Abstract
The studies so far reported on the metabolic clearance rate of insulin in human diabetes mellitus have given conflicting results, probably because they have been conducted on few patients and have used a variety of experimental techniques and data treatments. We investigated the kinetics of insulin distribution and degradation in 35 normal subjects and in 42 nonketotic, nonobese, overtly diabetic patients, of whom 26 were above 40 yr old and 16 were 40 yr old or less at diagnosis. The design of the study combined (a) the use of a tracer to perturb minimally the steady state and to avoid glucose infusion; (b) the preparation of purified [125I]-monoiodoinsulin, which has a metabolic behavior similar to that of native insulin; and (c) noncompartmental analysis of the plasma immunoprecipitable 125I-insulin disappearance curves, which were recorded for 2 h after pulse i.v. injection of the tracer.
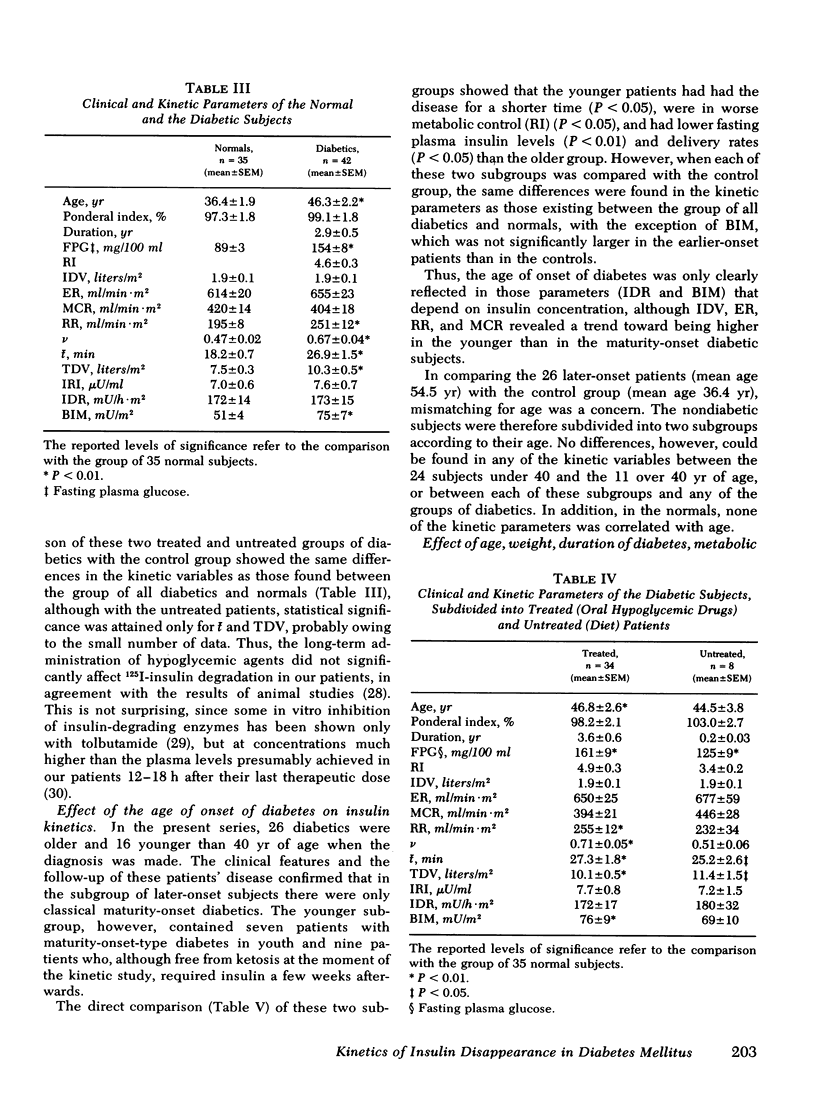
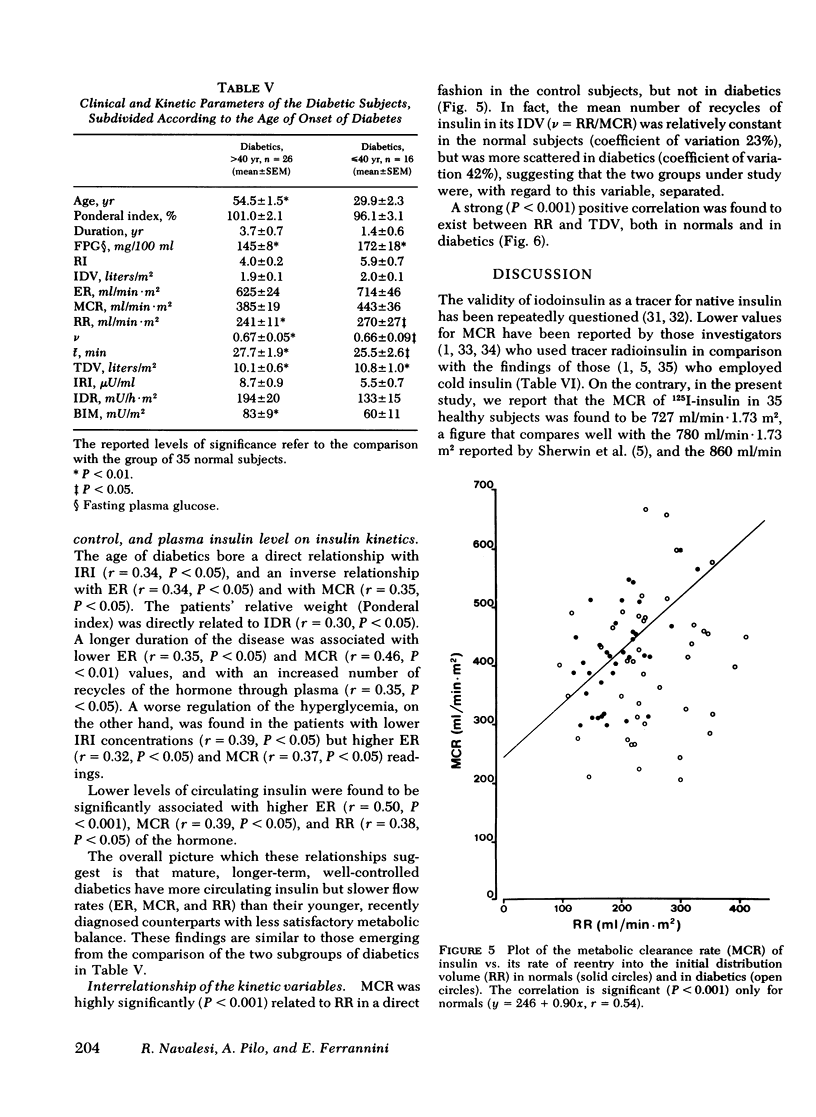
Metabolic clearance rate was found to be similar in diabetics (404±18 ml/min·m2, mean±SEM) and in normals (420±14), although the latter-onset patients had slightly, if not significantly, lower metabolic clearance rate values than the earlier-onset diabetics (385±19 and 443±36, respectively). The initial distribution volume of the hormone also did not significantly differ in diabetics and normals and was similar to plasma volume. The reentry rate into the initial distribution volume of the hormone and the total, plasma-equivalent distribution volume of insulin were both significantly raised in diabetics (251±12 ml/min·m2 and 10.3±0.5 liters/m2) in comparison with normals (195±8 and 7.5±0.3). The posthepatic delivery rate of insulin was found to be slightly raised in later-onset diabetics (194±20 mU/h·m2), but somewhat reduced in earlier-onset diabetics (133±15) in comparison with normals (172±14); these differences reflected the different basal plasma insulin concentrations in these three groups. Chronic treatment with oral hypoglycemic drugs, age, duration of the disease, and degree of metabolic control appeared to have only little effect on the kinetics of insulin.
