Abstract
Previous studies have demonstrated that bone marrow (BM)-derived cells differentiate into nonhematopoietic cells of multiple tissues. To date, it remains unknown which population(s) of BM cells are primarily responsible for this engraftment. To test the hypothesis that nonhematopoietic stem cells in the BM are the primary source of marrow-derived lung epithelial cells, either wild-type hematopoietic or nonhematopoietic BM cells were transplanted into irradiated surfactant-protein-C (SPC)-null mice. Donor-derived, SPC-positive type 2 pneumocytes were predominantly detected in the lungs of mice receiving purified nonhematopoietic cells and were absent from mice receiving purified hematopoietic stem and progenitor cells. We conclude that cells contained in the nonhematopoietic fraction of the BM are the primary source of marrow-derived lung epithelial cells. These nonhematopoietic cells may represent a primitive stem cell population residing in adult BM.
Keywords: Bone marrow, Epithelial cells, Cell differentiation
Introduction
In recent years, numerous reports have shown that bone marrow (BM)-derived cells can give rise to differentiated cells of multiple nonhematopoietic organs including the lung, a phenomenon that has become known as bone marrow cell plasticity [1]. In one of these studies, Krause et al. [2] used a BM cell population that was enriched for small cells that do not express markers of differentiated blood cells (lineage negative) and are able to home to the BM. This population was enriched for hematopoietic stem cell activity [3] but was not a pure hematopoietic stem cell population. In another study, Wagers et al. [4] reported that Thy1loLineagenegSca-1posc-Kitpos cells, a purely hematopoietic cell population that is highly enriched for hematopoietic stem cells, engraft only very rarely as nonhematopoietic cells. However, multiple other studies have consistently reported lung epithelial cells derived from the donor after transplantation of whole bone marrow (WBM) [5-7] or lineage negative cells [7, 8].
So far, it has remained unresolved which subpopulation of BM cells is capable of giving rise to cells of nonhematopoietic lineages. This has created tremendous controversy in the field, which has been exacerbated by the fact that many of the recent studies on BM cell plasticity have not used highly definitive and stringent approaches to identify BM-derived cells in nonhematopoietic tissues. Thus, very stringent analyses need to be performed to definitively identify BM-derived epithelial cells [8]. This includes the ability to clearly identify cells derived from the donor BM, the use of markers identifying them as epithelial cells, and confocal microscopy to exclude cell overlay and closely assess cell morphology. In the present study, we have combined all of these, and in addition used single cell analysis and polymerase chain reaction (PCR) to definitively identify BM-derived type 2 pneumocytes in the lung.
We hypothesized that nonhematopoietic stem cells residing in the BM are the major cell type responsible for engraftment as epithelial cells. To directly test this hypothesis, we assessed the capacity of hematopoietic versus nonhematopoietic BM cells to give rise to epithelial cells in the lung. Using a vav-Cre lineage tracing approach, we separated hematopoietic from nonhematopoietic BM cells, and assessed for donor-derived epithelial cells in the lungs of recipient mice. We demonstrate that surfactant-protein-C (SPC) expressing type 2 pneumocytes derived from the donor BM were identified in SPC-null recipients predominantly after transplantation of nonhematopoietic BM cells, while hematopoietic cells only in extremely rare cases gave rise to lung epithelial cells. In addition, purified hematopoietic stem and progenitor cells (HSPCs) did not give rise to lung epithelial cells. We conclude from these findings that cells contained in the nonhematopoietic fraction of the BM are the primary source of marrow-derived type 2 pneumocytes, while hematopoietic BM cells have only very limited capacity to give rise to lung epithelial cells.
Materials and Methods
Mice
Homozygous vav-Cre mice (kind gift from Thomas Graf, Center for Genomic Regulation and ICRIA, Barcelona, Spain) were crossed to homozygous ROSA26R-YFP reporter mice (B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J Jackson Laboratory), and heterozygous pups, referred to as “vav-YFP,” were used as BM donors. At the time of weaning, blood and skin were assessed for specificity of YFP expression exclusively in hematopoietic cells. Mice with ubiquitous expression of YFP due to activation of Cre in the blastocyst, as described previously [9], were not used in experiments. SPC-KO mice [10] were a kind gift from J. Whitsett (Cincinnati Childrens Hospital). All animal work was approved by the Yale Institutional Animal Care and Use Committee. Animals were housed in the Yale Animal Resource Center.
Sorting of vavYFP+ and vavYFP−BM Cells, BM Transplant
BM was flushed from femurs and tibias using RPMI medium, resuspended and filtered through a 70-μm cell strainer. Lineage marker positive cells were removed by magnetic depletion using the Mouse Hematopoietic Progenitor Cell Enrichment Set (BD Biosciences), which uses a cocktail of biotinylated monoclonal antibodies to mouse CD3e (CD3 ε chain), CD11b (Integrin αM chain), CD45R/B220, Ly-6G and Ly-6C (Gr-1), and TER-119/Erythroid Cells (Ly-76) followed by BD IMag Streptavidin Particles. After lineage depletion and washing, cells were again filtered and sorted on a MoFlo cell sorter (Cytomation) into IMDM medium (Gibco). Ten percentage of lineage depleted BM cells were negative for YFP, with 90% of cells falling into the vav-YFP positive fraction (Fig. 1A). For each independent experiment (n = 3), Lin-YFP+ cells (450,000-800,000 per mouse) or Lin-YFP− cells (50,000-150,000 per mouse) from five donors were pooled and injected into the retro-orbital plexus of SPC-KO (n = 10 per condition) recipient mice that had been lethally irradiated with 1,000 cG from a Cs-137 source. Note that the number of YFP+ and YFP− cells transplanted represented the same proportion in which they are found in the BM. Each group of 10 recipients received sorted YFP+ or YFP− cells pooled from five donors. For recipients of YFP negative cells, 1 million SPC-KO (recipient-type) WBM cells were coinjected to provide hematopoietic recovery. As negative controls, irradiated SPC-KO mice were transplanted with 2 million WBM cells from SPC-KO mice and analyzed in the same fashion as mice receiving vav-YFP BM cells. As positive controls, 2 million unfractionated WBM cells from a vav-YFP donor were injected. Vav-YFP donor engraftment was analyzed after 2-6 months by flow cytometry for YFP in the peripheral blood, comparing the frequency of YFP positive blood cells in a vav-YFP mouse to the frequency of YFP positive cells in BMT recipient mice. In a separate experiment, Lineage-negative, Sca-1-positive, and CD45-positive HSPCs were sorted from male wild-type BM and transplanted into female SPC-KO recipient mice (50,000 cells per mouse). Engraftment was analyzed by Y-chromosome FISH on BM cytospins as previously described [5].
