Abstract
Multiple studies have reported both functional and neuroanatomical differences between adults who stutter and their normally fluent peers. However, the reasons for these differences remain unclear although some developmental data suggest that structural brain differences may be present in school-age children who stutter. In the present study, the corpus callosum of children with persistent stuttering, children who recovered from stuttering and typically developing children between 9 and 12 years of age was compared to test if the presence of aberrant callosal morphology is implicated in this disorder. The total corpus callosum midsagittal area and area of each subsection consisting of the rostrum, anterior midbody, posterior midbody and splenium were measured using MIPAV (Medical Image Processing, Analysis, and Visualization). Voxel-based morphometry (VBM) was also used to compare white matter volume. No differences were detected in the corpus callosum area or white matter volume between children with persistent stuttering, children who recovered from stuttering and typically developing children. These results agree with dichotic listening studies that indicate children who stutter show the typical right ear advantage. Therefore, the neural reorganization across the midline shown in adults who stutter may be the result of long-term adaptations to persistent stuttering.
Learning outcomes
Educational objectives: After reading this article, the reader will be able to: (1) summarize research findings on corpus callosum development; and (2) discuss the characteristics of corpus callosum anatomy in stuttering.
Keywords: Stuttering, Corpus callosum, White matter, Voxel-brain morphometry, Interhemispheric transfer
1. Introduction
The typical onset of developmental stuttering is around 3 years of age coinciding with a period of rapid speech and language development (Månsson, 2000; Reilly et al., 2009). Approximately 70% of children who stutter will recover within 2 years of onset (Månsson, 2000; Yairi & Ambrose, 2005). Children who begin stuttering at an earlier age are more likely to recover compared to those with later onsets (Buck, Lees, & Cook, 2002; Yairi & Ambrose, 2005), with boys more likelyto have persistent stuttering (Craig, Hancock, Tran, Craig, & Peters, 2002; Van Borsel, Moeyaert, Rosseel, Van Loo, & Van Renterghem, 2006; Yairi & Ambrose, 1999). The performance of children with persistent stuttering also differs from children who recovered from stuttering across several dimensions including temperament, sensory and motor (Howell, Davis, & Williams, 2008). Understanding the physiological factors that differentiate persistence and recovery remains a primary challenge for stuttering research as this could provide insight into the cause(s) of the disorder and facilitate clinical interventions that increase recovery rates.
Recovery and persistency seem to be mediated genetically to some extent as children with documented recovery or persistency are likely to have parents or siblings who respectively recovered from or persisted in stuttering (Ambrose, Cox, & Yairi, 1997). Neurological explanations are also thought to be centrally involved in persistency and recovery as stuttering is expressed as subtle alterations in the structure and function of speech-language relevant brain regions (Cykowski et al., 2008; Cykowski, Fox, Ingham, Ingham, & Robin, 2010; De Nil, Kroll, & Houle, 2001; De Nil, Kroll, Lafaille, & Houle, 2003; De Nil, Kroll, Kapur, & Houle, 2000; Foundas, Bollich, Corey, Hurley, & Keilman, 2001; Kell et al., 2009; Lu et al., 2010; Sommer, Koch, Paulus, Weiller, & Büchel, 2002; Watkins, Smith, Davis, & Howell, 2008).
We recently reported that the overall area of the corpus callosum (CC) and anterior portion of the CC was larger in adults who stutter (AWS) compared to normally fluent adults (Choo et al., 2011). Generally, a larger CC is associated with right hemisphere dominance or reduced hemispheric asymmetry for speech (Dorion et al., 2000; Gootjes et al., 2006; O’Kusky et al., 1988), which is consistent with neurological reports of increased right hemisphere activation or a lack of left hemisphere dominance in stuttering (De Nil et al., 2001; Fox et al., 1996; Neumann et al., 2003, 2005). The anterior portions of the CC including the rostrum, genu, and anterior body connect the prefrontal, premotor and supplementary motor areas. A larger anterior midbody may indicate greater interhemispheric communication between the left and right hemisphere motor cortices involved in speech production, which may be related to the rightward shift in motor activity reported in certain functional imaging studies of stuttering. The anterior callosa has also been implicated in auditory processing. A larger anterior CC has been associated with decreased right ear performance in right-handed individuals. One interpretation is that decreased performance results from greater competition for resources from the left ear or inhibition from the right hemisphere (Clarke, Lufkin, & Zaidel, 1993; Westerhausen & Hugdahl, 2008). The posterior regions of the CC including the isthmus and splenium which connect the parietal and temporal cortical regions are also involved in speech and language processing (Hofer & Frahm, 2006). A smaller posterior CC is thought to be linked to greater hemispheric lateralization. Patients with multiple sclerosis showed an increased right ear advantage in verbal dichotic listening tasks with progressive posterior CC loss affecting the isthmus and splenium (Gadea et al., 2009).
The importance of the CC for inter-hemispheric connections in support of language and cognition comes from different perspectives (see Gazzaniga, 2000; Pujol et al., 2006). Disruptions in language performance may be directly correlated with deficits in coordination of language pathways mediated by the CC (Paul, 2011). For example, children with developmental language disorder characterized by impairments in language production and/or comprehension (Bishop, 1992) have been reported to show disproportionate CC size relative to brain volume. Although the absolute size of the CC was similar between children with developmental language delay and typically developing children, the relative size of the CC to brain volume was disproportionally smaller in children with developmental language delay, and consequently, may result in greater constraints on inter-hemispheric communication (Herbert et al., 2003, 2005). In addition to developmental language delay, atypical CC development has also been associated with other developmental disorders including dyslexia, attention-deficit hyperactive disorder and autism (see Paul, 2011).
In this study, we compared the midsagittal area and white matter volume of the CC in school-age children with persistent stuttering, children who recovered and typically developing children to determine whether an enlarged CC is present in childhood stuttering. If the anterior CC is larger or has a greater volume in children who stutter, it could conceivably be associated with other aberrant early developmental processes that result in the unusual right hemisphere anatomy or function in persistent stuttering. Alternatively, enlargement of the CC could emerge as part of a neuroplastic response to prolonged stuttering, thus deviations from a typical pattern of CC development may not be present in younger children. In that case, an enlarged CC would be expressed in AWS along with the unusual brain findings reported in previous studies including atypical structural symmetry in the auditory cortex (Foundas et al., 2001; Jäncke, Hänggi, & Steinmetz, 2004).
