Abstract
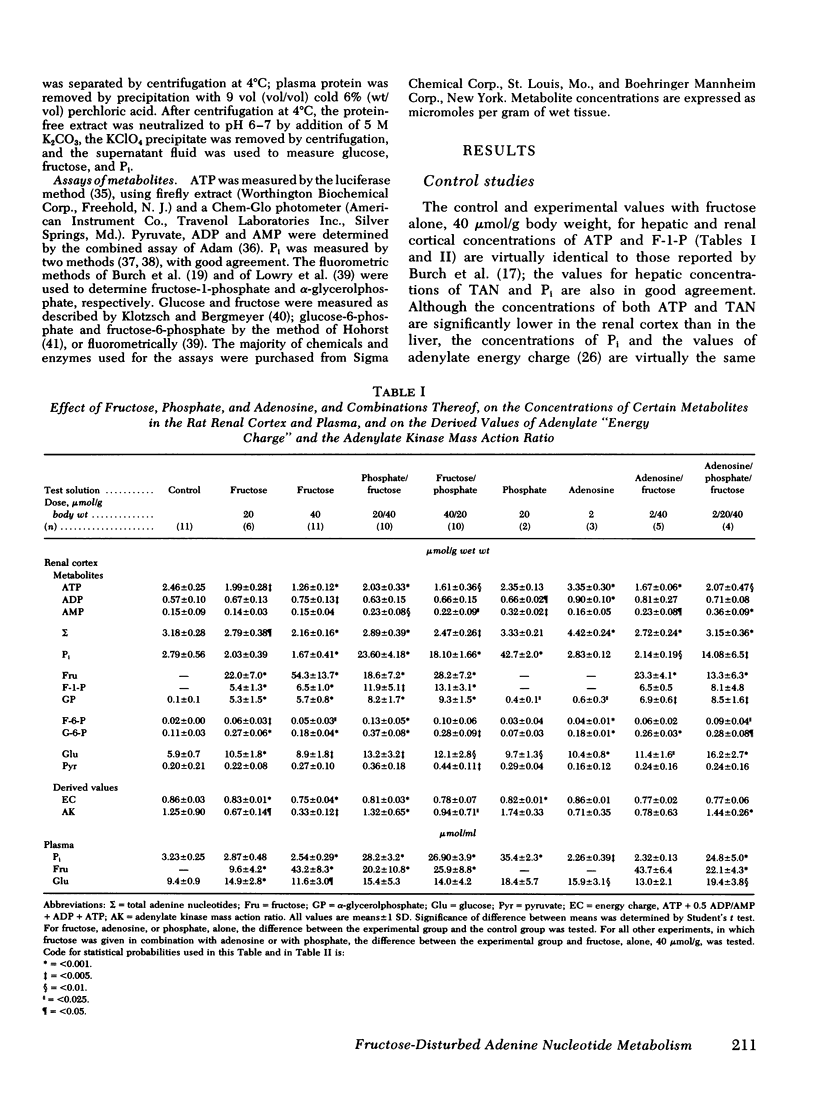
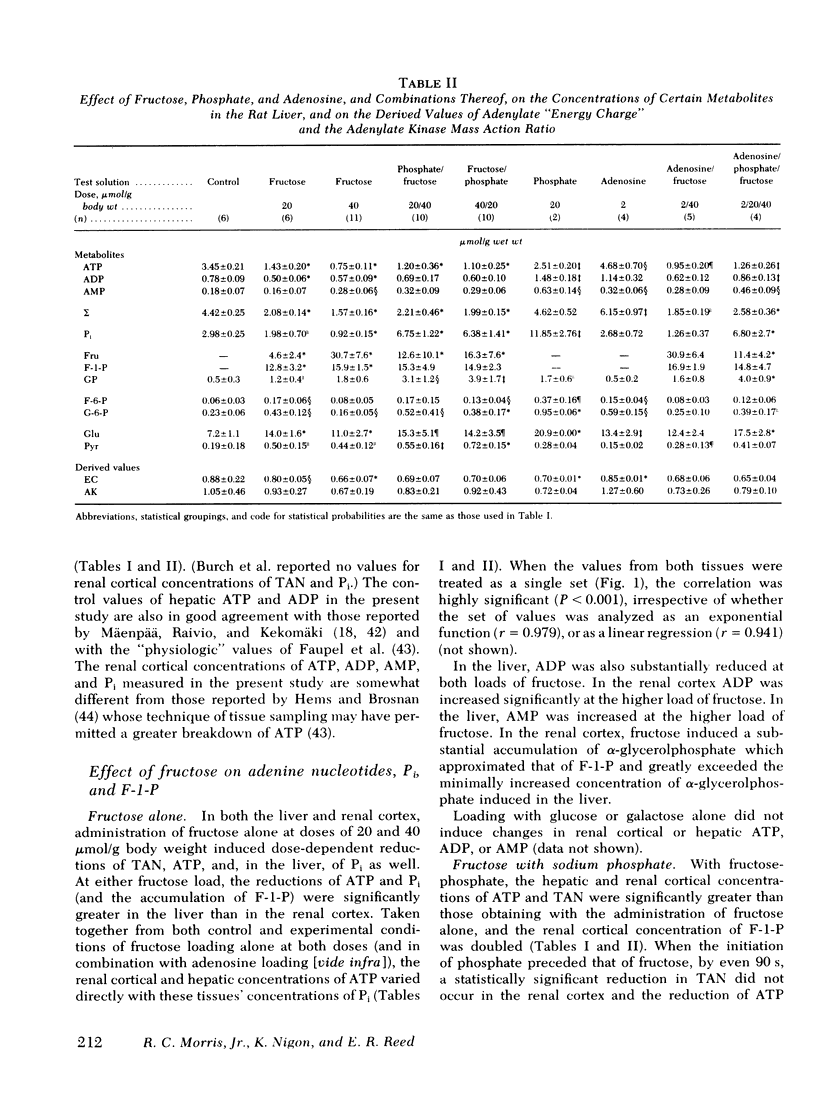
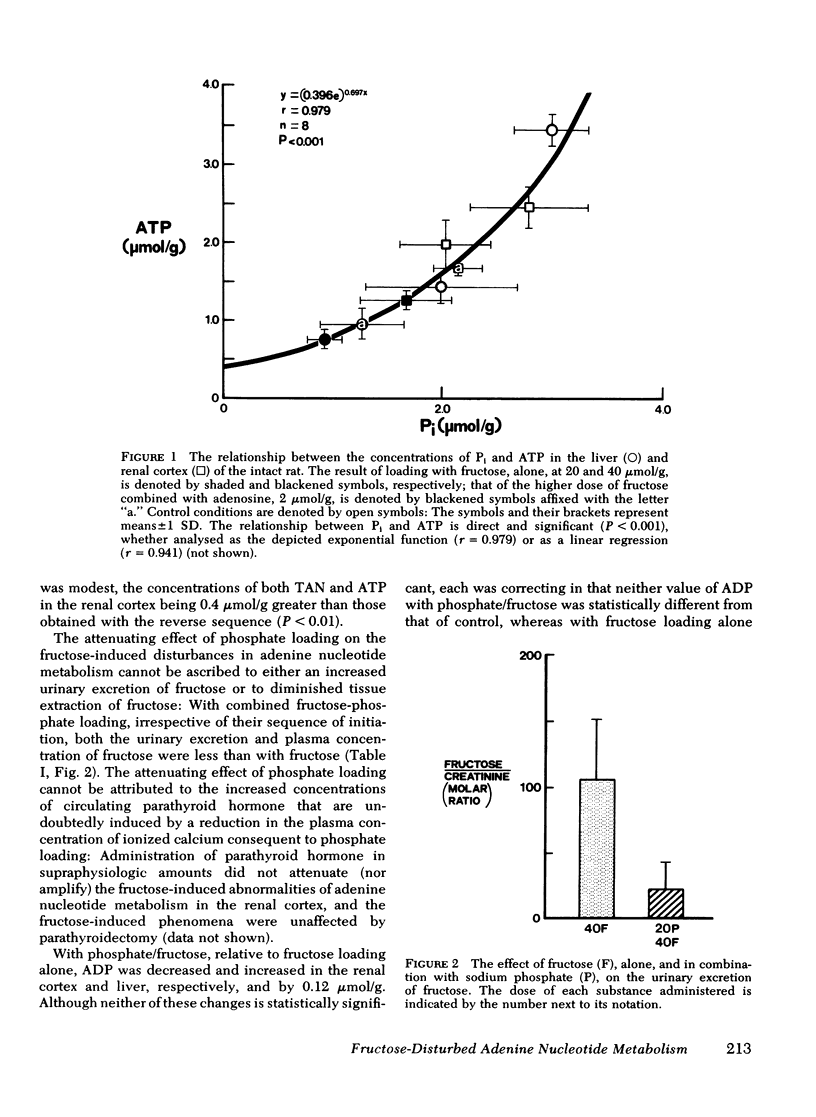
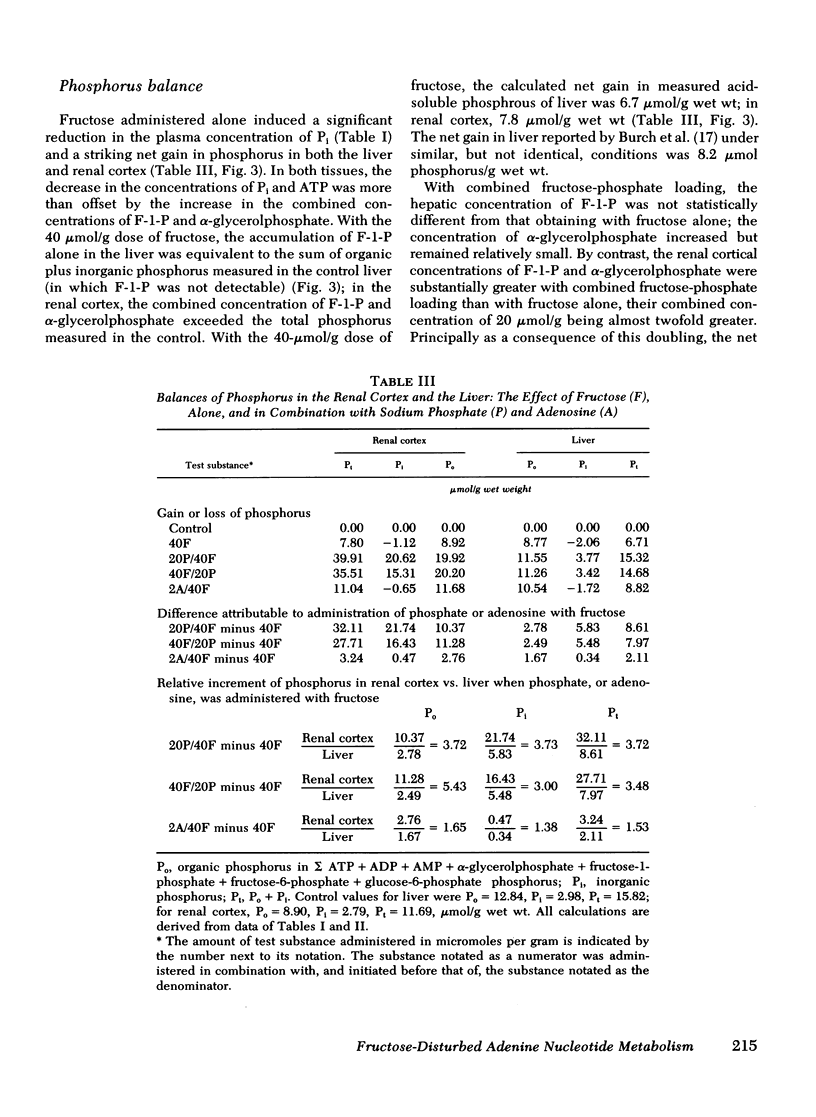
To test the hypothesis that in both the liver and renal cortex of the fructose-loaded rat, severity of depletion of inorganic phosphate (Pi), and not the magnitude of accumulation of fructose-1-phosphate (F-1-P), determines the severity of the dose-dependent reduction of ATP, we intraperitoneally injected fed rats with fructose, 20 and 40 μmol/g, alone, and at the higher load, in combination with (a) sodium phosphate, 20 μmol/g, administered shortly beforehand or subsequently or, (b) adenosine, 2 μmol/g, administered beforehand. The following observations were made: (a) With fructose loading alone, at the higher load, both Pi and total adenine nucleotides (TAN) were