semin Reprod Med. 2009;27:443–9. doi: 10.1055/s-0029-1241053. [DOI] [PubMed] [Google Scholar]
- 12.Borini A, Gambardella A, Bonu MA, et al. Comparison of IVF and ICSI when only few oocytes are available for insemination. Reprod Biomed Online. 2009;19:270–5. doi: 10.1016/S1472-6483(10)60084-3. [DOI] [PubMed] [Google Scholar]
- 13.Brinster RL. Lactate dehydrogenase activity in the preimplanted mouse embryo. Biochim Biophys Acta. 1965;110:439–41. doi: 10.1016/S0926-6593(65)80056-X. [DOI] [PubMed] [Google Scholar]
- 14.Brown KI, Graham EF, Crabo BG. Effect of some hydrogen ion buffers on storage and freezing of turkey spermatozoa. Poultry Sci. 1972;51:840–9. doi: 10.3382/ps.0510840. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Xing Q, Zhang Z-G, et al. Cryopreservation of immature and in-vitro matured human oocytes by vitrification. Reprod Biomed Online. 2009;19:369–73. doi: 10.1016/S1472-6483(10)60170-8. [DOI] [PubMed] [Google Scholar]
- 16.Checura CM, Seidel GE. Effect of macromolecules in solutions for vitrification of mature bovine oocytes. Theriogenology. 2007;67:919–30. doi: 10.1016/j.theriogenology.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 17.Chian R-C, Quinn P (2010) Fertility Cryopreservation.
- 18.Chung HM, Hong SW, Lim JM, et al. In vitro blastocyst formation of human oocytes obtained from unstimulated and stimulated cycles after vitrification at various maturational stages. Fertil Steril. 2000;73:545–51. doi: 10.1016/S0015-0282(99)00546-4. [DOI] [PubMed] [Google Scholar]
- 19.Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96:277–85. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Coticchio G, Borini A, Distratis V, et al. Qualitative and morphometric analysis of the ultrastructure of human oocytes cryopreserved by two alternative slow cooling protocols. J Assist Reprod Gen. 2010;27:131–40. doi: 10.1007/s10815-010-9394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crabo BG, Brown KI, Graham EF. Effect of some buffers on storage and freezing of boar spermatozoa. J Anim Sci. 1972;35:377–82. doi: 10.2527/jas1972.352377x. [DOI] [PubMed] [Google Scholar]
- 22.Dib LA, de Araújo MCPM, Giorgenon RC, et al. Apparently matured oocytes injected in telophase I have worse outcomes from assisted reproduction. Rev Bras Ginecol Obstetrícia: Rev Federação Bras Sociedades Ginecol Obstetrícia. 2012;34:203–8. doi: 10.1590/S0100-72032012000500003. [DOI] [PubMed] [Google Scholar]
- 23.Dumoulin JC, Bergers-Janssen JM, Pieters MH, et al. The protective effects of polymers in the cryopreservation of human and mouse zonae pellucidae and embryos. Fertil Steril. 1994;62:793–8. doi: 10.1016/s0015-0282(16)57006-x. [DOI] [PubMed] [Google Scholar]
- 24.El-Danasouri I, Selman H, Strehler E. Comparison of MOPS and HEPES buffers during vitrification of human embryos. Hum Reprod. 2004;14:i136. [Google Scholar]
- 25.Eroglu A, Bailey SE, Toner M, Toth TL. Successful cryopreservation of mouse oocytes by using low concentrations of trehalose and dimethylsulfoxide. Biol Reprod. 2009;80:70–8. doi: 10.1095/biolreprod.108.070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahy GM. Vitrification: a new approach to organ cryopreservation. Prog Clin Biol Res. 1986;224:305–35. [PubMed] [Google Scholar]
- 27.Fahy GM, Lilley TH, Linsdell H, et al. Cryoprotectant toxicity and cryoprotectant toxicity reduction: in search of molecular mechanisms. Cryobiology. 1990;27:247–68. doi: 10.1016/0011-2240(90)90025-Y. [DOI] [PubMed] [Google Scholar]