Differences in cortical gray matter (GM) volume and white matter (WM) integrity have been found previously in a comparison of children with persistent stuttering, children who recovered from stuttering and typically developing children using a combination of voxel-based morphometry (VBM) and fractional anisotropy (FA) analyses using diffusion tensor imaging (DTI) (Chang, Erickson, Ambrose, Hasegawa-Johnson, & Ludlow, 2008). Children who recovered from stuttering and children with persistent stuttering featured reduced GM volume in the left inferior frontal gyrus (IFG) and superior temporal gyrus. The left IFG has also been implicated in AWS. In terms of WM integrity, Kell et al. (2009) reported increased FA values in the left inferior frontal region (and forceps minor of the CC) of adults with persistent stuttering. In terms of GM, there was reduced volume in the left IFG in both adults with persistent stuttering and adults who recovered from stuttering. Interestingly, greater stuttering severity was associated with lower GM volume than less severe stuttering. Additionally, other studies have reported reduced functional connectivity between the IFG and left motor regions, and reduced FA in the left (and right posterior) IFG in stuttering (Chang et al., 2011; Lu et al., 2009; Watkins et al., 2008). In contrast to these reports of left IFG anomalies, other investigations have reported increased or no GM differences in the left IFG in AWS compared to normally fluent adults (Beal et al., 2007; Jäncke et al., 2004; Lu et al., 2010). There is clear controversy over the status of the left IFG in stuttering that calls for more studies, particularly developmental studies that identify early neurological changes that could mark clinically relevant factors in stuttering. In addition to the IFG, the left arcuate fasiculus, which links Broca’s and Wernicke’s regions is also associated with reduced WM integrity in both children with persistent stuttering and children who recovered from stuttering (Chang et al., 2008). Although differences in the CC were not investigated by Chang et al. (2008), anomalies in the largest collection of WM fibers which integrate activities of the right and left hemispheres could be implicated in the atypical right hemisphere expression of brain structure and function in AWS (Brown, Ingham, Ingham, Laird, & Fox, 2005; Cykowski et al., 2008).
Our previous finding of CC hypertrophy and increased WM in the anterior portion of the CC of AWS needs to be considered in light of the finding of lower WM integrity in the callosal body by Cykowski et al. (2010) as measured with FA. These two studies used different measures to examine WM so reconciling the findings may be difficult given that our knowledge of the biological bases of these measurements is still limited. However, increased CC volume and decreased FA need not be contradictory because increased or decreased tissue volume of WM does not necessarily indicate whether the connections are highly organized (high FA) or less coherent (lower FA). The current study cannot reconcile these findings but directs a focus on children who stutter to assess whether atypical CC development is a feature of early stuttering.
In normal development, CC maturation continues into early adulthood with prominent size increases occurring during early childhood and adolescence (Keshavan et al., 2002; Pujol, Vendrell, Junque, Marti-Vilalta, & Capdevila, 1993). Development of the CC proceeds in an anterior to posterior direction, with the exception of the rostrum which develops last (Georgy, Hesselink, & Jernigan, 1993). Anterior sections of the CC typically reach adult size by early childhood followed by a second growth period in the rostrum which coincides with frontal lobe maturation in late adolescent males (Giedd et al., 1996; Luders, Thompson, & Toga, 2010; Sowell, Thompson, Tessner, & Toga, 2001). In contrast, myelination of CC fibers which promotes greater speed of neuronal processing follows a posterior to anterior trajectory beginning in late fetal development and continuing postnatally into adulthood (Georgy et al., 1993; Giedd, 2004; Pujol et al., 1993). Patterns and degree of myelination may also be a plastic process with prolonged experience/ activity typically stimulating greater rates of myelination (Bengtsson et al., 2005; Grossman, Churchill, Bates, Kleim, & Greenough, 2002).
The prolonged development of the CC and sensitivity to experience suggests that a communication disorder such as stuttering, that affects a widespread cortical network could elicit compensatory plastic response(s) that affect the trajectory of CC development. In this study, we predicted that the anterior CC midsagittal area and WM volume would be larger in school-age children with persistent stuttering compared to children who recovered and typically developing children. Data from these children were previously reported by Chang et al. (2008), but that study did not investigate midsagittal CC area and did not analyze WM volume with VBM. A larger anterior CC in children with persistent stuttering could indicate this is a feature of early developmental stuttering and perhaps a neurological marker of persistent stuttering.
2. Method
2.1. Participants
A total of 21 right-handed boys between 9 and 12 years of age were recruited as part of a larger study investigating brain anatomy in children who stutter, using advertisements or by referral from the University of Illinois Speech Language Pathology Clinic. The participants included boys who were persistent in stuttering, those who had recovered from stuttering and typically fluent controls. Besides stuttering participants did not have other concomitant disorders. All participants except one were initially participants in the previous MRI study by Chang et al. (2008). The study was approved by the Institutional Review Board at the University of Illinois at Urbana-Champaign.
The general characteristics of participants in each group including mean age, age at stuttering onset (range), time between onset and recovery from stuttering, stuttering severity score based on the Illinois Clinician Stuttering Severity Scale (ICSSS) (Yairi & Ambrose, 2005), mean Edinburgh Handedness Inventory score (Oldfield, 1971), mean Peabody Picture Vocabulary Test scores (PPVT-Dunn & Dunn, 1997) and mean Test of Language Development-3 (TOLD:I-3-Newcomer & Hamill, 1997) are listed in Table 1.
Table 1.
General characteristics of participants in each group including mean age, age at stuttering onset (range), time between onset and recovery from stuttering, stuttering severity score based on the Illinois Clinician Stuttering Severity Scale (ICSSS), mean Edinburgh Handedness Inventory score, mean Peabody Picture Vocabulary Test score and mean Test of Language Development-3.
| Group | n | Mean age (SD) |
Age at stuttering onset (range in years) |
Time between onset and recovery (range in years) |
Stuttering severity score (SD) (0 = normal; 4 = moderate 7 = very severe) |
Mean Edinburgh Handedness Inventory (SD) (Oldfield, 1971) |
Mean Peabody Picture Vocabulary Test (SD) (PPVT - Dunn & Dunn, 1997) |
Mean Test of Language Development-3 TOLD:I-3 (SD) (Newcomer & Hamill, 1997) |
|---|---|---|---|---|---|---|---|---|
| Children with persistent stuttering |
8 | 10.95 (1.7) | 2–5 | – | 2.58 (0.6) | 94.68 (8.40) | 104.13 (15.69) | 8.57 (1.90) |
| Children who recovered from stuttering |
6 | 11.13 (1.44) | 2–5 | 2–3 | – | 99 (2.24) | 123.00 (19.01) | 9.50 (2.07) |
| Typically developing children |
7 | 10.69 (1.06) | – | – | – | 91 (12.73) | 109.86 (18.41) | 10.29 (4.35) |
2.2. Language scores
Multiple independent t-tests were conducted to compare language scores specifically the PPVT-III (Dunn & Dunn, 1997) and TOLD-I:3 (Newcomer & Hamill, 1997) between the three groups.
