Abstract
In testis mRNA stability and translation initiation are extensively under the control of poly(A)-binding proteins (PABP). Here we have cloned a new human testis-specific PABP (PABP3) of 631 amino acids (70.1 kDa) with 92.5% identical residues to the ubiquitous PABP1. A northern blot of multiple human tissues hybridised with PABP3- and PABP1-specific oligonucleotide probes revealed two PABP3 mRNAs (2.1 and 2.5 kb) detected only in testis, whereas PABP1 mRNA (3.2 kb) was present in all tested tissues. In human adult testis, PABP3 mRNA expression was restricted to round spermatids, whereas PABP1 was expressed in these cells as well as in pachytene spermatocytes. PABP3-specific antibodies identified a protein of 70 kDa in human testis extracts. This protein binds poly(A) with a slightly lower affinity as compared to PABP1. The human PABP3 gene is intronless with a transcription start site 61 nt upstream from the initiation codon. A sequence of 256 bp upstream from the transcription start site drives the promoter activity of PABP3 and its tissue-specific expression. The expression of PABP3 might be a way to bypass PABP1 translational repression and to produce the amount of PABP needed for active mRNA translation in spermatids.
INTRODUCTION
In testis mRNA stabilisation is of crucial importance during the different stages of differentiation from spermatogonia to spermatozoa. In fact, gene transcription is mainly active in the first steps of this process (meiosis) and most of the mRNAs are stored as mRNP particles and translated at later stages (1–5). The maximal levels of poly(A)-binding protein (PABP) parallel the presence of mRNP, suggesting that PABP maintains the stability and the translational potentiality of long-lived mRNA.
In eukaryotic cells mRNA and pre-mRNA associate with at least 20 different specific proteins to form mRNP (6,7). The predominant protein of this complex is a PABP, which binds to the 3′-poly(A) tail of mRNA (8). In mammals the PABP family comprises a nuclear isoform and several cytoplasmic forms coded by different genes. Two mouse cytoplasmic isoforms, the ubiquitous PABP1 and testis-specific PABP2, have been characterised (9,10). In human a ubiquitous PABP (PABP1) has been cloned (11) and a cytoplasmic inducible isoform (iPABP) has been characterised following activation of T cells (12) and platelets (13). In addition, three PABP pseudogenes are present in the human genome (14–16). As initially described for Saccharomyces cerevisiae (15,16), mammalian cytoplasmic PABPs stabilise mRNA and enhance translation in association with the eukaryotic initiation factor eIF-4G subunit (17) or PABP-binding protein (PAIP-1) (18). Shuttling of PABP1 between the nucleus and the cytoplasm suggests an involvement of this protein in nuclear mRNP formation and cytoplasmic export (19). These key roles have recently been highlighted by the observation that PABP1 is one of the targets of enteroviruses (20), coxsackieviruses (21) and rotaviruses (22) for host protein synthesis shut-off.
In the current study we describe a new testis-specific PABP isoform (PABP3) specifically expressed in round spermatids. Its mRNA is transcribed from a retroposon under the control of a tissue-specific promoter.
MATERIALS AND METHODS
The nucleotide sequences for PABP3, PABP4 mRNA and the PABP3 promoter have been deposited in the GenBank database under accession nos AF132026, AF132027 and AF315079, respectively. The other PABP sequences mentioned in this study, PABP1 mRNA, the splicing variant PABPII, iPABP mRNA, the PABP1 gene and mouse PAPB2 mRNA, have accession nos AH007272, Z48501, U33818, U68093–U68105 and AF001290, respectively. All the oligonucleotides used in this study are described in Table 1.
Table 1. Primers used in this study, as detailed in Materials and Methods.
 |
Name, name of the oligonucleotide primer; Sequence, 5′→3′ sequence of each oligonucleotide; Position, position in nt of the oligonucleotide on each specific sequence (these positions refer to sequences and GenBank accession numbers specified in the last column, PABP type and Acc. N°). The different 5′PROM and 3′PROM oligonucleotides included BglII and HindIII restriction sites (lower case letters) in their 5′-end sequences.
cDNA cloning
The insert of one clone (EST h08023t), corresponding to a new potential PABP (23), was used as a probe to screen a human testis library (24,25) as previously described (26).
DNA sequencing and analysis
Plasmids were prepared and sequenced as previously reported (23,27).
Northern blot analysis
Human multiple tissue northern blots (Clontech, CA) were probed with oligonucleotides PABP1, PABP3, PABP4 and iPABP specific for each PABP (Table 1). Blots were pre-hybridised and hybridised in ExpressHyb hybridisation solution (Clontech) at 37°C. They were washed in 2× SSC (1× SSC = 150 mM NaCl, 15 mM sodium citrate, pH 7), 0.1% SDS at room temperature for 15 min then in 2× SSC, 0.1% SDS at 43°C twice for 1 h. The specific signals were detected using a Storm 840 PhosphorImager (Molecular Dynamics, CA).
Semi-quantitative RT–PCR on human testis RNA
Total RNA from human adult testis was extracted using acid guanidium thiocyanate (28). RT–PCR was performed on 5 ng total RNA using the Access RT–PCR System (Promega, WI) and the two oligonucleotides 3′RT and 5′RT (the latter being labelled with 6FAM) (Table 1). This set of primers frames a region with insertions or deletions in the PABP3, PABP4, PABP1 and iPABP fragments, which exhibited sizes of 513, 519, 528 and 555 bp, respectively. RT–PCR was carried out in a Perkin-Elmer GeneAmp 9600 Thermocycler under the following incubation conditions: 45 min of reverse transcription at 48°C, denaturation for 2 min at 94°C and 30 cycles of PCR (1 min at 94°C, 1 min at 50°C and 1 min at 72°C). The amplified products were detected in an Applied Biosystems 373A automated DNA sequencer and analysed using the GeneScan program. Under these experimental conditions, as determined using various mixes of the four PABP RNAs synthesised in vitro, the surface under each peak was proportional to the amount of specific mRNA in the assayed samples.
In situ hybridisation
Human testis samples were taken during surgery for orchidectomy, fixed overnight in 10% formol and embedded in paraffin using routine procedures. Sections of 6 µm thickness were mounted on polylysine-coated slides and stored at room temperature until used. The HPLC-purified PABP1 and PABP3 primers (Table 1) were used as antisense PABP1 and PABP3 probes, respectively, with primer 5′RT being used as the sense probe. These oligonucleotides were labelled using a digoxigenin (DIG)-11-dUTP oligonucleotide tailing kit (Roche Diagnostics, France) and kept at –20°C. Following deparaffinisation in xylene and rehydratation, sections were incubated in 50% acetic acid for 30 s, rinsed in sterile water and digested with proteinase K (10 µg/ml) in 100 mM Tris–HCl pH 7.5, 1 mM CaCl2 for 15 min at 37°C. The sections were then washed in sterile water, post-fixed in 2% paraformaldehyde for 20 min at room temperature, washed in sterile water, dehydrated in graded ethanol baths and air dried. Each section was pre-hybridised for 2 h at 37°C in 100 µl of hybridisation buffer (4% deionised formamide, 1× Denhardt’s solution, 1.5× SSC and 330 µg/ml yeast tRNA) in a humid chamber. In situ hybridisations were performed for 16 h at 37°C in 20 µl of hybridisation buffer containing 10% dextran sulphate and 25 ng DIG-labelled oligonucleotide probe. After hybridisation the sections were briefly rinsed in 2× SSC at room temperature, in 2× SSC for 1 h at 37°C and, finally, in 1× SSC for 30 min at room temperature. Hybridisation signals were detected as previously described (29). Sections were counterstained using methyl green in order to visualise the nuclei.
Preparation of antibodies
A 9 amino acid peptide (CAVPNQRAP) specific for PABP3 (peptide 3), corresponding to amino acids 391–398 of this protein with a Cys residue at its N-terminal end for coupling to keyhole limpet hemocyanin, was used as immunogen (Syntem SA, France). Peptide 3 antibodies were generated by immunisation of rabbits with this antigen. An 11 amino acid peptide (CPNPVINPYQP) specific for PABP1 (peptide 1), corresponding to amino acids 393–402 of the PABP1 protein sequence with an additional Cys residue at its N-terminal end, was used as control in competition experiment.