- 28.Fasano G, Demeestere I, Englert Y. In-vitro maturation of human oocytes: before or after vitrification? J Asst Repod Gen. 2012;29:507–12. doi: 10.1007/s10815-012-9751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzharris G, Baltz JM. Granulosa cells regulate intracellular pH of the murine growing oocyte via gap junctions: development of independent homeostasis during oocyte growth. Development (Cambridge, England). 2006;133(4):591–9. [DOI] [PubMed]
- 30.FitzHarris G, Siyanov V, Baltz JM. Granulosa cells regulate oocyte intracellular pH against acidosis in preantral follicles by multiple mechanisms. Development (Cambridge, England) 2007;134:4283–95. doi: 10.1242/dev.005272. [DOI] [PubMed] [Google Scholar]
- 31.Fu X-W, Shi W-Q, Zhang Q-J, et al. Positive effects of Taxol pretreatment on morphology, distribution and ultrastructure of mitochondria and lipid droplets in vitrification of in vitro matured porcine oocytes. Anim Reprod Sci. 2009;115:158–68. doi: 10.1016/j.anireprosci.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Fuchinoue K, Fukunaga N, Chiba S, et al. Freezing of human immature oocytes using cryoloops with Taxol in the vitrification solution. J Asst Reprod Gen. 2004;21:307–9. doi: 10.1023/B:JARG.0000043705.63523.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia MA, Graham EF. Development of a buffer system for dialysis of bovine spermatozoa before freezing. I. Effect of zwitterion buffers. Theriogenology. 1989;31:1021–8. doi: 10.1016/0093-691X(89)90485-8. [DOI] [PubMed] [Google Scholar]
- 34.Gardner DK, Sheehan CB, Rienzi L, et al. Analysis of oocyte physiology to improve cryopreservation procedures. Theriogenology. 2007;67:64–72. doi: 10.1016/j.theriogenology.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Ghetler Y, Skutelsky E, Ben Nun I, et al. Human oocyte cryopreservation and the fate of cortical granules. Fertil Steril. 2006;86:210–6. doi: 10.1016/j.fertnstert.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 36.Gomes C, Merlini M, Konheim J, et al. Oocyte meiotic-stage-specific differences in spindle depolymerization in response to temperature changes monitored with polarized field microscopy and immunocytochemistry. Fertil Steril. 2012;97:714–9. doi: 10.1016/j.fertnstert.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Gomes CM, Silva CA, Acevedo N, et al. Influence of vitrification on mouse metaphase II oocyte spindle dynamics and chromatin alignment. Fertil Steril. 2008;90:1396–404. doi: 10.1016/j.fertnstert.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Gook DA, Osborn SM, Johnston WI. Cryopreservation of mouse and human oocytes using 1,2-propanediol and the configuration of the meiotic spindle. Hum Reprod. 1993;8:1101–9. doi: 10.1093/oxfordjournals.humrep.a138201. [DOI] [PubMed] [Google Scholar]
- 39.Graham EF, Crabo BG, Brown KI. Effect of some zwitter ion buffers on the freezing and storage of spermatozoa. I. Bull J Dairy Sci. 1972;55:372–8. doi: 10.3168/jds.S0022-0302(72)85499-7. [DOI] [PubMed] [Google Scholar]
- 40.Gualtieri R, Iaccarino M, Mollo V, et al. Slow cooling of human oocytes: ultrastructural injuries and apoptotic status. Fertil Steril. 2009;91:1023–34. doi: 10.1016/j.fertnstert.2008.01.076. [DOI] [PubMed] [Google Scholar]
- 41.Heo YS, Lee H-J, Hassell BA, et al. Controlled loading of cryoprotectants (CPAs) to oocyte with linear and complex CPA profiles on a microfluidic platform. Lab Chip. 2011;11:3530–7. doi: 10.1039/c1lc20377k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horvath G, Seidel GE., Jr Use of fetuin before and during vitrification of bovine oocytes. Reprod Domest Anim. 2008;43:333–8. doi: 10.1111/j.1439-0531.2007.00905.x. [DOI] [PubMed] [Google Scholar]