2.3. Imaging
Details on imaging were reported in Chang et al. (2008). Briefly, all children were imaged on a 3T Siemens Magnetom Allegra MR Headscanner with a single channel head coil at the Biomedical Imaging Center of the Beckman Institute at the University of Illinois, Urbana-Champaign. High resolution anatomical volumes encompassing the cerebrum, cerebellum and brainstem were collected using a T1-weighted MPRAGE (magnetization-prepared rapid acquisition gradient echo) sequence (parameters: TR/TE = 21 s/4.38 ms, FOV 256 mm, matrix 256 mm × 160 mm ×128 mm, slice thickness 1.3 mm, flip angle 8°, bandwidth 130 Hz/pixel). Children viewed a movie of their choice while being scanned. To minimize movement, heads and shoulders were padded with cushions.
2.4. Segmentation of CC area
In the first quantitative analysis, the midsagittal CC image was divided into four segments and the area of each segment was calculated using MIPAV (Medical Image Processing, Analysis, and Visualization) (McAuliffe, McGarry, Gandler, Csaky, & Trus, 2001). Segmentation of the CC was performed in a similar method as described in Sullivan et al. (2001). Using MIPAV, the midline CC was visualized and subdivided into four regions in the midsagittal plane: rostrum, anterior midline body, posterior midline body and splenium (Fig. 1a and b). Before the segments were identified, each rater independently selected the sagittal slice from each participant that was considered most representative of the midsagittal plane.In 19/21 cases, both raters independently chose the same midline image. In the other two cases, there was only a difference in one anatomical slice (difference of 1 mm) and the midline image selected by rater 1 was used. As per Fig. 1, the caudal border of the rostrum was marked by a tangent line at the bend of the genu. An identical line dividing the rostral boundary of the body and splenium was placed at the concave bend of the genu. The length of the midline body between these lines was divided exactly in half to mark the anterior and posterior sections. Each segment was filled manually using the MIPAV-FILL tool, which also determined the area of the filled region. Twenty five percent of the data from each group were randomly selected and re-evaluated to measure intra-rater reliability. For inter-rater reliability, 100% of the data was re-traced by a second investigator who was blinded to the gender, identity and diagnosis of each participant and analyzed using Intra-class Correlation Coefficients (ICC).
Fig. 1.
(a) Schematic midsagittal corpus callosum indicating the division into four subregions: rostrum, anterior midbody, posterior midbody and splenium. The caudal border of the rostrum was marked by a tangent line at the bend of the genu. An identical line dividing the rostral boundary of the body and splenium was placed at the concave bend of the genu. The length of the midline body between these lines was divided in half to mark the anterior and posterior body. (b) Midsagittal corpus callosum MR images of a participant showing the callosal subdivisions.
2.5. Area analysis
Two different measures of the CC were analyzed: absolute area and relative area. The relative area was determined by dividing the area of the subregion over the total CC area using a similar method described by Preis, Steinmetz, Knorr and Ja¨ncke (2000). One-way ANOVA’s were performed to compare the (1) absolute area of the total CC, (2) absolute area of each subregion and (3) relative area of each subregion across the three groups.
2.6. VBM analysis – regions of interest
A voxel-based morphometry analysis of the CC was conducted with SPM5 using the recommended default options (Wellcome Trust Centre for Neuroimaging, London, UK). Spatial normalization, segmentation and modulation were performed with the unified segmentation algorithm in SPM5. Initial processing followed a similar protocol to Beal, Gracco, Lafaille and DeNil (2007) with the exception of the region of interest (ROI) which only focused on the CC in the present study. Images were registered on an adult template. Several studies have shown that spatial normalization of brains of children over 6 years of age to an adult template can be successfully performed (Beal et al., 2011; Burgund et al., 2002; Kang, Burgund, Lugar, Petersen, & Schlaggar, 2003; Muzik, Chugani, Juhász, Shen, & Chugani, 2000). The normalized WM images of each subject were masked with the CC ROI mask from the WFU PickAtlas toolbox developed by the ANSIR laboratory at Wake Forest University (Department of Radiology, Wake Forest University School of Medicine, Winston-Salem, NC). Between group comparisons were performed using voxel-wise two-sample t-test with a statistical threshold of p < 0.001 uncorrected.
3. Results
3.1. Language scores
Results of the multiple independent t-tests indicate that the PPVT scores were not significantly different between children with persistent stuttering and children who recovered (t(23) = –1.98, p = 0.77), children with persistent stuttering and typically developing children (t(23) = –0.64, p = 0.23), or children who recovered from stuttering and typically developing children (t(23) = 1.26, p = 0.77). Similarly, the TOLD-I:3 scores were not significantly different between children with persistent stuttering and children who recovered (t(23) = –0.84, p = 0.42), children with persistent stuttering and typically developing children (t(23) = –0.96, p = 0.37), or children who recovered from stuttering and typically developing children (t(23) = –0.43, p = 0.77).
3.2. Reliability
An intra-class correlation analysis for the average measures indicated high intra-rater reliability for all segments comprising the rostrum (ICC = 0.98), anterior midbody (ICC = 0.94), posterior midbody (ICC = 0.93) and splenium (ICC = 0.98). Intra-class correlations for inter-rater reliability were also high for the rostrum (ICC = 0.92), anterior midbody (ICC = 0.73), posterior midbody (ICC = 0.89) and splenium (ICC = 0.94).