Western blot analysis
Human testis and liver tissues were homogenised in 40 mM Tris–HCl buffer pH 8.5, containing 250 mM sucrose, 1 mM EDTA, 1 mM phenylmethylsulphonyl fluoride, 1 µg/ml leupeptin. After centrifugation (13 000 g for 2 min) the supernatants were kept frozen until assayed for protein by the Bradford method (Bio-Rad, CA). Following denaturation for 5 min at 90°C, protein extracts (70 µg/lane) were separated by 9.2% SDS–PAGE, blotted onto a PVDF transfer membrane (Millipore, France) and probed with either anti-peptide 3 serum (1/100) or anti-peptide 3 serum pre-absorbed with peptide 3 (200 µg) or peptide 1 (200 µg). Treatment of the membrane was according to the enhanced chemifluorescence protocol (ECF; Amersham, UK). Sheep anti-rabbit IgG, conjugated with alkaline phosphatase (1/10 000) was used as secondary antibody. The membrane was incubated for 10 min in ECF solution and fluorescent signals were visualised using a Storm 840 PhosphorImager (Molecular Dynamics).
Protein synthesis and RNA binding assays
The open reading frames (ORFs) of PABP1 and PABP3 were amplified using the primers 5′ORF and 3′ORF (Table 1), while iPABP was amplified using primers 5′iORF and 3′iORF. Each PCR product was cloned in both orientations into the vector pTargeT (Promega). The inserts of the plasmids, linearised with KpnI restriction enzyme, were transcribed and translated using the Linked in vitro T7 Transcription/Translation radioactive kit (Roche Diagnostics) in the presence of [35S]methionine (Amersham) (1000 Ci/mmol). One-tenth of the translation reaction was analysed on a 10% acrylamide (29:1) denaturing gel. The specific signals from dried gels were detected using a Storm 840 PhosphorImager (Molecular Dynamics).
Binding of in vitro translated PABP to RNA homopolymers coupled to Sepharose was performed as described (12,30,31), with the following changes. For each binding experiment poly(A)–Sepharose [50 ng poly(A)/reaction] was equilibrated in 500 µl of KCl (0.1 M) binding buffer (10 mM Tris pH 7.4, 2.5 mM MgCl2, 0.1 M KCl and 0.5% Triton X-100). The standard binding reaction was performed for 1 h at 4°C with 3 µl of the in vitro translated products in a final volume of 80 µl of KCl (0.1–2 M) binding buffer containing 20 U RNasin (Promega), 20 µg yeast tRNA (Sigma-Aldrich, France) and 8 µg poly(dI·dC) (Pharmacia Biotech, Sweden) as non-specific competitors. In competition experiments a 30 min preincubation with 5 µg HPLC-purified oligonucleotides, poly(A)40, poly(U)40, poly(C)40 or poly(G)40 (Genset SA, France), was performed before poly(A)–Sepharose addition to the binding reaction (1 M KCl). The poly(A)–Sepharose was then washed twice with 500 µl of KCl (0.1–2 M) binding buffer, resuspended in SDS sample buffer (50 mM Tris pH 6.8, 100 mM DTT, 2% SDS and 10% glycerol), boiled for 3 min and loaded on a 10% acrylamide (29:1) denaturing gel. The dried gels were exposed overnight on a phosphor screen and the signal detected on a Storm 840 PhosphorImager (Molecular Dynamics).
Gene structure analysis
Standard PCR reactions were carried out on a Perkin-Elmer GeneAmp 9600 Thermocycler using the Advantage cDNA PCR Kit (Clontech) in 50 µl containing either high molecular weight human DNA (60 ng) from blood peripheral leukocyte cells or PABP1 and PABP3 cDNA (1 ng) as positive controls. These DNA sequences were amplified with the 5′ORF/3′ORF (PABP3), 5′PCR/3′ORF (PABP1) or T7 (Promega)/3′ORF primer pairs. This last set of primers, which is able to amplify PABP sequence cloned in plasmids, was used to check that the genomic DNA was not contaminated with PABP-containing plasmids. The PCR cycles for PABP3 started with a DNA polymerase activation step (94°C for 1 min), followed by 10 cycles of ‘touchdown’ from 60 to 50°C (32), 30 cycles of PCR (5 s at 94°C, 10 s at 50°C and 3 min at 72°C) and a final extension for 10 min at 72°C. The PCR for PABP1 was performed under the following incubation conditions: 94°C for 1 min, followed by 35 cycles of PCR including two steps (95°C for 15 s and 68°C for 22 min). The amplified products were loaded on a 1% agarose gel in TBE buffer (90 mM Tris–borate pH 8, 1 mM EDTA) for electrophoresis. The gel was then blotted onto a Hybond N+ membrane (Amersham) and probed with the specific PABP1 and PABP3 primers as described for the northern blot.
Cloning of the 5′-region of PAPB3 mRNA
5′-RACE cloning was carried out on 1 µg human testis mRNA (Clontech) using the Smart RACE Cloning Kit (Clontech) and the RACE primer (Table 1). The resulting products were subcloned into vector pCR4-TOPO using the TOPO-TA Cloning Kit (Invitrogen, ND). Positive clones were sequenced as mentioned for PABP3 cDNA.
PABP3 promoter cloning and sequencing
The PABP3 gene was isolated from the Single Human BAC Library (Research Genetics, AL) according to the supplier’s protocols. Briefly, two PABP3-specific oligonucleotides (5′BAC and 3′BAC) were used to amplify a fragment which was detected by the BAC oligonucleotide probe. PCR reactions were performed as previously described (33) on 1 µl of DNA from the ‘superpool’ plate (PCR step 1), then on 2 µl of DNA from the ‘plate pool’ plates (PCR step 2). The final screening was achieved by hybridisation of the selected 384 colony BAC DNA membranes with the [α-32P]dCTP-labelled PABP3 insert as probe. DNA from the positive BAC clones was extracted and purified using the Qiagen Large-Construct Kit (Qiagen, CA). The putative PABP3 promoter sequence was obtained directly from 2 µg of SphI-digested BAC DNA using a dye terminator sequencing kit (Applied Biosystems, CA) and oligonucleotides RACE, Seq1, Seq2 and Seq3, designed from the sequences of PABP3, the 5′-RACE products and the results of RACE and Seq1 sequence reactions, respectively. The PABP3 putative promoter sequence was analysed using the program MatInspector (core similarity = 1; matrix similarity > 0.87) (34) in order to identify consensus binding sites for transcription factors.
S1 nuclease mapping analysis
A 737 bp genomic DNA fragment was amplified from 10 ng BAC DNA using oligonucleotides 5′PROM and RACE (Table 1) as 5′ and 3′ primers, respectively, under conditions previously described (33). This PCR fragment was gel purified using Qiaex II (Qiagen) and then used as the matrix for S1 mapping probe synthesis. Briefly, the 6FAM-labelled RACE antisense primer (Genset SA) was used to generate a 5′-labelled single-stranded DNA fragment using 50 ng of the 737 bp genomic fragment. The probe was checked on an agarose gel, purified using a Qiaex II kit and quantified by ethidium bromide staining. Poly(A)+ RNA from either testis or liver (2 µg) (Clontech SA, France) was hybridised to the end-labelled probe (50 ng) in 80% formamide, 100 mM sodium citrate, 300 mM sodium acetate and 1 mM EDTA, pH 6.5, at 37°C overnight. S1 nuclease (1 U/µl) (Life Technologies, France) was then added to the digestion buffer (50 mM sodium acetate, 200 mM sodium chloride, 1 mM zinc chloride and 0.5% glycerol) for 30 min at 37°C. As a control, the probe was also hybridised to 10 ng PABP3 synthetic RNA, produced using the Riboprobe Gemini II kit (Promega) and the corresponding PABP3 plasmid as template according to the supplier’s protocol. Protected fragments were detected on an Applied Biosystems 373A automated DNA sequencer and analysed using the program GeneScan.
