Abstract
Esophageal adenocarcinoma is preceded by the development of reflux-related intestinal metaplasia or Barrett’s esophagus which is a response to inflammation of the esophageal squamous mucosa, reflux esophagitis. Gastroesophageal reflux impairs the mucosal barrier in the distal esophagus, allowing chronic exposure of the squamous epithelium to the diverse microbial ecosystem or microbiome, and inducing chronic inflammation. The esophageal microbiome is altered in both esophagitis and Barrett's esophagus, characterized by a significant decrease in Gram-positive bacteria and an increase in Gram-negative bacteria in esophagitis and Barrett's esophagus. Lipopolysaccharides (LPS), a major structure of the outer membrane in Gram-negative bacteria, can up-regulate gene expression of proinflammatory cytokines via activation of the TLR4 and NF-kB pathway. The potential impact of LPS on reflux esophagitis may be through relaxation of the lower esophageal sphincter via iNOS and by delaying gastric emptying via COX-2. Chronic inflammation may be play a critical role in the progression from benign to malignant esophageal disease. Therefore analysis of the pathways leading to chronic inflammation in the esophagus may help to identify biomarkers in Barrett's esophagus patients for neoplastic progression and provide insight into molecular events suitable for therapeutic intervention in prevention of esophageal adenocarcinoma development in patients with reflux esophagitis and Barrett's esophagus.
Keywords: Barrett's Esophagus, esophagitis, adenocarcinoma, microbiome, inflammation
BACKGROUND
Esophageal adenocarcinoma, the malignant transformation at the end of a spectrum of diseases related to gastroesophageal reflux, is now the most rapidly increasing cancer in the Western world. Barrett’s esophagus is defined as the metaplastic columnar epithelium that replaces squamous mucosa and predisposes to cancer development (1). The rate of progression from Barrett’s esophagus to esophageal adenocarcinoma is approximately 0.12–0.4% per patient-year (2–4). For unclear reasons the incidence of esophageal adenocarcinoma in the US has risen approximately 600% since the 1970s (5–7), and since its development is not universal among patients with Barrett’s esophagus, it is important to understand and to gauge the factors that influence risk of progression to dysplasia and cancer. The current review aims to highlight the new insights into mechanisms underlying host-bacterium interaction in the context of reflux-induced inflammation and esophageal carcinogenesis. In particular the influence of microbial lipopolysaccharides (LPS) on the molecular pathways involved in inflammation-associated esophageal tumorigenesis will be examined.
Microbiome alteration in GERD
Microbiome
The term “microbiome”, coined by Joshua Lederberg, refers to the collection of all members in a complex microbial community (8). The host relationship with the microbiome can be commensal, symbiotic, or pathogenic. Bacterial mutualists within the gastrointestinal tract aid digestion benefit from the host as they assist in the synthesis of vitamins, promote development of the gut immune system, and provide competitive barriers to pathogen invasion. This complex microbial population influences an estimated 10% of all metabolites in our body (9). In return the host provides bacteria with safe housing and food during lean times. In order for this symbiotic relationship to be sustained, the immune system has to balance permissive, tolerogenic responses to food antigens and commensal microbes with potentially damaging, inflammatory responses to ward off pathogens. This delicate balance is maintained by the constant interplay among the microbiome, the gastrointestinal barrier, and the mucosal immune system, which is a prerequisite for normal gut homeostasis. Imbalance of this system may lead to innate immune (inflammation) and adaptive immune (infectious pathology) responses.
Microbiome alteration in Barrett’s esophagus
Reflux esophagitis and Barrett's esophagus represent phenotypes of inflammation of the esophageal mucosa induced by long-term gastric acid and bile reflux into the esophagus. The gastroesophageal reflux impairs the mucosal barrier and exposes the squamous epithelium and lamina propria to 1) the microbes swallowed from the oral cavity, colonized in the esophagus, and regurgitated from the stomach, 2) acidic gastric contents, and 3) bile from the duodenum.