3.3. Absolute area comparisons
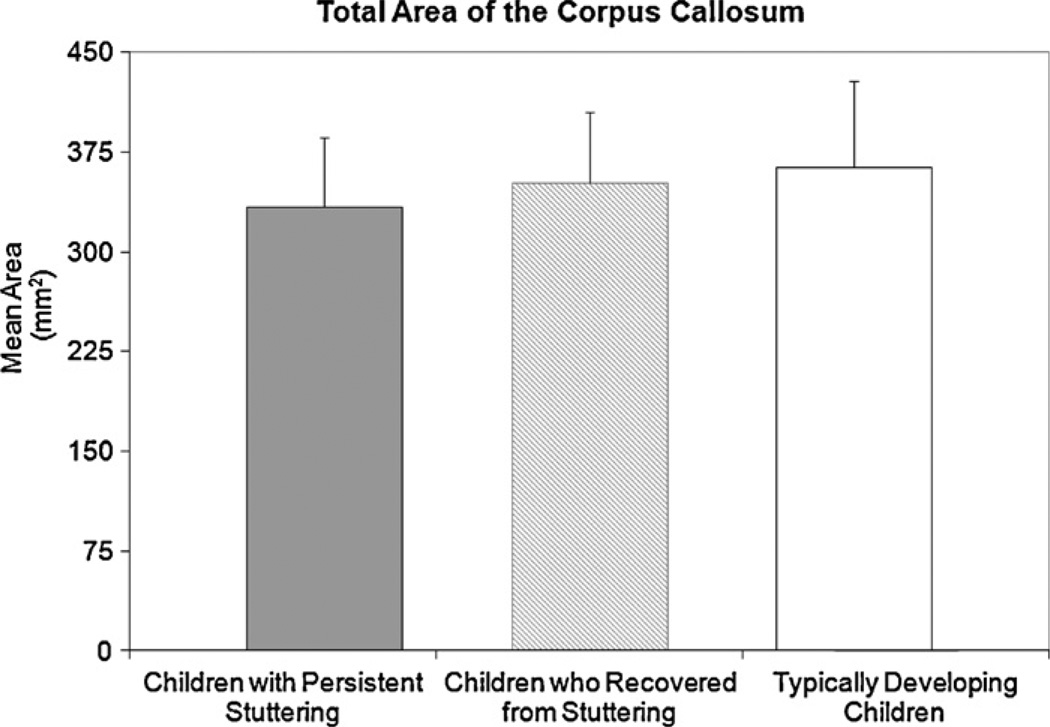
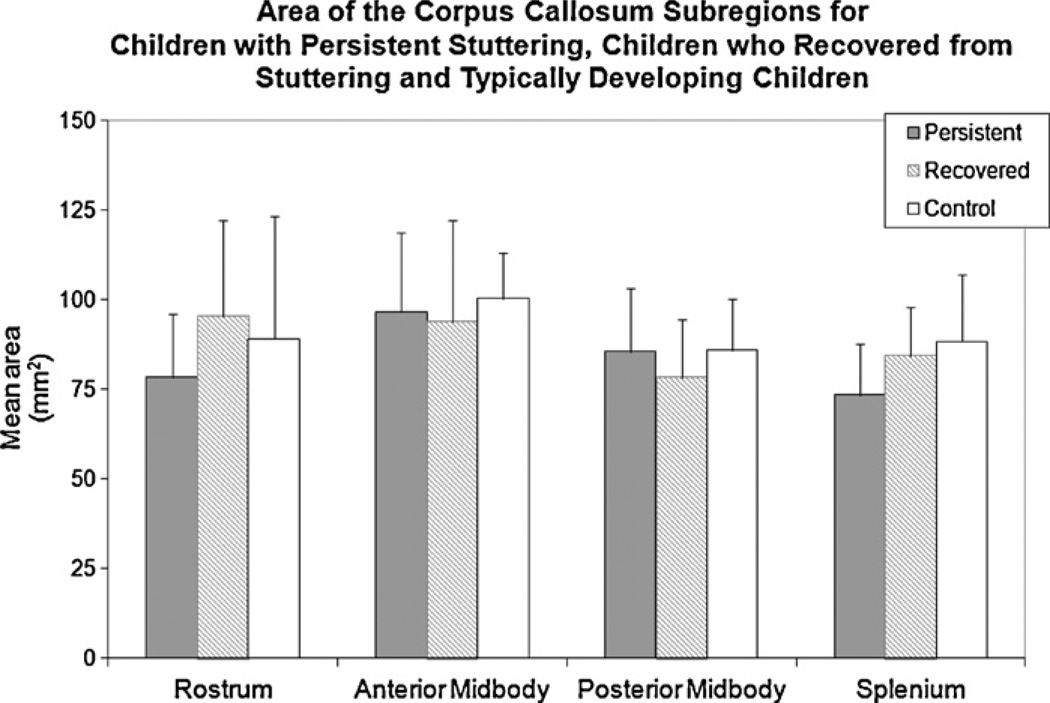
No significant between-group-differences were found for the total CC area although it was smallest for children with persistent stuttering (333.88 mm2, SD = 52.64), intermediate for children who recovered (351.67 mm2, SD = 53.27) and largest for controls (363.71 mm2, SD = 64.39)(F(2,18) = 0.522, p = 0.602) (Fig. 2). In addition, no significant differences were found between groups for the CC subsections: rostrum (F(2,18) = 0.747, p = 0.488), anterior midbody (F(2,18) = 0.153, p = 0.859), posterior midbody (F(2,18) = 0.448, p = 0.646)], or splenium (F(2,18) = 1.842, p = 0.187) (Fig. 3). Differences were also non-significant in a post hoc comparison between ‘ever stuttered’ (children with persistent stuttering and children who recovered) and the control group.
Fig. 2.
The average total corpus callosum area for children with persistent stuttering, children who recovered from stuttering and typically developing children is shown. Differences between groups were not significant. Error bars indicate standard deviations.
Fig. 3.
The average area for each CC subregion for children with persistent stuttering, children who recovered from stuttering and typically developing children is shown. Differences between groups were not significant. Error bars indicate standard deviations.