Plasmid constructs for promoter analysis
The PCR products corresponding to different regions of the promoter were subcloned upstream from the enhanced green fluorescent protein (EGFP) reporter gene in vector pEGFP-1 (Clontech) under conditions previously described (33). These inserts were generated by PCR on 10 ng BAC DNA using an Advantage cDNA PCR Kit (Clontech). The four oligonucleotide pairs used for PCR were 5′PROM/3′PROM, 5′PROM1/3′PROM, 5′PROM2/3′PROM and 5′PROM3/3′PROM, in order to amplify fragments PC (528 bp), P1 (473 bp), P2 (286 bp) and P3 (160 bp), respectively. The resulting products were digested with BglII and HindIII (see Table 1) and then subcloned into the promoter-less reporter vector pEGFP-1. Plasmids were purified using the Maxiprep reagent system (Qiagen) and verified by sequence analysis. Plasmid CMV/pEGFP-C1 (Clontech), which contains the human cytomegalovirus (CMV) promoter, was used as a positive control in transfection experiments.
Cell culture and transfection assays
The human NTERA-2/D1 cell line (NTERA-2/clone D1, a human pluripotent embryonic carcinoma cell line derived from a lung metastasis of a testicular teratocarcinoma) was obtained from the American Type Culture Collection (no. CRL 1973; ATCC, Biovalley, France). NTERA-2/D1 and HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (Life Technologies, France) containing 10% (v/v) foetal calf serum (Life Technologies), penicillin/streptomycin and 2 mM glutamine with a pCO2 of 10% (NTERA-2 cells) or 5% (HeLa cells) at 37°C in humidified air. Cells were transfected using the LipofectAMINE+ protocol (Life Technologies). Briefly, HeLa or NTERA-2/D1 cells, at 60 and 90% confluence, respectively, were washed twice with serum-free medium. They were then transfected with 400 ng of either CMV/pEGFP-C1 (Clontech) as a positive control or the PABP3/pEGFP-1 constructs with 4 µl of LipofectAMINE and 1 µl of PLUS reagent (Life Technologies) in 200 µl of serum-free medium. After 3 h incubation the medium was replaced with 500 µl of medium supplemented with 10% serum. Cells were harvested after 48 h culture by trypsin/EDTA digestion and resuspended in PBS containing 0.5 µg/ml propidium iodide (PI). EGFP fluorescence was detected with a FACS flow cytometer (Coulter EPICS XL-MCL; Beckman Coulter, Switzerland) equipped with an argon laser emitting at 488 nm. The FL1 emission channel (normally used to detect FITC) was used to monitor EGFP fluorescence; the FL3 channel was used to identify dead or dying cells with PI red fluorescence.
RESULTS
Isolation and sequence of PABP3 and PABP4
Recently we identified a large series of human testis ESTs (23). One of them (EST h08023t), corresponding to a new PABP isoform, was used as probe in order to screen a human testis cDNA library. Seven of 10 positive clones corresponded to PABP1 and three of them revealed two new PABP sequences, PABP3 and PABP4. The PABP3 cDNA (2397 nt) exhibits an ORF of 1893 nt encoding a 631 amino acid protein (Fig. 1). The initiation codon (ATG), at position 24, is flanked by a Kozak consensus sequence favourable for translation initiation (35,36). Three polyadenylation consensus signal sequences (AATAAA) are detected in the 3′-untranslated region (UTR) at positions 2015, 2035 and 2373. PABP3 exhibits four RNA-binding domains (RBDs), each of them containing the two ribonucleoprotein consensus motifs (RNP1 and RNP2). Alignment of the PABP3 and PABP1 amino acid sequences reveals 92.5% identical residues (data not shown). The major differences between these two species are a deletion of six amino acids (PVINPY) in the PABP3 sequence, corresponding to positions 395–400 of PABP1, and four amino acid substitutions in RBDs I, II and III, at positions 52, 53, 102 and 191.
Figure 1.
cDNA and predicted amino acid sequences of PABP3. Lower case letters indicate the non-coding regions. The upper case letters correspond to the detected ORF. White and shaded boxes frame the consensus RNP2 and RNP1 sequences, respectively. The bold letters in each RNP consensus highlight the conserved amino acid residues as compared to similar regions in the PABP1 sequence. The three polyadenylation signal sequences (AATAAA) are underlined. The vertical bar represents the site of deletion of six amino acids (PVINPY) in PABP3 as compared to PABP1.
The sequence of the PABP4 cDNA (accession no. AF132027) reveals two ORFs corresponding to incomplete PABP proteins (data not shown). The first starts at nt 81, with an initiation codon in a favourable consensus sequence for translation initiation (35,36). It codes for a peptide of 56 amino acids which contains RNP2 of RBD1. The second ORF (269 amino acids) starts at nt 548 with an ATG in a poor context for initiation of translation. It might code for a protein of 269 amino acids containing only one-and-a-half RBDs. In the 3′-UTR an imperfect polyadenylation consensus signal sequence (AATTAA) (37,38) is located 52 bases upstream from the poly(A) tail.
Analysis of PABP mRNA expression in human tissues
Since the nucleotide sequences of the different PABPs are very well conserved, we designed oligonucleotides specific for PABP1, iPABP, PABP3 and PABP4 (named PABP1, iPABP, PABP3 and PABP4, respectively) (Table 1) in order to differentiate expression of their respective mRNAs. The specificity of these oligonucleotides was checked by hybridisation to in vitro transcribed RNA of the four PABP isoforms (data not shown). Hybridisation of the PABP1-specific probe to a multiple tissue northern blot revealed one major band at 3.2 kb, corresponding to PABP1 mRNA, in all the tissues tested (Fig. 2A). In contrast, hybridisation to PABP3 revealed two weak but distinct bands at 2.1 and 2.5 kb only in human testis. Oligonucleotides specific for PABP4 and iPABP did not reveal any specific signal (data not shown).
Figure 2.
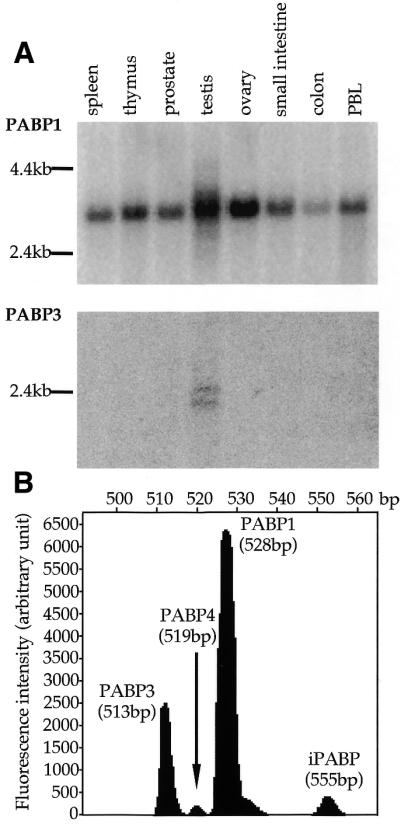
Northern blot analysis of PABP3 expression using specific oligonucleotide probes. (A) A multiple tissue blot was sequentially hybridised with oligonucleotides PABP1 and PABP3, exposed to a phosphor screen for 16 and 48 h, respectively, and analysed on a Storm 840 PhosphorImager. PBL, peripheral blood leukocytes. The size of the markers is indicated on the left. (B) Oligonucleotides 3′RT and 5′RT were used in RT–PCR on 5 ng total RNA from normal human adult testis. The amplified products were migrated on a sequencing gel and analysed using the GeneScan program as described in Materials and Methods. The surface under each peak reflects the amount of each PABP mRNA.
Due to the weakness of the signal detected on the northern blot, a more accurate quantification of PABP mRNA isoforms expressed in human testis was achieved using semi-quantitative RT–PCR on total RNA. To that end we designed a pair of specific primers, 5′RT and 3′RT, which were able to amplify fragments of different sizes for the four human PABPs in the same reaction sample (see Materials and Methods). A characteristic electrophoretic profile of the amplified products is presented in Figure 2B. As expected, PABP1 and PABP3 are the two major PABP mRNA isoforms expressed in human testis. Based on 11 independent experiments, the amount of PABP3 mRNA represents 18.8 ± 5.2% of the PABP1 messenger level, whereas PABP4 and iPABP mRNA expression represent only 1.77 ± 0.57 and 5.5 ± 0.06% of those of PABP1, respectively. These results are in agreement with the northern blot analyses, in which these latter two mRNAs were undetectable.
