Abstract
Background
Many patients with Parkinson disease (PD) develop dementia (PDD), a syndrome that overlaps clinically and pathologically with dementia with Lewy bodies (DLB); PDD and DLB differ chiefly in the relative timing of dementia and parkinsonism. Brain amyloid deposition is an early feature of DLB and may account in part for its early dementia. We sought to confirm this hypothesis and also to determine whether amyloid accumulation contributes to cognitive impairment and dementia in the broad range of parkinsonian diseases.
Methods
29 cognitively normal PD, 14 PD subjects with mild cognitive impairment (PD-MCI), 18 with DLB, 12 with PDD and 85 healthy control subjects (HCS) underwent standardized neurologic and neuropsychological examinations and PiB imaging with PET. Apolipoprotein (APOE) genotypes were obtained in many patients. PiB retention was expressed as the distribution volume ratio using a cerebellar tissue reference.
Results
PiB retention was significantly higher in DLB than in any of the other diagnostic groups. PiB retention did not differ across PDD, PD-MCI, PD, and HCS. Amyloid burden increased with age and with the presence of the APOEε4 allele in all patient groups. Only in the DLB group was amyloid deposition associated with impaired cognition.
Conclusions
DLB subjects have higher amyloid burden than subjects with PDD, PD-MCI, PD or HCS; amyloid deposits are linked to cognitive impairment only in DLB. Early amyloid deposits in DLB relative to PDD may account for their difference in the timing of dementia and parkinsonism.
Keywords: dementia, Lewy, Parkinson, amyloid, PiB
INTRODUCTION
Isolated cognitive impairments are common in Parkinson disease (PD); these increase in number and severity with advancing age and across stage of motor disability (1,2). Mild cognitive impairment (MCI) is viewed as an intermediate stage between normal cognition and dementia, and PD patients with cognitive complaints and documented cognitive impairments that fall short of a diagnosis of dementia are often called PD-MCI (3,4). Frank dementia is also common in PD, with incidences between 5%–10% and prevalences between 30%–80% (5–7). When the dementia manifests itself years after the onset of motor signs, the condition is called Parkinson disease dementia or PDD (8). When the onset of dementia occurs before or within 1 year of the parkinsonian motor signs, the clinical diagnosis is dementia with Lewy bodies (DLB) (9). PDD and DLB have clinically similar manifestations with dementia and associated parkinsonism, hallucinations, and fluctuations in arousal and cognition. The pathologic basis for PDD and for DLB appear to overlap as well, with evidence supporting cortical Lewy body accumulation/α-synuclein toxicity (10), accumulation of Aβ amyloid (10,11), dopamine system dysfunction (12,13), and loss of basal forebrain cholinergic neurons (14). The development of positron emission tomographic (PET) tracers that bind Aβ enables ante mortem detection of amyloid in humans. One such agent, Pittsburgh Compound B (11C-PiB), specifically binds fibrillar forms of brain amyloid and affords an opportunity to assess pre-mortem the contribution of Aβ amyloid to cognitive impairment and dementia in the parkinsonian spectrum of Lewy body diseases (15,16,17,18). Several groups, including our own, have found higher amyloid burden in DLB than in PDD (15,16,18; but see 19). Given the similar clinical phenotype of DLB and PDD with the notable exception of the relative timing of motor and cognitive decline, we previously suggested that amyloid burden may hasten the onset of dementia in subjects with parkinsonism (15; see also 20). It still remains uncertain whether amyloid burden contributes to cognitive impairment in PD, or whether it may relate to the relative timing of motor and cognitive symptoms.
In order to explore the hypothesis that Aβ toxicity contributes to cognitive impairment and dementia in Lewy body diseases, we enrolled 73 patients with PD, PD-MCI, PDD, DLB and 85 cognitively intact healthy control subjects (HCS), tested them with comprehensive neurological and neuropsychological examinations, and imaged them with PiB-PET brain scans. We evaluated the extent of Aβ accumulation in these subjects and analyzed the relation of Aβ deposition to cognitive test scores and to apolipoprotein E (APOE) genotype. We tested these specific hypotheses: (1) PiB uptake will be higher in DLB than the other parkinsonian disorders, (2) PiB uptake will be higher in PD-MCI than in PD with normal cognition (PD-nl), (3) PiB uptake will relate to cognitive function, and (4) the APOE ε4 allele will predict amyloid burden in this population.
METHODS
Study design
We enrolled 73 patients with a range of Lewy body diseases: 29 PD-nl, 14 PD-MCI, 12 PDD and 18 DLB (Table 1). All patients were recruited from the Massachusetts General Hospital (MGH) Movement Disorders and Memory Disorders Units and gave informed consent to participate in this research study according to the protocol approved by the Partners HealthCare Inc. Institutional Review Board. Acquired data from these parkinsonian subjects were compared with those of a separately collected cohort of 85 HCS individuals who were enrolled in a separate but related research study; the parkinsonian patients and HCS all underwent identical standardized neurological examinations, cognitive testing and PiB PET imaging. All HCS subjects had a normal neurological examination, no subjective complaint, a Clinical Dementia rating (CDR) Sum of Boxes (21; 22) scale score of 0, and normal cognition. All but one HCS subject had a Mini-Mental Status Examination (MMSE) score > 27 (23). That subject performed better than 2 standard deviations below the mean for ability level on a majority of the tests. All PD subjects fulfilled criteria for a diagnosis of idiopathic PD according to the UK Parkinson Disease Society Brain Bank Research Center’s clinical diagnostic criteria (24). Individual cognitive tests were grouped into four aggregate cognitive domain scores (ACD scores; see below) and were used to distinguish a population of PD-nl from a population of PD-MCI subjects. PD-nl subjects had no ACD score in excess of 1.5 standard deviations above the group mean of PD subjects without cognitive complaints. All but one PD-nl subject had CDR scores of 0 (that subject had very mild complaints and a CDR-SB of 0.5, with CDR-SB scores of 0 in three subsequent years). The diagnosis of PD-MCI required an individual’s subjective complaint coupled with at least one abnormal ACD score (exceeding 1.5 standard deviations above the group mean of PD subjects without cognitive complaints), in the absence of dementia. One subject had two markedly abnormal ACD scores yet denied subjective complaints and was included in the PD-MCI group. The diagnosis of PDD was based on the movement disorders criteria of Emre et al. (8); the diagnosis of DLB was based on the consensus criteria of McKeith et al. (9).
Table 1.