3.4. Relative area comparisons
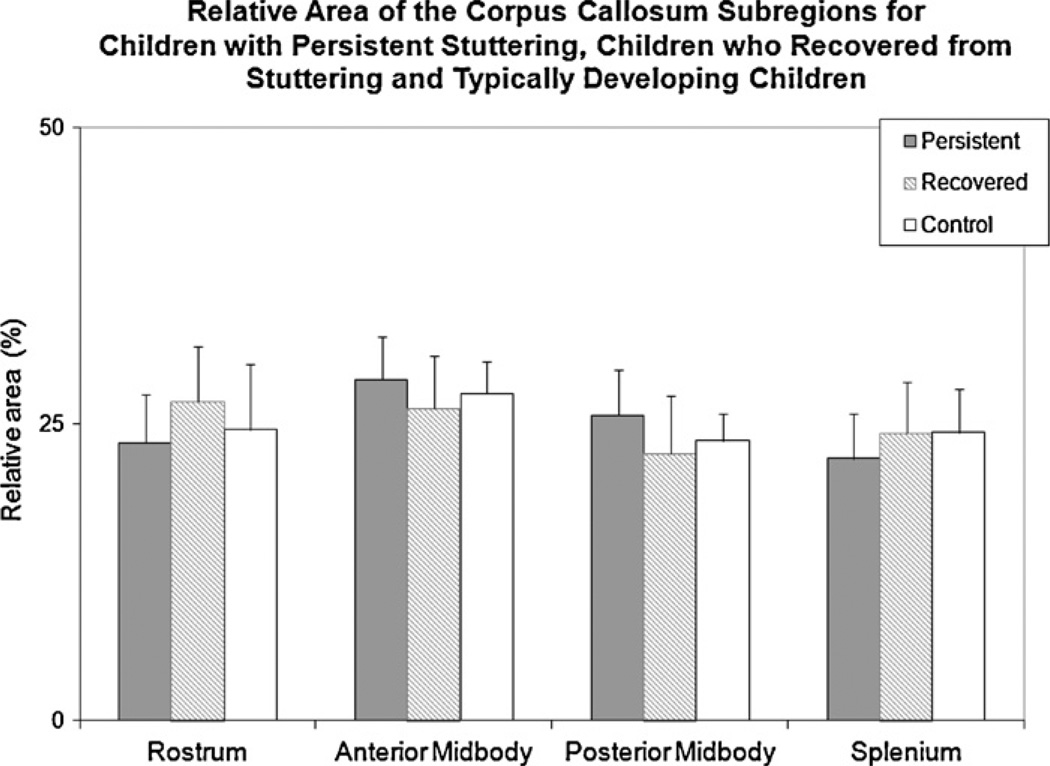
No significant between-group-differences were found for the relative area of the rostrum (F(2,18) = 1.001, p = 0.387), anterior midbody (F(2,18) = 0.787, p = 0.470), posterior midbody (F(2,18) = 1.304, p = 0.296), or splenium (F(2,18) = 0.829, p = 0.452) between the three groups (Fig. 4). Also as above, the post hoc comparison between ‘ever stuttered’ and controls was not significant.
Fig. 4.
The relative area of each CC subregion for children with persistent stuttering, children who recovered from stuttering and typically developing children is shown. Differences between groups were not significant. Error bars indicate standard deviations.
3.5. VBM comparison
No significant differences in WM volume were detected between the three groups. Post hoc comparison between ‘ever-stuttered’ and controls also did not detect a statistical difference in WM volume.
4. Discussion
The purpose of the study was to explore the neurodevelopmental basis of stuttering by investigating CC morphology and volume in children with persistent stuttering and children who recovered from stuttering. Unlike our previous study that found a larger anterior CC in AWS (Choo et al., 2011), the current study did not detect any statistically significant differences in CC size and WM volume between children with persistent stuttering, children who recovered from stuttering and typically developing children. Reconciling these findings between children and adults is challenging but could involve the protracted and nonlinear growth pattern of the CC. Namely, aberrant callosal development could emerge in later childhood or adolescence as stuttering progresses. The results of the present study are consistent with investigations using dichotic listening in stuttering. Children who stutter and typically developing children show similar right ear advantage in dichotic listening tasks, however in stuttering this right ear advantage has been observed to decrease in adulthood whereas right ear advantage is maintained or becomes more pronounced in normally fluent adults (Ambrose, Chang, Chon, King, & Steger, 2007; Brosch, Haege, Kalehne, & Johnson, 1999; Cimorell-Strong, Gilbert, & Frick, 1983; Blood, Blood, & Hood, 1987). This observation suggests that neural organization across the midline has not been completely established in early childhood but becomes increasingly biased towards the right hemisphere with age in stuttering.
The right ear advantage is one manifestation of functional lateralization of language to the left hemisphere. In normal development, language becomes increasingly left hemisphere lateralized with the strongest lateralization occurring between 20 and 25 years of age (Szaflarski, Holland, Schmithorst, & Byars, 2006). This progression is reflected in CC maturation in which the growth asymptote is reached during adolescence followed by modest growth into early adulthood (Keshavan et al., 2002; Pujol et al., 1993). Individuals with less consistent or weaker hemispheric lateralization have a larger CC compared to those who display a greater degree of left lateralization (Gootjes et al., 2006; Witelson, 1989). It is possible that the presence of deficits or perturbations in childhood or adolescence during the period of rapid CC development before lateralization is firmly entrenched may alter the mechanism(s) that directs callosal development. By implication, the nonsignificant differences in CC size and WM volume between the school-age child groups in this study suggest that gross deviations from normal callosal development may not be expected before or during recovery from stuttering.
In stuttering, left hemisphere deficits or dysfunction could result in compensatory neural responses including reorganization of inter-hemispheric connections and consequently, a larger callosa to permit increased participation of the right hemisphere (Geschwind & Galaburda, 1985; O’Kusky et al., 1988). Such stuttering related brain changes might reduce axonal pruning that spares a larger number of callosal fibers which eventually results in a larger callosa (O’Kusky etal., 1988). Also, potentially slower WM growth in children with persistent stuttering could lead to a delayed and protracted maturation of the CC and possibly other WM connections. Considering that the rostrum is the last part of the callosum to mature, it may reflect the protracted increase in WM volume, and accordingly is larger in AWS. This pattern of CC development in stuttering is similar to Tourette’s Syndrome (TS) which is associated with motor and vocal tics. In adulthood both disorders show CC hypertrophy despite a smaller callosa in childhood TS (Moriarty et al., 1997; Plessen et al., 2004) and minimal differences in callosa size in childhood stuttering – there was also a nonsignificant trend towards a smaller CC size and reduced WM volume in children with persistent stuttering. Overall, enlargement of the CC may be part of a general process that serves to increase compensatory responses or the capacity to mitigate the long-term effects of developmental disorders such as stuttering or TS. The results of the present study are based on a small sample size. It is possible that the current study lacked sufficient statistical power to detect group differences in the volume of the corpus callosum. An exploratory analysis using a less stringent threshold of p = 0.05 (uncorrected) did indicate differences in WM volume between children with persistent stuttering and typically developing children. Children with persistent stuttering featured two clusters of decreased WM in the anterior midbody and splenium compared to typically developing children. However, these differences did not exceed the relatively stringent threshold for significance (p = 0.001) that was adopted to limit false positive rates. There is certainly a possibility these differences would have been significant with a larger sample size. Accordingly, follow-up studies are needed to better determine the pattern of CC development in stuttering.