reduced by one third in the renal cortex and (as previously observed) by two thirds in the liver; and at either load, the reduction of ATP (and TAN) and the accumulation of F-1-P were less severe in the renal cortex than in the liver. (b) Prior phosphate loading largely prevented the reductions of ATP and TAN in the renal cortex and significantly attenuated them in the liver, yet doubled the renal cortical accumulation of F-1-P. (c) Adenosine loading substantially attenuated the reductions of ATP, TAN, and Pi only in the renal cortex. (d) ATP varied directly with Pi (P < 0.001, r = 0.98) in the domain of control and reduced values of Pi taken from both liver and renal cortex. (e) As judged from tissue and plasma concentrations of fructose and glucose, and tissue concentrations of fructose-6-phosphate and glucose-6-phosphate, the rate at which the renal cortex and liver converted fructose to glucose was much lower at the higher fructose load. (f) Prior phosphate loading prevented this decrease in rate in the renal cortex and attenuated it in the liver; adenosine loading attenuated it only in the renal cortex. We conclude that in both the renal cortex of the fructose-loaded rat: (a) Depletion of Pi is critical to the causation of the reductions in both ATP and TAN and, at the higher fructose load, of a decrease in the rate at which ATP is regenerated. (b) The severity of depletion of Pi determines the severity of these disturbances. (c) By differentially mitigating the depletion of Pi, prior phosphate loading largely prevents these disturbances in the renal cortex, and attenuates them in the liver; and adenosine loading attenuates them only in the renal cortex.