A recent study of human distal esophageal microbiome linked inflammation and Barrett's esophagus to the change in the microbiome. The study used 16S rRNA gene survey to characterize the bacterial communities in biopsy samples taken from the distal esophagus (10). With an unsupervised approach, samples of the microbiome form two distinct clusters or two microbiome types, type I and II, based on combined genetic distance among samples. Although neither of the two types of clusters correlated exclusively with esophageal phenotypes, the type I microbiome is more closely associated with normal esophagus (11/12, 91.7%), whereas the type II microbiome is mainly associated with abnormal esophagus (13/22, 59.1%) (p=0.0173 among group comparison), including both esophagitis (7/12, 58.3%, odds radio=15.4) and Barrett's esophagus (6/10, 60.0%, odds radio=16.5). Thus alteration of the microbiome from type I to type II in the distal esophagitis is associated with host phenotypes and its disease progression. The type I microbiome is dominated by Gram-positive bacteria representing the Firmicutes phylum. In contrast the type II microbiomes composed of larger numbers of Gram-negative bacteria in phyla Bacteroidetes, Proteobacteria, Fusobacteria, and Spirochaetes. Streptococcus is the most dominant genus in the esophageal microbiome and its relative abundance is significantly higher in the type I microbiome (78.8%) than in the type II microbiome (30%). In the type II microbiome the decrease in the relative abundance of Streptococcus is compensated by an increase in the relative abundance of 24 other genera. Specifically, the most prominent increase involves Veillonella, Prevotella, Haemophilus, Neisseria, Rothia, Granulicatella, Campylobacter, Porphyromonas, Fusobacterium, and Actinomyces, many of which are Gram-negative anaerobes or microaerophiles and are putative pathogens for periodontal disease. Overall, Gram-negative bacteria comprise 53.4% of type II microbiome but only 14.9% of type I microbiome.
The type II microbiome with its larger content of Gram-negative bacteria might engage innate immune functions of the epithelial cells in a different way than the type I microbiome, owing to their production of a larger amounts of Gram-negative microbial components, for instance LPS. The bacterial products may directly or indirectly stimulate pattern receptors (i.e., Toll-like receptors) in the epithelial or inflammatory cells to promote expression of proinflammatory cytokines and persistent innate immune responses in the esophagus. Since many of the periodontal pathogens in the type II microbiome are known to cause inflammation in the mouth, it is plausible that they may similarly contribute to the development and maintenance of chronic inflammation in the esophagus. The change from microbiome type I to type II might thus prove to be an important step in the pathogenesis of esophageal tumorigenesis in progression of reflux esophagitis and Barrett's esophagus and development of esophageal adenocarcinoma.
CLINICAL-TRANSLATIONAL ADVANCES
Potential clinical applications
The current strategy for Barrett’s esophagus screening and surveillance may not be cost-effective and has not been shown to reduce esophageal adenocarcinoma incidence or mortality (4, 11). Indeed, data regarding risk factors for Barrett’s esophagus have not been systematically applied to screening guidelines, and the current state of the art for screening focuses primarily on endoscopic evaluation of individuals with chronic reflux symptoms. A surveillance interval of 3–5 years has been suggested for individuals without dysplasia, 6–12 months for low grade dysplasia, and every 3 months for high grade dysplasia (1). Much investigation is currently underway to identify prognostic biomarkers that may determine the best diagnostic and therapeutic course in esophageal adenocarcinoma.
