Summary
A key tool in neuroscience is the ability to transiently inactivate specific neurons on timescales of milliseconds to minutes. In Drosophila, there are two available techniques for doing this (shibirets and halorhodopsin [1–3]), but both have shortcomings [4–9]. Here we describe a complementary technique using a native histamine-gated chloride channel (Ort). Ort is the receptor at the first synapse in the visual system. It forms large-conductance homomeric channels that desensitize only modestly in response to ligand [10]. Many regions of the central nervous system are devoid of histaminergic neurons [11, 12], raising the possibility that Ort could be used to artificially inactivate specific neurons in these regions. To test this idea, we performed in vivo whole-cell recordings from antennal lobe neurons misexpressing Ort. In these neurons, histamine produced a rapid and reversible drop in input resistance, clamping the membrane potential below spike threshold, and virtually abolishing spontaneous and odor-evoked activity. Every neuron type in this brain region could be inactivated in this manner. Neurons that did not misexpress Ort showed negligible responses to histamine. Ort also performed favorably in comparison to the available alternative effector transgenes. Thus, Ort misexpression is a useful tool for probing functional connectivity among Drosophila neurons.
Results
Many regions of the Drosophila central nervous system contain no histaminergic fibers, or else only sparse histaminergic innervation (Fig. S1, Movie S1, Movie S2). The antennal lobe is one region that contains no histaminergic innervation, and it is one of the best-studied regions of the Drosophila brain. For this reason, we chose antennal lobe neurons for testing the effect of histamine on Ort-misexpressing neurons.
There are two major cell types in the antennal lobe: projection neurons (PNs) and local neurons (LNs). About two-thirds of antennal lobe LNs are GABAergic, and about one-third are glutamatergic [13, 14]. We targeted expression of Ort to PNs and LNs with the Gal4/UAS system, using Gal4 lines that drive expression specifically in these cell types. We performed targeted in vivo patch clamp recordings from these cells. In order to target our electrodes to the Ort+ neurons, we co-expressed CD8:GFP along with Ort.
In the absence of histamine, the electrophysiological properties of Ort+ neurons were no different from those of Ort- neurons (i.e., cells recorded in flies that lacked the UAS-ort transgene). The resting membrane potential of these cells (−64 ± 1 mV) was not significantly different from controls (−65 ± 2 mV). Similarly, the input resistance of these cells (920±130 MΩ) was not significantly different from normal (1040±150 MΩ, p=0.58, n=8 and 5, t-test).
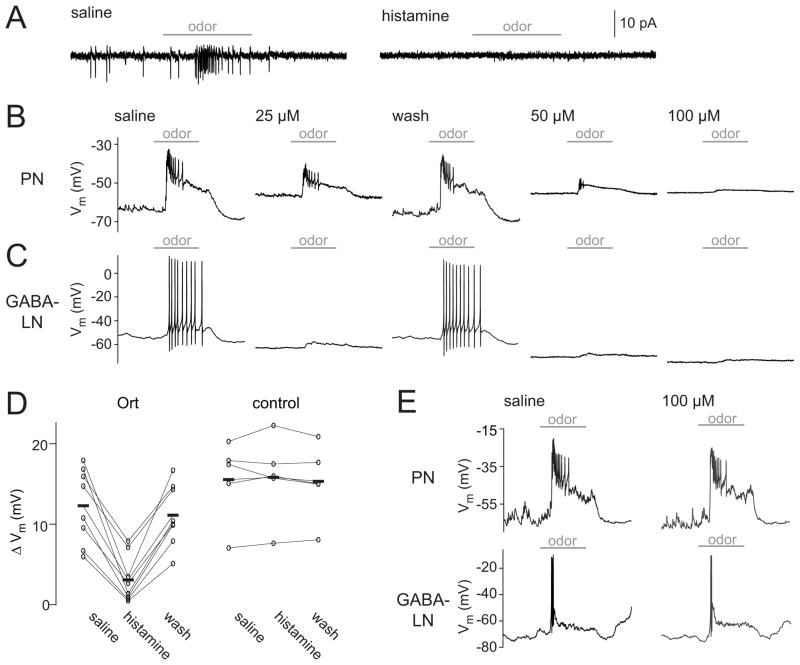
Upon bath application of histamine, Ort+ neurons were potently inhibited. Odor-evoked activity was almost completely suppressed, and this was true of both Ort+ PNs and Ort+ LNs (Fig. 1A–C). The effects of histamine on Ort+ neurons were dose-dependent and consistent with the known sensitivity of Ort to histamine [10, 15–17]. At the highest dose (100 μM), histamine completely eliminated all spiking, and substantially reduced odor-evoked depolarization (Fig. 1B,C). Importantly, histamine had essentially no effect on the odor responses of PNs and LNs recorded in flies that lacked the UAS-ort transgene, even at the highest histamine concentration (Fig. 1D,E).
Figure 1. Histamine suppresses stimulus-evoked activity in Ort-expressing neurons.
(A) A cell-attached recording showing spikes in an Ort-expressing antennal lobe PN (left) in a regular saline bath. Subsequent bath application of histamine (100 μM) abolishes both spontaneous and odor-evoked spikes (right). Horizontal bars indicate the period (500 msec) of the odor stimulus. For all sample traces in this figure, the odor was pentyl acetate (10−2 dilution).
(B) A whole-cell recording of an Ort-expressing PN. Increasing concentrations of histamine produce greater suppression of activity, and suppression is reversed by wash-out. Note that this particular PN is depolarized by histamine, which we observed in several PNs; others were hyperpolarized or showed no change in resting membrane potential (see Discussion).