Group demographics
| Dx | DLB | PDD | PD-MCI | PD | HCS |
|---|---|---|---|---|---|
| N | 18 | 12 | 14 | 29 | 85 |
| Sex, M:F | 17:1 | 10:2 | 11:3 | 20:9 | 30:55 |
| Age, y | 72.6 ± 6.7 | 71.7 ± 4.7 | 69.4 ± 7.3 | 69.6 ± 6.4 | 73.2 ± 6.7 |
| Education, y | 16.1 ± 2.7 | 16.3 ± 2.7 | 16.4 ± 2.6 | 16.9 ± 2.8 | 16.1± 2.8 |
| H&Y | 1.9 ± 1.2 a | 2.9 ± 0.9 b | 2.7 ± 0.7 b | 2.2 ± 0.5 a | 0.0 ± 0.2 c |
| CDR-SB | 6.4 ± 3.5 d | 6.9 ± 3.4 d | 1.5 ± 1.2 e | 0.2 ± 0.4 f | 0.0 ± 0.0 g |
| MMSE | 20.7 ± 7.3 d | 22.8 ± 4.8 d | 26.2 ± 3.1 c | 29.1 ± 1.2 g | 29.2 ± 0.9 g |
| On levodopa | 9 | 11 | 14 | 26 | 0 |
| On DA agonist | 1 | 2 | 7 | 14 | 0 |
Values are listed as mean ± SD unless otherwise stated. Significance tests for contrasts were made after adjusting for age and for multiple comparisons within (Tukey) but not across cognitive tests. The sex ratio differed between groups, with an increased proportion of males in the disease groups compared with the HCS group (Fisher exact test, p = 1.7e-7). Average ages of the dementia with Lewy bodies (DLB), Parkinson disease dementia (PDD), Parkinson disease (PD), and healthy control subject (HCS) cohorts were similar. Education levels did not differ significantly across groups. Unadjusted Hoehn and Yahr (H&Y), Clinical Dementia Rating Sum of Boxes (CDR-SB), and Mini-Mental State Examination (MMSE) are shown, along with the number of subjects taking levodopa or dopamine agonist medications.
= different from HCS, PD-MCI, and PDD;
= different from DLB, PD, and HCS;
= different from all other groups;
= different from HCS, PD, and PD-MCI;
= different from DLB, PDD, and HCS;
= different from DLB and PDD;
= different from DLB, PDD, and PD-MCI.
Clinical evaluation
The clinical evaluation of motor function included the Hoehn & Yahr (H&Y) stage (25) and the motor subscale of the Unified Parkinson Disease Rating Scale (UPDRS) (26) testing in the “on” state. Evaluation of cognition included these neuropsychological tests: Logical Memory I and II (LM I and LM II) (27), Free and Cued Selective Reminding Test (free recall: FRsrt and cued recall: FCsrt) (28), Digit Span Forward and Backward (29), the Digit Symbol component of the Wechsler Adult Intelligence Scale–Revised (30), Trailmaking Tests A and B (31), Verbal Fluency (F-A-S (32); animals & vegetables (33)), the 30 item Boston Naming Test (34), Benton Visual Form Discrimination (32)and the American version of the National Adult Reading Test (AMNART) Verbal IQ assessment (35). Functional status was assessed by the Clinical Dementia Rating Scale Sum of Boxes (CDR_SB) and the Global CDR (36,37). Psychiatric state was assessed by the Neuropsychiatric Inventory Questionnaire (38). Clinical fluctuations were quantified with the Mayo fluctuations screen (39). We also queried the medical record, the subject, and an informant to derive the age of onset of parkinsonian motor symptoms and the age of onset of cognitive symptoms. Eighteen demented subjects were unable to complete some cognitive tests due to their degree of cognitive impairment. We used multiple regression to estimate performance on those tests using the predictors of MMSE and CDR_SB, and we added a slight perturbation based on the error variance from the regression model for each respective measure so as to avoid biasing error variance downward. Depending on the measure, 1 to 12% of scores subsequently analyzed were so estimated. We applied correlated factor analysis to the cognitive test performance of subjects with PD, PD-MCI, PDD, and DLB to identify and form the following 4 aggregate cognitive domain factors: executive (trails B, digit symbol), episodic memory (logical Ia, logical IIa), semantic memory and language (FRsrt, FCsrt, BNT, FAS, verbal fluency), and visuospatial skill (Benton Vfdt). Cognitive domain factor scores were calculated as the average z-score of the nonmissing component tests (no more than half of each factor’s component tests were allowed to be missing for a given subject).
Blood tests
We acquired APOE genotype (40) in 65 subjects for regression with PiB imaging and cognitive test performance.
PET imaging acquisition and analyses
N-Methyl-[11C]2-(4methylaminophenyl)-6-hydroxybenzothiazole (PiB) was prepared at MGH as previously described (41). Subjects were positioned in a Siemens HR+ scanner (three-dimensional mode; 63 image planes; 15.2 cm axial field of view; 5.6 mm transaxial resolution; 2.4 mm slice interval; 69 frames: 12 × 15 seconds, 57 × 60 seconds; Knoxville, TN). After a transmission scan, 8 – 15 mCi of 11C PiB was injected as a bolus, followed immediately by a 60 minute dynamic acquisition. PET data were reconstructed, corrected for attenuation and each frame was evaluated to verify adequate count statistics and absence of head movement.
PiB retention is highly correlated between brain regions. As PiB retention is particularly high in the precuneus region, and as precuneus PiB retention correlates very highly with global retention (r=0.939), we used precuneus PiB retention for our analyses. Each subject’s precuneus was identified with the Automated Anatomic Labeling template following SPM spatial transformation (see below), as described previously (15). Precuneus PiB retention was calculated using the Logan graphical analysis method (42,43), with cerebellar cortex as the reference tissue input function; specific PiB retention was expressed as the distribution volume ratio (DVR), as in previous studies (44–47).
SPM analyses
PET DVR data were also analyzed using a voxel-based method, Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, Function Imaging Laboratory, London) (48), which was implemented using Matlab R2010b (MathWorks Inc, Sherborn, MA). Each dataset was spatially transformed to the SPM template PET image and smoothed with a 12mm Gaussian kernel to account for individual anatomic differences. The threshold for statistical significance was set at a p-value of 0.05, corrected for multiple comparisons at the voxel level, using the False Discovery Rate. Statistical significance for clusters was also recorded. The cluster extent threshold (k) was set at 50 voxels. Anatomical localization of clusters reaching a peak height significance threshold of 0.05 corrected for multiple comparisons was ascertained using the coplanar stereotaxic atlas (49,50). Voxel-wise two sample t-tests for PiB retention comparison between-group were performed, adjusting for the age at PiB-PET. Two subjects with PD-MCI were excluded from SPM analysis due to truncation error, leaving 18 DLB, 12 PDD, 12 PD-MCI, 29 PD and 85 HCS.
Analyses examining the association of precuneus amyloid with clinical, motor and cognitive variables
To assess the relation of various dependent variables to diagnostic groups (PD-nl, PD-MCI, PDD, DLB, and HCS) and other predictors, we ran a backward elimination general linear model (GLM) with cutoff p values for removal from the model set at 0.05. Patterns of residuals from the final retained model were examined graphically for reasonable conformance to significance test assumptions of normality and homoscedasticity. Log transformations were applied to dependent variables when assumptions appeared questionable. Tukey adjusted post hoc tests were performed where required to follow up significant main effects. Curvilinear quadratic and interaction terms were included for some predictors in initial runs. Even when such terms were significant, a second analysis was run in which all predictors were constrained to be linear because the gain in parsimony and robustness appeared to offset any slight degradation in fit.