Lipopolysaccharides induces the signaling and modulates cytokine production
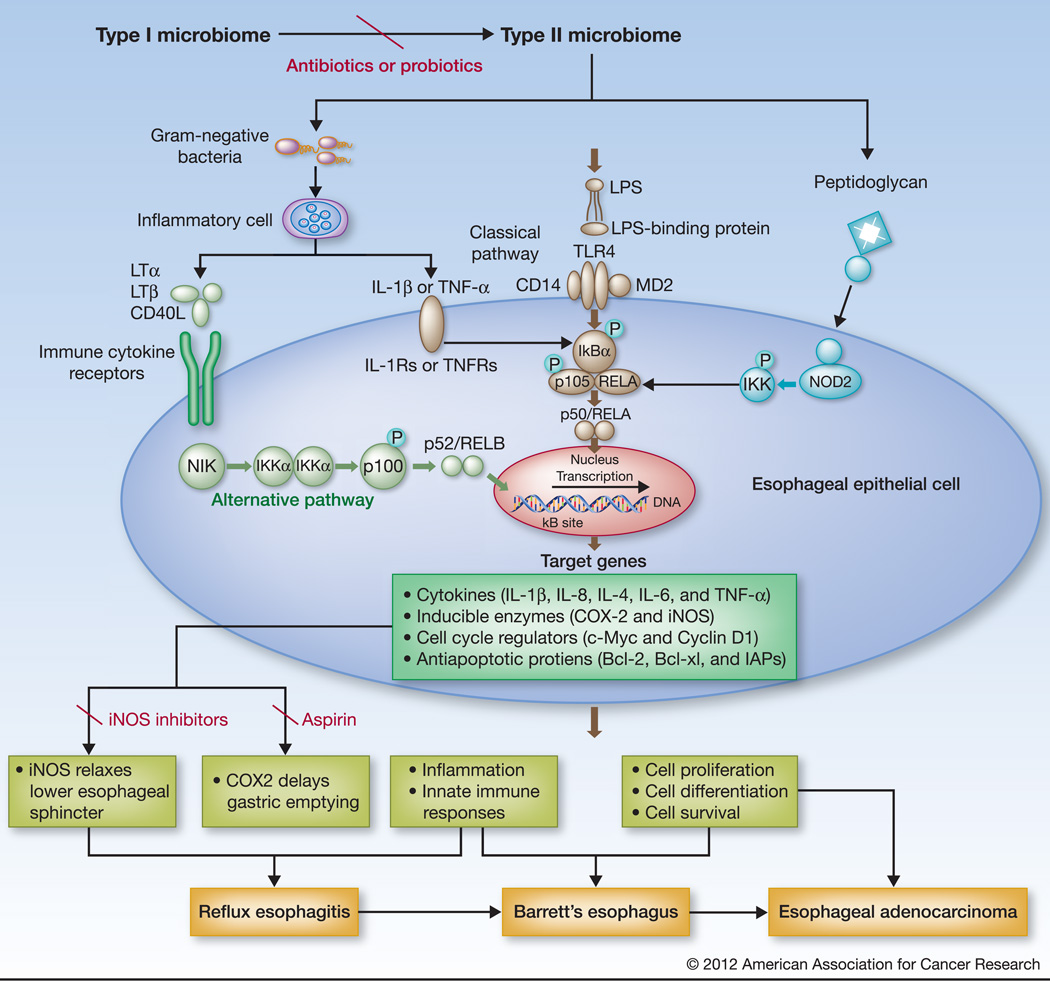
LPS, the major outer membrane component present in Gram-negative bacteria, consists of a lipid core and polysaccharide side chain joined by a covalent bond. LPS acts as the prototypical endotoxin to promote the secretion of pro-inflammatory cytokines in many cell types. Host responses to Gram-negative LPS are mediated mainly through activation of toll-like receptor 4 (TLR4) (Figure 1). LPS molecules first bind plasma derived LPS-binding protein and then interact with cluster of differentiation 14 (CD14) expressed mainly by macrophages, neutrophil granulocytes, dendritic cells, and local gastrointestinal epithelial cells to form a ternary complex, LPS: LPS-binding protein:CD14, which further transfers LPS to TLR4 accessory protein MD2 complex (12). This leads to the activation of TLR4 and the downstream nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway to evoke an inflammatory response. LPS might indirectly activate the NF-κB pathway of the epithelial cells by inducing inflammatory cells to produce interleukin-1 beta (IL-1β), or tumor necrosis factors-alpha (TNF-α) that may then engage cytokine receptor(s) through alternative pathway (non-canonical)(13–14)(Figure 1).
Figure 1.