On the basis of these results, we conclude that insulin-independent adult diabetics show, already in the fasting state, a combination of insulin resistance and insulin deficiency and a derangement in insulin distribution, the precise significance of which is uncertain.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N. Metabolism of single-component and high-molecular-weight radioactive insulin in rats. Endocrinology. 1976 Aug;99(2):481–489. doi: 10.1210/endo-99-2-481. [DOI] [PubMed] [Google Scholar]
- Arquilla E. R., Ooms H., Mercola K. Immunological and biological properties of iodoinsulin labeled with one or less atoms of iodine per molecule. J Clin Invest. 1968 Mar;47(3):474–487. doi: 10.1172/JCI105744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christlieb A. R. Renin, angiotensin, and norepinephrine in alloxan diabetes. Diabetes. 1974 Dec;23(12):962–970. doi: 10.2337/diab.23.12.962. [DOI] [PubMed] [Google Scholar]
- Citti L., Ferrannini E., Battini L., Navalesi R. Radioimmunoassay of labeled and unlabeled insulin mixtures. J Nucl Biol Med. 1976 Apr-Jun;20(2):75–78. [PubMed] [Google Scholar]
- Covelli I., Wolff J. Iodination of the normal and buried tyrosyl residues of lysozyme. I. Chromatographic analysis. Biochemistry. 1966 Mar;5(3):860–866. doi: 10.1021/bi00867a008. [DOI] [PubMed] [Google Scholar]
- Dussault J. H., Turcotte R., Guyda H. The effect of acetylsalicylic acid on TSH and PRL secretion after TRH stimulation in the human. J Clin Endocrinol Metab. 1976 Jul;43(1):232–235. doi: 10.1210/jcem-43-1-232. [DOI] [PubMed] [Google Scholar]
- FAJANS S. S., CONN J. W. The early recognition of diabetes mellitus. Ann N Y Acad Sci. 1959 Sep 25;82:208–218. doi: 10.1111/j.1749-6632.1959.tb44901.x. [DOI] [PubMed] [Google Scholar]
- Feldman J. M., Lebovitz H. E. Appraisal of the extrapancreatic actions of sulfonylureas. Arch Intern Med. 1969 Mar;123(3):314–322. [PubMed] [Google Scholar]
- Franckson J. R., Vanroux R., Leclercq R., Brunengraber H., Ooms H. A. Labelled insulin catabolism and pancreatic responsiveness during long-term exercise in man. Horm Metab Res. 1971 Nov;3(6):366–373. doi: 10.1055/s-0028-1094123. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Frost D. P., Srivastava M. C., Jones R. H., Nabarro J. D., Sonksen P. H. The kinetics of insulin metabolism in diabetes mellitus. Postgrad Med J. 1973 Dec;49(Suppl):949–954. [PubMed] [Google Scholar]
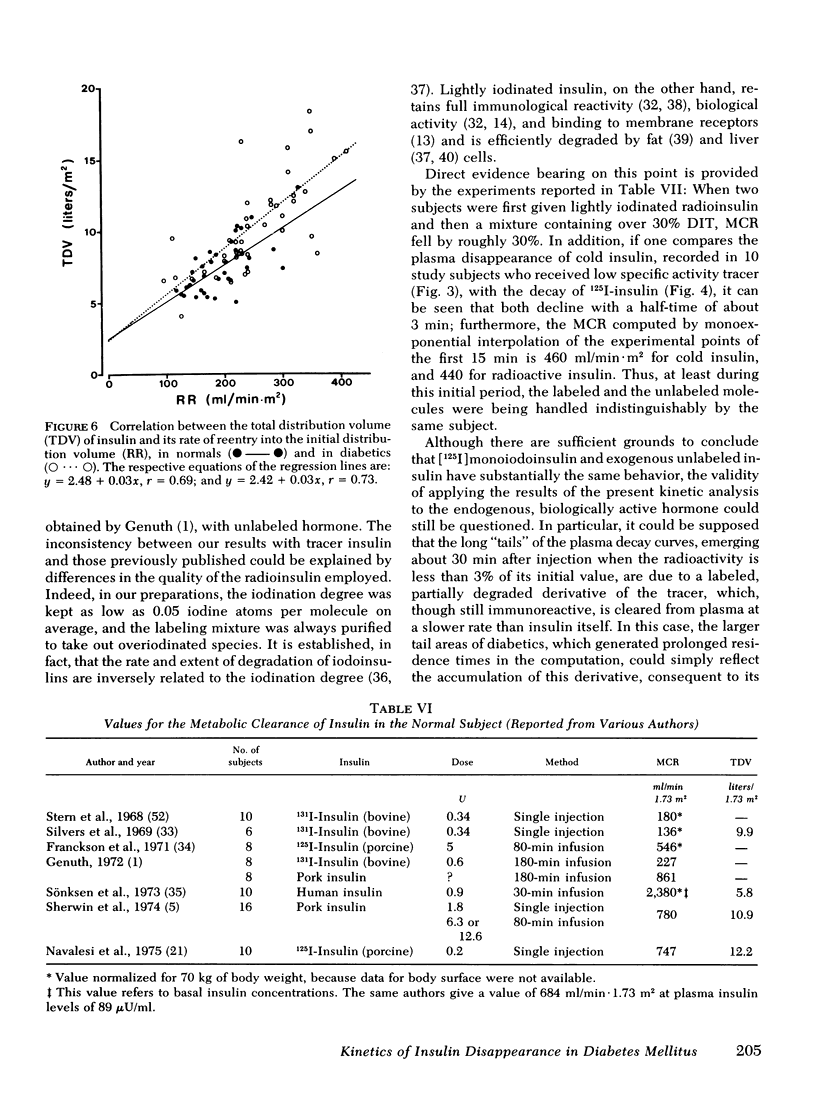
- Genuth S. M. Metabolic clearance of insulin in man. Diabetes. 1972 Oct;21(10):1003–1012. doi: 10.2337/diab.21.10.1003. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Gammeltoft S., Vinten J. Time course of insulin-receptor binding and insulin-induced lipogenesis in isolated rat fat cells. J Biol Chem. 1975 May 10;250(9):3368–3374. [PubMed] [Google Scholar]
- Gundersen H. J., Christensen N. J. Intravenous insulin causing loss of intravascular water and albumin and increased adrenergic nervous activity in diabetics. Diabetes. 1977 Jun;26(6):551–557. doi: 10.2337/diab.26.6.551. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L., Arquilla E. R. Monoiodoinsulin. Preparation, purification, and characterization of a biologically active derivative substituted predominantly on tyrosine A14. J Biol Chem. 1974 Jan 10;249(1):21–32. [PubMed] [Google Scholar]