Although reports of atypical CC morphology in terms of size and volume (Choo et al., 2011), along with reduced integrity (Cykowski et al., 2010), suggest that stuttering is associated with unusual CC development, further investigations are needed to ascertain the relationship between these anomalies. The seemingly diametric results of these reports highlight the complexities in understanding a developmental disorder such as stuttering. However, reports of increased CC size and WM volume but reduced WM integrity may not be contradictory. Anisotropy is influenced by a number of factors including packing of fibers, fiber orientation, and fiber crossings (Chepuri et al., 2001; Leow et al., 2009). Higher FA values are typically associated with tighter packing of fibers and fewer obliquely oriented axons, while regions with complex, multi-directional fiber crossings tend to have lower FA values (Chepuri et al., 2001; Leow et al., 2009). Thus, the higher number of obliquely oriented axons in the body of the CC along with greater concentrations of less densely packed axons in the posterior portion of the callosal midbody may result in lower FA values (Chepuri et al., 2001; Moody, Bell, & Challa, 1988; Wahl et al., 2007) that are not necessarily due to decreased WM volume. Although VBM has been used to assess WM as in the present study, other tools including DTI can provide more robust methods to characterize and visualize WM in both two and three dimensions. Evaluations of neuronal pathways in the CC using DTI can offer insight into the relationship between WM volume and fiber orientation in stuttering.
In the present study, no significant differences in the CC were found between school-age children with persistent stuttering, school-age children who recovered from stuttering, and typically developing children school-age children. A dynamic interplay between development and adaptation in children who stutter may result in brain activity and structural changes observed in AWS as reported in previous studies. Together, the adult and children studies of the CC in stuttering suggests that temporally modulated changes including CC hypertrophy and WM alterations that possibly emerge in late adolescence or early adulthood most plausibly reflect adaptations that are a result of persistent stuttering. In other words, reorganization of neural interconnectivity is likely occurring as a consequence of long-term brain responses to stuttering and ongoing development. Individual variations in the rate of and capacity for neural reorganization could also exist and may be an important aspect in recovery from or persistency in stuttering. Inter-mixed with development and individual variation, treatment-dependent plastic responses may also be driving neural reorganization that is able to attenuate deficits associated with stuttering, particularly at an early stage of development when reorganization has not been completely established, and the capacity of the neural system to adapt and reorganize connections is most prominent. The discrepancy in differences in the CC between adults and children who stutter that we have reported is impetus for larger studies of how early intervention may prevent maladaptive neural reorganization in response to childhood stuttering.
5. Conclusions
The present study did not identify factors in CC development that can be related to recovery or persistency, so there is no evidence for unusual CC development as a marker of stuttering in school-age children. This could imply that differences in CC morphology or area are also not present at the age of recovery from stuttering. However, CC hypertrophy in adulthood and a trend towards reduced area of the rostrum and splenium in childhood could be part of an aberrant trajectory of brain development. The CC could be part of a prolonged process of neural adaptation to persistent stuttering that involves widespread connections with multiple brain areas. Future neurological investigations of stuttering should include multi-modal assessments of callosal size, volume, and white matter integrity.
Acknowledgments
The authors wish to acknowledge the volunteers who participated in this study. The study was partially supported by: (1) an NIH RO1 DC 05210 award – Subtypes and Associated Risk Factors in Stuttering (P.I. Nicoline Ambrose); (2) an ASH Foundation New Investigator Award (P.I. Torrey Loucks); and, (3) a University of Illinois Research Board Award (P.I. Torrey Loucks).
Appendix A. Continuing education
-
The onset of developmental stuttering typically occurs during periods of rapid language development.
True
False
-
The corpus callosum (CC) is the largest white matter structure in the brain. The results of the present study indicates that differences in CC area__________________
are present between children with persistent stuttering and typically developing children
are present between children who recovered from stuttering and typically developing children
were observed between all groups of children
were not observed between any of the groups of children
-
The rates of recovery in children who stutter is around:
25%
50%
70%
75%
-
A previous study investigating the CC of adults who stutter (AWS) found that:
The anterior CC area was larger in AWS than in normally fluent adults
The anterior CC area was larger in normally fluent adults than in AWS
The posterior CC area was smaller in AWS than in normally fluent adults
No differences were observed between AWS and normally fluent adults
-
In the present study, no difference in white matter volume was found between children with persistent stuttering,children who recovered from stuttering and typically developing children.
True
False
Contributor Information
Ai Leen Choo, Email: choo1@illinois.edu.
Soo-Eun Chang, Email: schang7@msu.edu.
Hatun Zengin-Bolatkale, Email: hatun.zengin@vanderbilt.edu.
Nicoline G. Ambrose, Email: nambrose@illinois.edu.
Torrey M. Loucks, Email: tloucks@illinois.edu.