Figure 1. Vav-Cre-ROSA-YFP lineage tracing and experimental approach.
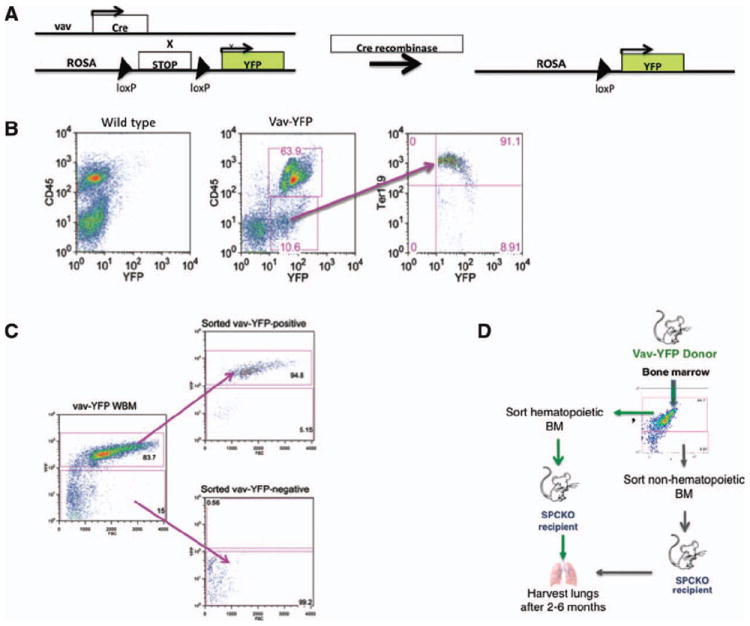
(A): Expression of Cre recombinase under the hematopoietic vav-promoter leads to excision of the Stop-codon with subsequent expression of YFP in vav-Cre/ROSA26R-YFP (vav-YFP) transgenic mice. (B): Fluorescence-activated cell sorting (FACS) analysis of bone marrow from vav-YFP mice. (C): FACS-sorting of vav-YFP-negative and vav-YFP-positive BM cell populations and purity after sorting. (D): Experimental approach. Hematopoietic (YFP positive) or nonhematopoietic (YFP negative) BM cells were sorted by flow cytometry from vav-YFP transgenic mice and transplanted into irradiated SPC-KO recipient mice. Lung tissue was harvested for analysis 2-6 months after transplant. Abbreviations: BM, bone marrow; WBM, whole bone marrow.
Lung Harvest and Lung Single Cell Suspension
Two to six months post-transplant, mice were anesthetized with ketamine/xylazine and bronchoalveolar lavage was performed to remove blood cells from the alveolar space. Mice underwent thoracotomy and right ventricular perfusion as described previously [5]. The left lung lobe was tied off and processed for paraffin embedding. The remaining lung was inflated with 3 ml dispase in Dulbecco’s modified Eagle’s medium (DMEM) followed by 0.5% low melting agarose. After cooling the agarose, the lung was digested with dispase for 45 minutes at room temperature and incubated with DNase (100 units/ml) for 10 minutes before dissociation using program B on a GentleMACS tissue dissociator (Miltenyi Biotec). Cells were then filtered through 100-μm, 70-μm, and 40-μm cell strainers. Cells were washed with DMEM and processed for either ImageStream analysis or cell sorting.
ImageStream Analysis
After suspension of single lung cells in DMEM with 10% fetal bovine serum (FBS), blood cells were removed by incubation for 2 hours at 37°C on a plastic dish coated with anti-CD45, anti-CD16, and anti-CD32 antibodies. The resulting cell population is enriched for type 2 pneumocytes [11]. Comparable number of epithelial cells were recovered from each dish, and viability was similar between samples. Cells were washed with phosphate-buffered saline (PBS) EDTA to avoid clumping, fixed with 4% paraformaldehyde, washed in PBS, permeabilized with 0.5% saponin and then stained with guinea pig anti-SPC (kind gift from J. Whitsett), rabbit anti-bovine wide spectrum cytokeratin (DAKO), rat anti-mouse CD45 (BD Pharmingen) and rat anti-mouse F4/80 (EBiosciences), followed by Alexa 555 conjugated donkey anti-guinea pig, Alexa 594 conjugated donkey anti-rabbit and Alexa 647 conjugated donkey anti-rat secondary antibodies (Invitrogen). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue; Invitrogen). Cells were washed twice with PBS, filtered through a 40-μm mesh and acquired on a two-camera ImageStream imaging cytometer (Amnis Corporation, Seattle) using 405, 488, and 561 excitations. Raw image files were electronically compensated and analyzed using IDEAS image analysis software version 4.0 (Amnis Corporation, Supporting Information Fig. S2). Compensation matrices were created using single color control files. None of the secondary antibodies produced a signal when the primary antibody was omitted (not shown). Remaining CD45 and F4/80 positive cells were excluded from analysis, as well as cell fragments and cells that were out of focus (Supporting Information Fig. S2). Only cells with positive fluorescence for SPC featuring at least two bright spots (vesicles) inside the cell and no colocalization with the fluorescent signal in the channel for cytokeratin were included in the final analysis. The percentage of donor-derived type 2 pneumocytes was normalized to the total number of intact, SPC-positive type 2 cells in a corresponding wild-type sample, which was normalized to 100%. p values were determined using a two-tailed Student’s t test.