Hypothetical activation of NF-kB pathway by type II microbiome in the esophagus. The type I microbiome, more closely associated with normal esophagus, is dominated by Gram-positive bacteria, while the type II microbiome, mainly associated with abnormal esophagus, including reflux esophagitis and Barrett's esophagus, contains a larger proportion of Gram-negative bacteria. The increased Gram-negative bacterial components, such as LPS might thus directly stimulate TLRs (mainly TLR4) leading to activation of the classical NF-kB pathway (p50/RELA; middle); LPS might also stimulate inflammatory cells, like macrophages, to release cytokines that bind to cytokine receptors on esophageal epithelial cells to trigger the alternative NF-B pathway (p52/RelB; left). Moreover, Peptidoglycan from Gram-negative bacteria might act on NOD-like receptors to activate the NF-kB pathway (right). NF-kB activation up-regulates the expression of its downstream genes encoding a variety of cytokines, inducible enzymes, and proteins that provoke inflammation, relaxes smooth muscles, and regulate cell proliferation and apoptosis. Known NF-kB regulated enzymes include iNOS that relaxes the lower esophageal sphincter and COX2 that delays gastric emptying. The end effects could be the induction of gastroesophageal reflux, metaplasia, and/or neoplasia. The effect could be prevented by reversion of the type II to type I microbiome using antibiotics or probiotics, NF-kB inhibitors, or selective inhibitors to iNOS or COX2 (Aspirin).
NF-κB activation engages inflammation to cancer in the distal esophagus
NF-κB activation is important in the initial cellular response to chemical, bacterial, or viral stimuli. It is a major transcription factor that regulates genes responsible for both the innate and adaptive immune response. It is normally predominantly located in the cytoplasm, but translocates to the nucleus upon activation. While the normal esophagus has no detectable active NF-κB, high levels of active NF-κB are found in esophageal adenocarcinoma in the setting of reduced levels of IκB-α (a known inhibitor of NF-kB) (15). There is a stepwise increase in the activation of NF-κB pathway along the spectrum of reflux esophagitis (16–17), Barrett’s epithelium (18–19), and adenocarcinoma (16, 19–20), parallel to an increase in IL-1β, IL-6, IL-8, and TNF-α.
NF-κB Activation
The NF-κB pathway can be triggered by exposingcells to LPS from Gram-negative bacteria, Peptidoglycan from Gram-positive bacteria, inflammatory cytokines (such as TNF-α or IL-1β), or by other physiological and non physiological stimuli (Figure 1).Microbial components activate the NF-κB pathway via Toll-like receptors (TLRs) signaling through the classical pathway (canonical), while cytokines via cytokine receptors (such as IL-1Rs, TNFRs, and other TNFR-like receptors) through the alternative pathway (non-canonical)(21). Peptidoglycan can also activate the NF-κB pathway by stimulating the Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) 1 (22–23) and 2 (24–26). Moreover, the observations of NF-κB activation in responses to inflammatory signaling through mutated NOD2 gene in Crohn’s disease (24–25) and through NOD1 in infection with Helicobacter pylori (23) and Chlamydophila pneumoniae (22) raise some interesting possibilities in relation to human cancers developed in these inflammatory diseases. Despite evidence of TLRs 1, 2, 3, 4, 5, 7, 9 expression in human esophageal epithelial cells (27–28), their degree of expression varies among individuals and their roles in NF-kB activation in reflux disorders remain poorly defined.
NF-κB regulated genes in reflux disorders
NF-κB is important in reflux disorders because of its broad role in up-regulating its downstream target gene expressions involved in inflammation, innate immune responses, adaptive immune responses, apoptosis blocking, cell proliferation, and cell differentiation. It directly contributes to innate immune responses in reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma, and may ultimately determine the rate of progression to esophageal adenocarcinoma. NF-κB-regulated pro-inflammatory cytokines, IL-1β, IL-8 are stepwise increased in reflux esophagitis, Barrett's esophagus towards esophageal adenocarcinoma (14, 18, 24), and IL-4, IL-6 are also increased in reflux esophagitis and Barrett's esophagus (14, 19). It is notable that Fitzgerald et al. found no difference in the levels of IL-1β and IL-8 between non-inflamed squamous mucosa and Barrett's esophagus in the same patient (29). The secreted cytokines, including TNF and IL-1β, may also start a feedback loop for a second phase of NF-κB activation that continues the induction of robust innate immune responses. The cellular pattern recognition receptors (PRRs) such as TLRs, retinoic acid-inducible gene Ilike receptors (RLRs) and NOD-like receptors, which all sense microbial opportunistic pathogens or pathogens, and Pathogen-associated molecular patterns, use distinct signaling pathways that eventually converge to activate NF-κB, leading to the production of inflammatory mediators(21) (Figure 1).