References
- Ambrose NG, Chang S-E, Chon HC, King A, Steger SJ. Right ear advantage and laterality in stuttering children and adults. ASHA Leader. 2007 [Google Scholar]
- Ambrose NG, Cox NJ, Yairi E. The genetic basis of persistence and recovery in stuttering. Journal of Speech, Language, and Hearing Research. 1997;40:567–580. doi: 10.1044/jslhr.4003.567. [DOI] [PubMed] [Google Scholar]
- Beal DS, Gracco VL, Lafaille SJ, De Nil L. Voxel-based morphometry of auditory and speech-related cortex in stutterers. NeuroReport. 2007;18:1257–1260. doi: 10.1097/WNR.0b013e3282202c4d. [DOI] [PubMed] [Google Scholar]
- Beal DS, Quraan MA, Cheyne DO, Taylor MJ, Gracco VL, De Nil LF. Speech-induced suppression of evoked auditory fields in children who stutter. NeuroImage. 2011;54:2994–3003. doi: 10.1016/j.neuroimage.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ulle´n F. Extensive piano playing has regionally specific effects on white matter development. Nature Neuroscience. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bishop D. The underlying nature of specific language impairment. Journal of Child Psychology and Psychiatry. 1992;33:3–66. doi: 10.1111/j.1469-7610.1992.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Blood GW, Blood IM, Hood SB. The development of ear preference in stuttering and nonstuttering children: a longitudinal study. Journal of Fluency Disorders. 1987;12:119–131. [Google Scholar]
- Brosch S, Haege A, Kalehne P, Johanssen HS. Stuttering children and the probability of remission – the role of cerebral dominance and speech production. International Journal of Pediatric Otorhinolaryngology. 1999;47:71–76. doi: 10.1016/s0165-5876(98)00178-5. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: an ALE meta-analysis of functional neuroimaging studies. Human Brian Mapping. 2005;25:105–117. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck S, Lees R, Cook F. The influence of family history of stuttering on the onset of stuttering in young children. Folia Phoniatrica et Logopaedica. 2002;54:117–124. doi: 10.1159/000063407. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies for development. NeuroImage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Chang S-E, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. NeuroImage. 2008;39:1333–1344. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Horwitz B, Ostuni J, Reynolds R, Ludlow C. Evidence of left ventral premotor–motor structural and functional connectivity deficits in adults who stutter. Cerebral Cortex. 2011;21(11):2507–2518. doi: 10.1093/cercor/bhr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepuri NB, Yen YF, Burdette JH, Li H, Moody DM, Maldjian JA. Diffusion anisotropy in the corpus calloum. American Society of Neuroradiology. 2001;23:803–808. [PMC free article] [PubMed] [Google Scholar]
- Choo AL, Kraft SJ, Olivero W, Ambrose NG, Sharma H, Chang S-E, et al. Corpus callosum differences associated with persistent stuttering in adults. Journal of Communication Disorders. 2011;44(4):470–477. doi: 10.1016/j.jcomdis.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimorell-Strong JM, Gilbert HR, Frick JV. Dichotic speech perception: a comparison between stuttering and nonstuttering children. Journal of Fluency Disorders. 1983;8:77–91. [Google Scholar]
- Clarke JM, Lufkin RB, Zaidel E. Corpus callosum morphometry and dichotic listening performance: individual differences in functional interhemispheric inhibition? Neuropsychologia. 1993;31(6):547–557. doi: 10.1016/0028-3932(93)90051-z. [DOI] [PubMed] [Google Scholar]
- Craig A, Hancock K, Tran Y, Craig M, Peters K. Epidemiology of stuttering in the community across the entire life span. Journal of Speech, Language, and Hearing Research. 2002;45:1097–1105. doi: 10.1044/1092-4388(2002/088). [DOI] [PubMed] [Google Scholar]
- Cykowski MD, Fox PT, Ingham RJ, Ingham JC, Robin DA. A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: a potential role for the impaired myelination. NeuroImage. 2010;52:1495–1504. doi: 10.1016/j.neuroimage.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cykowski MD, Kochunov P, Ingham RJ, Ingham JC, Mangin JF, Rivie`re JL, et al. Perisylvian sulcal morphology and cerebral asymmetry patterns in adults who stutter. Cerebral Cortex. 2008;18(3):571–583. doi: 10.1093/cercor/bhm093. [DOI] [PubMed] [Google Scholar]
- De Nil L, Kroll RM, Houle S. Functional neuroimaging of cerebellar activation during single word reading and verb generation in stuttering and nonstuttering adults. NeuroScience Letters. 2001;302:77–80. doi: 10.1016/s0304-3940(01)01671-8. [DOI] [PubMed] [Google Scholar]
- De Nil L, Kroll RM, Kapur S, Houle S. A positron emission tomography study of silent and oral reading of single words in stuttering and nonstuttering adults. Journal of Speech, Language and Hearing Research. 2000;43:1038–1053. doi: 10.1044/jslhr.4304.1038. [DOI] [PubMed] [Google Scholar]
- De Nil LF, Kroll RM, Lafaille SJ, Houle S. A positron emission tomography study of short- and long-term treatment effects on functional brain activation in adults who stutter. Journal of Fluency Disorders. 2003;28(4):357–380. doi: 10.1016/j.jfludis.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Dorion AA, Chantôme M, Hasboun D, Zouaoui A, Marsault C, Capron C, et al. Hemispheric asymmetry and corpus callosum morphometry: a magnetic resonance imaging study. Neuroscience Research. 2000;36:9–13. doi: 10.1016/s0168-0102(99)00102-9. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test. 3rd edition. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Foundas AL, Bollich AM, Corey D, Hurley M, Heilman K. Anomalous anatomy of speech-language areas in adults with persistent developmental stuttering. Neurology. 2001;57:207–215. doi: 10.1212/wnl.57.2.207. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Hunter Downs J, Martin C, et al. A PET study of the neural systems of stuttering. Nature. 1996;382:158–162. doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- Gadea M, Marti-Bonmati L, Arana E, Espert R, Salvador A, Casanova B. Corpus callosum function in verbal dichotic listening: inferences from a longitudinal follow-up of relapsing-remitting multiple sclerosis patients. Brain and Language. 2009;110:101–105. doi: 10.1016/j.bandl.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Georgy BA, Hesselink JR, Jernigan TL. MR imaging of the corpus callosum. American Journal of Roentgenology. 1993;160:949–955. doi: 10.2214/ajr.160.5.8470609. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations and pathology. I. A hypothesis and a program for research. Archives of Neurology. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rumsey JM, Castellanos FX, Rajapakse JC, Kaysen D, Vaituzis AC, et al. A quantitative MRI study of the corpus callosum in children and adolescents. Developmental Brain Research. 1996;91:274–280. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Gootjes L, Bouma A, Van Strien JW, Van Schijndel R, Barkhof F, Scheltens P. Corpus callosum size correlates with asymmetric performance on a dichotic listening task in healthy aging but not in Alzheimer’s disease. Neuropsychologia. 2006;44:208–217. doi: 10.1016/j.neuropsychologia.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Churchill JD, Bates KE, Kleim JA, Greenough WT. A brain adaptation view of plasticity: is synaptic plasticity an overly limited concept? Progress in Brain Research. 2002;138:91–108. doi: 10.1016/S0079-6123(02)38073-7. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Kennedy DN, Caviness VS. Brain asymmetries in autism and developmental language disorders: a nested whole brain analysis. Brain. 2005;128:213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Bakardjiev A, Hodgson J, Caviness VS. Larger brain and white matter volumes in children with developmental language disorder. Developmental Science. 2003;6:11–22. [Google Scholar]
