Abstract
Recent results from high-throughput and other screening approaches reveal that small molecules can directly interact with recombinant full-length tau monomers and fibrillar tau aggregates in three distinct modes. First, in the high concentration regime (>10 micromolar), certain anionic molecules such as Congo red efficiently promote tau filament formation through a nucleation-elongation mechanism involving a dimeric nucleus and monomer-mediated elongation. These compounds are useful for modeling tau aggregation in vitro and in biological models. Second, in the low concentration regime (<1 micromolar), other ligands, including cyanine dyes, display aggregation antagonist activity. Compounds that can prevent or reverse fibrillization are candidate modifiers of disease pathology. Finally, certain compounds bind mature tau fibrils with varying affinities at multiple binding sites without modulating the aggregation reaction. For some ligands, >10-fold selectivity for tau aggregates relative to filaments composed of beta-amyloid or alpha-synuclein can be demonstrated at the level of binding affinity. Together these observations suggest that small-molecules have utility for interrogating the tau aggregation pathway, for inhibiting neuritic lesion formation, and for selective pre-mortem detection of neurofibrillary lesions through whole brain imaging.
Keywords: Alzheimer’s disease, frontotemporal lobar degeneration, tau, neurofibrillary tangle, paired helical filaments, aggregation
INTRODUCTION
Alzheimer’s disease (AD) is definitively diagnosed on the basis of pathology. Characteristic neuritic brain lesions include neurofibrillary tangles (corresponding to affected nerve cell bodies), neuropil threads (affected neuronal processes), and dystrophic neurites (swollen and misshapen nerve cell processes associated with Aβ plaques). All neuritic morphologies involve intracellular deposition of aggregated forms of tau, a microtubule-associated protein that normally functions as a monomer in conjunction with its physiological binding partner, tubulin. Because the transformation of tau from monomer to aggregate along with changes in its state of post-translational modification correlates with neurodegeneration and cognitive decline, neuritic lesion formation can serve as a marker of AD neurodegeneration. In fact, the hierarchical appearance of neuritic lesions is commonly used to stage AD progression (1). Alzheimer’s like changes in tau structure occur in certain frontotemporal lobar degeneration (FTLD) diseases as well, where they can involve glial cells in addition to neurons, and also in inclusion body myositis, where they accumulate in muscle cells. In some familial cases of FTLD, disease is conferred by mutations in the tau gene, suggesting a closer link between tau misfunction and neurodegeneration than implied solely by histological correlation studies, and raising the possibility that tau structural transitions contribute to pathogenesis through gain-of-function or loss-of-function effects. Because of tau’s established role as a marker of AD progression, and its putative role as a mediator of neurodegeneration, small molecules that directly interact with tau protein are being investigated in three contexts: as a means of leveraging an established marker of neurodegeneration for premortem diagnosis of AD and other tauopathic neurodegenerative diseases, as a means of modeling the biological effects of intracellular tau aggregation in biological models, and as a potential avenue to therapeutics that attack a root cause of neurodegeneration. Recent progress in these areas is summarized below.