For the analysis of PiB as the dependent variable, the initial pool of predictor variables included diagnostic group, age at PiB scan, gender, and their interactions. This GLM analysis was re-run with inclusion of APOE genotype for the subset of subjects with APOE measurement. Parallel GLM analyses were run separately for the dependent variables of MMSE and cognitive domain factors, except that PiB DVR was now also included among the pool of predictors along with its interactions with other terms. GLM analyses provide a comprehensive and flexible technique to exhaustively examine both quantitative and qualitative predictor variables as well as their interactions. GLM provides a net of tests that theoretically allows nothing of interest to escape detection.
RESULTS
Group demographics
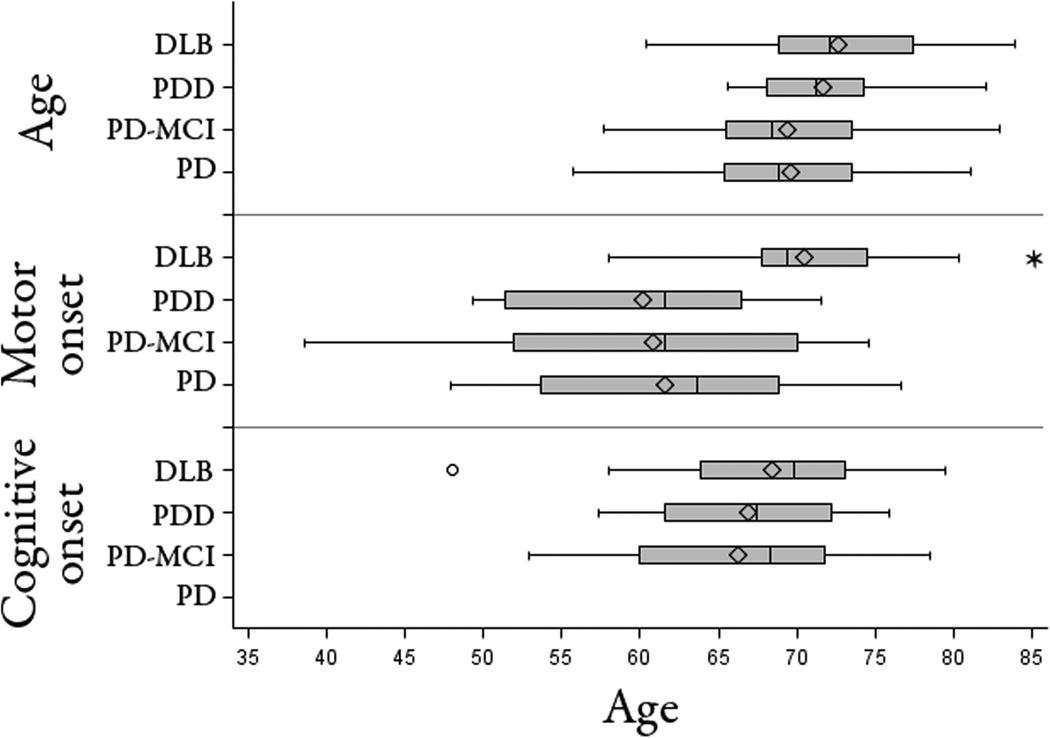
The groups did not differ significantly in age or in educational level (Table 1). Most of the participants in the groups with parkinsonism were men. However, there were more women than men in the HCS group. The PDD and DLB groups had similar CDR and MMSE scores; these were lower than the nondemented group scores (p<0.001; Table 1). The PD-MCI CDR and MMSE scores were intermediate, significantly lower than the PD-nl (p<0.05) but higher than the PDD and DLB groups (p<0.05). Performance on the full set of cognitive tests differed similarly between groups (Table 2). Clinical fluctuations (39), were higher in PDD and DLB than in the other groups (p<0.003). Motor impairment, as measured by the H&Y stage, was greater for the PDD and PD-MCI groups than for the DLB or PD groups (for all pairwise comparisons, p<0.04). Age at the time of PiB imaging was similar across the four parkinsonian groups, and the age of onset of cognitive impairment was likewise similar for the DLB, PDD and PD-MCI groups. The DLB group however had a significantly later onset of motor symptoms than the other parkinsonian groups (p<0.02 for all comparisons; Figure 1). In other words, the PD, PD-MCI and PDD groups all had the motor signs of parkinsonism years before the onset of cognitive signs, and approximately a decade before motor signs appeared in the DLB group.
Table 2.
Neuropsychologic test performance
| DLB | PDD | PD-MCI | PD | HCS | |
|---|---|---|---|---|---|
| CDR | 1.1 ± 0.7 a | 1.2 ± 0.7 a | 0.4 ± 0.2 b | 0.1 ± 0.2 c | 0.0 ± 0.0 c |
| Logical Memory IA | 5.9 ± 4.1 d | 5.8 ± 4.6 d | 9.6 ± 3.4 d | 13.8 ± 4.2 b | 16.7 ± 3.5 b |
| Logical Memory IIA | 4.9 ± 4.3 d | 5.5 ± 3.7 d | 7.9 ± 3.9 d | 13.1 ± 4.2 c | 15.7 ± 3.9 c |
| Digits forward | 5.9 ± 1.5 d | 6.1 ± 0.9 e | 6.8 ± 1.2 e | 6.9 ± 1.1 f | 6.9 ± 1.0 f |
| Digits backward | 3.4 ± 0.9 d | 3.4 ± 1.2 d | 4.4 ± 1.4 g | 5.0 ± 1.2 h | 5.7 ± 1.1 b |
| Trails A | 99.7 ± 43.5 a | 104.8 ± 52.5 a | 60.0 ± 26.0 b | 35.3 ± 11.8 c | 30.8 ± 9.3 c |
| Trails B | 261.9 ± 68.7 d | 218.8 ± 76.1 d | 220.0 ± 86.0 d | 88.4 ± 29.0 c | 78.1 ± 44.4 c |
| Category fluency | 16.7 ± 7.7 a | 15.8 ± 6.2 a | 24.5 ± 5.1 b | 35.4 ± 7.5 c | 32.9 ± 5.8 c |
| Letter fluency | 23.0 ± 13.0 d | 20.2 ± 12.1 d | 32.6 ± 11.5 g | 44.8 ± 11.9 i | 49.0 ± 14.4 c |
| WAIS-R | 14.7 ± 12.7 j | 15.3 ± 11.6 j | 30.3 ± 9.6 b | 43.4 ± 8.4 b | |
| Boston Naming Test | 23.4 ± 7.2 d | 21.8 ± 6.0 d | 25.9 ± 3.4 e | 28.6 ± 1.3 i | 28.6 ± 1.3 i |
| Blessed Dementia Scale | 12.2 ± 10.8 j | 8.5 ± 6.0 k | 3.9 ± 2.2 f | 1.8 ± 1.9 i | |
| FRSRT | 15.9 ± 11.3 a | 11.7 ± 8.4 a | 24.2 ± 4.1 b | 31.8 ± 5.4 b | 35.1 ± 5.6 b |