The PABP1 and PABP3 mRNAs were localised by in situ hybridisation on human adult testis sections using PABP1- and PABP3-specific oligonucleotide probes, respectively. PABP1 mRNA was strongly expressed in round spermatids and to a lesser extent in pachytene spermatocytes (Fig. 3A and B). In contrast, PABP3 mRNA was only detected in round spermatids and at a lower level as compared to PABP1 (24 h revelation as compared to 6 h for PABP1) (Fig. 3C and D). No signal was observed with the control sense oligonucleotide 5′RT (Fig. 3E and F).
Figure 3.
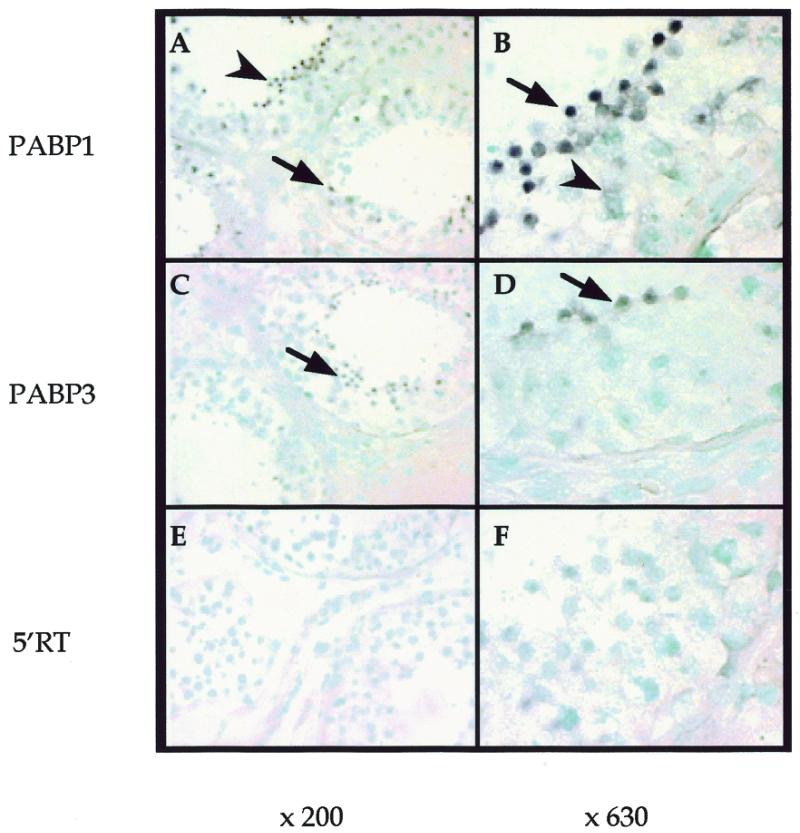
Localisation of PABP1 and PABP3 mRNA in human adult testis sections. Paraffin-embedded human testis sections were hybridised with the PABP1-specific antisense oligonucleotide probe (PABP1) (A and B), the PABP3-specific antisense oligonucleotide probe (PABP3) (C and D) or the common sense oligonucleotide probe (5′RT) (E and F) and counterstained with methyl green. Original magnifications: (A, C and E) 200×; B, D and F) 630×. Signals were confined to the seminiferous tubules in germinal cells (A–D). After 6 h detection the PABP1 mRNA-specific signal appeared in pachytene spermatocytes (arrowheads) and round spermatids (arrows) (A and B). The PABP3 mRNA signal appeared after 24 h reaction only in round spermatids (arrows) (C and D). No signal was observed with the control 5′RT probe after 24 h detection (E and F).
Western blot analysis of PABP3
In order to demonstrate the presence of PABP3 in testis we raised antibodies against oligopeptide 3 (CAVPNQRAP, amino acids 391–398 of PABP3) elongated with a Cys at the N-terminal end. This sequence is specific for PABP3 as compared to PABP1, since six amino acids (PVINPY) are inserted in PABP1 at position 391. These antibodies were used to detect PAPB3 on a western blot of testis and liver extracts. Liver was used as a negative control, since no PABP3 mRNA was detected in this tissue (Fig. 2). As shown in Figure 4, these antibodies recognised a band in testis at 70 kDa but no signal in liver extracts. This band was competed out by an excess of peptide 3 (used to raise the antibodies), whereas an excess of peptide 1 (specific for PABP1) did not alter the specific signal. This analysis clearly established the presence of PABP3 in human testis.
Figure 4.
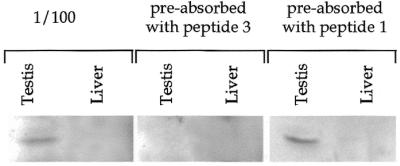
Detection of PABP3 by western blot analysis. Western blot of human testis protein extracts incubated with serum raised against a 9 amino acid specific PABP3 peptide (peptide 3). A human liver extract was used as negative control. In competition experiments the serum was pre-absorbed with peptide 1 or peptide 3.
PABP synthesis and binding to RNA homopolymers
In order to demonstrate the functionality of PABP3, we compared its ability to bind RNA homopolymers with that of PABP1 and iPABP. We synthesised mRNAs corresponding to PABP1, iPABP and PABP3 and to the luciferase control. These mRNAs were translated in the presence of [35S]methionine and the labelled proteins were analysed on SDS–acrylamide gels (data not shown). For each sense RNA we observed a band corresponding to a protein of the expected size from the length of the different ORFs (PABP1, 70.3 kDa; iPABP, 72.4 kDa; PABP3m 70.1 kDa). No labelled protein was detected when antisense RNA was used as template.
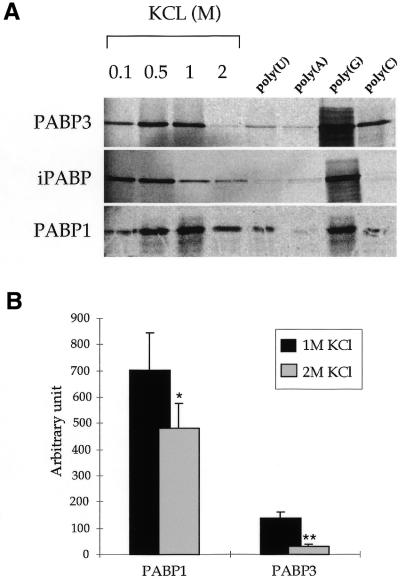
In a second step we determined whether these proteins were able to bind poly(A)–Sepharose. In order to estimate the relative affinities of each PABP for the polymer each of the three PABPs was incubated in the presence of poly(A)–Sepharose in a buffer containing increasing KCl concentrations of 0.1, 0.5, 1 and 2 M (Fig. 5A). PABP3 exhibited optimal binding at 0.5–1 M KCl and a strong decrease in affinity at 2 M KCl. iPABP exhibited maximal binding activity at 0.1 and 0.5 M KCl and a sharp decrease at 1 and 2 M KCl. Binding of PABP1 was optimal at 1 M KCl and was only slightly affected at 2 M KCl (Fig. 5A). The decrease in binding of PABP3 and PABP1 to the poly(A) homopolymer in the presence of 1 and 2 M KCl was evaluated as 78 and 32%, respectively (Fig. 5B). The binding properties of PABP1 and iPABP were compatible with the results obtained by other groups (12,30,31). The three PABPs were also incubated with poly(A)–Sepharose in 1 M KCl binding buffer in the presence of a 100-fold molar excess of ribohomopolymers [poly(U), poly(A), poly(G) and poly(C)] (Fig. 5A). The competition by purified soluble poly(U) for binding of PABP to poly(A)–Sepharose appears slightly more efficient for PABP3 and iPABP than for PABP1. For each PABP an increased binding to poly(A)–Sepharose was observed in the presence of poly(G). Under these experimental conditions we did not observe any non-specific binding of luciferase to poly(A)–Sepharose or of PABP to Sepharose 4B (data not shown).
Figure 5.
RNA binding assays of PABP to poly(A)–Sepharose. (A) Binding of in vitro [35S]methionine-labelled PABP proteins to poly(A)–Sepharose was performed in the presence of 0.25 mg/ml yeast tRNA and 0.1 mg/ml poly(dI·dC) as non-specific competitors. In the four first lanes on the left the KCl concentration of the binding buffer varied from 0.1 to 2 M. In the four lanes on the right, binding reactions were carried out in 1 M KCl and in the presence of a 100-fold excess of the indicated competitors. (B) Analysis of five independent binding experiments of PABP1 and PABP3 to poly(A)–Sepharose in the presence of 1 or 2 M KCl. *P < 0.05; **P < 0.001.