- 43.Horvath G, Seidel GE. Vitrification of bovine oocytes after treatment with cholesterol-loaded methyl-beta-cyclodextrin. Theriogenology. 2006;66:1026–33. doi: 10.1016/j.theriogenology.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Hu W, Marchesi D, Qiao J, Feng HL. Effect of slow freeze versus vitrification on the oocyte: an animal model. Fertil Steril. 2012;98:752–760.e3. doi: 10.1016/j.fertnstert.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 45.Hyun C-S, Cha J-H, Son W-Y, et al. Optimal ICSI timing after the first polar body extrusion in in vitro matured human oocytes. Hum Reprod. 2007;22:1991–5. doi: 10.1093/humrep/dem124. [DOI] [PubMed] [Google Scholar]
- 46.Jasensky J, Boughton A, Khmaladze A, et al. Title/Author Year Live-cell intra-oocyte lipid analysis and quantification with hyperspectral imaging by multiplex coherent anti-stockes Raman scattering microscopy (CARS-M) Fertil Steril. 2012;98:S79. doi: 10.1016/j.fertnstert.2012.07.286. [DOI] [Google Scholar]
- 47.Jeyendran RS, Graham EF. An evaluation of cryoprotective compounds on bovine spermatozoa. Cryobiology. 1980;17:458–64. doi: 10.1016/0011-2240(80)90055-3. [DOI] [PubMed] [Google Scholar]
- 48.Jones A, Van Blerkom J, Davis P, Toledo AA. Cryopreservation of metaphase II human oocytes effects mitochondrial membrane potential: implications for developmental competence. Hum Reprod. 2004;19:1861–6. doi: 10.1093/humrep/deh313. [DOI] [PubMed] [Google Scholar]
- 49.Karran G, Legge M. Non-enzymatic formation of formaldehyde in mouse oocyte freezing mixtures. Hum Reprod. 1996;11:2681–6. doi: 10.1093/oxfordjournals.humrep.a019191. [DOI] [PubMed] [Google Scholar]
- 50.Keefe D, Liu L, Wang W, Silva C. Imaging meiotic spindles by polarization light microscopy: principles and applications to IVF. Reprod Biomed Online. 2003;7:24–9. doi: 10.1016/S1472-6483(10)61724-5. [DOI] [PubMed] [Google Scholar]
- 51.Kuwayama M, Fujikawa S, Nagai T. Ultrastructure of IVM-IVF bovine blastocysts vitrified after equilibration in glycerol 1,2-propanediol using 2-step and 16-step procedures. Cryobiology. 1994;31:415–22. doi: 10.1006/cryo.1994.1051. [DOI] [PubMed] [Google Scholar]
- 52.Lane M, Gardner DK. Vitrification of mouse oocytes using a nylon loop. Mol Reprod Dev. 2001;58:342–7. doi: 10.1002/1098-2795(200103)58:3<342::AID-MRD13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 53.Lane M, Lyons EA, Bavister BD. Cryopreservation reduces the ability of hamster 2-cell embryos to regulate intracellular pH. Hum Reprod. 2000;15:389–94. doi: 10.1093/humrep/15.2.389. [DOI] [PubMed] [Google Scholar]
- 54.Lane M, Maybach JM, Hooper K, et al. Cryo-survival and development of bovine blastocysts are enhanced by culture with recombinant albumin and hyaluronan. Mol Reprod Dev. 2003;64:70–8. doi: 10.1002/mrd.10210. [DOI] [PubMed] [Google Scholar]
- 55.Larman MG, Katz-Jaffe MG, Sheehan CB, Gardner DK. 1,2-propanediol and the type of cryopreservation procedure adversely affect mouse oocyte physiology. Hum Reprod. 2007;22:250–9. doi: 10.1093/humrep/del319. [DOI] [PubMed] [Google Scholar]
- 56.Larman MG, Minasi MG, Rienzi L, Gardner DK. Maintenance of the meiotic spindle during vitrification in human and mouse oocytes. Reprod Biomed Online. 2007;15:692–700. doi: 10.1016/S1472-6483(10)60537-8. [DOI] [PubMed] [Google Scholar]