| FCSRT | 43.4 ± 6.1 l | 38.7 ± 12.4 b | 46.4 ± 1.5 m | 47.5 ± 1.0 i | 47.7 ± 0.9 i |
| Benton | 23.1 ± 4.4 d | 22.9 ± 4.9 d | 27.6 ± 4.2 e | 29.5 ± 3.3 i | 28.4 ± 5.3 i |
| AMNART Verbal IQ | 118.3 ± 11.7 e | 116.4 ± 11.3 k | 112.7 ± 12.7 d | 125.8 ± 6.4 j | 123.0 ± 7.9 n |
| UPDRS | 22.6 ± 14.7 m | 34.8 ± 14.5 b | 22.2 ± 10.7 m | 19.4 ± 7.2 m | |
| NPI-Q | 9.2 ± 5.0 a | 8.2 ± 4.4 d | 4.1 ± 3.1 h | 1.9 ± 2.1 i | 0.4 ± 1.1 c |
| Geriatric Depression Scale | 4.3 ± 3.0 e | 4.9 ± 4.6 e | 4.1 ± 2.1 e | 2.0 ± 2.1 e | 2.5 ± 3.2 e |
| Motor symptom duration, y | 2.5 ± 1.2 b | 11.5 ± 7.3 f | 8.7 ± 6.2 f | 8.1 ± 4.6 f | N/A |
| Cognitive symptom duration, y | 4.2 ± 3.1 e | 4.8 ± 4.2 e | 3.2 ± 2.8 e | N/A | N/A |
| Fluctuations score | 2.2 ± 1.2 j | 2.3 ± 1.2 j | 0.9 ± 1.0 i | 0.2 ± 0.5 i | N/A |
Values are listed as mean ± SD. Unadjusted clinical and neuropsychological test score means are shown. Significance tests for contrasts were made after adjusting for age and for multiple comparisons within (Tukey) but not across cognitive tests. Significant contrasts (p = 0.05) are indicated with superscript letters and defined as follows:
= different from HCS, PD, and PD-MCI;
= different from all other groups;
= different from DLB, PDD, PD-MCI;
= different from HCS and PD;
= different from no other group;
= different from DLB;
= different from HCS;
= different from DLB, PDD, and HCS;
= different from DLB and PDD;
= different from PD and PD-MCI;
= different from PD;
= different from HCS, PD, and PDD;
= different from PDD;
= different from PD-MCI;
= different from PDD.
CDR = Clinical Dementia Rating; WAIS-R=Wechsler Adult Intelligence Scale–Revised; FRSRT = Free Selective Reminding Test; FCSRT=Cued Selective Reminding Test; AMNART = American version of the National Adult Reading Test.; UPDRS = United Parkinson’s Disease Rating Scale; NPIQ=Neuropsychiatric Inventory Questionnaire.
Figure 1. Distribution of onset of motor and cognitive symptoms across the diagnostic groups.
Age at acquisition of PiB PET, age at onset of motor symptoms and age at onset of cognitive symptoms for each diagnostic group are displayed using the box-whiskers convention. Box edges show the inter-quartile range. The vertical line within each box is the median value, and the diamond designates the mean. Box whiskers extend to as much as 1.5 interquartiles above and below the edges. The DLB group had a significantly later onset of motor symptoms than the other groups (p <0.01), whereas all groups associated with cognitive symptoms had similar ages at onset of cognitive impairment.
Group comparisons of amyloid burden
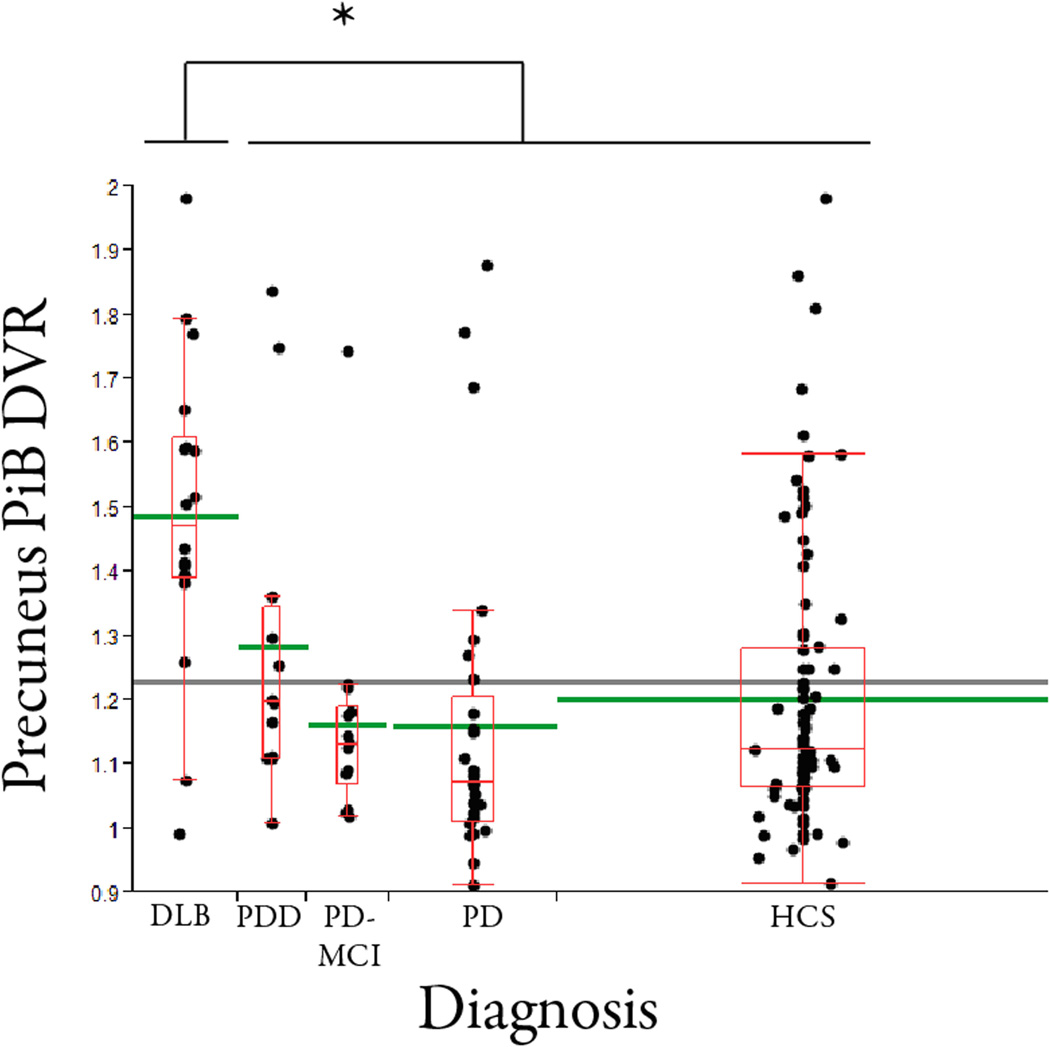
We assessed amyloid burden using PiB PET in PD-nl, PD-MCI, PDD, DLB and HCS subjects. Amyloid burden, measured as precuneus PiB DVR normalized to the cerebellum, differed across the diagnostic groups (with or without a linear adjustment for age; ANCOVA, p< 0.0001; Figure 2). The mean PiB uptake in the precuneus region for DLB was 1.49 and for PDD was 1.28 (p = 0.033 on head to head comparison, linearly adjusting for age). PiB uptake was also significantly higher in DLB than in the PD-nl, PD-MCI, and HCS groups (all comparisons, p<0.002). The mean PiB DVR for the PD-nl group was 1.16 and for the PD-MCI group was 1.16, indicating that amyloid burden at baseline did not distinguish these two non-demented parkinsonian groups. PiB DVR values in PD, PD-MCI, and PDD were not significantly different from each other. PiB retention did not differ between males and females in any group.