NEUROFIBRILLARY LABELS
Filamentous tau adopts cross-β-sheet conformation, consisting of parallel, in-register β-sheets oriented perpendicular to the filament axis, with two molecules of tau per β-sheet spacing (2). Mature tau filaments having this architecture are termed paired helical filaments when isolated from AD brain, but other morphologies have been observed in AD and certain FTLD diseases (3). The cross-β-sheet folding pattern, which is shared with Aβ protofilaments, generates channels along the filaments (4) to which heterocyclic small molecules can bind (5) (Fig. 1). However, molecular dynamics simulations have identified other potential binding modes, suggesting that cross-β-sheet conformations harbor multiple non- or partially overlapping binding sites for small molecules (6,7). These sites have been directly observed through ligand binding experiments carried out in three distinct formats. Radiometric methods involve direct incorporation of a radionuclide into a candidate ligand, followed by direct measurement of specific binding stoichiometry and affinity in filter trap assays. In contrast, fluorescent ligands that change fluorescence intensity or undergo a Stokes shift in excitation and/or emission optima when bound to filamentous aggregates are assayed by measurement of intrinsic fluorescence. Finally, either of these approaches can be used in competition format with unlabeled compounds to assess binding affinity and relative occupancy of binding sites. The competition strategy frequently involves thioflavine dyes as intrinsic fluorescent probes (8), in part because these agents label authentic lesions in brain sections (9). Together, these assays have identified at least three distinct sites on synthetic Aβ(1–40) fibrils that are capable of binding diverse scaffold classes with varying stoichiometries and affinities (10,11). Certain derivatives of these molecules, including 11C-PIB, 11C-SB-13, 18F-AV-45, and 18F-BAY94-9172, are under development as contrast agents for β-amyloid plaques in whole-brain PET imaging (12). This approach is especially attractive for assessing the activity of investigational therapies designed to clear brain Aβ deposits (13). However, contrast agents capable of selectively binding tau lesions may better reflect AD stage and progression than those based on Aβ plaque formation. This is because total plaque density in neocortical regions correlates poorly with cognitive decline, whereas the appearance of neuritic lesions correlates well with neurodegeneration and cognitive decline (1,14,15).
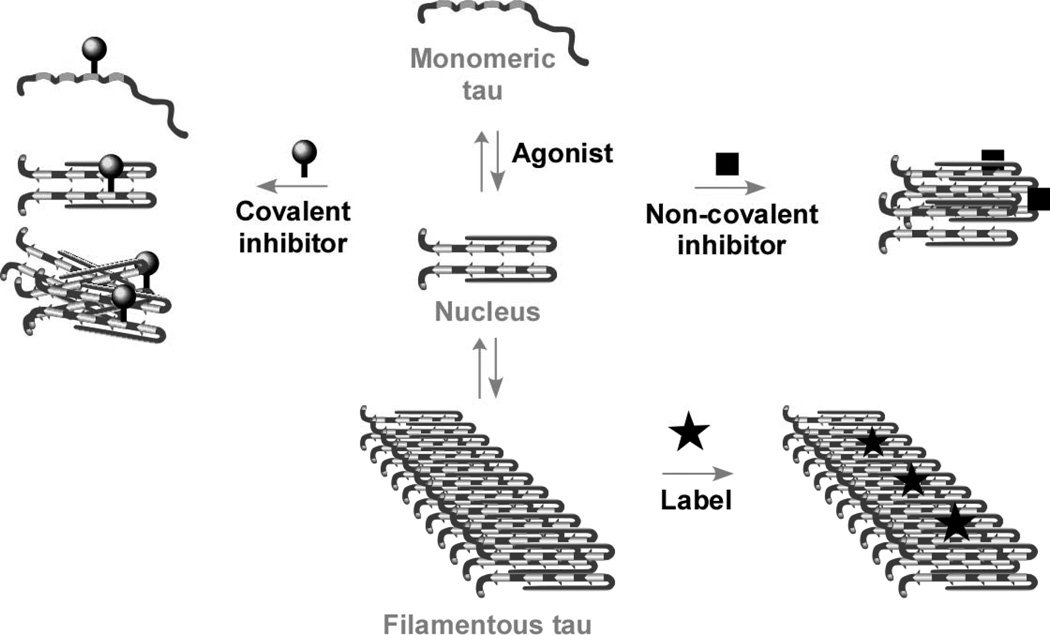
Fig. (1).
Hypothetical model of the tau aggregation pathway. Soluble full-length tau isoforms exist as natively disordered monomers that aggregate poorly at physiological concentrations. Agonists allow the aggregation reaction to proceed through a dimeric nucleus under physiological conditions over a period of hours. Antagonists inhibit fibrillization through covalent modification of nucleophilic tau residues or through non-covalent stabilization of off-fibrillization pathway species, both of which act to decrease free tau available to support filament formation. Labels bind mature tau filaments with high affinity but do not necessarily modulate the aggregation reaction.