NF-κB activation also up-regulates the expression of genes encoding proinflammatory enzymes, such as Cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS) (Figure 1). COX-2 protein expresses in the epithelial cells in Barrett’s metaplasia and its level of expression is elevated in esophageal adenocarcinoma (30–33). The elevation in expression occurs along the progression from low-grade dysplasia to high-grade dysplasia in Barrett's esophagus and esophageal adenocarcinoma (31). iNOS expression is also increased in esophageal adenocarcinoma (32) and in the lower esophageal sphincter in mouse model (34). Increased expressions of both iNOS and COX-2 have been demonstrated in inflammatory bowel disease, such as ulcerative colitis (35), and Crohn's disease (24–26).
LPS relaxes lower esophageal sphincter
Two major opposing factors stand out among the mechanisms that determine the development of pathological reflux. One is the lower esophageal sphincter which serves as a gatekeeper against reflux and the other is increased intragastric pressure which promotes reflux. Studies in a mouse model for sepsis illustrates that in normal mice, the lower esophageal sphincter maintains a basal tone, but LPS causes a dose-dependent fall in the basal tone, and the LPS's effect can be blocked by L-canavanine which is a selective iNOS inhibitor (34). In this rodent model, LPS caused a selective increase in iNOS protein and mRNA in both the lower esophageal sphincter and internal anal sphincter without significant changes in the expression of other NOS isozymes. In LPS-treated mice, the increased iNOS activates mitogen-activated protein kinase signaling pathway by phosphorylation of mitogen-activated protein kinases (36). Mitogen-activated protein kinases, including a family of serine and threonine kinases of ERK, JNK, and p38, convert external stimuli into a wide range of cellular responses, such as proliferation, survival, differentiation and migration. Because of these critical functions, deregulated mitogen-activated protein kinases are often found to contribute to the development of many cancers (37). The increased Gram-negative bacteria in the type II microbiome in reflux esophagitis and Barrett's esophagus could serve as the trigger of the NF-kb pathway and be responsible for the up-regulation of the iNOS gene. Thus, the type II microbiome might cause abnormal relaxation of the lower esophageal sphincter and contribute to the etiology of gastroesophageal reflux and carcinogenesis of esophageal adenocarcinoma.
LPS delays gastric emptying
Forward flow of gastric contents reduces the pressure and reduces the opportunity for reflux. In normal mice the stomach is mostly empty between meals but is nearly full in LPS-treated mice (38). The LPS-delayed gastric emptying can be blocked by NS398 which is a selective COX-2 inhibitor (38). Thus, by reducing gastric emptying the type II microbiome might cause an increase in the intragastric pressure that contributes to the development of gastroesophageal reflux.
Microbiomic biomarker and clinical interventions
The type II microbiome could serve as a marker as well as an important target of intervention in clinical practice. If proven to play a critical role in disease progression from reflux esophagitis to esophageal adenocarcinoma, the use of the type II microbiome as a biomarker might help to better stratify reflux esophagitis and Barrett's esophagus patients into high vs. low risk groups which would improve the sensitivity as well as specificity of a surveillance strategy for early detection of esophageal adenocarcinoma. Furthermore, the cancer risk may be reduced by reversion from the type II microbiome to type I microbiome with the use of selective antibiotics or probiotics.
NF-kB inhibitors
Because its association with a number of inflammatory and neoplastic diseases, the NF-κB pathway has been the target of drug development (39). Several drugs have been reported to block the pathway at various steps and their clinical use has been described (40–41). Many of these inhibitors could be effective in reduction of inflammation caused by NF-κB activation in reflux disorders. Drugs that are currently used in treating inflammatory diseases, such as glucocorticoids, nonsteroidal anti-inflammatory drugs, sulfasalazine, immunosuppressive agents (cyclosporin A and tacrolimus) often interfere the NF-κB pathway at multiple steps (Figure 1). Since they interfere with normal cellular function necessary to mount immune responses, inhibitors of NF-kB may also cause significant side effects such as increased susceptibility to infections, and liver dysfunction. Although limited by clinical side effects, interest remains in the therapeutic potential of NF-kB inhibitors to halt the metaplastic progression of Barrett's esophagus or to treat esophageal adenocarcinoma by inhibiting inflammation. Curcumin (diferuloylmethane) a naturally occurring NF-kB inhibitor was recently shown to increase apoptosis in two esophageal adenocarcinoma cell lines and to enhance their sensitivity to chemotherapeutic agents (42).