Figure 2. Amyloid burden varies across the diagnostic groups.
Precuneus PiB DVR values are displayed for each of the diagnostic groups, using the box-whiskers convention. Dots (jittered slightly laterally) represent actual values. The red horizontal line within each box is the group median PiB DVR value, and the green horizontal line extending beyond the box is the diagnostic group mean. The horizontal line extending across the entire graph indicates the grand mean. The widths of the boxes are proportional to group sample sizes. Group means were not substantially altered by adjustment for age. The DLB group had higher PiB retention than each of the other groups (p<0.04 for each comparison). The PDD, PD-MCI, PD, and HCS group means were not significantly different from each other.
Within diagnostic groups, older subjects had higher amyloid burden (p=0.0009, GLM analysis). Six percent of the variance of (log) PiB burden across subjects could be attributed to its linear relation to age uniquely; 15% of the variance was separately explained by differences among diagnostic groups in their mean levels.
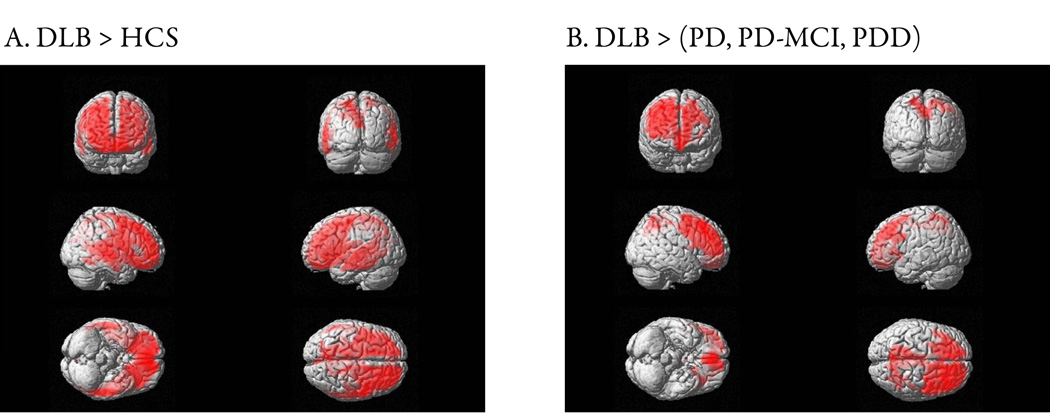
SPM analyses at the voxel level revealed significantly higher PiB retention in DLB compared to HCS in frontal and parietal lobes with a peak in the right putamen (p (voxel-level) < 0.001, z = 6.33; Figure 3A). Amyloid burden in PDD, PD-MCI, PD, and HCS groups were indistinguishable. Compared to the aggregated PD-nl, PD-MCI and PDD groups, PiB uptake in DLB was higher in frontal and parietal regions (p (voxel-level) = 0.025, z = 5.13; Figure 3B).
Figure 3. Topography of amyloid burden in DLB.
SPM contrasts of PiB DVR in DLB with other diagnostic groups. Regional PiB retention differences are shown in red. A. Amyloid burden in DLB compared with HCS was significantly higher in the frontal, parietal and superior temporal lobes (p (voxel-level) < 0.001, z = 6.33). B. Compared with the aggregated PD, PD-MCI and PDD groups, DLB had higher PiB retention in both frontal and parietal lobes (p (voxel-level) = 0.025, z = 5.13).
Amyloid burden and cognitive function
The relation between amyloid burden and global cognitive function as measured by the MMSE varied by diagnostic group (p<0.004 for the interaction of diagnosis and PiB DVR). In the DLB group, higher amyloid was associated with worse performance (p < 0.001). In contrast, PiB uptake was not significantly related to MMSE score for any of the PD-nl, PD-MCI, PDD, or HCS groups.
We also assessed the relation of amyloid deposition to performance on the four aggregate cognitive domain factors that we identified by factor analysis: executive, semantic memory and language, episodic memory and visuospatial skill. For each cognitive factor, significant diagnostic group mean differences were found in expected directions (HCS and PD higher than the others). However, of more interest was the finding that in the DLB group alone, performance on the semantic memory factor worsened with increasing amyloid deposition (p=0.0012 for interaction of diagnostic group and PiB). In contrast to the relation of PiB to semantic memory among DLB subjects, PiB burden did not significantly relate to cognitive performance on the episodic memory, executive, or visuospatial factors in any of the five diagnostic groups.
Amyloid burden and motor function in the parkinsonian groups
We found no significant relation of PiB burden to motor impairment as measured either by the Hoehn and Yahr (H&Y) score or by the UPDRS score within any of the diagnostic groups.