Genomic analysis of PABP3 and PABP4 sequences
Recently Kleene et al. (39) reported that mouse testis PABP2 is encoded by an intronless gene. In order to determine whether the human testis-specific PABP3 gene might also be an intronless gene, we amplified the PABP1 and PABP3 genomic sequences using a set of oligonucleotides designed to clone the ORFs. The specific fragments were revealed by hybridisation with oligonucleotides specific for PABP1 and PABP3 (Fig. 6). We obtained a 23 kb genomic PABP1 fragment as expected from the human PABP1 gene sequence. The same set of oligonucleotides amplified a 2 kb band on the PABP1 plasmid. In contrast, a fragment of 1.9 kb was amplified from genomic DNA or plasmid cDNA using the PAPBP3-specific oligonucleotide, a result which is compatible with the presence of an intronless gene. The lack of an amplified fragment on genomic DNA using primers T7 and 3′ORF indicated that the signal cannot arise from genomic DNA contamination by the plasmid. The recent availability of a working draft of human chromosome 13 (accession no. AL359757.4), on which the PABP3 gene is located (33), confirmed that this gene is intronless. We also determined that the PABP4 pseudogene is intronless (data not shown)
Figure 6.
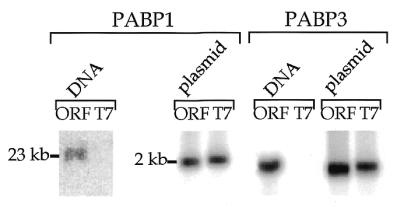
PCR analysis of PABP1 and PABP3 genomic sequences. PCR amplification reactions of human genomic DNA (DNA) were performed using a 3′ primer (3′ORF) common for PABP1 and PABP3 and a 5′ primer (5′ORF) specific for PABP1 and PABP3 or a T7 sequence-specific primer which generates a fragment from a plasmid (plasmid). The lanes ORF contain samples amplified with oligonucleotides 3′ORF and 5′ORF, the lanes T7 contain the samples amplified with oligonucleotides 3′ORF and T7.
5′-End cloning of PABP3 mRNA
In order to determine whether PABP3 cDNA corresponded to a full-length mRNA the oligonucleotide RACE (Table 1) was hybridised to human testis poly(A) mRNA and the extension products cloned. The sequence of 25 clones revealed four potential initiation start sites located 26, 40, 42 and 58 bp upstream from the initiator ATG, extending the PABP3 cDNA by a maximum of 35 nt (Fig. 7). Therefore, the 5′-UTR of PABP3 is expected to be very short and it should be noted that it does not contain any poly(A) stretches as described for PABP1 (14).
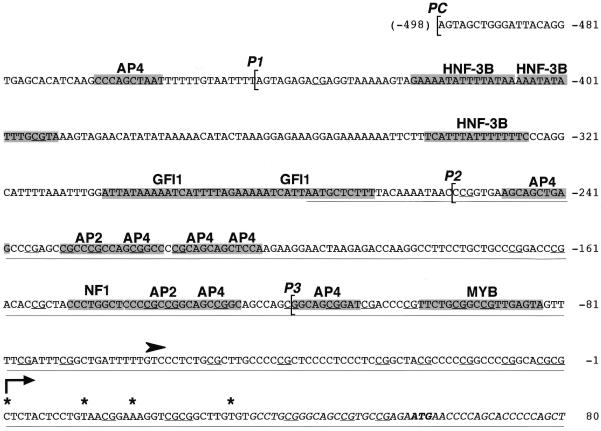
Figure 7.
Sequence of the human PABP3 promoter. Grey boxes represent the location of potential binding sites for transcription factors identified using the program MatInspector (core similarity = 1; matrix similarity > 0.87). Other motifs meeting the above criteria are not shown: BRN2, CETS1P54, E47, FREAC7, IK2, LMO2COM, SRY, NF1, ROX and XFD2. The transcription start site identified by S1 mapping and corresponding to the 5′-end of the longest RACE product is indicated by an arrow at position +1. Asterisks indicate the 5′-end of cDNA sequences obtained by 5′-RACE. The translation start site is in bold and the CpG are underlined. Brackets indicate the 5′-end of the four constructs used in the transfection experiments, PC, P1, P2 and P3, respectively. The black arrowhead indicates the relative position of the transcription start site in the mouse testis-specific PABP2 gene (accession no. AF001290). The underlined sequence exhibits 91% identical nucleotide residues with the 5′-UTR of human PABP1 (accession no. AH007272).
Isolation and characterisation of the promoter region of the PABP3 gene
Screening of a human genomic library for the PABP3 gene resulted in isolation of two positive BAC clones. They contained a 100 and a 150 kb insert, respectively, each including a 3.5 kb SphI fragment that hybridised to oligonucleotide Seq1 specific for the extremity of the 5′-UTR of the PAPB3 cDNA. This fragment rearranged systematically during subcloning. Therefore, we sequenced the BAC insert as described in Materials and Methods up to 556 bp upstream from the initiator ATG. This fragment was cloned by PCR and its sequence is reported in Figure 7. Alignment of the PABP3 cDNA with this genomic fragment showed that 23 nt of its 5′-UTR and a further 35 nt determined by primer extension were contiguous on the gene and lay within a single exon.
S1 nuclease protection analysis of this genomic DNA fragment by hybridisation to poly(A) mRNA from human testis resulted in protection of a 239 bp fragment (Fig. 8). The 5′-end of this fragment maps exactly at the 5′-end of the longest PABP3 cDNA obtained by primer extension of the PABP3 mRNA, located 58 nt upstream of the initiation codon. As expected, no DNA fragment was protected by hybridisation of the probe with liver poly(A) mRNA, which does not contain PABP3 mRNA. The specificity of this S1 nuclease experiment was also confirmed by the protection obtained by hybridisation to a synthetic PABP3 mRNA (240 bp) and not to PABP1 mRNA (data not shown). This experiment identifies the start site of PABP3 mRNA and therefore the promoter region of this gene. As shown in Figure 7, this region revealed several consensus sequences: AP4 (–456, –241, –216, –205, –202, –125, –108); HNF3β (–406, –391, –326); GFI1 (–281, –267); NF1 (–227, –220); AP2 (–219, –131); MYB (–83). Other consensus sequences are mentioned in the legend to Figure 7. CpG residues represent 21.6% of the nucleotides between bases 1 and –256 of the promoter and 0.8% further upstream. Alignment of the human PABP3 promoter with the human PABP1 and mouse PABP2 promoters revealed that the first 277 bp of the promoter of the PAPB3 gene exhibit 91.4% identity with the 5′-UTR of PABP1 mRNA and that the promoter of the human testis PABP3 has an initiation start site located 100 bp downstream as compared to the putative start site of the mouse PABP2 gene (Fig. 7).
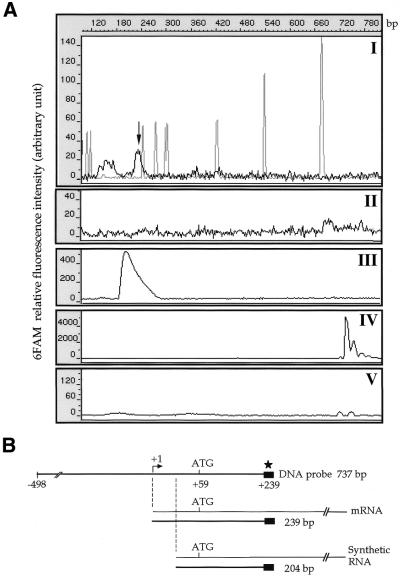
Figure 8.