- Izzo J. L., Bartlett J. W., Roncone A., Izzo M. J., Bale W. F. Physiological processes and dynamics in the disposition of small and large doses of biologically active and inactive 131-I-insulins in the rat. J Biol Chem. 1967 May 25;242(10):2343–2355. [PubMed] [Google Scholar]
- Izzo J. L., Roncone A., Izzo M. J., Foley R., Bartlett J. W. Degradation of 131 I-insulins by rat liver. Studies in vitro. J Biol Chem. 1972 Feb 25;247(4):1219–1226. [PubMed] [Google Scholar]
- Katz M. A. Hyperglycemia-induced hyponatremia--calculation of expected serum sodium depression. N Engl J Med. 1973 Oct 18;289(16):843–844. doi: 10.1056/NEJM197310182891607. [DOI] [PubMed] [Google Scholar]
- Le Cam A., Freychet P., Lenoir P. Degradation of insulin by isolated rat liver cells. Diabetes. 1975 Jun;24(6):566–573. doi: 10.2337/diab.24.6.566. [DOI] [PubMed] [Google Scholar]
- MIRSKY I. A., PERISUTTI G., DIENGOTT D. The inhibition of insulinase by hypoglycemic sulfonamides. Metabolism. 1956 Mar;5(2):156–161. [PubMed] [Google Scholar]
- Navalesi R., Pilo A., Ferrannini E. Insulin kinetics after portal and peripheral injection of [125I] insulin: II. Experiments in the intact dog. Am J Physiol. 1976 Jun;230(6):1630–1636. doi: 10.1152/ajplegacy.1976.230.6.1630. [DOI] [PubMed] [Google Scholar]
- Navalesi R., Pilo A., Lenzi S., Donato L. Insulin metabolism in chronic uremia and in the anephric state: effect of the dialytic treatment. J Clin Endocrinol Metab. 1975 Jan;40(1):70–85. doi: 10.1210/jcem-40-1-70. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Decreased insulin binding to lymphocytes from diabetic subjects. J Clin Invest. 1974 Dec;54(6):1323–1328. doi: 10.1172/JCI107878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms H. A., Arnould Y., Rosa U., Pennisi G. F., Franckson J. R. Clearances métaboliques globales de l'insuline cristalline et d'insulines substituées au radioiode. Pathol Biol. 1968 Mar;16(5):241–245. [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Determination of common parameters fo iodothyronine metabolism and distribution in man by noncompartmental analysis. J Clin Endocrinol Metab. 1975 Aug;41(2):319–324. doi: 10.1210/jcem-41-2-319. [DOI] [PubMed] [Google Scholar]
- Orskov H., Christensen N. J. Plasma disappearance rate of injected human insulin in juvenile diabetic, maturity-onset diabetic and nondiabetic subjects. Diabetes. 1969 Oct;18(10):653–659. doi: 10.2337/diab.18.10.653. [DOI] [PubMed] [Google Scholar]
- P ARVING H. H., Rasmussen S. M. Transcapillary escape rate of albumin and plasma volume in short- and long-term juvenile diabetics. Scand J Clin Lab Invest. 1973 Aug;32(1):81–87. doi: 10.3109/00365517309082454. [DOI] [PubMed] [Google Scholar]
- Parving H. H. Increased microvascular permeability to plasma proteins in short- and long-term juvenile diabetics. Diabetes. 1976;25(2 Suppl):884–889. [PubMed] [Google Scholar]
- Pilo A., Navalesi R., Ferrannini E. Insulin kinetics after portal and peripheral injection of [125I] insulin. I. Data analysis and modeling. Am J Physiol. 1976 Jun;230(6):1626–1629. doi: 10.1152/ajplegacy.1976.230.6.1626. [DOI] [PubMed] [Google Scholar]