(C) Same as above, but in an Ort-expressing antennal lobe GABA-LN.
(D) Odor responses in saline, 100 μM histamine, and after wash-out (measured as the odor-evoked change in membrane potential). Each connected set of circles represents a different PN recording, and bars represent means (n = 8 Ort+ and 5 control). Histamine significantly reduces the odor response in Ort+ flies (p < 0.001, paired t-test). In control flies that lack the UAS-ort transgene, the odor response before and during histamine application is not significantly different (p = 0.69, paired t-test).
(E) Whole-cell recordings from a PN and a GABA-LN that did not express Ort. Histamine had no effect on these cells.
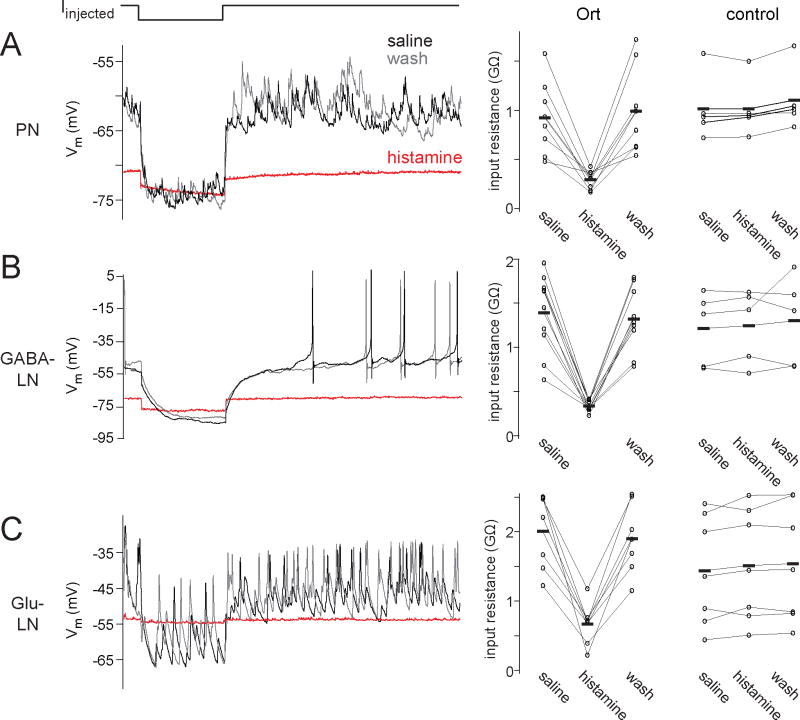
The effects of histamine are consistent with the opening of a massive chloride conductance in Ort+ neurons. First, histamine dramatically reduced the input resistance of these cells (Fig. 2). Moreover, histamine abolished all spontaneous activity, as would be expected from a large decrease in input resistance. Histamine had no effect on the input resistance of control PNs or LNs (Fig. 2).
Figure 2. Histamine reduces input resistance and suppresses spontaneous activity in Ort-expressing neurons.
(A) A whole-cell recording from an antennal lobe PN showing that histamine (100 μM) reduces input resistance, quantified as the membrane potential change elicited by hyperpolarizing current injection, divided by the magnitude of the current step (500 msec duration). Spontaneous EPSPs are also suppressed by histamine. These effects are reversed upon histamine washout. The plot at right shows the input resistance in histamine, for all PN experiments (n = 8 Ort+ and 5 control).
(B) Same as above, but for a GABA-LNs (n = 10 Ort+ and 5 control).
(C) Same as above, but for a glutamatergic LN (Glu-LN; n = 7 Ort+ and 7 control). In this set of experiments, we used a lower concentration of histamine (25 μM) because this concentration produced near-maximal effects in Glu-LNs. This may reflect a higher level of Gal4 expression in these cells.
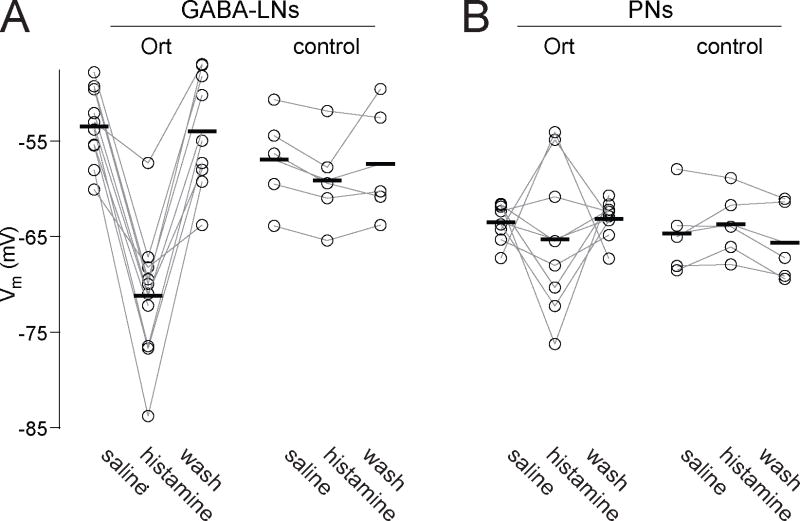
Histamine also affected the membrane potential of Ort+ neurons. The initial resting potential of Ort+ GABA-LNs was −53 ± 1 mV, and these cells were consistently hyperpolarized by histamine, to a new value of −71 ± 2 mV (Fig. 3A). PNs had an initial resting potential which was more hyperpolarized than that of GABA-LNs (−64 ± 1 mV), and accordingly the effects of histamine on membrane potential were more modest and varied, with some cells becoming slightly hyperpolarized by histamine, and others becoming slightly depolarized (Fig. 3B; see Discussion). On average, histamine caused Ort+ PNs to rest at −65 ± 3 mV (Fig. 3B). There was again no significant effect on the membrane potential of control PNs or GABA-LNs (Fig. 3A,B).