Fluorescence-Activated Cell Sorting and Immunofluorescence on Sorted Lung Cells
For antibody staining, single lung cells were washed with PBS and resuspended in PBS with 2% FBS and 25 mM EDTA. Cells were stained for 30 minutes at 37°C with APC labeled rat anti-mouse CD45, rat anti-mouse CD11b, and rat anti-mouse CD31 (BD Pharmingen). After washing, cells were placed on ice and APC-negative cells were sorted on a MoFlo cell sorter (Cytomation) using low pressure settings. Sorted cells were resuspended in DMEM 20% FBS and allowed to attach to poly-l-lysine-coated coverslips. From each sorted sample, comparable numbers of cells attached to each slide (10-28,000 cells). The medium was removed and cells were fixed with 2% paraformaldehyde for 10 minutes at room temperature. Fixed cells were permeabilized and blocked with 0.03% Triton X-100 and donkey-serum in PBS and stained with polyclonal guinea pig anti-SPC (kind gift from J. Whitsett), rabbit anti-bovine wide spectrum cytokeratin (DAKO), rat anti-mouse CD45 (BD Pharmingen) and rat anti-mouse F4/80 (EBiosciences), followed by Alexa 555 conjugated donkey anti-guinea pig, Alexa 488 conjugated donkey anti-rabbit and Alexa 647 conjugated donkey anti-rat secondary antibodies. Nuclei were stained with DAPI (Invitrogen). Coverslips were mounted on microscope slides and analyzed by fluorescence microscopy for the presence of SPC-positive, cytokeratin-positive type 2 pneumocytes. Double positive cells were analyzed in detail on a Leica SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) using 405, 488, 543, and 633 laser excitations and sequential scanning. From each cell, images of optical slices were acquired every 0.4 μm from the top to the bottom of the cell (z-stacks).
Immunofluorescence for SPC and Thyroid Transcription Factor 1 on Lung Tissue Sections
One lobe of the lung was tied off and fixed in 4% paraformaldehyde for 4 hours and embedded in paraffin. Then, 5-μm sections were cut, deparaffinized, and treated with antigen retrieval solution (Retrievagen A, BD Biosciences). After blocking with donkey-serum and mouse-on-mouse blocking reagent (MOM-kit, Vectorlabs), sections were stained with polyclonal rabbit anti-surfactant-protein-C (Millipore) and mouse anti-thyroid transcription factor 1 (anti-TTF1; clone 8G7G3/1, DAKO) in the presence of 0.03% Triton X-100 and donkey-serum, followed by Alexa-555-conjugated donkey anti-rabbit and Alexa-488 conjugated donkey anti-mouse secondary antibodies (Invitrogen), and imaged after adding Vectashield mounting medium with DAPI (Vectorlabs). Two to four sections (one section contains approximately 1,056 type 2 cells in WT mice) per mouse were analyzed by fluorescence microscopy for the presence of SPC-positive and TTF1 positive type 2 pneumocytes, and double positive cells were analyzed in detail on a Leica SP5 confocal microscope (Leica Microsystems) using 405, 488, and 543 laser excitations and sequential scanning. From each cell, images of optical slices were acquired as described above. Three-dimensional (3D) projections and optical x and y cross-sections were created using LAS-AF software (Leica Microsystems). The percentage of donor-derived type 2 pneumocytes was normalized to the fraction of intact, SPC-positive type 2 cells in corresponding wild-type controls, which was normalized to 100%. p values were determined using a two-tailed Student’s t test.
Nested PCR for SPC-mRNA
RNA was extracted either directly from lung tissue or from digested lung cells and transcribed into cDNA using random primers and Superscript RTII enzyme (Invitrogen). The first round of PCR used primers binding in exons 1 and 3 of the SPC-mRNA (SPC-Ex1F-GGT CCT GAT GGA GAG TCC ACC G and SPC-Ex3R-TTC CTG GAG CTG GCT TAT ACG CC), which in the wild type yields a product of 334 base pairs and in SPC-KO mice, in which the mRNA lacks exon 2, a product of 174 base pairs. For the second round of PCR, 1 μl of the first reaction was amplified using primers in exons 2 and 3 (SPC-nestedF-CTC GTT GTC GTG GTG ATT GT and SPCnestedR- AAA GGT AGC GAT GGT GTC TG), which in wild-type mice yields a product of 149 base pairs, and no product in SPC-KO mice. All PCRs were performed using Hot-Star-Taq-Polymerase (Qiagen) using the following conditions: initial denaturation at 95°C for 15 minutes followed by 30 seconds at 95°C, 30 seconds at 56°C annealing temperature, 15 seconds at 72°C, for 35 cycles.
Results
Transplantation of Hematopoietic Versus Nonhematopoietic BM Cells into Surfactant-Protein-C-Knockout Mice
To determine whether BM-derived type 2 pneumocytes are derived from the nonhematopoietic versus the hematopoietic fraction of BM cells, we used a lineage tracing approach in which Cre recombinase under the control of the hematopoiesis-specific vav-promoter activates expression of YFP from a ROSA-YFP reporter transgene (Fig. 1A). Vav is expressed in all cells committed to the hematopoietic lineage, including fetal and adult HSC [9, 12, 13], and is not expressed outside the hematopoietic system. Thus, in vav-Cre-ROSA-YFP mice, all hematopoietic cells are permanently labeled with YFP. CD45-positive cells are uniformly YFP-positive, and YFP-positive cells not expressing CD45 are Ter119-positive red blood cells that have lost CD45 expression (Fig. 1B). YFP positive (hematopoietic) or YFP negative (nonhematopoietic) BM cells were separated by FACS (purity: 94.8 and 99.2%, respectively, Fig. 1C), and transplanted into lethally irradiated SPC-KO mice (Fig. 1D). SPC is exclusively expressed by type 2 pneumocytes in the lung, and its expression is completely absent in SPC-KO mice. Thus, type 2 pneumocytes derived from donor BM cells can be specifically identified based on expression of SPC in the lungs of SPC-KO recipient mice.