- Pilo A., Zucchelli G. C. Automatic treatment of radioimmunoassay data: an experimental validation of the results. Clin Chim Acta. 1975 Oct 1;64(1):1–9. doi: 10.1016/0009-8981(75)90137-0. [DOI] [PubMed] [Google Scholar]
- ROSA U., SCASSELLATI G. A., PENNISI F. LABELLING OF HUMAN FIBRINOGEN WITH 131-I BY ELECTROLYTIC IODINATION. Biochim Biophys Acta. 1964 Jun 8;86:519–526. doi: 10.1016/0304-4165(64)90091-1. [DOI] [PubMed] [Google Scholar]
- Rasio E. A., Hampers C. L., Soeldner J. S., Cahill G. F., Jr Diffusion of glucose, insulin, inulin, and Evans blue protein into thoracic duct lymph of man. J Clin Invest. 1967 Jun;46(6):903–910. doi: 10.1172/JCI105596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven G. M., Bernstein R., Davis B., Olefsky J. M. Nonketotic diabetes mellitus: insulin deficiency or insulin resistance? Am J Med. 1976 Jan;60(1):80–88. doi: 10.1016/0002-9343(76)90536-2. [DOI] [PubMed] [Google Scholar]
- Rescigno A., Gurpide E. Estimation of average times of residence, recycle and interconversion of blood-borne compounds using tracer methods. J Clin Endocrinol Metab. 1973 Feb;36(2):263–276. doi: 10.1210/jcem-36-2-263. [DOI] [PubMed] [Google Scholar]
- Roy C. C., Shapcott D. J., O'Brien D. The case for an "abnormal" insulin in diabetes mellitus. Diabetologia. 1968 Jun;4(3):111–117. doi: 10.1007/BF01219430. [DOI] [PubMed] [Google Scholar]
- Schneider B., Straus E., Yalow R. S. Some considerations in the preparation of raioiodoinsulin for radioimmunoassay and receptor assay. Diabetes. 1976 Apr;25(4):260–267. doi: 10.2337/diab.25.4.260. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Kramer K. J., Tobin J. D., Insel P. A., Liljenquist J. E., Berman M., Andres R. A model of the kinetics of insulin in man. J Clin Invest. 1974 May;53(5):1481–1492. doi: 10.1172/JCI107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers A., Swenson R. S., Farquhar J. W., Reaven G. M. Derivation of a three compartment model describing disappearance of plasma insulin-131-I in man. J Clin Invest. 1969 Aug;48(8):1461–1469. doi: 10.1172/JCI106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodoyez J. C., Sodoyez-Goffaux F., Goff M. M., Zimmerman A. E., Arquilla E. R. [127-I]- or carrier-free [125-I]monoiodoinsulin. J Biol Chem. 1975 Jun 10;250(11):4268–4277. [PubMed] [Google Scholar]
- Stern M. P., Farquhar J. W., Silvers A., Reaven G. M. Insulin delivery rate into plasma in normal and diabetic subjects. J Clin Invest. 1968 Sep;47(9):1947–1957. doi: 10.1172/JCI105884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimmler L., Mashiter K., Snodgrass G. J., Boucher B., Abrams M. Insulin disappearance after intravenous injection and its effect on blood glucose in diabetic and non-diabetic children and adults. Clin Sci. 1972 Mar;42(3):337–344. doi: 10.1042/cs0420337. [DOI] [PubMed] [Google Scholar]
- Sönksen P. H., Tompkins C. V., Srivastava M. C., Nabarro J. D. A comparative study on the metabolism of human insulin and porcine proinsulin in man. Clin Sci Mol Med. 1973 Nov;45(5):633–654. doi: 10.1042/cs0450633. [DOI] [PubMed] [Google Scholar]
- Turner R. C., Grayburn J. A., Newman G. B., Nabarro J. D. Measurement of the insulin delivery rate in man. J Clin Endocrinol Metab. 1971 Aug;33(2):279–286. doi: 10.1210/jcem-33-2-279. [DOI] [PubMed] [Google Scholar]