- Hofer S, Frahm J. Topography of the corpus callosum revisited – comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:898–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Howell P, Davis S, Williams R. Late childhood stuttering. Journal of Speech, Language and Hearing Research. 2008;51:669–687. doi: 10.1044/1092-4388(2008/048). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja¨ncke L, Hanggi J, Steinmetz H. Morphological brain differences between adult stutterers and non-stutterers. BioMed Central Neurology. 2004;4:23. doi: 10.1186/1471-2377-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CH, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. NeuroImage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kell CA, Neumann K, von Kriegstein K, Posenenske C, von Gudenberg AW, Euler H, et al. How the brain repairs stuttering. Brain. 2009;132:2747–2760. doi: 10.1093/brain/awp185. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, DeBellis M, Dick M, Kotwal R, Rosenberg DR, et al. Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sciences. 2002;70:1909–1922. doi: 10.1016/s0024-3205(02)01492-3. [DOI] [PubMed] [Google Scholar]
- Leow AD, Zhan L, Zhu S, Hageman N, Chiang M, Barysheva M, et al. White matter integrity measured by fractional anisotropy correlates poorly with actual fiber anisotropy. Biomedical imaging: from nano to macro. Boston, MA: IEEE; 2009. pp. 622–625. [Google Scholar]
- Lu C, Chen C, Ning N, Ding G, Guo T, Peng D, et al. The neural substrates for atypical planning and execution of word production in stuttering. Experimental Neurology. 2010;221:146–156. doi: 10.1016/j.expneurol.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Lu C, Ning N, Peng D, Ding G, Li K, Yang Y, et al. The role of large-scale neural interactions for developmental stuttering. Neuroscience. 2009;161:1008–1026. doi: 10.1016/j.neuroscience.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. The Journal of Neuroscience. 2010;30:10985–10990. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson H. Childhood stuttering: incidence and development. Journal of Fluency Disorders. 2000;25:47–57. [Google Scholar]
- McAuliffe MJ, McGarry D, Gandler W, Csaky K, Trus BL. 14th IEEE symposium on computer-based medical systems. Bethesda, MD: IEEE Computer Society; 2001. Medical image processing, analysis and visualization in clinical research; pp. 381–386. [Google Scholar]
- Moody DM, Bell MA, Challa VR. The corpus callosum, a unique white-matter tract: anatomic features that may explain sparing in Binswanger disease and resistance to flow of fluid masses. American Journal of Neuroradiology. 1988;9:1051–1059. [PMC free article] [PubMed] [Google Scholar]
- Moriarty J, Varma AR, Stevens J, Fish M, Trimble MR, Robertson MM. A volumetric MRI study of Gilles de la Tourette’s syndrome. Neurology. 1997;49:410–415. doi: 10.1212/wnl.49.2.410. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhász C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. NeuroImage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Neumann K, Euler HA, von Gudenberg AW, Giraud A-L, Lanfermann H, Gall V, et al. The nature and treatment of stuttering as revealed by fMRI. A withing- and between-group comparison. Journal of Fluency Disorders. 2003;28:381–410. doi: 10.1016/j.jfludis.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Neumann K, Preibisch C, Euler HA, von Gudenberg AW, Landermann H, Gall V, et al. Cortical plasticity associated with stuttering therapy. Journal of Fluency Disorders. 2005;30:23–29. doi: 10.1016/j.jfludis.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Newcomer PL, Hamill DD. Test of language development-primary. 3rd edition. Austin, TX: ProED; 1997. (TOLD-I:3) [Google Scholar]
- O’Kusky J, Strauss E, Kosaka B, Wada J, Li D, Druhan M, et al. The corpus callosum is larger in right-hemisphere cerebral speech dominance. Annals of Neurology. 1988;23(3):379–383. doi: 10.1002/ana.410240305. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paul LK. Developmental malformation of the corpus callosum: a review of typical callosal development and examples of developmental disorders with callosal involvement. Journal of Neurodevelopmental Disorders. 2011;3:3–27. doi: 10.1007/s11689-010-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Wentzel-Larsen T, Hugdhal K, Feineigle P, Klein J, Staib L, et al. Altered interhemispheric connectivity in individuals with Tourette’s Disorder. American Journal of Psychiatry. 2004;161:2028–2037. doi: 10.1176/appi.ajp.161.11.2028. [DOI] [PubMed] [Google Scholar]
- Preis S, Steinmetz H, Knorr U, Ja¨ncke L. Corpus callosum size in children with developmental language disorder. Cognitive Brain Research. 2000;10:37–44. doi: 10.1016/s0926-6410(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Ortiz H, Sebastian-Galles N, Losilla JM, Deus J. Myelination of language-related areas in the developing brain. Neurology. 2006;66:339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Annals of Neurology. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Reilly S, Onslow M, Packman A, Wake M, Bavin EL, Prior M, et al. Predicting stuttering onset by the age of 3 years: a prospective, community cohort study. Pediatrics. 2009;123:270–277. doi: 10.1542/peds.2007-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Human Brain Mapping. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Buchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet. 2002;360:380–383. doi: 10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation? The Journal of Neuroscience. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, et al. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. NeuroReport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Van Borsel J, Moeyaert E, Rosseel M, Van Loo , Van Renterghem L. Prevalence of stuttering in regular and special school population in Belgium based in teacher perception. Folia Phoniatrica et Logopaedica. 2006;58:289–302. doi: 10.1159/000093185. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, et al. Human motor corpus callosum: topography, somatotopy and link between microstructure and function. The Journal of Neuroscience. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131:50–59. doi: 10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R, Hugdahl K. The corpus callosum in dichotic listening studies of hemispheric asymmetry: a review of clinical and experimental evidence. Neuroscience and Behavioral Reviews. 2008;32:1044–1054. doi: 10.1016/j.neubiorev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Yairi E, Ambrose NG. Early childhood stuttering I: persistency and recovery rates. Journal of Speech, Language, and Hearing Research. 1999;42:1097–1112. doi: 10.1044/jslhr.4205.1097. [DOI] [PubMed] [Google Scholar]
- Yairi E, Ambrose NG. Early childhood stuttering. Austin: Pro Ed.; 2005. [Google Scholar]