Relation of APOE genotype to amyloid burden
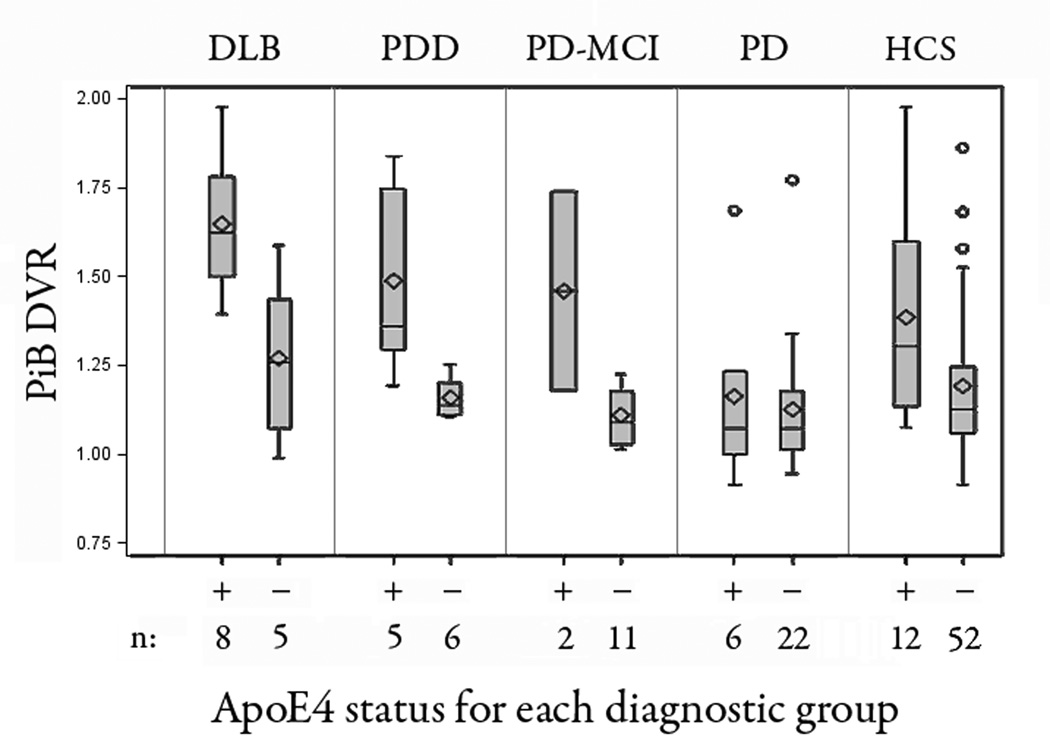
We acquired APOE genotype in 65 of the 74 parkinsonian subjects and in 64 of the 85 HCS. As anticipated, PiB burden positively correlated with the number of APOE ε4 alleles (r = +.49; p < 0.0001) in the sample as a whole (Figure 4). The number of ε4 alleles (0, 1, 2) was significantly linked to PiB burden (p<0.0001), with the strongest association in the HCS, PD-MCI, PDD and DLB groups. On examining the relative contributions of factors impacting amyloid burden, we uncovered three independent features: diagnostic group (p=0.013), number of APOE ε4 alleles (p<0.0001), and advancing age (p=0.0001). In these analyses, the number of APOE ε4 alleles accounted for 14% of the variance in (log) PiB burden (unbiased population estimate). Adjusting for diagnostic group and age, the presence of at least one APOE ε4 allele (treated as binary: present/absent) was associated with a two-fold higher log amyloid load (0.33 vs 0.17; p = 0.0001) or approximately a 20% higher amyloid load (1.39 vs 1.18). Conversely, after adjusting for number of APOE ε4 alleles and age, the mean PiB uptake in the precuneus region for DLB was 1.48 and for PDD was 1.28 (p = 0.05) on head to head comparison).
Figure 4. Amyloid burden varies with apolipoprotein E genotype.
Box-whisker plots showing PiB retention in each diagnostic group differentiated by the presence (+) or absence (−) of at least one ApoE4 allele. There was a significant main effect of ApoE4 versus PiB retention for the entire cohort (p=0.0001), with the strongest associations in the HCS, PD-MCI, PDD and DLB groups.
DISCUSSION
The results of this study confirm and extend our initial report of high PiB amyloid signal in DLB compared to PDD, PD-MCI, PD and HCS. These results are consistent with several prior studies, including our own (15,16,18; but see 19) that cortical amyloid deposits are a common feature of DLB that can be detected during life. Although prior reports have identified age as a risk factor for amyloid burden (for example, 51), this would not explain the higher amyloid burden seen in our DLB cohort as their mean age did not differ significantly from the other parkinsonian groups. While diagnostic groups were well matched for age, the age of onset of motor illness was higher for DLB than for the other Parkinson disease groups. This finding is compatible with the known epidemiology of DLB and PD (52).
Differential amyloid accumulation in PDD and DLB suggests differences in how neuropathological lesions evolve in these diseases. In PD, the clinical history of early motor dysfunction followed by later cognitive impairment correlates with nigral Lewy body accumulation and neuronal death, followed by diffuse Lewy-related pathology in cortex, possibly influenced by late amyloid deposition (53, 54). In contrast, in dementia with Lewy bodies, the underlying neuropathology appears to take a rostral to caudal course, with an amyloid-driven cortex-specific pathology present early and likely related to early cognitive dysfunction, with concomitant or subsequent motor decline due to midbrain Lewy related pathology. The observation that the severity and duration of motor deficits were greater in the PDD than DLB groups supports this formulation. However, as PiB binds amyloid fibrils but not soluble amyloid oligomers (55), it remains possible that DLB and PDD share high concentrations of toxic amyloid oligomers, and that these oligomers underlie cognitive impairment in both DLB and PDD.
Diffuse plaques with amorphous amyloid deposits have been observed in both PDD and DLB (56–58). PiB labels neuritic plaques as well as some diffuse plaques, and PiB retention in cases of DLB and PDD appears to relate to diffuse plaque burden (43, 56, 57, 59). The extent to which diffuse plaques contribute to cognitive impairment in Lewy body diseases remains to be determined.
The DLB and PDD groups had similar degrees of cognitive impairment, yet amyloid burden was linked to indices of dementia only in the DLB cases. The difference in amyloid burden, and its cognitive consequences, is surprising, given the similar clinical phenotype and neuropathology findings in DLB and PDD. The low amyloid burden of PDD may hinder our ability to resolve a relation between amyloid and cognitive function in PDD. Another possibility is that some of the other pathologic processes that cause dementia in PD, such as diffuse Lewy body/α-synuclein accumulation, loss of cholinergic neurons and/or loss of medial nigral dopaminergic neurons exert greater effects in PDD than in DLB. Alternatively, it is possible that amyloid burden accelerates cognitive decline, thereby narrowing the temporal window between motor and cognitive deterioration. This possibility is supported by recent neuropathological studies in PD, which have shown that late onset of parkinsonism is associated with higher amyloid burden (18) and dementia in PD (18, 54). It is noteworthy that cognitively normal PD subjects and PD-MCI subjects were indistinguishable on the basis of amyloid burden, with levels similar to HCS. Amyloid burden therefore is not an important factor for cognitive impairments in PD-MCI.
Consistent with prior reports (15, 60), PiB retention varied widely in the HCS, with amyloid burden in some subjects elevated into the DLB and AD range. Whether higher amyloid burden in cognitively normal subjects represents a risk factor for future cognitive decline is an active area of investigation.