Figure 3. Histamine changes the membrane potential of Ort-expressing neurons.
(A) Ort+ GABA-LNs are consistently hyperpolarized by histamine (100 μM). There is no effect on control GABA-LNs (n = 10 Ort+ and 5 control). (B) Some Ort+ PNs are hyperpolarized by histamine (100 μM), whereas others are depolarized (n = 8 Ort+ and 5 control). Note that the initial resting potential of PNs is more hyperpolarized than that of GABA-LNs.
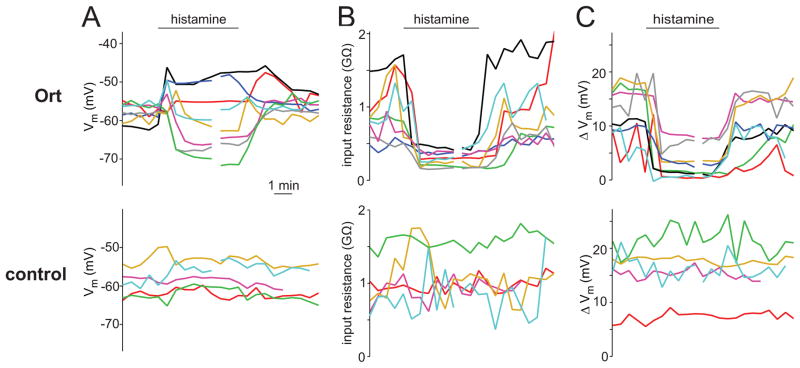
As one would expect from a small and hydrophilic ligand, the effects of histamine were rapid and reversible. The onset and offset of histamine’s effects occurred within the several minutes required for the bath solution to completely equilibrate with the incoming perfusate (Fig. 4A–C). The histamine-induced changes in input resistance, membrane potential, and odor responses had similar kinetics, as expected. Prolonged applications of histamine (up to 15 minutes) produced constant effects on all these metrics, without appreciable decay in potency (data not shown).
Figure 4. Time course of the effect of histamine.
(A) Time course of the effect of histamine (100 μM) on the membrane potential for all recorded Ort+ and control PNs. The bar indicates the period when histamine began to enter and exit the bath. Each trace represents a different PN recording. Broken traces indicate that a period of time is omitted from the display, so that all traces could be aligned at their wash-on and wash-out times. The time base is the same for all panels.
(B) Same as above, but for input resistances.
(C) Same as above, but for odor responses.
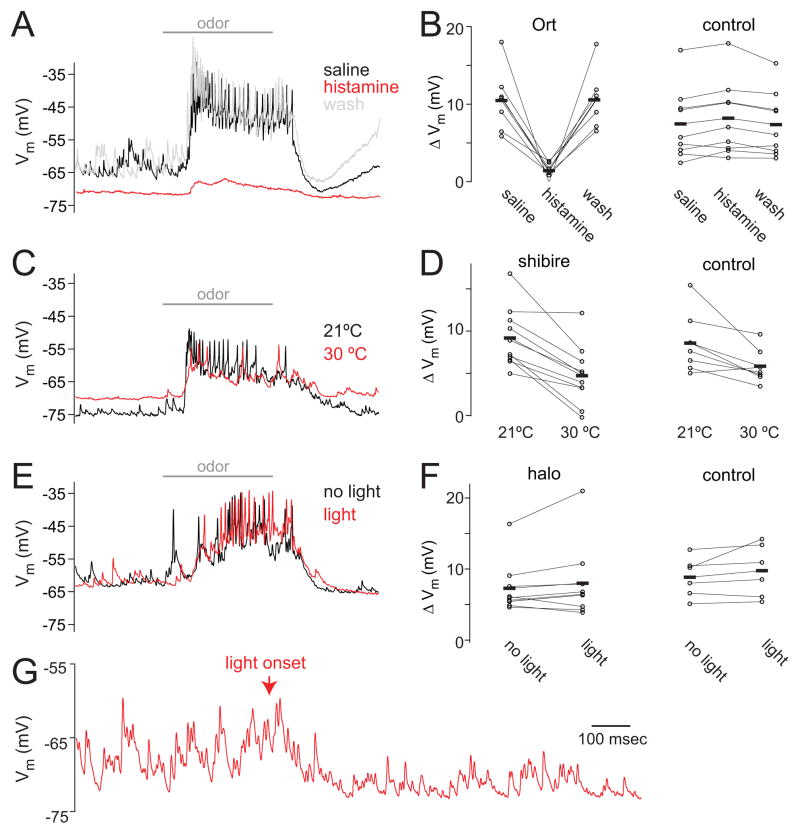
Finally, we directly compared Ort with the two published alternative techniques designed to transiently silence Drosophila neurons, shibirets and halorhodopsin. The shibirets transgene encodes a form of dynamin which misfolds at restrictive temperatures, thereby preventing synaptic vesicle formation [1]. Halorhodopsin is a light-activated chloride pump which hyperpolarizes cells [2, 3]. The ideal technique for silencing neurons would be both potent (i.e., it would completely silence activity) and selective (it would directly affect only the neurons expressing the effector molecule). To compare the potency and selectivity of Ort with shibirets and halorhodopsin, we employed all three techniques at silencing olfactory receptor neurons (ORNs). We chose ORNs as our targets in these experiments because shibirets affects neurotransmitter release rather than spike initiation, and so its effects are only visible in neurons postsynaptic to the neurons expressing Gal4. In other words, in order to assess the ability of shibirets to silence activity in the antennal lobe, we needed to express Gal4 in neurons presynaptic to the antennal lobe.