For recipients of YFP negative cells, 1 million recipient-type SPC-KO WBM cells were cotransplanted to provide hematopoietic rescue following irradiation. Engraftment levels were calculated based on expression of YFP in peripheral blood, which was compared to expression levels in vav-YFP mice. In recipients of vav-YFPpos hematopoietic BM cells, engraftment levels were between 75 and 98%, whereas vav-YFPneg nonhematopoietic BM cells did not engraft in the hematopoietic system (Supporting Information Fig. S1A).
Detection of Lung Epithelial Cells Derived from Nonhematopoietic BM Cells by Confocal Microscopy on Tissue Sections
Lung tissue from transplanted SPC-KO mice was analyzed 2-6 months after transplant. In initial experiments analyzing recipients of WBM, there were no differences in the numbers of BM-derived epithelial cells between mice analyzed at 2 and 6 months post-transplant. Therefore, all quantification results from different time points were averaged for the remaining studies. For confocal microscopy, paraffin sections were stained by immunofluorescence for alveolar type 2 cell-specific SPC, for the epithelial cell-specific nuclear TTF1 (which is predominantly expressed in type 2 pneumocytes), and YFP. In vav-YFP control lungs, YFP is exclusively expressed in blood cells distinct from epithelial cells; no YFP positive cells expressed either TTF1 or SPC (Fig. 2A). In wild-type mice, type 2 pneumocytes, which are localized within alveoli at alveolar junctions, express SPC within lamellar bodies, resulting in a vesicular staining pattern, and express TTF1 in the nuclei (Fig. 2A). High-power confocal microscopy images and cross-sections through a series of images taken in different planes from the top to the bottom of a type 2 pneumocyte (z-stack) show that the nucleus containing the TTF1 signal is surrounded by SPC contained in lamellar bodies (Fig. 2A). This staining pattern and morphology was used as a standard to identify SPC and TTF1 double positive cells in SPC-KO recipient mice. In control SPC-KO mice and SPC-KO mice transplanted with SPC-KO BM, SPC expression is absent, but TTF1 positive type 2 pneumocytes can be identified at alveolar junctions (Fig. 2C).
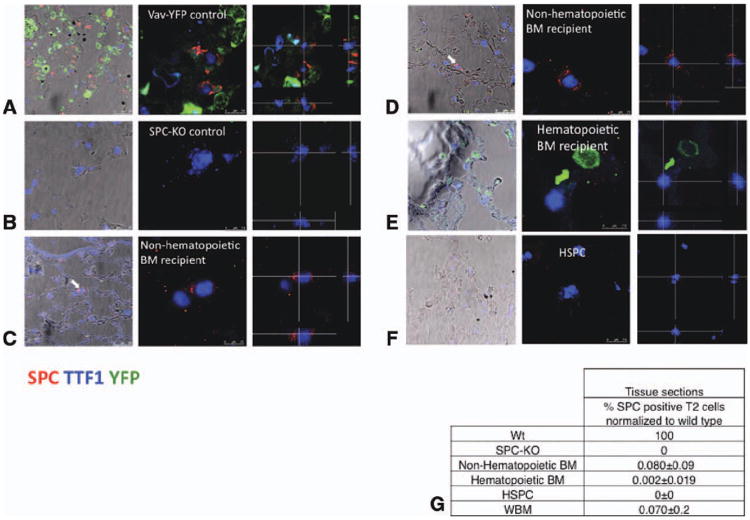
Figure 2. Analysis of lung tissue sections for donor-derived, SPC-positive cells.
(A)–(F): Confocal microscopic images of lung tissue sections stained for SPC, YFP, and TTF1. The left-most panels show images taken at low zoom, merged with bright-field images to show localization of type 2 cells within lung architecture. Donor-derived type 2 cells are marked with arrows. Middle panel: confocal images, ×630. Right panel: images were taken in 0.4-μm steps from the top to the bottom of each cell. Optical cross-sections through one focal plane show SPC expression in cytoplasmic vesicles surrounding the nucleus, excluding cell overlay. Cross-hairs indicate planes of optical x and y sections projected on bottom and right side. (A): Type 2 pneumocytes from vav-YFP control lungs coexpress vesicular SPC and nuclear TTF1. YFP is expressed in blood cells but not epithelial cells. (B): Type 2 pneumocytes in SPC-KO lungs express nuclear TTF1 but not SPC. (C) and (D): Lungs from two different SPC-KO mice that received nonhematopoietic BM cells contain donor-derived type 2 pneumocytes (vesicular cytoplasmic SPC and nuclear TTF1) located at the alveolar junction (arrows). (E): Lungs from SPC-KO mice transplanted with vav-YFP positive, hematopoietic BM cells contain vav-YFP positive blood cells. (F): Lung from SPC-KO mouse transplanted with wild-type HSPC. (G): The percentage of donor-derived, SPC-positive type 2 pneumocytes on tissue sections was calculated based on the percentage of type 2 pneumocytes among nucleated cells in corresponding wild-type samples, which was set to 100%. Abbreviations: BM, bone marrow; HSPC, hematopoietic stem and progenitor cell; TTF1, thyroid transcription factor 1; WBM, whole bone marrow; and Wt, wild type.
Donor-derived type 2 pneumocytes exhibiting SPC expression in intracellular vesicles, while coexpressing TTF1 in the nucleus, were detected in four out of six mice receiving vav-YFP negative, nonhematopoietic BM cells (Fig. 2C, 2D). These cells were localized to alveolar junctions (Fig. 2C, 2D, arrows), and cross-sections through a z-stack show expression of SPC in lamellar bodies surrounding the nucleus. None of these cells expressed YFP. An average of 0.08% ± 0.09% (SD) of type 2 pneumocytes were donor derived in mice receiving nonhematopoietic cells (Fig. 1F). In 19 out of 20 mice receiving vav-YFPpos, hematopoietic cells, no SPC-positive, donor-derived type 2 pneumocytes were detected, and YFP was expressed in blood cells, but not epithelial cells (Fig. 1F). However, in 1 out of 20 mice that received vav-YFPpos hematopoietic BM cells, one SPC-positive cell was detected (Supporting Information Fig. S2A), corresponding to a frequency of 0.002% ± 0.019% of type 2 cells derived from vav-YFPpos hematopoietic BM cells (Fig. 1G). When purified Linneg Sca-1pos CD45pos HSPCs isolated from wild-type mice were transplanted into SPC-KO recipients, in four out of four mice, no SPC-positive cells were detected, indicating that HSPCs do not give rise to lung epithelial cells. All four mice had more than 90% donor bone marrow engraftment based on Y chromosome FISH. The differences between vav-YFPneg and vav-YFPpos BM cells (p < .001) and vav-YFPneg BM cells and HSPCs (p < .05) were statistically significant.