Prior reports identified APOE e4 allele (reviewed in 61) and age (51) as risk factors for amyloid burden. We confirmed these findings. As expected, we found that the ε4 allele conferred significant risk for amyloid burden in our entire cohort. Remarkably, the explanatory power of APOE genotype in predicting amyloid burden was comparable to the explanatory power of diagnostic group (in terms of percent variance of log amyloid accounted for). Nonetheless, after taking into account APOE status and age as independent variables, amyloid burden was still substantially higher in the DLB group compared to the other parkinsonian groups, including PDD. Further, large population studies have not consistently found a relation between APOE genotype and dementia in PD (62–67) or in DLB (68–72). We conclude therefore that amyloid plays an early and central role in the pathology and clinical manifestations of dementia that is unique to DLB, and helps explain the difference from PDD in the timing of dementia.
Acknowledgments
Funding sources:
Michael J. Fox Foundation for Parkinson’s Research (S.N.G., J.H.G)
National Alzheimer's Coordinating Center Collaborative Project 5 U01 AG016976-11
(S.N.G., K.A.J., J.H.G)
NINDS (K.A.J.)
NIA (K.A.J.)
Alzheimer’s Disease Association (K.A.J.)
Caja Madrid Foundation - Scholarship for postgraduate studies (M.M.)
Full financial disclosure for the previous 12 months
S.N. Gomperts receives research support from the Michael J. Fox Foundation for Parkinson’s Research, NIH/NIA, and NIH/NIMH.
J. J. Locascio receives research support from the Michael J. Fox Foundation for Parkinson’s Research, and NIH/NIA.
M. Marquie receives research support from the Caja Madrid Foundation - Scholarship for postgraduate studies.
A. L. Santarlasci is supported through the Michael J. Fox Foundation for Parkinson’s Research.
D. M. Rentz receives research support from NIH/NIA and the Alzheimer’s Association J. Maye is supported through grants from NIH/NIA and the Alzheimer’s Association, K. A. Johnson receives research support from Michael J. Fox Foundation for Parkinson’s Research, NIH/NIA, American Health Assistance Foundation, and Alzheimer Association. He has consulted for GE Healthcare, Bayer, Avid, Bristol Myers Squibb, Janssen, and Pfizer.
J. H. Growdon receives research support from the Michael J. Fox Foundation for Parkinson’s Research, NIH/NIA. He serves on the Scientific Advisory Board of Neurimmune Therapeutics.
Footnotes
Financial Disclosure/Conflict of Interest: None
- Research project: A. Conception, B. Organization, C. Execution;
- Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
- Manuscript Preparation: A. Writing of the first draft, B. Review and Critique;
| S.N. Gomperts | 1ABC, 2AC, 3AB |
| J. J. Locascio | 2AB, 3B |
| M. Marquie | 1C, 2AB, 3AB |
| A. L. Santarlasci | 1BC, 3B |
| D. M. Rentz | 1BC, 2AB, 3B |
| J. Maye | 1C, 3B |
| K. A. Johnson | 1ABC, 2AC, 3B |
| J. H. Growdon | 1ABC, 2AC, 3AB |
References
- 1.Locascio JJ, Corkin S, Growdon JH. Relation between clinical characteristics of parkinson's disease and cognitive decline. J Clin Exp Neuropsychol. 2003;25:94–109. doi: 10.1076/jcen.25.1.94.13624. [DOI] [PubMed] [Google Scholar]
- 2.Mortimer JA, Pirozzolo FJ, Hansch EC, Webster DD. Relationship of motor symptoms to intellectual deficits in parkinson disease. Neurology. 1982;32:133–137. doi: 10.1212/wnl.32.2.133. [DOI] [PubMed] [Google Scholar]
- 3.Litvan I, Aarsland D, Adler CH, et al. MDS task force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Mov Disord. 2011 Jun 9; doi: 10.1002/mds.23823. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jellinger K. Heterogeneous mechanisms of mild cognitive impairment in Parkinson’s disease. J Neural Transm. 2011 doi: 10.1007/s00702-011-0716-4. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5.Hughes TA, Ross HF, Musa S, et al. A 10-year study of the incidence of and factors predicting dementia in parkinson's disease. Neurology. 2000;54:1596–1602. doi: 10.1212/wnl.54.8.1596. [DOI] [PubMed] [Google Scholar]
- 6.Mayeux R, Chen J, Mirabello E, et al. An estimate of the incidence of dementia in idiopathic parkinson's disease. Neurology. 1990;40:1513–1517. doi: 10.1212/wnl.40.10.1513. [DOI] [PubMed] [Google Scholar]
- 7.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in parkinson disease: An 8-year prospective study. Arch Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 8.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 9.McKeith IG, Dickson DW, Lowe J Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 10.Harding AJ, Halliday GM. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001;102:355–363. doi: 10.1007/s004010100390. [DOI] [PubMed] [Google Scholar]
- 11.Mattila PM, Röyttä M, Torikka H, Dickson DW, Rinne JO. Cortical Lewy bodies and Alzheimer-type changes in patients with Parkinson’s disease. Acta Neuropathol. 1998;95:576–582. doi: 10.1007/s004010050843. [DOI] [PubMed] [Google Scholar]
- 12.Torack RM, Morris JC. The association of ventral tegmental area histopathology with adult dementia. Arch Neurol. 1998;45:497–501. doi: 10.1001/archneur.1988.00520290025008. [DOI] [PubMed] [Google Scholar]
- 13.Rinne JO, Rummukainen J, Paljarvi L, Rinne UK. Dementia in parkinson's disease is related to neuronal loss in the medial substantia nigra. Ann Neurol. 1989;26:47–50. doi: 10.1002/ana.410260107. [DOI] [PubMed] [Google Scholar]
- 14.Tiraboschi P, Hansen LA, Alford M, et al. Cholinergic dysfunction in diseases with Lewy bodies. Neurology. 2000;54:407–411. doi: 10.1212/wnl.54.2.407. [DOI] [PubMed] [Google Scholar]
- 15.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edison P, Rowe CC, Rinne JO, et al. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79:1331–1338. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- 17.Claassen DO, Lowe VJ, Peller PJ, Petersen RC, Josephs KA. Amyloid and glucose imaging in dementia with Lewy bodies and multiple systems atrophy. Parkinsonism Relat Disord. 2011;17:160–165. doi: 10.1016/j.parkreldis.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 19.Foster ER, Campbell MC, Burack MA, et al. Amyloid imaging of Lewy body-associated disorders. Mov Disord. 2010;25:2516–2523. doi: 10.1002/mds.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks DJ. Imaging amyloid in Parkinson's disease dementia and dementia with Lewy bodies with positron emission tomography. Mov Disord. 2009;24(Suppl 2):S742–S747. doi: 10.1002/mds.22581. [DOI] [PubMed] [Google Scholar]
- 21.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 22.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol. 1993;50:140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 25.Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 26.Lang A, Fahn S. Assesment of Parkinson's disease. In: Munstat TL, editor. Quantification of Neurologic Deficit. Boston: Butterworths; 1989. pp. 285–309. [Google Scholar]