We crossed flies expressing each UAS transgene with flies expressing Gal4 in a broad population of ORNs [18]. In each genotype, we recorded from postsynaptic PNs while silencing ORNs. If ORNs were silenced completely, we would expect essentially all spontaneous and odor-evoked activity in PNs to disappear. This is because ORNs are the only major source of excitatory input to PNs, and ORNs normally spike spontaneously, producing excitatory postsynaptic potentials in PNs [19, 20]. Note that all these techniques likely affect only the ORN axons in the brain, as the ORN somata and dendrites are housed in the antennae and so are likely inaccessible to any of these manipulations. Neurotransmitter release from ORNs should be inhibited by either hyperpolarizing their axons (using Ort or halorhodopsin) or preventing synaptic vesicle formation in axons (with shibirets).
We found that Ort proved effective in silencing ORNs. As one would expect if ORN terminals were clamped at a hyperpolarized potential, we observed that odor-evoked activity in PNs was severely reduced by histamine. On average, the magnitude of PN odor response dropped by 84% (Fig. 5A,B). Histamine also eliminated the barrage of spontaneous excitatory postsynaptic potentials in PNs that arises from normal spontaneous ORN spiking [19, 20], and as a consequence, PNs were hyperpolarized. Importantly, histamine had little effect in recordings from PNs in control flies (Fig. 5B).
Figure 5. Comparison between Ort, shibirets, and halorhodopsin in silencing ORN input to PNs.
(A) A recording from a PN in a fly where Ort is selectively expressed in ORNs. Horizontal bar indicates the period (500 ms) of odor application. Histamine dramatically reduces odor-evoked activity in the PN, indicating that it is effective in silencing ORNs. Histamine also eliminates spontaneous EPSPs. In this example, the odor is pentyl acetate.
(B) Odor response in regular saline, 100 μM histamine, and wash (measured as the odor-evoked change in membrane potential, n = 7 Ort and 9 control genotype). Histamine significantly reduces the odor response in flies where ORNs express Ort (p < 0.005, paired t-test). In control flies, histamine has little effect, although the small amount of potentiation is in this case statistically significant (p = 0.02, paired t-test).
(C) A recording from a PN in a fly where shibirets is selectively expressed in ORNs. Shifting the bath to the restrictive temperature inhibits odor-evoked activity in this recording, albeit partially, and eliminates most of the spontaneous activity. The membrane potential also depolarizes. In this example, the odor is a blend of fenchone, pentyl acetate, benzaldehyde, ethyl acetate and ethyl butyrate.
(D) Odor response at the restrictive and permissive temperature (n = 10 shibirets and 7 control genotype). Raising the temperature significantly reduces the odor response in shibirets flies (p < 0.001, paired t-test). Shifting the bath temperature also modestly reduces the odor response of control flies (p < 0.05, paired t-test).
(E) A recording from a PN in a fly where halorhodopsin is selectively expressed in ORNs. Illuminating the preparation with green light had little effect. Here the odor is trans-2-hexenal.
(F) Odor response in interleaved trials with light versus no light (n = 9 halorhodopsin and 6 control genotype). Light does not have a significant effect in either halorhodopsin flies or control flies (p = 0.26 and 0.22, paired t-tests).
(G) At higher light intensities than those used above (E,F), we saw that light produced a nonspecific suppression of activity. This example PN was recorded in a fly where the Gal4 transgene was omitted, so no halorhodopsin should be expressed. The magnitude of this effect was similar in flies with and without the transgene. The light intensity here was four times that used in (E,F); see Experimental Procedures.
By comparison, shibirets was less consistent and specific. In flies where ORNs expressed shibirets, raising the bath to a restrictive temperature (29–30 °C) to inactivate ORNs produced variable effects. On average, the temperature shift reduced PN odor responses by 52% (Fig. 5D). Spontaneous activity was also reduced. The membrane potential was depolarized by the temperature shift (generally by > 10 mV), and in several experiments the recording was lost. Shifting the bath temperature also depolarized control cells, indicating that this effect was unrelated to shibirets. Moreover, in control cells, the temperature shift reduced PN odor responses by 28% (Fig. 5D). Overall, the effect of the temperature shift was still significantly different in control and shibirets flies, indicating that shibirets was acting as intended, but the nonspecific effects of the temperature shift were sizeable.
Halorhodopsin was the least effective technique in this experimental context. In flies where ORNs expressed halorhodopsin, illuminating the brain produced no significant change at intensities that were low enough to avoid nonspecific effects (Fig. 5E,F). In our pilot experiments, we found that higher light intensities strongly hyperpolarized PNs in control flies (i.e., flies that lacked the UAS-halorhodopsin transgene; Fig. 5G).