Pursuit of this strategy will require molecules with selectivity for aggregates composed of tau protein relative to those composed of Aβ or other proteins. But because of the folding commonalities among filamentous aggregates composed of different proteins, it is likely that binding sites are shared among different lesions. Indeed, both β-amyloid plaques and tau-bearing neuritic lesions can be detected in situ by 18F-FDDNP (16), 18F-FENE (17), 18F-BF-108 (18), non-radiolabeled X-34 (19,20), and a family of iodinated flavones (21). Therefore, these agents may be suboptimal for selective detection of neuritic lesions. In the absence of a selective ligand for neuritic lesions, Shin and colleagues suggest both PIB and FDDNP be employed in the same subject for visualization of total AD pathology, with the net difference between them used to selectively assess the neuritic component (16).
Two in vitro studies have identified compounds that selectively bind tau aggregates. The first by Kudo and colleagues demonstrated that the benzimidazole BF-126 and quinolines BF-158 and BF-170 exhibited 2- to 3-fold selectivity for synthetic 1N4R tau aggregates compared to those composed of Aβ(1–42) (22). Despite modest selectivity, neurofibrillary lesions were preferentially stained compared to β-amyloid plaques in AD hippocampal brain sections. Because these measurements were carried out at nominally saturating concentrations of ligand, the observations may reflect higher binding stoichiometry on tau filaments, or the higher concentration of tau protomers (120 pmol/mg frontal cortex protein (23)) relative to protomeric Aβ (3–4 pmol/mg midfrontal, parietal, or temporal cortex protein (24,25)) reported in other areas of late-stage AD brain. Interestingly, these compounds did not detect neuropathological lesions in brain sections prepared from Pick’s disease or progressive supranuclear palsy brains, suggesting that these compounds favor the tau isoform composition and post-translational modification signature associated with AD. The second selectivity study identified small molecules that preferentially bound synthetic tau aggregates (composed of 2N4R human tau) over aggregates composed of Aβ(1–42) or α-synuclein (8). A library of 70,000 compounds was screened in competition binding format to identify compounds with submicromolar binding affinity. A secondary screen revealed that Thiazine Red R bound tau aggregates with greater than 10-fold selectivity compared to the other two substrate proteins tested. These data suggest that at least one order of magnitude selectivity can be generated at the major Thioflavine S binding site. The presence of multiple binding sites suggests that additional scaffold classes potentially capable of supporting selective binding await discovery.
Still, the approach faces additional challenges beyond binding selectivity. First, early stage tau aggregates appear within cells, as opposed to Aβ plaques which appear in the extracellular space. Thus, tau proteins are exposed to an extensive array of post-translational modifications and immersed in a crowded molecular environment. Indeed, authentic Lewy bodies (composed of α-synuclein as the aggregating protein) fail to bind 3H-PIB, although high affinity binding sites for this compound reside on synthetic α-synuclein filaments prepared in vitro (26). It will be essential to confirm the activity of all ligands discovered on the basis of in vitro binding assays against authentic tissue since binding sites may differ in protein protomers associated with lesions (27,28). Second, the rate of uptake into cells will influence the pharmacokinetic profile of each ligand, and hence the apparent selectivity for neuritic lesions versus other types of lesions. In silico pharmacokinetic modeling may clarify the kinetic properties that favor detection of intracellular tau aggregates. Finally, tau consists of multiple isoforms that may differentially interact with certain ligands. For example, aggregates composed of human Aβ(1–40) doped with small amounts of rodent Aβ(1–40) displayed fewer high affinity binding sites, suggesting that filament microheterogeneity arising from protein isoform mixtures influences binding site structure (29). This issue may be especially important for tau aggregates, which are composed of up to six distinct isoforms, each of which contributes different sequences to the cross-β-sheet structure at the core of each filament (30). Different isoform ratios predominate in other tauopathies (31), and this may change binding site characteristics in ways that are difficult to predict.