iNOS inhibitors
iNOS has been an attractive drug target as it is related to a variety of human diseases (43–46). Although L-canavanine, a selective iNOS inhibitor present in alfalfa can block the LPS-induced lower esophageal sphincter relaxation in rodents (34), its relationship with lupus-like autoimmunity limits its direct use in humans (47). The prodrug L-N6-(1–iminoethyl)lysine 5-tetrazole amide (SC-51), another selective iNOS inhibitor causes marked suppression of exhaled breath NO levels both in healthy control subjects and in patients without significant side effect (48). It may have therapeutic potential for controlling reflux/microbiome-induced esophageal inflammation.
COX2 inhibitors
COX-2 selective inhibitors represent a form of non-steroidal anti-inflammatory drug, such as Aspirin, that directly targets COX-2, an enzyme expressed in inflammation of reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma (Figure 1). Ingestion of non-steroidal anti-inflammatory drug decreases the inflammatory complications of gastroesophageal reflux disease (49) and may reduce the risk of neoplastic progression in patients with Barrett's esophagus (50–51). Thus, aspirin and other non-steroidal anti-inflammatory drug could protect against esophageal adenocarcinoma by either preventing the development of its primary precursor (i.e., Barrett's esophagus) or by diminishing the likelihood of Barrett's esophagus progressing to esophageal adenocarcinoma. The mechanism of potential risk reduction is related to these agents’ inhibition of the COX-2 enzyme, which is expressed in reflux esophagitis and Barrett's esophagus and is induced early in the development esophageal carcinomas. Like NF-kB inhibitors, COX-2 inhibitors represent a potential therapeutic option in controlling reflux/microbiome-induced esophageal inflammation which might trigger a progressive cascade to esophageal adenocarcinoma.
Conclusion
With these data, we can speculate the roles of the type II microbiome in the diseases of reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma. The type II microbiome with stepwise increase in Gram-negative bacteria in the reflux esophagitis - Barrett's esophagus and probably in esophageal adenocarcinoma could contribute to carcinogenesis by induction of chronic inflammation and cause gastric reflux by induction of abnormal relaxation of the lower esophageal sphincter and increase in intragastric pressure by delaying gastric emptying. These pathological effects could be explained in part by the activation of LPS/TLR4/NF-kB pathway. The type II microbiome could be used as novel biomarkers for risk assessment in clinical management. Antibiotic/probiotic treatment could reverse the type II microbiome back to the type I microbiome and decrease the detrimental effects of Gram-negative bacteria on LPS/TLR4/NF-kB pathway. The negative effects could also be alleviated using specific inhibitors to NF-kB and/or the downstream components, such as COX-2 and iNOS.
Acknowledgements
Supported by grants from the National Cancer Institute and the National Institute forAllergy and Infectious Diseases UH3CA140233, R01CA159036, R01AI063477,U19DE018385, K23CA107123, as well as the RWJ Amos Medical Faculty Development Program.