The findings provide some basis for the observation that in patients with hereditary fructose intolerance experimentally exposed to fructose, prior loading with sodium phosphate substantially attenuates the renal but not hepatic dysfunction.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman R. C., Ballard F. J., Weinhouse S. Purification and properties of rat liver fructokinase. J Biol Chem. 1967 Jul 25;242(14):3360–3365. [PubMed] [Google Scholar]
- Bode J. C., Zelder O., Rumpelt H. J., Wittkamp U. Depletion of liver adenosine phosphates and metabolic effects of intravenous infusion of fructose or sorbitol in man and in the rat. Eur J Clin Invest. 1973 Sep;3(5):436–441. doi: 10.1111/j.1365-2362.1973.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Bogusky R. T., Lowenstein L. M., Lowenstein J. M. The purine nucleotide cycle. A pathway for ammonia production in the rat kidney. J Clin Invest. 1976 Aug;58(2):326–335. doi: 10.1172/JCI108476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch H. B., Lowry O. H., Meinhardt L., Max P., Jr, Chyu K. Effect of fructose, dihydroxyacetone, glycerol, and glucose on metabolites and related compounds in liver and kidney. J Biol Chem. 1970 Apr 25;245(8):2092–2102. [PubMed] [Google Scholar]
- Chagoya de Sánchez V., Brunner A., Piña E. In vivo modification of the energy charge in the liver cell. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1441–1445. doi: 10.1016/s0006-291x(72)80138-4. [DOI] [PubMed] [Google Scholar]
- Chagoya de Sánchez V., Brunner A., Sánchez M. E., López C., Piña E. Utilization of adenosine as a tool in studies on the regulation of liver glycogen biosynthesis. Arch Biochem Biophys. 1974 Jan;160(1):145–150. doi: 10.1016/s0003-9861(74)80019-6. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Stabilization of adenylate energy charge by the adenylate deaminase reaction. J Biol Chem. 1973 Dec 10;248(23):8309–8312. [PubMed] [Google Scholar]
- Desbuquois B., Lardinois R., Gentil C., Odievre M. Effects d'une surcharge en phosphate de sodium sur l'hypoglucosémie dans onze observations d'intolérance héréditaire au fructose. Arch Fr Pediatr. 1969 Jan;26(1):21–35. [PubMed] [Google Scholar]
- Faupel R. P., Seitz H. J., Tarnowski W., Thiemann V., Weiss C. The problem of tissue sampling from experimental animals with respect to freezing technique, anoxia, stress and narcosis. A new method for sampling rat liver tissue and the physiological values of glycolytic intermediates and related compounds. Arch Biochem Biophys. 1972 Feb;148(2):509–522. doi: 10.1016/0003-9861(72)90170-1. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism. 1972 Aug;21(8):713–721. doi: 10.1016/0026-0495(72)90120-5. [DOI] [PubMed] [Google Scholar]
- HERS H. G., JOASSIN G. [Anomaly of hepatic aldolase in intolerance to fructose]. Enzymol Biol Clin (Basel) 1961;1:4–14. [PubMed] [Google Scholar]
- HERS H. G. The conversion of fructose-1-C14 and sorbitol-1-C14 to liver and muscle glycogen in the rat. J Biol Chem. 1955 May;214(1):373–381. [PubMed] [Google Scholar]
- Hassinen I., Ylikahri R. H. Absorption spectrophotometry of perfused rat liver applied to fructose-induced inhibition of respiration. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1091–1097. doi: 10.1016/0006-291x(70)90351-7. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970 Nov;120(1):105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuckenkamp P. U., Zöllner N. Fructose-induced hyperuricaemia. Lancet. 1971 Apr 17;1(7703):808–809. doi: 10.1016/s0140-6736(71)91259-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann N., Thees M., Kinne R. Phosphate transport by isolated renal brush border vesicles. Pflugers Arch. 1976 Mar 30;362(2):147–156. doi: 10.1007/BF00583641. [DOI] [PubMed] [Google Scholar]