- 57.Larman MG, Sheehan CB, Gardner DK. Calcium-free vitrification reduces cryoprotectant-induced zona pellucida hardening and increases fertilization rates in mouse oocytes. Reproduction. 2006;131:53–61. doi: 10.1530/rep.1.00878. [DOI] [PubMed] [Google Scholar]
- 58.Leibo SP, Pool TB. The principal variables of cryopreservation: solutions, temperatures, and rate changes. Fertil Steril. 2011;96:269–76. doi: 10.1016/j.fertnstert.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 59.Matson PL, Graefling J, Junk SM, et al. Cryopreservation of oocytes and embryos: use of a mouse model to investigate effects upon zona hardness and formulate treatment strategies in an in-vitro fertilization programme. Hum Reprod. 1997;12:1550–3. doi: 10.1093/humrep/12.7.1550. [DOI] [PubMed] [Google Scholar]
- 60.Men H, Monson RL, Parrish JJ, Rutledge JJ. Degeneration of cryopreserved bovine oocytes via apoptosis during subsequent culture. Cryobiology. 2003;47:73–81. doi: 10.1016/S0011-2240(03)00070-1. [DOI] [PubMed] [Google Scholar]
- 61.Meng Q, Wu X, Bunch TD, et al. Enucleation of demecolcine-treated bovine oocytes in cytochalasin-free medium: mechanism investigation and practical improvement. Cell Reprogram. 2011;13:411–8. doi: 10.1089/cell.2011.0012. [DOI] [PubMed] [Google Scholar]
- 62.Morató R, Izquierdo D, Albarracín JL, et al. Effects of pre-treating in vitro-matured bovine oocytes with the cytoskeleton stabilizing agent taxol prior to vitrification. Mol Reprod Dev. 2008;75:191–201. doi: 10.1002/mrd.20725. [DOI] [PubMed] [Google Scholar]
- 63.Morató R, Mogas T, Maddox-Hyttel P. Ultrastructure of bovine oocytes exposed to Taxol prior to OPS vitrification. Mol Reprod Dev. 2008;75:1318–26. doi: 10.1002/mrd.20873. [DOI] [PubMed] [Google Scholar]
- 64.Nottola SA, Coticchio G, De Santis L, et al. Ultrastructure of human mature oocytes after slow cooling cryopreservation with ethylene glycol. Reprod Biomed Online. 2008;17:368–77. doi: 10.1016/S1472-6483(10)60220-9. [DOI] [PubMed] [Google Scholar]
- 65.Nottola SA, Coticchio G, Sciajno R, et al. Ultrastructural markers of quality in human mature oocytes vitrified using cryoleaf and cryoloop. Reprod Biomed Online. 2009;19(Suppl 3):17–27. doi: 10.1016/S1472-6483(10)60280-5. [DOI] [PubMed] [Google Scholar]
- 66.Nottola SA, Macchiarelli G, Coticchio G, et al. Ultrastructure of human mature oocytes after slow cooling cryopreservation using different sucrose concentrations. Hum Reprod. 2007;22:1123–33. doi: 10.1093/humrep/del463. [DOI] [PubMed] [Google Scholar]
- 67.Park K-E, Kwon I-K, Han M-S, Niwa K. Effects of partial removal of cytoplasmic lipid on survival of vitrified germinal vesicle stage pig oocytes. J Repro Dev. 2005;51:151–60. doi: 10.1262/jrd.51.151. [DOI] [PubMed] [Google Scholar]
- 68.Parmegiani L, Bertocci F, Garello C, et al. Efficiency of human oocyte slow freezing: results from five assisted reproduction centres. Repro Biomed Online. 2009;18:352–9. doi: 10.1016/S1472-6483(10)60093-4. [DOI] [PubMed] [Google Scholar]
- 69.Porcu E, Fabbri R, Seracchioli R, et al. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997;68:724–6. doi: 10.1016/S0015-0282(97)00268-9. [DOI] [PubMed] [Google Scholar]
- 70.Regula CS, Pfeiffer JR, Berlin RD. Microtubule assembly and disassembly at alkaline pH. J Cell Biol. 1981;89:45–53. doi: 10.1083/jcb.89.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rizos D, Gutiérrez-Adán A, Pérez-Garnelo S, et al. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol Reprod. 2003;68:236–43. doi: 10.1095/biolreprod.102.007799. [DOI] [PubMed] [Google Scholar]