Detection of Lung Epithelial Cells Derived from Nonhematopoietic BM Cells by ImageStream Analysis
Absolute quantification of donor-derived type 2 cells on tissue sections is difficult and may be underestimated, because every tissue contains different amounts of blood cell infiltration, and the levels of autofluorescence, which also differ, can interfere with detection of rare donor-derived cells. Thus, BM-derived type 2 cells might be missed. To more accurately quantitate bone marrow-derived type 2 pneumocytes, we used imaging flow cytometry (Amnis ImageStream), which is very sensitive and allows for single cell analysis in flow without interference of cell overlay or background fluorescence, as well as detailed image analysis, including calculation of fluorescence levels and the number of bright spots within a cell.
Lung tissue from SPC-KO mice that had been transplanted with either nonhematopoietic or hematopoietic BM cells was analyzed 2-6 months after transplant by dissociation into single cells with enzymatic digestion and enrichment for type 2 cells as described in Materials and Methods. Single cells were fixed, permeabilized, and stained for alveolar type 2 cell-specific SPC, epithelial-specific cytokeratin, and blood cell markers (CD45 and F4/80). Only cells negative for both CD45 and F4/80 were imaged. Images were then assessed in detail by image analysis software (Amnis IDEAS application) for cells exhibiting intracellular expression of SPC and cytokeratin (Supporting Information Fig. S3 for detailed schematic of analysis). In type 2 pneumocytes from wild-type mice, SPC is expressed in intracellular lamellar bodies, resulting in a vesicular staining pattern (Fig. 3A). Cells with a fluorescent signal in the SPC-channel (yellow) and positive staining for cytokeratin (orange) were further analyzed only if the signals in the cytokeratin and SPC channels did not colocalize (colocalization feature in IDEAS application), to exclude autofluorescence (Supporting Information Fig. S3). SPC-positive cells were then compared individually to the phenotype seen in wild-type cells, and only counted as donor derived if they contained at least two distinct, SPC-positive vesicles (bright detail intensity and spot count feature). With this approach, donor-derived alveolar type 2 cells expressing SPC were never detected in untransplanted SPC-KO mice (Fig. 3B). They were detected in four out of five SPC-KO mice that received vav-YFP negative, nonhematopoietic BM cells (Fig. 3C). SPC-positive cells were entirely absent from 11 mice transplanted with vav-YFPpos hematopoietic BM cells and 4 mice transplanted with purified HSPC (Fig. 3D, 3E). None of the SPC-positive cells expressed YFP (not shown).
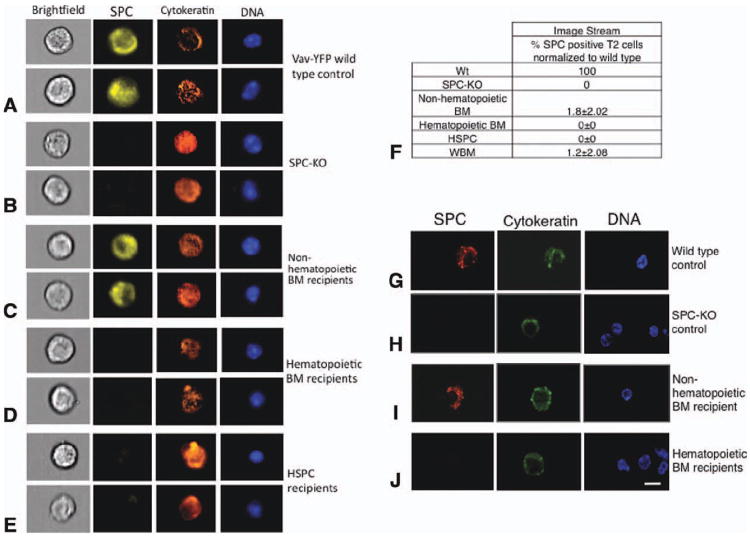
Figure 3. Detection of bone marrow-derived lung epithelial cells by ImageStream and confocal microscopy on single cells.
(A)–(E): Lung tissues from control (Wt and SPC-KO) mice or SPC-KO mice transplanted with the indicated donor BM population were digested with dispase, fixed, and analyzed for SPC (yellow), cytokeratin (orange), and YFP (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Cells were analyzed using an ImageStream imaging flow cytometer with ×40 magnification (Amnis ImageStreamX). Examples of two cells from each group are shown. (A): Type 2 pneumocytes from a WT control mouse. (B): Lung cells from SPC-KO control mice. (C)–(E): Donor-derived, type 2 pneumocytes expressing SPC and cytokeratin were detected in SPC-KO mice transplanted with nonhematopoietic BM cells but not in mice receiving hematopoietic BM cells or purified HSPC. (F): The percentage of donor-derived, SPC-positive type 2 pneumocytes detected by ImageStream was calculated based on the number of type 2 pneumocytes among CD45-negative, round cells in corresponding wild-type samples, which was set to 100%. (G)–(J): Confocal microscopic analysis of sorted lung epithelial cells. Sorted cells were adhered to slides, fixed, and stained for SPC and cytokeratin. Nuclei were stained with DAPI. All cells shown were also CD45 negative (not shown). SPC-positive cells were detected in SPC-KO mice transplanted with nonhematopoietic BM cells but not in mice receiving hematopoietic BM cells. Scale bar = 7.5 μm is the same for all images. Abbreviations: BM, bone marrow; HSPC, hematopoietic stem and progenitor cell; WBM, whole bone marrow; and Wt, wild type.