- Hultman E., Nilsson L. H., Sahlin K. Adenine nucleotide content of human liver. Normal values and fructose-induced depletion. Scand J Clin Lab Invest. 1975 May;35(3):245–251. doi: 10.1080/00365517509095736. [DOI] [PubMed] [Google Scholar]
- Kaufmann U., Froesch E. R. Inhibition of phosphorylase-a by fructose-1-phosphate, alpha-glycerophosphate and fructose-1,6-diphosphate: explanation for fructose-induced hypoglycaemia in hereditary fructose intolerance and fructose-1,6-diphosphatase deficiency. Eur J Clin Invest. 1973 Sep;3(5):407–413. doi: 10.1111/j.1365-2362.1973.tb02208.x. [DOI] [PubMed] [Google Scholar]
- Kranhold J. F., Loh D., Morris R. C., Jr Renal fructose-metabolizing enzymes: significance in hereditary fructose intolerance. Science. 1969 Jul 25;165(3891):402–403. doi: 10.1126/science.165.3891.402. [DOI] [PubMed] [Google Scholar]
- LEE J. B., VANCE V. K., CAHILL G. F., Jr Metabolism of C14-labeled substrates by rabbit kidney cortex and medulla. Am J Physiol. 1962 Jul;203:27–36. doi: 10.1152/ajplegacy.1962.203.1.27. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Landau B. R., Marshall J. S., Craig J. W., Hostetler K. Y., Genuth S. M. Quantitation of the pathways of fructose metabolism in normal and fructose-intolerant subjects. J Lab Clin Med. 1971 Oct;78(4):608–618. [PubMed] [Google Scholar]
- Lund P., Cornell N. W., Krebs H. A. Effect of adenosine on the adenine nucleotide content and metabolism of hepatocytes. Biochem J. 1975 Dec;152(3):593–599. doi: 10.1042/bj1520593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. C., Jr An experimental renal acidification defect in patients with hereditary fructose intolerance. I. Its resemblance to renal tubular acidosis. J Clin Invest. 1968 Jun;47(6):1389–1398. doi: 10.1172/JCI105830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. C., Jr An experimental renal acidification defect in patients with hereditary fructose intolerance. II. Its distinction from classic renal tubular acidosis; its resemblance to the renal acidification defect associated with the Fanconi syndrome of children with cystinosis. J Clin Invest. 1968 Jul;47(7):1648–1663. doi: 10.1172/JCI105856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. C., Jr, McSherry E. Symposium on acid-base homeostasis. Renal acidosis. Kidney Int. 1972 May;1(5):322–340. doi: 10.1038/ki.1972.44. [DOI] [PubMed] [Google Scholar]
- Morris R. C., Jun, Ueki I., Loh D., Eanes R. Z., McLin P. Absence of renal fructose-1-phosphate aldolase activity in hereditary fructose intolerance. Nature. 1967 May 27;214(5091):920–921. doi: 10.1038/214920b0. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H., Raivio K. O., Kekomäki M. P. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968 Sep 20;161(3847):1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- NIKIFORUK G., COLOWICK S. P. The purification and properties of 5-adenylic acid deaminase from muscle. J Biol Chem. 1956 Mar;219(1):119–129. [PubMed] [Google Scholar]
- Nisell J., Lindén L. Fructose-1-phosphate aldolase and fructose-1-6-diphosphate aldolase activity in the mucosa of the intestine in hereditary fructose intolerance. Scand J Gastroenterol. 1968;3(1):80–82. doi: 10.3109/00365526809180103. [DOI] [PubMed] [Google Scholar]
- Nivelon J. L., Mathieu M., Kissin C., Collombel C., Cotte J., Béthenod M. Intolérance au fructose. Observation et mécanisme physiopathologique de l'hypoglucosémie. Ann Pediatr (Paris) 1967 Dec 2;14(12):817–828. [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Watanabe T. Isozymes of rat AMP deaminase. Biochim Biophys Acta. 1975 Oct 22;403(2):530–537. doi: 10.1016/0005-2744(75)90081-9. [DOI] [PubMed] [Google Scholar]