S1 mapping of the PAPB3 promoter on human testis and liver mRNA. (A) S1 nuclease protection assay. A 737 bp gel-purified 6FAM-labeled RACE antisense probe containing potential transcription start sites identified by mRNA primer extension analysis was hybridised to 2 µg poly(A)+ RNA from either testis (I) or liver (II). As a positive control the S1 probe was also hybridised to 10 ng PABP3 synthetic RNA (accession no. AF132026) lacking nt 1–36 (see Fig. 7) (III). The undigested probe or probe digested with S1 in the absence of RNA is shown in (IV) and (V), respectively. The arrow indicates the DNA fragment protected by hybridisation to poly(A)+ mRNA. The grey peaks in (I) correspond to ROX-1000 DNA standards (Perkin Elmer, CA). The 6FAM relative fluorescence intensity (arbitrary units) is reported on the left. (B) The position of the single-stranded PABP3 DNA probe with respect to the endogenous and synthetic PABP3 mRNA, as well as the protected fragment, is diagrammed at the bottom. Numbering is relative to the transcription start site.
Promoter activity of the genomic sequence flanking the PABP3 mRNA start site
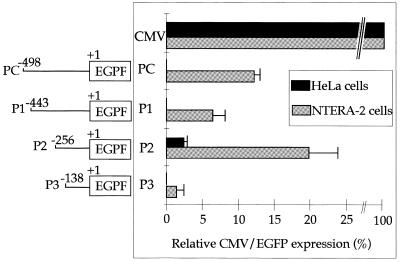
The promoter activity of the 498 bp sequence flanking the PABP3 mRNA start site was assessed for its ability to drive expression of an EGFP reporter gene in transiently transfected NTERA-2 cells, which express endogenous PABP3 mRNA as demonstrated by RT–PCR (data not shown). The shortest construct (–130, +1) drove low but significant activity in NTERA-2 cells, which represented 1.42 ± 1.02% of the activity driven by the CMV promoter in the same cells (Fig. 9). Extension of this construct up to residue –256 resulted in a 14-fold increase (19.87 ± 3.99%) in EGFP fluorescence in NTERA-2 cells, activity of the construct dropped to 6.43 ± 1.8% of the control when it was extended to –443 bp, whereas a further extension of the sequence up to –498 bp induced an increase in EGFP fluorescence up to 12.2 ± 0.77% of the activity driven by the CMV promoter. As a negative control we used HeLa cells, which do not express PABP3 mRNA as assessed by RT–PCR (data not shown). Transfection of these cells with EGFP under control of the CMV promoter exhibited four times more activity than in NTERA-2 cells. Following transfection of the different PABP3 promoter constructs into HeLa cells only the P2 construct exhibited a very weak promoter activity (2.42 ± 0.44% of the control). These data demonstrate that the –498 nucleotide sequence upstream from the transcription start site of the PAPB3 mRNA contains all the elements necessary for cell-specific expression of the PABP3 promoter.
Figure 9.
Expression of EGFP protein in cells transiently transfected with EGFP under the control of PABP3 promoter sequences. PABP3 genomic sequences extending from nt –498 to +30 were amplified by PCR, as described in Materials and Methods, and then inserted into a promoter-less EGFP vector (pEGFP-1). These constructs were transiently transfected into HeLa (black boxes) or NTERA-2 (grey boxes) cells. The numbers of cells counted were identical in the two transfection assays. EGFP fluorescence driven by each construct was normalised to that obtained with the CMV promoter/pEGFP-C1 vector (fluorescence of 100%). Results are the means of triplicate determinations obtained in two independent experiments. CMV corresponds to the control construct of the CMV promoter upstream from EGFP. PC, P1, P2 and P3 refer to the different PABP3 promoter constructs upstream from the EGFP reporter gene as indicated on the left.
DISCUSSION
We have characterised two novel PABP mRNAs (PABP3 and PABP4), isolated from human testis, which exhibit strong similarities to human PABP1. Sequence analysis of the PABP4 mRNA reveals an ORF encoding a truncated protein of 56 amino acids which contains only half of RBDI (RNP2) and is unable to bind to ribohomopolymers. Therefore, the PABP4 gene, which encodes a non-functional protein, should be considered as a pseudogene.
More interestingly, we identified a new functional PABP in human testis (PABP3) which is under the control of a cell-specific promoter. PABP3 is highly similar to the ubiquitous human PABP1. These two proteins differ mainly by four amino acid changes in the RBDs and by deletion of 18 nt in PABP3 as compared to PABP1, which eliminates the PVINPY sequence in PABP3. This motif is located immediately upstream from the proline-rich region involved in protein–protein interactions (18). Therefore, PABP3 might display specific properties in its interaction with other proteins, such as translation initiation factors or viral proteins.
Northern blot revealed two testis-specific PABP3 mRNAs (2.5 and 2.1 kb). Three polyadenylation signals are present in the PAPB3 cDNA sequence located at positions 2015, 2035 and 2373. The mRNA that we cloned was polyadenylated at the last site, but we cloned a shorter PABP3 cDNA which used the first polyadenylation signal located 358 bp upstream. Therefore, it is very likely that the 2.1 and the 2.5 kb PABP3 mRNA species are generated by alternative polyadenylation. A similar situation exists for PABP1 mRNA, which contains two polyadenylation signals at nt 2507 and 2823 (11). Of 270 PABP1 ESTs in the dbest database, 90 were generated from transcripts polyadenylated at the upstream site and 180 were generated from transcripts polyadenylated at the downstream site.
Due to high similarities among the human PABP species we analysed PABP expression using specific oligonucleotides for detection of specific PABP transcripts by northern blotting and by semi-quantitative RT–PCR. PABP1 mRNA is the major transcript in human testis, as well as in other tissues, whereas PABP3 expression appears to be testis-specific, where it represents 18% of PABP mRNA; no signals were observed for PABP4 and iPABP. Using semi-quantitative RT–PCR it was determined that iPABP mRNA represented <7% of the PABP1 mRNA level in human testis. This result differs from those of Yang et al. (12), who reported that both PABP1 and iPABP mRNA were expressed at similar levels in all human tissues tested, including testis. This discrepancy might result from cross-hybridisation between the PABP probes, since they used a cDNA probe.
We have demonstrated that PABP1 and PABP3 are expressed in spermatogenic cells at different differentiation stages. Human PABP1 is expressed in pachytene spermatocytes and round spermatids, whereas expression of the specific testis isoform appears later in spermatogenesis and is restricted to round spermatids. The distribution of PAPB mRNA in human testis differs slightly from the PABP mRNA expression reported in mouse testis, where PABP1 and testis-specific PABP2 mRNA co-localised in pachytene spermatocytes and round spermatids (10).
Human PABP3 binds to poly(A) sequences with a lower affinity than PABP1. This can be related to the amino acid substitutions detected in the different RBDs, however, we did not observe any of the tyrosine substitutions described in mouse testis PABP (10) which were reported to decrease binding to poly(A). As for other PABPs (10,12,30,31,40), poly(A) binding to PABP3 is adversely affected by poly(C) and poly(U), whereas poly(G) enhances poly(A) binding activity. This might result from a poly(G)-binding site on PABP, located in RBD III and/or IV and distinct from the poly(A) sites (30). Binding of poly(G) would promote conformational changes in the protein leading to an increased affinity of the poly(A) sites of the first two RBDs.
Human testis-specific PABP3 and PABP4 mRNAs are transcribed from intronless genes which very likely correspond to retroposons. Expression of functional retroposons is a common phenomenon in meiotic and haploid spermatogenic cells, as pointed out by Kleene et al. (39). The gene tree of 21 PABP sequences from various species (data not shown) revealed that the human PABP3 and PABP4 genes appeared independently of the mouse testis-specific retroposon PABP2. These genes likely derive from PABP1, which exhibits 94 and 87% identity with the human PABP3 and PABP4 sequences, respectively. Based on these similarities, PABP4 might have appeared before PABP3.