Discussion
In mammalian systems, several techniques have been developed in recent years for transiently inactivating neurons using non-native neurotransmitter receptor [21–25]. The goal in using a non-native receptor is to avoid activation by endogenous ligands. Here, we took a different approach: we exploited the fact that histamine is present only sparsely in the nervous system outside of the eye, where it is the native neurotransmitter of photoreceptors [26]. Indeed, there are only about 20 histaminergic neurons outside of the optic lobes, and many brain regions are devoid of histaminergic processes. These areas include the antennal lobe, mushroom bodies, and the central complex ([11, 12] and Fig. S1, Movie S1, Movie S2). In these brain regions, histamine can potentially be used as an artificial neurotransmitter, because it is not a native neurotransmitter. Using a receptor which is native to Drosophila is convenient for achieving high levels of surface expression without further transgene optimization. Moreover, Ort is an excellent candidate for a transgenic effector molecule because it forms homomeric channels – thereby avoiding the need for multiple transgenes – and because the Ort channel has a large conductance and shows little desensitization [10]. Importantly, Ort channels are highly selective for histamine over GABA [17]. These properties motivated our investigation of Ort as a candidate effector molecule.
We found that the histamine/Ort system can be a potent and selective method for neural inactivation. It can produce essentially complete inactivation, and its effects are similar in diverse cell types, under the control of various Gal4 drivers. This is important because it shows that the technique is robust to the properties of the Gal4 driver used to control transgene expression. Moreover, the effects of histamine are completely reversible.
How does the histamine/Ort system actually work? Ort is a chloride channel, and the nominal chloride reversal potential in these experiments was −121 mV, given the compositions of the external and pipette solutions. One might therefore expect histamine to dramatically hyperpolarize Ort+ neurons. This is not what we observed. Histamine did clearly open a massive conductance – more than doubling the resting conductance – but it generally produced a modest hyperpolarization, and some cells were even modestly depolarized. In a typical cell, histamine clamped the membrane potential between −60 and −75 mV. This suggests that opening Ort channels causes a large amount of chloride to enter the cell, thereby depolarizing the chloride reversal potential. In other words, the chloride gradient partially collapses. Strong activation of ligand-gated chloride channels is known to be capable of partially collapsing chloride gradients in mammalian neurons [27]. When a ligand-gated invertebrate chloride channel is misexpressed in mammalian neurons, application of its ligand produces effects on the membrane potential which are similar to what we observe here [21]. In sum, these techniques appear to work largely by shunting inhibition, rather than hyperpolarizing inhibition.
The motivation for these experiments arose from the limitations of currently available techniques intended to produce transient neural inactivation in Drosophila. The shibirets technique has been very widely used, but its limitations are also well-known [4, 5]. First, temperature changes alter virtually every aspect of a fly’s physiology, which can make it difficult to interpret negative controls. Second, in order to verify that this technique has inactivated a neuron, one cannot record from that neuron itself; rather, one would need to record postsynaptic to that neuron. Third, the mis-folded dynamin will only inhibit synaptic vesicle recycling if it is present in high copy number, and so this technique relies on achieving high transgene expression levels [6]. Fourth, this technique should not affect release of peptidergic vesicles, which do not depend on rapid endocytotic recycling. Finally, because dynamin has multiple functions within cells, overexpressing this transgene can cause necrosis, even at permissive temperatures [7].
Similarly, there are also limitations associated with the current generation of optogenetic reagents for hyperpolarizing neurons. These reagents are light-activated microbial chloride or proton pumps which should generate an outward pump current which hyperpolarizes neurons [8]. However, these pumps have a relatively low single-molecule conductance, meaning that they must be expressed at high levels, and they must also be trafficked efficiently to the cell membrane, which has been difficult to achieve [8], particularly in Drosophila [9]. Recently, halorhodopsin has been found to be effective at inactivating neurons in the Drosophila larva in vivo [2, 3], demonstrating that it can be useful in experimental contexts different from those of our study. The differential efficacy of halorhodopsin in those cases and in our case may reflect differences in the cell types, Gal4 drivers, or other methodological differences.
The immediate application for which we developed this technique is to inactivate specific neurons with histamine, while simultaneously recording from other neurons in vivo. In this way, one can study how circuit physiology is affected by silencing specific neurons within the circuit. Several studies have transiently activated specific neurons in this experimental configuration [28–31]; what has been lacking is a robust and highly-selective method of transient inactivation.
This technique does have limitations. First, it cannot be used in a Drosophila brain region where histamine has major endogenous effects, such as the visual system. Several regions of the central nervous system receive sparse histaminergic innervation, including much of the lateral and dorsal protocerebrum (Fig. S1, Movie S1, Movie S2), but it is not known whether histamine has major endogenous effects in these regions. A necessary and sufficient control for endogenous effects will be to compare flies with and without Ort misexpression.
Second, this technique requires delivery of exogenous histamine, and so cannot be used in intact flies. In principle, this might be circumvented by injecting caged histamine, and then illuminating the intact fly to photo-uncage the ligand. A similar approach has been used previously to transiently activate specific neurons in vivo, in that case using caged ATP and transgenic expression of purinergic receptors [32].
Ultimately, the desired properties of a genetic effector system depend on the experimental setting [4, 5, 33]. For this reason, it is useful to develop multiple complementary systems. Recent years have seen new techniques to monitor neural activity in vivo in Drosophila, as well new reagents for selectively expressing transgenes in specific neurons [5, 9]. The Ort/histamine system is a promising component of this toolkit for probing functional connectivity between identified neurons in vivo.