- PARKS R. E., Jr, BEN-GERSHOM E., LARDY H. A. Liver fructokinase. J Biol Chem. 1957 Jul;227(1):231–242. [PubMed] [Google Scholar]
- Perheentupa J., Raivio K. O., Nikkilä E. A. Hereditary fructose intolerance. Acta Med Scand Suppl. 1972;542:65–75. doi: 10.1111/j.0954-6820.1972.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Perheentupa J., Raivio K. Fructose-induced hyperuricaemia. Lancet. 1967 Sep 9;2(7515):528–531. doi: 10.1016/s0140-6736(67)90494-1. [DOI] [PubMed] [Google Scholar]
- Raivio K. O., Becker 7. A., Meyer L. J., Greene M. L., Nuki G., Seegmiller J. E. Stimulation of human purine synthesis de novo by fructose infusion. Metabolism. 1975 Jul;24(7):861–869. doi: 10.1016/0026-0495(75)90133-x. [DOI] [PubMed] [Google Scholar]
- Raivio K. O., Kekomäki M. P., Mäenpä P. H. Depletion of liver adenine nucleotides induced by D-fructose. Dose-dependence and specificity of the fructose effect. Biochem Pharmacol. 1969 Oct;18(10):2615–2624. doi: 10.1016/0006-2952(69)90192-0. [DOI] [PubMed] [Google Scholar]
- Rambaud P., Joannard A., Bost M., Marchal A., Rachail M., Roget J. Trouble de la glycogénolyse dans l'intolerance héréditaire au fructose. Etude de deux observations chez l'enfant. Arch Fr Pediatr. 1973 Dec;30(10):1051–1062. [PubMed] [Google Scholar]
- SALOMON L. L., LANZA F. L., SMITH D. E. Renal conversion of fructose to glucose. Am J Physiol. 1961 Apr;200:871–877. doi: 10.1152/ajplegacy.1961.200.4.871. [DOI] [PubMed] [Google Scholar]
- STREHLER B. L., TOTTER J. R. Determination of ATP and related compounds: firefly luminescence and other methods. Methods Biochem Anal. 1954;1:341–356. doi: 10.1002/9780470110171.ch13. [DOI] [PubMed] [Google Scholar]
- Sestoft L. Regulation of fructose metabolism in the perfused rat liver. Interrelation with inorganic phosphate, glucose, ketone body and ethanol metabolism. Biochim Biophys Acta. 1974 Mar 20;343(1):1–16. doi: 10.1016/0304-4165(74)90235-9. [DOI] [PubMed] [Google Scholar]
- Sánchez J. J., González N. S., Pontis H. G. Fructokinase from rat liver. II. The role of K+ on the enzyme activity. Biochim Biophys Acta. 1971 Jan 13;227(1):79–85. doi: 10.1016/0005-2744(71)90169-0. [DOI] [PubMed] [Google Scholar]
- Thurston J. H., Jones E. M., Hauhart R. E. Decrease and inhibition of liver glycogen phosphorylase after fructose. An experimental model for the study of hereditary fructose intolerance. Diabetes. 1974 Jul;23(7):597–604. doi: 10.2337/diab.23.7.597. [DOI] [PubMed] [Google Scholar]
- Till U., Brox D., Frunder H. Orthophosphate turnover in the extracellular and intracellular space of mouse liver. Eur J Biochem. 1969 Dec;11(3):541–548. doi: 10.1111/j.1432-1033.1969.tb00806.x. [DOI] [PubMed] [Google Scholar]
- Van Den Berghe G., Hue L., Hers H. G. Effect of administration of the fructose on the glycogenolytic action of glucagon. An investigation of the pathogeny of hereditary fructose intolerance. Biochem J. 1973 Jun;134(2):637–645. doi: 10.1042/bj1340637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening J., Nowack J., Decker K. The dependence of glucose formation from lactate on the adenosine triphosphate content in the isolated perfused rat liver. Biochim Biophys Acta. 1975 Jun 12;392(2):299–309. doi: 10.1016/0304-4165(75)90011-2. [DOI] [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods H. F. Hepatic accumulation of metabolites after fructose loading. Acta Med Scand Suppl. 1972;542:87–103. doi: 10.1111/j.0954-6820.1972.tb05322.x. [DOI] [PubMed] [Google Scholar]
- Zalitis J., Oliver I. T. Inhibition of glucose phosphate isomerase by metabolic intermediates of fructose. Biochem J. 1967 Mar;102(3):753–759. doi: 10.1042/bj1020753. [DOI] [PMC free article] [PubMed] [Google Scholar]