We have identified the transcription start site on the PABP3 gene by 5′-RACE and S1 mapping. Several constructs of the genomic region with this start site were conjugated with an EGFP reporter gene and transfected into NTERA-2 and HeLa cells. From these experiments it appears that the PABP3 promoter is tissue-specific, since it was not functional in HeLa cells, although these cells expressed the control CMV/pEGFP construct at a very high level. The region (–130, +1) is sufficient to drive low, but significant activity in NTERA-2 cells, but not in HeLa cells. Interestingly, a myb-binding site, able to bind A-myb and possibly involved in the proliferation and differentiation of spermatogonia (41–43), is located in this sequence. Activation of this myb element could play a role in the tissue-specific expression of the PABP3 promoter, as has been suggested for other testis-specific genes such as PGK-2 (44) and Hsp70-2 (45). An extension of the promoter sequence up to nt 256 upstream from the initiation start site strongly induces promoter activity, up to 20% of the activity of the control CMV/pEGFP. This region contains four AP4, two AP2 and one NF1 binding sequence. Binding of ubiquitous factors to this region could depend on the presence of other interacting testis-specific factors, as proposed for the mouse Tcp-10bt gene (46). A further extension of the promoter sequence up to –446 inhibits promoter activity. This region contains a well-conserved site for Gf-1 (47), a transcriptional repressor described in a large series of genes encoding various cytokines and regulators of cell proliferation and differentiation. Therefore, it is tempting to speculate that the decrease in promoter activity of the longest construct might result from an interaction with Gfi-1. Finally, it should be pointed out that the PABP3 gene is transcribed from a CpG-rich promoter. Several germline-specific genes with CpG-rich promoters, such as MAGE-AI and LAGE-I, are largely unmethylated in male germ cells and methylated in somatic tissues (48). Such a lack of methylation in testis would be sufficient to explain the testis specificity of the PABP3 promoter.
Interestingly, the 256 bp upstream from the initiation start site of the PABP3 gene, which display the highest promoter activity, exhibit >91% identity with the 5′-UTR of PABP1 mRNA. This indicates that the 5′-UTR of this PAPB1 mRNA contains a potential promoter sequence for testis-specific expression. Until now there has been no evidence that this region functions as an alternative promoter for the PABP1 gene. Thus, it is likely that the region from 256 bp upstream of the PABP3 promoter plays a crucial role in commitment of this promoter in vivo. A mouse testis-specific PABP2 gene has also been described, but the promoter functionality and specificity of this gene has not yet been analysed. Its transcription start site would correspond to position –108 of the PABP3 promoter and, therefore, it lacks the region where we observed a myb-binding sequence. As for the PABP3 promoter, it exhibits AP2-binding sites susceptible to direct transcription in spermatogenic cells (49).
The presence of a functional PABP3 isoform in spermatogenic cells might contribute to testis-specific regulation of mRNA homeostasis. In meiotic and early haploid cells, stages at which many mRNAs are synthesised, PABP mRNAs are present at a high level (50). Nevertheless, most of the testicular PABP mRNAs are not engaged in polysomes, indicating that translation of PABP mRNAs is strongly repressed at these stages (10,51). However, this repression might be specific for PABP1, which contains an A-rich sequence in its 5′-UTR allowing binding of PABP and blockage of translation of its own mRNA (52–54). Clearly, human PABP3 does not contain an A-rich sequence in its 5′-UTR. Therefore, PAPB3 might represent the major functional species associated with polysomes in round spermatids. Expression of this testis-specific PABP mRNA might be a way for the cell to escape negative feedback control and to produce the amount of PABP protein needed for the active mRNA synthesis phase of spermatogenesis.
Acknowledgments
ACKNOWLEDGEMENTS
We are very grateful to Drs T.Grange and T.Lindsten for providing us with PABP1 and iPABP cDNA, respectively. We thank Drs J.Hanoune, Y.Laperche and S.Lotersztajn for critical reading of the manuscript. This work was partly funded by the Fondation de France and the Association pour la Recherche contre le Cancer. C.F. is the recipient of a grant from the Ministère de la Recherche et de la Technologie.
DDBJ/EMBL/GenBank accession nos+ To whom correspondence should be addressed. Tel: +33 1 49 81 35 30; Fax: +33 1 48 98 09 08; Email: AF132026, AF132027, AF315079
References
- 1.Yelick P.C., Kwon,Y.H., Flynn,J.F., Borzorgzadeh,A., Kleene,K.C. and Hecht,N.B. (1989) Mouse transition protein 1 is translationally regulated during the postmeiotic stages of spermatogenesis. Mol. Reprod. Dev., 1, 193–200. [DOI] [PubMed] [Google Scholar]
- 2.Steger K., Klonisch,T., Gavenis,K., Drabent,B., Doenecke,D. and Bergmann,M. (1998) Expression of mRNA and protein of nucleoproteins during human spermiogenesis. Mol. Hum. Reprod., 4, 939–945. [DOI] [PubMed] [Google Scholar]
- 3.Schafer M., Nayernia,K., Engel,W. and Schafer,U. (1995) Translational control in spermatogenesis. Dev. Biol., 172, 344–352. [DOI] [PubMed] [Google Scholar]
- 4.Penttila T.L., Yuan,L., Mali,P., Hoog,C. and Parvinen,M. (1995) Haploid gene expression: temporal onset and storage patterns of 13 novel transcripts during rat and mouse spermiogenesis. Biol. Reprod., 53, 499–510. [DOI] [PubMed] [Google Scholar]
- 5.Tafuri S.R., Familari,M. and Wolffe,A.P. (1993) A mouse Y box protein, MSY1, is associated with paternal mRNA in spermatocytes. J. Biol. Chem., 268, 12213–12220. [PubMed] [Google Scholar]
- 6.Dreyfuss G. (1986) Structure and function of nuclear and cytoplasmic ribonucleoprotein particles. Annu. Rev. Cell Biol., 2, 459–498. [DOI] [PubMed] [Google Scholar]
- 7.Dreyfuss G., Matunis,M.J., Pinol-Roma,S. and Burd,C.G. (1993) hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem., 62, 289–321. [DOI] [PubMed] [Google Scholar]
- 8.Blobel G. (1973) A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc. Natl Acad. Sci. USA, 70, 924–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M.Y., Cutler,M., Karimpour,I. and Kleene,K.C. (1992) Nucleotide sequence of a mouse testis poly(A) binding protein cDNA. Nucleic Acids Res., 20, 3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleene K.C., Wang,M.Y., Cutler,M., Hall,C. and Shih,D. (1994) Developmental expression of poly(A) binding protein mRNAs during spermatogenesis in the mouse. Mol. Reprod. Dev., 39, 355–364. [DOI] [PubMed] [Google Scholar]
- 11.Grange T., Martins de Sa,C., Oddos,J. and Pictet,R. (1987) Human mRNA polyadenylate binding protein: evolutionary conservation of a nucleic acid binding motif. Nucleic Acids Res., 15, 4771–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H., Duckett,C.S. and Lindsten,T. (1995) iPABP, an inducible poly(A)-binding protein detected in activated human T cells. Mol. Cell. Biol., 15, 6770–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houng A.K., Maggini,L., Clement,C.Y. and Reed,G.L. (1997) Identification and structure of activated-platelet protein-1, a protein with RNA-binding domain motifs that is expressed by activated platelets. Eur. J. Biochem., 243, 209–218. [DOI] [PubMed] [Google Scholar]
- 14.Hornstein E., Git,A., Braunstein,I., Avni,D. and Meyuhas,O. (1999) The expression of poly(A)-binding protein gene is translationally regulated in a growth-dependent fashion through a 5′-terminal oligopyrimidine tract motif. J. Biol. Chem., 274, 1708–1714. [DOI] [PubMed] [Google Scholar]
- 15.Tarun S.Z,.Jr and Sachs,A.B. (1996) Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J., 15, 7168–7177. [PMC free article] [PubMed] [Google Scholar]
- 16.Sachs A.B. and Davis,R.W. (1989) The poly(A) binding protein is required for poly(A) shortening and 60S ribosomal subunit-dependent translation initiation. Cell, 58, 857–867. [DOI] [PubMed] [Google Scholar]