REFERENCES
- 1.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 2.O'Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett's esophagus: report on the Cleveland Clinic Barrett's Esophagus Registry. Am J Gastroenterol. 1999;94:2037–2042. doi: 10.1111/j.1572-0241.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 3.Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92:212–215. [PubMed] [Google Scholar]
- 4.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of Adenocarcinoma among Patients with Barrett's Esophagus. New Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 5.Haggitt RC. Adenocarcinoma in Barrett's esophagus: a new epidemic? Hum Pathol. 1992;23:475–476. doi: 10.1016/0046-8177(92)90121-i. [DOI] [PubMed] [Google Scholar]
- 6.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 7.Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: clinical applications. JAMA. 2002;287:1982–1986. doi: 10.1001/jama.287.15.1982. [DOI] [PubMed] [Google Scholar]
- 8.Lederberg J, McCray AT. 'Ome sweet 'omics - A genealogical treasury of words. Scientist. 2001;15:8. [Google Scholar]
- 9.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett's oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10:87–101. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol. 2000;165:3541–3544. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
- 13.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Jacinto R, McCall C, Li L. Regulation of IL-1 receptor-associated kinases by lipopolysaccharide. J Immunol. 2002;168:3910–3914. doi: 10.4049/jimmunol.168.8.3910. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Latif MM, O'Riordan J, Windle HJ, Carton E, Ravi N, Kelleher D, et al. NF-kappaB activation in esophageal adenocarcinoma: relationship to Barrett's metaplasia, survival, and response to neoadjuvant chemoradiotherapy. Ann Surg. 2004;239:491–500. doi: 10.1097/01.sla.0000118751.95179.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colleypriest BJ, Ward SG, Tosh D. How does inflammation cause Barrett's metaplasia? Curr Opin Pharmacol. 2009;9:721–726. doi: 10.1016/j.coph.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Lee JS, Oh TY, Ahn BO, Cho H, Kim WB, Kim YB, et al. Involvement of oxidative stress in experimentally induced reflux esophagitis and Barrett's esophagus: clue for the chemoprevention of esophageal carcinoma by antioxidants. Mutat Res. 2001;480–481:189–200. doi: 10.1016/s0027-5107(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 18.Konturek PC, Nikiforuk A, Kania J, Raithel M, Hahn EG, Muhldorfer S. Activation of NFkappaB represents the central event in the neoplastic progression associated with Barrett's esophagus: a possible link to the inflammation and overexpression of COX-2, PPARgamma and growth factors. Dig Dis Sci. 2004;49:1075–1083. doi: 10.1023/b:ddas.0000037790.11724.70. [DOI] [PubMed] [Google Scholar]
- 19.O'Riordan JM, Abdel-latif MM, Ravi N, McNamara D, Byrne PJ, McDonald GS, et al. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol. 2005;100:1257–1264. doi: 10.1111/j.1572-0241.2005.41338.x. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Latif MMM, Kelleher D, Reynolds JV. Potential Role of NF-kappa B in Esophageal Adenocarcinoma: As an Emerging Molecular Target. Journal of Surgical Research. 2009;153:172–180. doi: 10.1016/j.jss.2007.12.755. [DOI] [PubMed] [Google Scholar]
- 21.Rahman MM, McFadden G. Modulation of NF-kappa B signalling by microbial pathogens. Nat Rev Microbiol. 2011;9:291-U1500. doi: 10.1038/nrmicro2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Opitz B, Forster S, Hocke AC, Maass M, Schmeck B, Hippenstiel S, et al. Nod1-mediated endothelial cell activation by Chlamydophila pneumoniae. Circ Res. 2005;96:319–326. doi: 10.1161/01.RES.0000155721.83594.2c. [DOI] [PubMed] [Google Scholar]
- 23.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 24.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 25.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 26.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 27.Lim DM, Narasimhan S, Michaylira CZ, Wang ML. TLR3-mediated NF-kappa B signaling in human esophageal epithelial cells. Am J Physiol-Gastr L. 2009;297:G1172–G1180. doi: 10.1152/ajpgi.00065.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheyhidin I, Nabi G, Hasim A, Zhang RP, Ainiwaer J, Ma H, et al. Overexpression of TLR3, TLR4, TLR7 and TLR9 in esophageal squamous cell carcinoma. World J Gastroenterol. 2011;17:3745–3751. doi: 10.3748/wjg.v17.i32.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. 2002;50:451–459. doi: 10.1136/gut.50.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirvani VN, Ouatu-Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487–496. doi: 10.1016/s0016-5085(00)70254-x. [DOI] [PubMed] [Google Scholar]