Experimental Procedures
Fly stocks
Flies were raised on standard cornmeal agar medium supplemented with rehydrated potato flakes on a 12 h light/dark cycle at 25°C. The two exceptions were flies expressing UAS-shibirets, which were raised at 18°C, and flies expressing halorhodopsin, which were raised in the dark on food supplemented with all-trans retinal. All-trans retinal was prepared as a 35mM stock solution in ethanol and diluted 10-fold in water before mixing with rehydrated potato flakes; this mix was layered on top of conventional food. All experiments were performed on adult female flies 1–3 days post eclosion, except for the experiments with shibirets, where some flies were male. The genotypes used were as follows:
| Fig. 1A,B – | GH146-Gal4,UAS-CD8:GFP/UAS-ort |
| Figs. 1C,3C – | UAS-ort/UAS-CD8:GFP;NP3056-Gal4/+ |
| Fig. 1D, 2A, 3B and 4 – | GH146-Gal4,UAS-CD8:GFP/+ (control) and GH146-Gal4,UAS-CD8:GFP/UAS-ort (Ort) |
| Fig. 1E – | GH146-Gal4,UAS-CD8:GFP/+ (PN) and UAS-CD8:GFP/+;NP3056-Gal4/+ (LN) |
| Figs. 2B,3A – | UAS-CD8:GFP/+;NP3056-Gal4/+ (control) and UAS-ort/UAS-CD8:GFP;NP3056-Gal4/+ (Ort) |
| Fig. 2C – | OK371-Gal4,UAS-CD8:GFP/+ (control) and OK371-Gal4,UAS-CD8:GFP/UAS-ort (Ort) |
| Fig. 5A – | pebbled-Gal4/+;UAS-ort/+ |
| Fig. 5B – | pebbled-Gal4/+ and UAS-ort/+ (control) and pebbled-Gal4/+;UAS-ort/+ (Ort) |
| Fig. 5C – | pebbled-Gal4/+;;UAS-UAS-shibirets/+ |
| Fig. 5D – | pebbled-Gal4 (control) and pebbled-Gal4/+;;UAS-shibirets/+ (shits) |
| Fig. 5E – | pebbled-Gal4/+;UAS-eNpHR-50C/+;UAS-eNpHR-19C,UAS-eNpHR-34B/+; |
| Fig. 5F – | UAS-eNpHR-50C;UAS-eNpHR-19C,UAS-eNpHR-34B (control) and pebbled-Gal4/+;UAS-eNpHR-50C/+;UAS-eNpHR-19C,UAS-eNpHR-34B/+ (halo) |
| Fig. 5G – | UAS-eNpHR-50C;UAS-eNpHR-19C,UAS-eNpHR-34B |
Fly stocks were previously published as follows: GH146-Gal4 (chromosome II) [34], NP3056-Gal4 (chromosome III) [13], OK371-Gal4 (chromosome II) [35], UAS-CD8:GFP (chromosome II or III) [36], UAS-ort (chromosome II) [37], UAS-eNpHR-50C;UAS-eNpHR-19C,UAS-eNpHR-34B (chromosomes II and III) [2], UAS-shibirets (chromosome III) [1], pebbled-Gal4(X chromosome) [18]. Stocks of OK371-Gal4 and UAS-CD8:GFP were obtained from the Bloomington Drosophila Stock Center.
Electrophysiological recordings
In vivo whole-cell patch clamp recordings were performed as previously described [38, 39]. In this preparation, the antennae and maxillary palps of the fly remained dry and accessible to odors, while the brain was bathed in saline and was accessible to patch-clamp electrodes. The saline contained (in mM): 103 NaCl, 3 KCl, 5 N-tris(hydroxymethyl) methyl-2-aminoethane-sulfonic acid, 8 trehalose, 10 glucose, 26 NaHCO3, 1 NaH2PO4, 1.5 CaCl2, and 4 MgCl2 (osmolarity adjusted to 270–275 mOsm). The saline was bubbled with 95% O2/5% CO2 to a pH of 7.3, and was flowed continuously over the preparation at a rate of 2 mL/min. Patch pipettes were filled with a solution containing the following (in mM): 140 potassium aspartate, 10 HEPES, 1 EGTA, 4 MgATP, 0.5 Na3GTP, 1 KCl, and 13 biocytin hydrazide. The pH of the internal solution was adjusted to 7.2 and the osmolarity was adjusted to ~265 mOsm. Recordings were performed with an Axopatch 200B amplifier (Axon Instruments). Voltages were low-pass filtered at 5 kHz and digitized at 10 kHz. Voltages were corrected for the measured liquid junction potential of +13 mV, which was subtracted from recorded voltages post hoc [40]. Series resistance was uncompensated. To record from PNs, GABA-LNs and Glu-LNs (in Fig. 1–3), we targeted our electrodes to cells labeled with GFP. To record from unlabeled PNs (in Fig. 5), we targeted our electrodes to the cluster of PN cell bodies immediately anterodorsal to the antennal lobe neuropil, and we confirmed that all these cells had small-amplitude action potentials (<12 mV), which is characteristic of PNs [38]. In these types of whole-cell recordings, the seal conductance is large enough (relative to the high input resistance of these cells) to produce a discernible depolarization in the resting potential; for this reason, we injected a small amount of constant hyperpolarizing current in order to bring the cell back down to its native resting potential [40]. The native resting potential of PNs and GABA-LNs was estimated by measuring spontaneous spiking in cell-attached mode prior to rupturing the seal, and then matching the spontaneous spike rate in whole-cell mode. Both Ort+ PNs and control PNs had seal resistances of >1GΩ prior to rupturing the seal. Because spontaneous spikes are typically not visible in cell-attached recordings from Glu-LNs, we are less confident about the native resting potential of these cells, and we did not inject any holding current into these cells. Cell-attached recordings were performed in voltage-clamp mode, and the command potential was adjusted so that no current was passed through the electrode. In these recordings, the patch pipettes were filled with external saline. Data was low-passed filtered at 1kHz. Histamine or histamine dihydrochloride was prepared as a 100 mM stock solution in water, and the stock was added to the reservoir feeding the bath flowing over the brain to achieve the desired final concentration. The stock solution was prepared fresh every week. For experiments using shibirets, the saline perfusate was heated from room temperature (~21 °C) to 29–30° C over a period of ~5 min using a TC-324B temperature controller equipped with an in-line solution heater (Warner Instruments). The temperature of the bath was monitored continuously with a submerged thermistor.