- 17.Imataka H., Gradi,A. and Sonenberg,N. (1998) A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J., 17, 7480–7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig A.W., Haghighat,A., Yu,A.T. and Sonenberg,N. (1998) Interaction of polyadenylate-binding protein with the eIF4G homologue PAIP enhances translation. Nature, 392, 520–523. [DOI] [PubMed] [Google Scholar]
- 19.Afonina E., Stauber,R. and Pavlakis,G.N. (1998) The human poly(A)-binding protein 1 shuttles between the nucleus and the cytoplasm. J. Biol. Chem., 273, 13015–13021. [DOI] [PubMed] [Google Scholar]
- 20.Joachims M., Van Breugel,P.C. and Lloyd,R.E. (1999) Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. J. Virol., 73, 718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerekatte V., Keiper,B.D., Badorff,C., Cai,A., Knowlton,K.U. and Rhoads,R.E. (1999) Cleavage of poly(A)-binding protein by coxsackievirus 2A protease in vitro and in vivo: another mechanism for host protein synthesis shutoff? J. Virol., 73, 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piron M., Vende,P., Cohen,J. and Poncet,D. (1998) Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J., 17, 5811–5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawlak A., Toussaint,C., Levy,I., Bulle,F., Poyard,M., Barouki,R. and Guellaen,G. (1995) Characterization of a large population of mRNAs from human testis. Genomics, 26, 151–158. [DOI] [PubMed] [Google Scholar]
- 24.Pawlak A., Chiannikulchai,N., Ansorge,W., Bulle,F., Weissenbach,J., Gyapay,G. and Guellaen,G. (1998) Identification and mapping of 26 human testis mRNAs containing CAG/CTG repeats. Mamm. Genome, 9, 745–748. [DOI] [PubMed] [Google Scholar]
- 25.Bulle F., Chiannilkulchai,N., Pawlak,A., Weissenbach,J., Gyapay,G. and Guellaen,G. (1997) Identification and chromosomal localization of human genes containing CAG/CTG repeats expressed in testis and brain. Genome Res., 7, 705–715. [DOI] [PubMed] [Google Scholar]
- 26.Pawlak A., Cohen,E.H., Octave,J.N., Schweickhardt,R., Wu,S.J., Bulle,F., Chikhi,N., Baik,J.H., Siegrist,S. and Guellaen,G. (1990) An alternatively processed mRNA specific for γ-glutamyl transpeptidase in human tissues. J. Biol. Chem., 265, 3256–3262. [PubMed] [Google Scholar]
- 27.Giuili G., Scholl,U., Bulle,F. and Guellaen,G. (1992) Molecular cloning of the cDNAs coding for the two subunits of soluble guanylyl cyclase from human brain. FEBS Lett., 304, 83–88. [DOI] [PubMed] [Google Scholar]
- 28.Chomczynski P. and Sacchi,N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem., 162, 156–159. [DOI] [PubMed] [Google Scholar]
- 29.Holic N., Suzuki,T., Corlu,A., Couchie,D., Chobert,M.N., Guguen-Guillouzo,C. and Laperche,Y. (2000) Differential expression of the rat gamma-glutamyl transpeptidase gene promoters along with differentiation of hepatoblasts into biliary or hepatocytic lineage. Am. J. Pathol., 157, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burd C.G., Matunis,E.L. and Dreyfuss,G. (1991) The multiple RNA-binding domains of the mRNA poly(A)-binding protein have different RNA-binding activities. Mol. Cell. Biol., 11, 3419–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson M.S. and Dreyfuss,G. (1988) Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol. Cell. Biol., 8, 2237–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Don R.H., Cox,P.T., Wainwright,B.J., Baker,K. and Mattick,J.S. (1991) ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res., 19, 4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feral C., Mattei,M.G., Pawlak,A. and Guellaen,G. (1999) Chromosomal localization of three human poly(A)-binding protein genes and four related pseudogenes. Hum. Genet., 105, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quandt K., Frech,K., Karas,H., Wingender,E. and Werner,T. (1995) MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res., 23, 4878–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozak M. (1986) Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell, 44, 283–292. [DOI] [PubMed] [Google Scholar]
- 36.Kozak M. (1987) An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res., 15, 8125–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birnstiel M.L., Busslinger,M. and Strub,K. (1985) Transcription termination and 3′ processing: the end is in site! Cell, 41, 349–359. [DOI] [PubMed] [Google Scholar]
- 38.Wickens M. and Stephenson,P. (1984) Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3′ end formation. Science, 226, 1045–1051. [DOI] [PubMed] [Google Scholar]
- 39.Kleene K.C., Mulligan,E., Steiger,D., Donohue,K. and Mastrangelo,M.A. (1998) The mouse gene encoding the testis-specific isoform of poly(A) binding protein (Pabp2) is an expressed retroposon: intimations that gene expression in spermatogenic cells facilitates the creation of new genes. J. Mol. Evol., 47, 275–281. [DOI] [PubMed] [Google Scholar]
- 40.Nietfeld W., Mentzel,H. and Pieler,T. (1990) The Xenopus laevis poly(A) binding protein is composed of multiple functionally independent RNA binding domains. EMBO J., 9, 3699–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trauth K., Mutschler,B., Jenkins,N.A., Gilbert,D.J., Copeland,N.G. and Klempnauer,K.H. (1994) Mouse A-myb encodes a trans-activator and is expressed in mitotically active cells of the developing central nervous system, adult testis and B lymphocytes. EMBO J., 13, 5994–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi T., Nakagoshi,H., Sarai,A., Nomura,N., Yamamoto,T. and Ishii,S. (1995) Human A-myb gene encodes a transcriptional activator containing the negative regulatory domains. FEBS Lett., 358, 89–96. [DOI] [PubMed] [Google Scholar]
- 43.Mettus R.V., Litvin,J., Wali,A., Toscani,A., Latham,K., Hatton,K. and Reddy,E.P. (1994) Murine A-myb: evidence for differential splicing and tissue-specific expression. Oncogene, 9, 3077–3086. [PubMed] [Google Scholar]
- 44.Goto M., Koji,T., Mizuno,K., Tamaru,M., Koikeda,S., Nakane,P.K., Mori,N., Masamune,Y. and Nakanishi,Y. (1990) Transcription switch of two phosphoglycerate kinase genes during spermatogenesis as determined with mouse testis sections in situ. Exp. Cell Res., 186, 273–278. [DOI] [PubMed] [Google Scholar]
- 45.Dix D.J., Rosario-Herrle,M., Gotoh,H., Mori,C., Goulding,E.H., Barrett,C.V. and Eddy,E.M. (1996) Developmentally regulated expression of Hsp70-2 and a Hsp70-2/lacZ transgene during spermatogenesis. Dev. Biol., 174, 310–321. [DOI] [PubMed] [Google Scholar]
- 46.Ewulonu U.K., Snyder,L., Silver,L.M. and Schimenti,J.C. (1996) Promoter mapping of the mouse Tcp-10bt gene in transgenic mice identifies essential male germ cell regulatory sequences. Mol. Reprod. Dev., 43, 290–297. [DOI] [PubMed] [Google Scholar]
- 47.Zweidler-Mckay P.A., Grimes,H.L., Flubacher,M.M. and Tsichlis,P.N. (1996) Gfi-1 encodes a nuclear zinc finger protein that binds DNA and functions as a transcriptional repressor. Mol. Cell. Biol., 16, 4024–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Smet C., Lurquin,C., Lethe,B., Martelange,V. and Boon,T. (1999) DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol. Cell. Biol., 19, 7327–7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleene K.C. and Mastrangelo,M.A. (1999) The promoter of the poly(A) binding protein 2 (Pabp2) retroposon is derived from the 5′-untranslated region of the Pabp1 progenitor gene. Genomics, 61, 194–200. [DOI] [PubMed] [Google Scholar]
- 50.Wolgemuth D.J. and Watrin,F. (1991) List of cloned mouse genes with unique expression patterns during spermatogenesis. Mamm. Genome, 1, 283–288. [DOI] [PubMed] [Google Scholar]
- 51.Gu W., Kwon,Y., Oko,R., Hermo,L. and Hecht,N.B. (1995) Poly(A) binding protein is bound to both stored and polysomal mRNAs in the mammalian testis. Mol. Reprod. Dev., 40, 273–285. [DOI] [PubMed] [Google Scholar]
- 52.de Melo Neto O.P., Standart,N. and Martins de Sa,C. (1995) Autoregulation of poly(A)-binding protein synthesis in vitro. Nucleic Acids Res., 23, 2198–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bag J. and Wu,J. (1996) Translational control of poly(A)-binding protein expression. Eur. J. Biochem., 237, 143–152. [DOI] [PubMed] [Google Scholar]
- 54.Wu J. and Bag,J. (1998) Negative control of the poly(A)-binding protein mRNA translation is mediated by the adenine-rich region of its 5′-untranslated region. J. Biol. Chem., 273, 34535–34542. [DOI] [PubMed] [Google Scholar]