Percentages were calculated by normalizing the number of BM-derived T2 cells in SPC-KO recipients to the number of T2 cells present within the cytokeratin positive cell population in wild-type controls assessed with the same technique. With this approach, 1.8% ± 2.02% of type 2 pneumocytes from five SPC-KO mice transplanted with nonhematopoietic BM cells were donor derived (Fig. 3F). The differences between vav-YFPneg and vav-YFPpos BM cells (p < .05) and vav-YFPneg BM cells and HSPCs (p < .05) were statistically significant.
Detection of Lung Epithelial Cells Derived from Nonhematopoietic BM Cells by Confocal Microscopy of Sorted Cells
The presence of SPC-positive lung cells derived from nonhematopoietic BM cells was confirmed using confocal microscopic analysis of single lung cells. Lung cells were isolated from SPC-KO recipient mice by enzymatic digestion and enriched by flow cytometric cell sorting for CD45− CD11b− and CD31− cells to eliminate hematopoietic and endothelial cells (Supporting Information Fig. S1B). Sorted cells were adhered to slides and analyzed by immunofluorescence for coexpression of donor-specific SPC and epithelial-specific cytokeratin. By this approach, donor-derived type 2 cells that expressed SPC in intracellular vesicles were found exclusively in the lungs of SPC-KO mice that received vav-YFP negative, nonhematopoietic BM cells (n = 6; Fig. 3I). No donor derived, SPC-positive cells were found in the lungs of SPC-KO mice that received vav-YFPpos hematopoietic cells (n = 4; Fig. 3J).
Expression of SPC-mRNA in Lungs of SPC-KO Mice Receiving Nonhematopoietic BM Cells
As an additional, potentially more sensitive analysis for rare SPC expressing cells, we performed RT-PCR for expression of SPC-mRNA from CD31-CD45− sorted cells of SPC-KO recipient mice. Since the frequencies of SPC-positive, donor-derived cells were relatively low even in recipients of nonhematopoietic cells, nonquantitative nested PCR was used to optimize detection of SPC-mRNA. Nested primers were designed to bind in exons 2 and 3 of the wild-type SPC-mRNA so that no PCR product would be generated from the SPC-KO allele, which lacks exon 2 (Fig. 4A). No SPC mRNA was detected in SPO-KO mice transplanted with SPC-KO BM cells. SPC-mRNA was detected in sorted lung cells from SPC-KO mice receiving unfractionated WT WBM (Supporting Information Fig. S3E), as well as in the lungs of seven out of nine mice that received nonhematopoietic BM cells (Fig. 4B). SPC mRNA was also detected in the one mouse (lane five) receiving vav-YFPpos hematopoietic BM cells in which an SPC-positive cell was detected on a tissue section (Supporting Information Fig. S3A). The detection of SPC-mRNA in lungs cells of this mouse suggests that very rare SPC-expressing cells were indeed present. A weak band of SPC-PCR product was also found in another mouse (lane four) that received vav-YFPpos hematopoietic BM cells; however, no SPC-expressing cells were detected by microscopy of sorted cells and paraffin sections from this mouse. Since nested PCR efficiently amplifies even very low amounts of template, we conclude that SPC-expressing cells in this mouse were very rare, and/or expressed very low levels of protein, which were not detectable by immunofluorescence. Nested PCR did not detect any SPC-mRNA in the lungs of SPC-KO mice transplanted with purified HSPC (lanes 1-3).
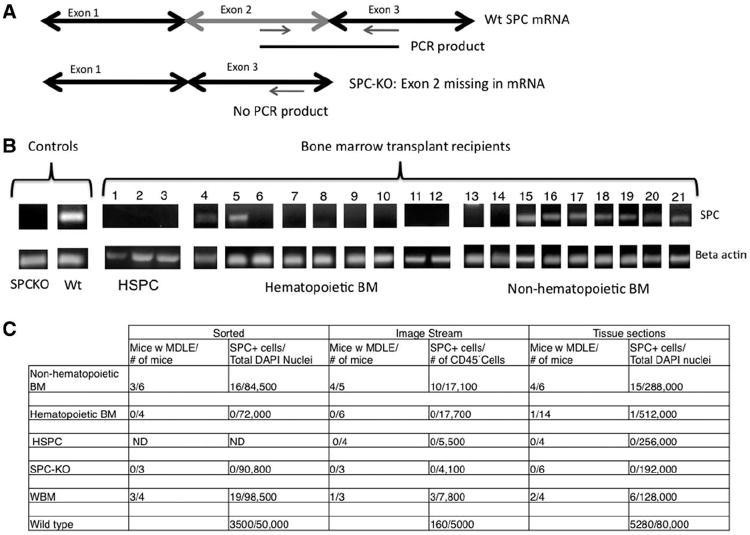
Figure 4. Detection of SPC-mRNA in SPC-KO recipients by nested PCR.
(A): Wild-type SPC-mRNA was detected by nested RT-PCR with primers designed to amplify the wild-type SPC-allele, with no PCR product generated in SPC-KO mice receiving bone marrow from SPC-KO donors. (B): SPC-mRNA was not expressed in mice receiving HSPC (lanes 1-3), and in only two out of nine mice receiving hematopoietic BM cells (lanes 4-12) but in seven out of nine lungs from SPC-KO mice receiving nonhematopoietic BM cells (lanes 13-21). (C): Total number of mice analyzed, number of SPC-positive cells detected, and number of cells analyzed with each indicated method. Total number of mice with SPC-positive cells detected (numerator) and total number of mice analyzed (denominator). Total number of SPC-positive cells detected (numerator) and total number of cells analyzed with each method (denominator). Denominators: ImageStream, CD45– intact cells; tissue sections, nucleated cells; sorted, CD45-CD31-CD11b– nucleated cells. Abbreviations: BM, bone marrow; DAPI, 4′,6-diamidino-2-phenylindole; HSPC, hematopoietic stem and progenitor cell; MDLE, marrow derived lung epithelial cells; ND, not done; PCR, polymerase chain reaction; WBM, whole bone marrow; and Wt, wild type.