Odor stimulation
Odors used were diluted 100-fold in paraffin oil (except for pentanoic acid, which was diluted 10,000-fold, and pentyl acetate, which was diluted 1,000-fold in some experiments) and delivered via a custom-built olfactometer, which further dilutes the headspace of the odor vial 10-fold in air [41]. Odorized air was delivered to the head of the fly at a flow rate of 2.2 mL/min. Odor stimuli were applied for 500-msec every 30 sec, with 5–10 trials per stimulus. Because we did not know in advance of obtaining a recording what odor(s) a cell might respond to, we prepared a small panel of odors that collectively are effective at stimulating many antennal lobe neurons (pentyl acetate, methyl salicylate, trans-2-hexenal, pentanoic acid, and also a blend of fenchone, pentyl acetate, benzaldehyde, ethyl acetate and ethyl butyrate). Once we obtained a recording, we tried odors from this set until we found an effective stimulus. If no response could be obtained with any of these stimuli, we discarded the cell.
Optogenetic stimulation
Light was delivered via a 100-W Hg arc lamp (Olympus) attenuated with a ND-25 neutral-density filter, band-pass filtered at 540–580 nm, and delivered to the specimen through the 40× water-immersion objective used to visualize the preparation for patch-clamp recording. The light intensity at the specimen was measured as 8.5 mW/mm2 using an optical power meter (Newport 1916-C) with a photodetector (818P-015-19, intensity reported at 560 nm) positioned behind a pinhole aperture. We chose this light intensity because it was the highest intensity that did not produce a nonspecific effect of light (see Fig. 5G for an example of a nonspecific effect at 37 mW/mm2). Pulses of light (2 sec in duration, beginning 1 sec before odor onset) were controlled with a shutter (Uniblitz) controlled by a TTL pulse. Odor presentations with and without light were interleaved, with a total of 12–20 presentations per odor per experiment.
Histochemistry
Histamine immunostaining (Fig. S1, Movie S1, Movie S2) we followed a modified version of previously published procedures [12, 42]. Briefly, the brain and ventral nerve cord were dissected out and fixed in 4% 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide in PBS for 5 hours at 4° C. Samples were rinsed with PBS, and incubated in blocking solution (5% normal goat serum [Vector Laboratories] in PBST [0.2% Triton X-100 in PBS]) for 30 min, and then incubated in 1:500 rabbit anti-histamine antibody (Abcam ab43870) and 1:50 mouse nc82 antibody (Developmental Studies Hybridoma Bank) in blocking solution at 4°C for 2 d. After washing for 20 min in PBST, samples were incubated with 1:250 goat anti-rabbit:Alexa Fluor 488 and 1:250 goat anti-mouse:Alexa Fluor 633 (Invitrogen) in blocking solution at room temperature for 1 d. Samples were then mounted in Vectashield (Vector Laboratories) and imaged with a laser-scanning confocal microscope (Zeiss LSM 510).
Data analysis
The odor-evoked membrane potential response was computed by low-pass filtering the membrane potential at 10 Hz (to remove spikes), and then taking the mean across trials over the 500-msec odor stimulus period, minus the mean in the period just before the odor stimulus. Input resistance was computed as the membrane potential change elicited by a step of negative current injected into the soma, divided by the magnitude of the current step. Group data in the text is reported as mean ± SEM, computed across experiments.
Supplementary Material
Highlights.
A histamine-gated chloride channel native to the retina is misexpressed in the CNS
Histamine rapidly clamps neurons misexpressing the channel below spike threshold
Neurons that do not misexpress the channel are not directly affected by histamine
Histamine-mediated silencing is a useful alternative to other techniques
Acknowledgments
We thank Chi-Hon Lee for UAS-ort flies, Toshi Kitamoto for UAS-shibirets flies, Akinao Nose for UAS-eNpHR flies, John Tuthill for performing ventral nerve cord dissections, and Pertti Panula for advice on histamine immunohistochemistry. This work was supported by a research project grant from the National Institutes of Health (R01DC008174). W.W.L. is supported by an HHMI International Research Fellowship and a Presidential Scholarship from the MD-PhD Program at Harvard Medical School. R.I.W. is an HHMI Early Career Scientist.