- 72.Röcken C, Ebert MPA, Roessner A. Proteomics in pathology, research and practice. Pathol Res Pract. 2004;200:69–82. doi: 10.1016/j.prp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Ruffing NA, Steponkus PL, Pitt RE, Parks JE. Osmometric behaavior, hydraulic conductivity, and incidence of intracellular ice formation in bovine oocytes at different developmental stages. Cryobiology. 1993;30:562–80. doi: 10.1006/cryo.1993.1059. [DOI] [PubMed] [Google Scholar]
- 74.Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction. 2011;141:1–19. doi: 10.1530/REP-10-0236. [DOI] [PubMed] [Google Scholar]
- 75.Sauvat F, Capito C, Sarnacki S, et al. Immature cryopreserved ovary restores puberty and fertility in mice without alteration of epigenetic marks. PLoS One. 2008;3:e1972. doi: 10.1371/journal.pone.0001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seidel GE. Modifying oocytes and embryos to improve their cryopreservation. Theriogenology. 2006;65:228–35. doi: 10.1016/j.theriogenology.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 77.Shau H, Chandler GS, Whitelegge JP, et al. Proteomic profiling of cancer biomarkers. Brief Funct Genomic Proteomic. 2003;2:147–58. doi: 10.1093/bfgp/2.2.147. [DOI] [PubMed] [Google Scholar]
- 78.Shehab-El-Deen MA, Leroy JLMR, Maes D, Van Soom A. Cryotolerance of bovine blastocysts is affected by oocyte maturation in media containing palmitic or stearic acid. Reprod Domest Anim. 2009;44:140–2. doi: 10.1111/j.1439-0531.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- 79.Shi W-Q, Zhu S-E, Zhang D, et al. Improved development by Taxol pretreatment after vitrification of in vitro matured porcine oocytes. Reproduction. 2006;131:795–804. doi: 10.1530/rep.1.00899. [DOI] [PubMed] [Google Scholar]
- 80.Sieracki NA, Hwang HJ, Lee MK, et al. (2008) A temperature independent pH (TIP) buffer for biomedical biophysical applications at low temperatures. Chem Commun 823–825. [DOI] [PMC free article] [PubMed]
- 81.Smith GD, Motta EE, Serafini P. Theoretical and experimental basis of oocyte vitrification. Reprod Biomed Online. 2011;23:298–306. doi: 10.1016/j.rbmo.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 82.Smith GD, Serafini PC, Fioravanti J, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril. 2010;94:2088–95. doi: 10.1016/j.fertnstert.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 83.Squirrell JM, Lane M, Bavister BD. Altering intracellular pH disrupts development and cellular organization in preimplantation hamster embryos. Biol Reprod. 2001;64:1845–54. doi: 10.1095/biolreprod64.6.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stachecki JJ, Cohen J, Willadsen S. Detrimental effects of sodium during mouse oocyte cryopreservation. Biol Reprod. 1998;59:395–400. doi: 10.1095/biolreprod59.2.395. [DOI] [PubMed] [Google Scholar]
- 85.Stachecki JJ, Cohen J, Willadsen SM. Cryopreservation of unfertilized mouse oocytes: the effect of replacing sodium with choline in the freezing medium. Cryobiology. 1998;37:346–54. doi: 10.1006/cryo.1998.2130. [DOI] [PubMed] [Google Scholar]
- 86.Sun X-F, Zhang W-H, Chen X-J, et al. Spindle dynamics in living mouse oocytes during meiotic maturation, ageing, cooling and overheating: a study by polarized light microscopy. Zygote. 2004;12:241–9. doi: 10.1017/S0967199404002850. [DOI] [PubMed] [Google Scholar]
- 87.Suo L, Zhou G-B, Meng Q-G, et al. OPS vitrification of mouse immature oocytes before or after meiosis: the effect on cumulus cells maintenance and subsequent development. Zygote. 2009;17:71–7. doi: 10.1017/S0967199408005091. [DOI] [PubMed] [Google Scholar]