To summarize the quantitative data (Fig. 4C), donor-derived type 2 pneumocytes were detected in 10 out of 12 SPC-KO mice receiving nonhematopoietic BM cells, in only 1 of 20 mice receiving hematopoietic BM cells and in 0 out of 4 mice receiving purified HSPC. On tissue sections, 0.08% of type 2 cells were donor derived in mice receiving nonhematopoietic BM cells, while only 0.0018% were donor derived in mice receiving vav-YFPpos hematopoietic BM cells. By ImageStream, 1.8% of type 2 cells were derived from nonhematopoietic BM cells, while no donor-derived cells were detected in recipients of hematopoietic cells or purified HSPC with this technique. On tissue sections, one SPC-positive cell was detected from a total of 512,000 cells analyzed from recipients of vav-YFPpos hematopoietic cells and none was found among 256,000 cells analyzed from HSPC recipients. By contrast, donor-derived SPC-positive cells were detected at a frequency of 1 in 19,200 nucleated cells on tissue from recipients of nonhematopoietic cells. In general, analysis of lung tissue sections confirmed the results obtained from the same mice by cell sorting or ImageStream. With all three approaches, donor-derived, SPC-positive cells were found in six out of seven mice receiving unfractionated WBM from wild-type donors (Supporting Information Fig. S2).
Discussion
From these findings, we conclude that nonhematopoietic BM cells are primarily responsible for the formation of marrow-derived lung epithelial cells. By contrast, the capacity of vav-YFPpos hematopoietic BM cells to form lung epithelial cells is very limited, and marrow-derived lung epithelial cells derived from the vav-YFPpos hematopoietic BM cell fraction are extremely rare. The fact that no type 2 cells were derived from purified HSPCs indicates that this cell population does not have the potential to give rise to lung epithelial cells.
Our data are consistent with two previous studies. Wagers et al. [4] reported a lack of BM-derived epithelial cells in the lungs of mice transplanted with purely hematopoietic Thy1loLineageneg Sca-1pos c-Kitpos population from the BM. By contrast, a previous study by Krause et al. [2] reported epithelial cells derived from a BM subpopulation enriched for small, lineage negative cells that are capable of homing to the BM. This population was not a purified hematopoietic stem cell population, although it did contain hematopoietic stem cell activity when cotransplanted with a short-term reconstituting population [3]. Our present study resolves the controversy that arose from these seemingly conflicting results by showing that the nonhematopoietic compartment of the BM is the primary source of BM-derived epithelial cells, and that LinnegSca-1posCD45pos cells, which are purely hematopoietic, do not give rise to lung epithelial cells.
The mechanism for engraftment of nonhematopoietic BM cells as epithelial cells is unknown, but our data support the hypothesis that stem cells contained in the nonhematopoietic fraction of the BM differentiate into lung epithelial cells. Alternatively, an epithelial progenitor population may be located in the BM [14]. Herzog et al. [15] showed that after BM transplantation with WBM, a significant fraction of marrow-derived lung epithelial cells arose from fusion events. Macrophages have been shown to be able to fuse with each other as well as other somatic cells such as hepatocytes [16-18]. However, in the present study, we used lineage depleted BM to isolate vav-YFP positive or negative cells. Lineage depleted BM does not contain macrophages or their immediate progenitors, which would otherwise be infused into the recipient at the time of acute radiation damage. On the basis of these facts and on our findings that lung epithelial cells derived from vav-YFPneg hematopoietic cells are extremely rare, and completely absent after transplantation of purified HSPC, we propose that in our study, marrow-derived lung epithelial cells arise from differentiation of an immature stem cell contained in the nonhematopoietic fraction of the BM.
The fact that we detected a single SPC-positive type 2 pneumocyte in 1 out of 20 recipients of hematopoietic BM cells, with 2 of these 20 recipients showing expression of SPC-mRNA, may be attributed to incomplete separation of YFP-positive and YFP-negative cells by FACS. With our sorting procedure, the purity of the YFP-positive fraction was approximately 96%, while the purity of the YFP-negative fraction was greater than 99% (Supporting Information Fig. S7). By ImageStream analysis, none of the SPC-positive cells was YFP-positive, no SPC-positive cells were detected in recipients of vav-YFPpos hematopoietic cells, and purified HSPC did not give rise to any SPC-positive cells. Together, these results demonstrate that hematopoietic cells do not give rise to epithelial cells.
The previously reported findings by Krause et al. [2] showed that a rare population of single BM-derived cells was able to repopulate the hematopoietic system and generate nonhematopoietic cell type in multiple tissues. However, there was no hematopoietic engraftment from the nonhematopoietic BM cell fraction in our study. In their experiments, Krause et al. [2] cotransplanted a recipient-type “rotor off” BM population that would provide only short-term radioprotection in addition to the one single BM stem cell. With this approach, the injected stem cell with the capacity to form cells of nonhematopoietic lineages may have been challenged to repopulate the hematopoietic system. By contrast, the mice receiving the nonhematopoietic BM cells in our study also received 1 million unfractionated WBM cells, which contain a high enough number of HSPC to repopulate the hematopoietic system. In this setting, stem cells contained in the nonhematopoietic fraction would lack the stimulus or challenge to differentiate toward the hematopoietic lineage. One potential nonhematopoietic BM subpopulation that can give rise to hematopoietic cells only under special circumstances are very small embryonic-stem cell like cells (VSELs) [19]. Future studies will be performed to assess whether VSELs can engraft as both hematopoietic and epithelial cells.
The number of BM-derived epithelial cells ranges between 0.08% and 1.8% of type 2 pneumocytes depending on the detection technique used. Analysis of tissue sections is the least sensitive method, as paraffin sections, while providing excellent preservation of morphology, contain high levels of autofluorescence. SPC expression levels may be lower in BM-derived type 2 cells compared to endogenous Wt cells, complicating their detection on tissue sections. Single cell analysis, especially ImageStream, allows for each cell to be analyzed independently, facilitating detection of low fluorescent, donor-derived SPC-positive cells. The single cell analysis methods used in this study allowed us to consistently and definitively detect small numbers of marrow-derived epithelial cells. With ImageStream technology, the sensitivity of detection was increased by an order of magnitude compared to tissue sections.