Footnotes
Author contributions: W.W.L. and R.I.W. designed research, W.W.L. performed research, W.W.L. analyzed data, and W.W.L. and R.I.W. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 2.Inada K, Kohsaka H, Takasu E, Matsunaga T, Nose A. Optical dissection of neural circuits responsible for Drosophila larval locomotion with halorhodopsin. PLoS One. 2011;6:e29019. doi: 10.1371/journal.pone.0029019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berni J, Pulver SR, Griffith LC, Bate M. Autonomous circuitry for substrate exploration in freely moving Drosophila larvae. Curr Biol. 2012;22:1861–1870. doi: 10.1016/j.cub.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson JH. Mapping and manipulating neural circuits in the fly brain. Adv Genet. 2009;65:79–143. doi: 10.1016/S0065-2660(09)65003-3. [DOI] [PubMed] [Google Scholar]
- 6.Kitamoto T. Targeted expression of temperature-sensitive dynamin to study neural mechanisms of complex behavior in Drosophila. J Neurogenet. 2002;16:205–228. doi: 10.1080/01677060216295. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Bellido PT, Wardill TJ, Kostyleva R, Meinertzhagen IA, Juusola M. Overexpressing temperature-sensitive dynamin decelerates phototransduction and bundles microtubules in Drosophila photoreceptors. J Neurosci. 2009;29:14199–14210. doi: 10.1523/JNEUROSCI.2873-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venken KJ, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–230. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantazis A, Segaran A, Liu CH, Nikolaev A, Rister J, Thum AS, Roeder T, Semenov E, Juusola M, Hardie RC. Distinct roles for two histamine receptors (hclA and hclB) at the Drosophila photoreceptor synapse. J Neurosci. 2008;28:7250–7259. doi: 10.1523/JNEUROSCI.1654-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nassel DR. Histamine in the brain of insects: a review. Microsc Res Tech. 1999;44:121–136. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<121::AID-JEMT6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Pollack I, Hofbauer A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res. 1991;266:391–398. doi: 10.1007/BF00318195. [DOI] [PubMed] [Google Scholar]
- 13.Chou YH, Spletter ML, Yaksi E, Leong JC, Wilson RI, Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neurosci. 2010;13:439–449. doi: 10.1038/nn.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das A, Chiang A, Davla S, Priya R, Reichert H, VijayRaghavan K, Rodrigues V. Identification and analysis of a glutamatergic local interneuron lineage in the adult Drosophila olfactory system. Neural Syst Circuits. 2011;1:4. doi: 10.1186/2042-1001-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gengs C, Leung HT, Skingsley DR, Iovchev MI, Yin Z, Semenov EP, Burg MG, Hardie RC, Pak WL. The target of Drosophila photoreceptor synaptic transmission is a histamine-gated chloride channel encoded by ort (hclA) J Biol Chem. 2002;277:42113–42120. doi: 10.1074/jbc.M207133200. [DOI] [PubMed] [Google Scholar]
- 16.Skingsley DR, Laughlin SB, Hardie RC. Properties of histamine-activated chloride channels in the large monopolar cells of the dipteran compound eye: a comparative study. J Comp Physiol [A] 1995;176:611–623. [Google Scholar]
- 17.Zheng Y, Hirschberg B, Yuan J, Wang AP, Hunt DC, Ludmerer SW, Schmatz DM, Cully DF. Identification of two novel Drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye. J Biol Chem. 2002;277:2000–2005. doi: 10.1074/jbc.M107635200. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney LB, Couto A, Chou YH, Berdnik D, Dickson BJ, Luo L, Komiyama T. Temporal target restriction of olfactory receptor neurons by Semaphorin-1a/PlexinA-mediated axon-axon interactions. Neuron. 2007;53:185–200. doi: 10.1016/j.neuron.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Kazama H, Wilson RI. Homeostatic matching and nonlinear amplification at genetically-identified central synapses. Neuron. 2008;58:401–413. doi: 10.1016/j.neuron.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazama H, Wilson RI. Origins of correlated activity in an olfactory circuit. Nat Neurosci. 2009;12:1136–1144. doi: 10.1038/nn.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slimko EM, McKinney S, Anderson DJ, Davidson N, Lester HA. Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels. J Neurosci. 2002;22:7373–7379. doi: 10.1523/JNEUROSCI.22-17-07373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl− channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 23.Lechner HA, Lein ES, Callaway EM. A genetic method for selective and quickly reversible silencing of Mammalian neurons. J Neurosci. 2002;22:5287–5290. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wulff P, Goetz T, Leppa E, Linden AM, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER, et al. From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAA receptors. Nat Neurosci. 2007;10:923–929. doi: 10.1038/nn1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011;333:1292–1296. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardie RC. A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature. 1989;339:704–706. doi: 10.1038/339704a0. [DOI] [PubMed] [Google Scholar]
- 27.Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- 28.Yaksi E, Wilson RI. Electrical coupling between olfactory glomeruli. Neuron. 2010;67:1034–1047. doi: 10.1016/j.neuron.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao Z, Macara AM, Lelito KR, Minosyan TY, Shafer OT. Analysis of functional neuronal connectivity in the Drosophila brain. J Neurophysiol. 2012;108:684–696. doi: 10.1152/jn.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Zhang W, Qiao W, Hu A, Wang Z. Functional connectivity and selective odor responses of excitatory local interneurons in Drosophila antennal lobe. Neuron. 2010;67:1021–1033. doi: 10.1016/j.neuron.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Thum AS, Knapek S, Rister J, Dierichs-Schmitt E, Heisenberg M, Tanimoto H. Differential potencies of effector genes in adult Drosophila. J Comp Neurol. 2006;498:194–203. doi: 10.1002/cne.21022. [DOI] [PubMed] [Google Scholar]
- 34.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Mahr A, Aberle H. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene expression patterns: GEP. 2006;6:299–309. doi: 10.1016/j.modgep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 37.Rister J, Pauls D, Schnell B, Ting CY, Lee CH, Sinakevitch I, Morante J, Strausfeld NJ, Ito K, Heisenberg M. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron. 2007;56:155–170. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- 39.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gouwens NW, Wilson RI. Signal propagation in Drosophila central neurons. J Neurosci. 2009;29:6239–6249. doi: 10.1523/JNEUROSCI.0764-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panula P, Happola O, Airaksinen MS, Auvinen S, Virkamaki A. Carbodiimide as a tissue fixative in histamine immunohistochemistry and its application in developmental neurobiology. J Histochem Cytochem. 1988;36:259–269. doi: 10.1177/36.3.3343510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.