- 27.Wechsler D. The Wechsler Memory Scale. Third Edition. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 28.Grober E, Lipton RB, Hall C, et al. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D. WAIS-III, Wechsler Adult Intelligence Scale—third edition, administration and scoring manual. New York, NY: The Psychological Corporation; 1997. [Google Scholar]
- 30.Wechsler D. WAIS-R manual. New York, NY: The Psychological Corporation; 1981. [Google Scholar]
- 31.Reitan R. Manual for administration of neuropsychological test batteries for adults and children. Tucson, AZ: Reitan Neuropsychology Laboratories; 1979. [Google Scholar]
- 32.Benton AL, Varney NR, Hamsher KD, et al. Contributions to neuropsychological assessment. Oxford, UK: Oxford University Press; 1983. [Google Scholar]
- 33.Monsch AU, Bondi MW, Butters N, et al. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer's type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test: assessment of aphasia and related disorders. 2nd ed. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 35.Ryan J, Paolo A. A screening procedure for estimating premorbid intelligence in the elderly. Clin Neuropsychol. 1992;6:53–62. [PubMed] [Google Scholar]
- 36.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;40:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 37.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 38.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 39.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62:181–187. doi: 10.1212/wnl.62.2.181. [DOI] [PubMed] [Google Scholar]
- 40.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 41.Mathis CA, Wang Y, Holt DP, et al. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 42.Logan J, Fowler JS, Volkow ND, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–1972. [PubMed] [Google Scholar]
- 44.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PiB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 45.Archer HA, Edison P, Brooks DJ, et al. Amyloid load and cerebral atrophy in Alzheimer’s disease: an (11)C-PiB positron emission tomography study. Ann Neurol. 2006;60:145–147. doi: 10.1002/ana.20889. [DOI] [PubMed] [Google Scholar]
- 46.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 47.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 48.Friston JK, Holmes AP, Worsley KJ. Statistical parametric maps in functional magnetic imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–198. [Google Scholar]
- 49.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Japan, Sendai: 2002. Jun 2–6, [Google Scholar]
- 50.Thalariach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain- 3-Dimensional Proportional System: An approach to cerebral Imaging. New York: Thieme Medical Publishers Inc.; 1988. pp. 3–9. [Google Scholar]
- 51.Becker JA, Hedden T, Carmasin, et al. Amyloid-β associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horimoto M, Matsumoto H, Nakazawa, et al. Cognitive conditions of pathologically confirmed dementia with Lewy bodies and Parkinson's disease with dementia. J Neurol Sci. 2003;216:105–108. doi: 10.1016/s0022-510x(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 53.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 54.Kempster PA, O'Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson's disease: a clinico-pathological study. Brain. 2010;133:1755–1762. doi: 10.1093/brain/awq059. [DOI] [PubMed] [Google Scholar]
- 55.Klunk WE, Lopresti BJ, Ikonomovic MD, et al. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer's disease brain but not in transgenic mouse brain. J Neurosci. 2005;25:10598–10606. doi: 10.1523/JNEUROSCI.2990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kantarci K, Yang C, Schneider JA, et al. Ante mortem amyloid imaging and β-amyloid pathology in a case with dementia with Lewy bodies. Neurobiol Aging. 2010 Oct 18; doi: 10.1016/j.neurobiolaging.2010.08.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burack MA, Hartlein J, Flores HP, Taylor-Reinwald L, Perlmutter JS, Cairns NJ. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology. 2010;74:77–84. doi: 10.1212/WNL.0b013e3181c7da8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dickson DW. Dementia with Lewy bodies: neuropathology. J Geriatr Psychiatry Neurol. 2002;15:210–216. doi: 10.1177/089198870201500406. [DOI] [PubMed] [Google Scholar]
- 59.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–1231. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang X, Chen PC, Poole, et al. APOE-ε2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology. 2004;62:2198–2202. doi: 10.1212/01.wnl.0000130159.28215.6a. [DOI] [PubMed] [Google Scholar]
- 63.Jasinska-Myga B, Opala G, Ochudlo S, Tustanowski J. Assessment of apolipoprotein E genotype in Parkinson disease patients with and without dementia. Wiad Lek. 2004;57:20–24. [PubMed] [Google Scholar]
- 64.Pankratz, Byder L, Halter C, et al. Presence of an APOE4 allele results in significantly earlier onset of Parkinson's disease and a higher risk with dementia. Mov Disord. 2006;21:45–49. doi: 10.1002/mds.20663. [DOI] [PubMed] [Google Scholar]
- 65.Jasinska-Myga B, Opala G, Goetz CG, et al. Apolipoprotein E gene polymorphism, total plasma cholesterol level, and Parkinson disease dementia. Arch Neurol. 2007;64:261–265. doi: 10.1001/archneur.64.2.261. [DOI] [PubMed] [Google Scholar]
- 66.Papapetropoulos S, Farrer MJ, Stone JT, et al. Phenotypic associations of tau and ApoE in Parkinson's disease. Neurosci Lett. 2007;414:141–144. doi: 10.1016/j.neulet.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Ezquerra M, Campdelacreu J, Gaig C, et al. Lack of association of APOE and tau polymorphisms with dementia in Parkinson's disease. Neurosci Lett. 2008;448:20–23. doi: 10.1016/j.neulet.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 68.Harrington CR, Louwagie J, Rossau R, et al. Influence of apolipoprotein E genotype on senile dementia of the Alzheimer and Lewy body types. Significance for etiological theories of Alzheimer's disease. Am J Pathol. 1994;145:1472–1484. [PMC free article] [PubMed] [Google Scholar]
- 69.Hardy J, Crook R, Prihar G, Roberts G, Raghavan R, Perry R. Senile dementia of the Lewy body type has an apolipoprotein E epsilon 4 allele frequency intermediate between controls and Alzheimer's disease. Neurosci Lett. 1994;182:1–2. doi: 10.1016/0304-3940(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 70.Morris CM, Massey HM, Benjamin R, et al. Molecular biology of APO E alleles in Alzheimer's and non-Alzheimer's dementias. J Neural Transm Suppl. 1996;47:205–218. doi: 10.1007/978-3-7091-6892-9_14. [DOI] [PubMed] [Google Scholar]
- 71.Nielsen AS, Ravid R, Kamphorst W, Jorgensen OS. Apolipoprotein E epsilon 4 in an autopsy series of various dementing disorders. J Alzheimers Dis. 2003;5:119–125. doi: 10.3233/jad-2003-5206. [DOI] [PubMed] [Google Scholar]
- 72.Carrillo Garcia F, Gil Neciga E, Alberca R, et al. Apolipoprotein E4 in dementia with Lewy bodies. Neurologia. 2008;23:152–156. [PubMed] [Google Scholar]