- 31.Morris CD, Armstrong GR, Bigley G, Green H, Attwood SE. Cyclooxygenase-2 expression in the Barrett's metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol. 2001;96:990–996. doi: 10.1111/j.1572-0241.2001.03599.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929–2934. [PubMed] [Google Scholar]
- 33.Buskens CJ, Van Rees BP, Sivula A, Reitsma JB, Haglund C, Bosma PJ, et al. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 2002;122:1800–1807. doi: 10.1053/gast.2002.33580. [DOI] [PubMed] [Google Scholar]
- 34.Fan YP, Chakder S, Gao F, Rattan S. Inducible and neuronal nitric oxide synthase involvement in lipopolysaccharide-induced sphincteric dysfunction. Am J Physiol Gastrointest Liver Physiol. 2001;280:G32–G42. doi: 10.1152/ajpgi.2001.280.1.G32. [DOI] [PubMed] [Google Scholar]
- 35.Verma R, Ahuja V, Paul J. Frequency of single nucleotide polymorphisms in NOD1 gene of ulcerative colitis patients: a case-control study in the Indian population. BMC Med Genet. 2009;10:82. doi: 10.1186/1471-2350-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puri RN, Fan YP, Rattan S. Role of pp60(c-src) and p(44/42) MAPK in ANG II-induced contraction of rat tonic gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2002;283:G390–G399. doi: 10.1152/ajpgi.00025.2002. [DOI] [PubMed] [Google Scholar]
- 37.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 38.Calatayud S, Garcia-Zaragoza E, Hernandez C, Quintana E, Felipo V, Esplugues JV, et al. Downregulation of nNOS and synthesis of PGs associated with endotoxin-induced delay in gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1360–G1367. doi: 10.1152/ajpgi.00168.2002. [DOI] [PubMed] [Google Scholar]
- 39.Sarkar FH, Li Y, Wang Z, Kong D. NF-kappaB signaling pathway and its therapeutic implications in human diseases. Int Rev Immunol. 2008;27:293–319. doi: 10.1080/08830180802276179. [DOI] [PubMed] [Google Scholar]
- 40.Calzado MA, Bacher S, Schmitz ML. NF-kappaB inhibitors for the treatment of inflammatory diseases and cancer. Curr Med Chem. 2007;14:367–376. doi: 10.2174/092986707779941113. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartojo W, Silvers AL, Thomas DG, Seder CW, Lin L, Rao H, et al. Curcumin promotes apoptosis, increases chemosensitivity, and inhibits nuclear factor kappaB in esophageal adenocarcinoma. Transl Oncol. 2010;3:99–108. doi: 10.1593/tlo.09235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qidwai T, Jamal F. Inducible nitric oxide synthase (iNOS) gene polymorphism and disease prevalence. Scand J Immunol. 2010;72:375–387. doi: 10.1111/j.1365-3083.2010.02458.x. [DOI] [PubMed] [Google Scholar]
- 44.Muscara MN, Wallace JL. Nitric Oxide. V. therapeutic potential of nitric oxide donors and inhibitors. Am J Physiol. 1999;276:G1313–G1316. doi: 10.1152/ajpgi.1999.276.6.G1313. [DOI] [PubMed] [Google Scholar]
- 45.Whittle BJ. Nitric oxide-modulating agents for gastrointestinal disorders. Expert Opin Investig Drugs. 2005;14:1347–1358. doi: 10.1517/13543784.14.11.1347. [DOI] [PubMed] [Google Scholar]
- 46.Hesslinger C, Strub A, Boer R, Ulrich WR, Lehner MD, Braun C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans. 2009;37:886–891. doi: 10.1042/BST0370886. [DOI] [PubMed] [Google Scholar]
- 47.Akaogi J, Barker T, Kuroda Y, Nacionales DC, Yamasaki Y, Stevens BR, et al. Role of non-protein amino acid L-canavanine in autoimmunity. Autoimmun Rev. 2006;5:429–435. doi: 10.1016/j.autrev.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Hansel TT, Kharitonov SA, Donnelly LE, Erin EM, Currie MG, Moore WM, et al. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 2003;17:1298–1300. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- 49.Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124:47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen DM, Richardson P, El-Serag HB. Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett's esophagus. Gastroenterology. 2010;138:2260–2266. doi: 10.1053/j.gastro.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kastelein F, Spaander MC, Biermann K, Steyerberg EW, Kuipers EJ, Bruno MJ. Nonsteroidal Anti-Inflammatory Drugs and Statins Have Chemopreventative Effects in Patients with Barrett's Esophagus. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.08.036. [DOI] [PubMed] [Google Scholar]