A previous study by Aliotta et al. [7] used 3D projections to convincingly show green fluorescent protein positive, cytokeratin positive lung epithelial cells derived from either lineage negative, Sca-1 positive or c-kit positive BM fractions. It is possible that these studies reflect epithelial differentiation from nonhematopoietic BM cells because the Lin− population contains both hematopoietic and nonhematopoietic cells, and the same is true for the Sca1-positive or c-kit-positive fractions (as CD45 was not included as a marker of hematopoietic cells). In the present study, for the first time, we definitively demonstrate that the nonhematopoietic fraction of the BM is the major source of marrow-derived epithelial cells. In addition, our technical approach is improved, as we use an epithelial cell-specific protein (SPC), which is otherwise absent from the SPC-KO recipient mice, as a marker for a marrow-derived cells adopting the phenotype of an epithelial cell. The vesicular pattern of SPC staining makes it appear different from cytoplasmic autofluorescence. In addition, because of potential autofluorescence, which can make cells appear double positive for two cytoplasmic markers especially on tissue sections, we used TTF1, a nuclear, epithelial cell-specific transcription factor, as an additional marker to identify donor-derived epithelial cells.
Summary
Our data suggest that the nonhematopoietic compartment of the BM contains cells with the capacity to differentiate into functional nonhematopoietic cell types such as lung epithelial cells. Therefore, we hypothesize that this cell type may be a rare pluripotent adult stem cell residing in the BM.
Differentiation of nonhematopoietic BM cells into lung epithelial cells in response to irradiation injury, based on the number of BM-derived epithelial cells found in the lung after 2-6 months, is likely to be too low to significantly contribute to regeneration. It is hoped that identification and characterization of the stem cell population responsible, as well as the mechanisms that govern the engraftment of these cells in nonhematopoietic tissues, may help to determine how this phenomenon can be exploited to enhance tissue regeneration and repair.
Supplementary Material
Acknowledgments
We thank Erica Herzog for helpful discussions and technical advice; Gouzel Tokmoulina for expert operation of the MoFlo cell sorter; Oriana Fisher for assistance with PCR; and Stephanie Donaldson for assistance with animal care and breeding. Sharon Lin is acknowledged for performing mouse irradiation. SHK was supported in part by a Brown-Coxe postdoctoral fellowship. This work was supported by the following NIH grants: R01-HL073742, R01-DK61846, P30-CA16359, and P30-DK072442. Cell sorting and ImageStream analysis was performed at the Yale School of Medicine Cell Sorter Core Facility.
Footnotes
Author contributions: S.K.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; E.B.: data analysis and interpretation and final approval of manuscript; P.-X.Z.: collection of data; and D.K.: conception and design, financial support, assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript.
See www.StemCells.com for supporting information available online.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22:487–500. doi: 10.1634/stemcells.22-4-487. [DOI] [PubMed] [Google Scholar]
- 2.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 3.Lanzkron SM, Collector MI, Sharkis SJ. Homing of long-term and short-term engrafting cells in vivo. Ann N Y Acad Sci. 1999;872:48–54. doi: 10.1111/j.1749-6632.1999.tb08452.x. discussion 54–46. [DOI] [PubMed] [Google Scholar]
- 4.Wagers AJ, Sherwood RI, Christensen JL, et al. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 5.Herzog EL, Van Arnam J, Hu B, et al. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells. 2006;24:1986–1992. doi: 10.1634/stemcells.2005-0579. [DOI] [PubMed] [Google Scholar]
- 6.MacPherson H, Keir PA, Edwards CJ, et al. Following damage, the majority of bone marrow-derived airway cells express an epithelial marker. Respir Res. 2006;7:145. doi: 10.1186/1465-9921-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aliotta JM, Keaney P, Passero M, et al. Bone marrow production of lung cells: The impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotype. Exp Hematol. 2006;34:230–241. doi: 10.1016/j.exphem.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassmer SH, Krause DS. Detection of bone marrow-derived lung epithelial cells. Exp Hematol. 2010;38:564–573. doi: 10.1016/j.exphem.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stadtfeld M, Graf T. Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non-invasive lineage tracing. Development. 2005;132:203–213. doi: 10.1242/dev.01558. [DOI] [PubMed] [Google Scholar]
- 10.Glasser SW, Burhans MS, Korfhagen TR, et al. Altered stability of pulmonary surfactant in SP-C-deficient mice. Proc Natl Acad Sci USA. 2001;98:6366–6371. doi: 10.1073/pnas.101500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 12.Katzav S, Martin-Zanca D, Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989;8:2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu P, Margolis B, Schlessinger J. Vav: A potential link between tyrosine kinases and ras-like GTPases in hematopoietic cell signaling. Bioessays. 1993;15:179–183. doi: 10.1002/bies.950150306. [DOI] [PubMed] [Google Scholar]
- 14.Wong AP, Keating A, Lu WY, et al. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009;119:336–348. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herzog EL, Van Arnam J, Hu B, et al. Lung-specific nuclear reprogramming is accompanied by heterokaryon formation and Y chromosome loss following bone marrow transplantation and secondary inflammation. FASEB J. 2007;21:2592–2601. doi: 10.1096/fj.06-7861com. [DOI] [PubMed] [Google Scholar]
- 16.Vignery A. Macrophage fusion: Are somatic and cancer cells possible partners? Trends Cell Biol. 2005;15:188–193. doi: 10.1016/j.tcb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Willenbring H, Bailey AS, Foster M, et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med. 2004;10:744–748. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- 18.Camargo FD, Finegold M, Goodell MA. Hematopoietic myelomonocytic cells are the major source of hepatocyte fusion partners. J Clin Invest. 2004;113:1266–1270. doi: 10.1172/JCI21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratajczak J, Wysoczynski M, Zuba-Surma E, et al. Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Exp Hematol. 2011;39:225–237. doi: 10.1016/j.exphem.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.