- 88.Swain JE, Smith GD. Cryoprotectants. In: Cihian RC, Quinn P, editors. Fertility cryopreservation. Cambrige: Cambridge University Press; 2010. pp. 24–39. [Google Scholar]
- 89.Toth TL, Baka SG, Veeck LL, et al. Fertilization and in vitro development of cryopreserved human prophase I oocytes. Fertil Steril. 1994;61:891–4. doi: 10.1016/s0015-0282(16)56702-8. [DOI] [PubMed] [Google Scholar]
- 90.Toth TL, Lanzendorf SE, Sandow BA, et al. Cryopreservation of human prophase I oocytes collected from unstimulated follicles. Fertil Steril. 1994;61:1077–82. [PubMed] [Google Scholar]
- 91.Trapphoff T, El Hajj N, Zechner U, et al. DNA integrity, growth pattern, spindle formation, chromosomal constitution and imprinting patterns of mouse oocytes from vitrified pre-antral follicles. Hum Reprod. 2010;25:3025–42. doi: 10.1093/humrep/deq278. [DOI] [PubMed] [Google Scholar]
- 92.Tucker M, Wright G, Morton P, et al. Preliminary experience with human oocyte cryopreservation using 1,2-propanediol and sucrose. Hum Reprod. 1996;11:1513–5. doi: 10.1093/oxfordjournals.humrep.a019428. [DOI] [PubMed] [Google Scholar]
- 93.Tucker MJ, Wright G, Morton PC, Massey JB. Birth after cryopreservation of immature oocytes with subsequent in vitro maturation. Fertil Steril. 1998;70:578–9. doi: 10.1016/S0015-0282(98)00205-2. [DOI] [PubMed] [Google Scholar]
- 94.Vincent C, Cheek TR, Johnson MH. Cell cycle progression of parthenogenetically activated mouse oocytes to interphase is dependent on the level of internal calcium. J Cell Sci. 1992;103:389–96. doi: 10.1242/jcs.103.2.389. [DOI] [PubMed] [Google Scholar]
- 95.Wang WH, Meng L, Hackett RJ, et al. The spindle observation and its relationship with fertilization after intracytoplasmic sperm injection in living human oocytes. Fertil Steril. 2001;75:348–53. doi: 10.1016/S0015-0282(00)01692-7. [DOI] [PubMed] [Google Scholar]
- 96.Will MA, Clark NA, Swain JE. Biological pH buffers in IVF: help or hindrance to success. J Assist Reprod Gen. 2011;28:711–24. doi: 10.1007/s10815-011-9582-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamaji Y, Seki S, Matsukawa K, et al. Developmental ability of vitrified mouse oocytes expressing water channels. J Reprod Dev. 2011;57:403–8. doi: 10.1262/jrd.10-201M. [DOI] [PubMed] [Google Scholar]
- 98.Zander-Fox D, Cashman KS, Lane M. The presence of 1 mM glycine in vitrification solutions protects oocyte mitochondrial homeostasis and improves blastocyst development. J Assist Reprod Gen. 2013;30:107–16. doi: 10.1007/s10815-012-9898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zander-Fox DL, Mitchell M, Thompson JG, Lane M. Alterations in mouse embryo intracellular pH by DMO during culture impair implantation and fetal growth. Reprod Biomed Online. 2010;21:219–29. doi: 10.1016/j.rbmo.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 100.Zeron Y, Pearl M, Borochov A, Arav A. Kinetic and temporal factors influence chilling injury to germinal vesicle and mature bovine oocytes. Cryobiology. 1999;38:35–42. doi: 10.1006/cryo.1998.2139. [DOI] [PubMed] [Google Scholar]
- 101.Zeron Y, Tomczak M, Crowe J, Arav A. The effect of liposomes on thermotropic membrane phase transitions of bovine spermatozoa and oocytes: implications for reducing chilling sensitivity. Cryobiology. 2002;45:143–52. doi: 10.1016/S0011-2240(02)00123-2. [DOI] [PubMed] [Google Scholar]
- 102.Zhou C, Baltz JM. JAK2 mediates the acute response to decreased cell volume in mouse preimplantation embryos by activating NHE1. J Cell Physiol. 2013;228:428–38. doi: 10.1002/jcp.24147. [DOI] [PubMed] [Google Scholar]