AGGREGATION AGONISTS
Unlike Aβ or α-synuclein, full-length tau proteins resist spontaneous aggregation in vitro under physiological conditions of protein concentration, temperature, pH, and ionic strength over tractable time periods (32). In biological models, this limitation has been overcome by high level tau overexpression (33–36), incorporation of aggregation-promoting missense mutations or deletions (37–43), or through aggressive post-translational modification (41,44). In vitro, resistance to aggregation can be overcome in the presence of polyanions (e.g., heparin (45,46)) or anionic surfactants (arachidonic acid or alkyl sulfate detergents). Although surfactants are small monomers in dilute solution, they readily aggregate to form micelles above a critical concentration. The presence of protein depresses surfactant critical micelle concentration and leads to formation of tau-surfactant complexes (47). These are predicted to vary in size depending on the surfactant employed and its concentration, ranging from small micelles arranged along the natively unfolded tau polypeptide chain (48) to larger protein-bound bodies visible in the electron microscope (47). Thus, despite their much smaller monomeric size relative to heparin, surfactants may share a limited ability to achieve aggregation-inducing concentrations in intact cells.
Recent studies show that the kinetic barrier to fibrillization also can be overcome by addition of agonist dyes such as Congo red, Thiazine red, and Thioflavine S (32). The resultant tau filaments display a twisted-ribbon morphology and a mass-per-unit-length similar to that of authentic tissue-derived filaments (49). Congo red can populate partially-folded amyloidogenic conformations of proteins (50), suggesting that agonists may function through thermodynamic linkage of binding with the aggregation reaction. Regardless of mechanism, the performance of small-molecule aggregation agonists differs substantially from macromolecular and micelle-based inducers. For example, the extent of the aggregation reaction does not depend on dye/tau ratios, thereby simplifying kinetic analysis (32). In addition, agonists facilitate fibrillization of full-length 4-repeat tau isoforms at submicromolar concentrations (49), which is well within physiological levels. These properties make small-molecule based tau aggregation inducers ideal for kinetic characterization of tau aggregation. In vitro, Thiazine red-induced aggregation follows a nucleation-elongation mechanism consistent with a dimeric nucleus, a monomeric extension reaction, and a submicromolar critical concentration (Fig. 1). Preliminary estimates of elementary rate constants derived from this model were consistent with the time dependent evolution of total filament length and also filament length distribution (49). These data suggest that tau fibrillization at physiological tau concentrations need not involve small soluble aggregates as intermediate species.
Wild-type tau aggregation provides a baseline for comparing the mechanism of action of FTLD-causing missense mutations, which act at different points in the aggregation pathway to promote fibril formation. Mutations that affect the Pro-Gly-Gly-Gly segments of microtubule binding repeats (e.g., G272V and P301L) lower the minimal concentration of tau necessary to support the aggregation reaction, while speeding reaction rate at the level of nucleation (51). In contrast, other mutations, such as R5L and V337M, accelerate the rate of filament formation at the level of nucleation without significantly altering the minimal tau concentration necessary to support the reaction. The differential activity of missense mutations on individual steps in the pathway may influence how tau misfunction leads to clinically and histopathologically distinct diseases.
Self association mechanism is but one constraint on the ability of tau to form filaments in vivo. Within cells, normal and misfolded tau interacts with chaperones and protein quality control circuitry as well. The complex interplay of these pathways, and whether tau aggregation is associated with toxicity, is best approached in biological models. Certain agonists, such as Congo red, are capable of passively crossing cell membranes (but not the blood brain barrier) so as to contact cytoplasmic tau, drive in situ aggregation, and create novel biological models of aggregation (52). The approach complements the strategy of expressing aggregation-promoting mutations/truncations in three ways. First, agonists drive robust aggregation, with up to 40% of bulk tau becoming insoluble at reaction plateau (52). Second, agonists offer superior control over aggregation initiation, which ensues once agonist is added to culture media. Aggregation is reversible after removal of agonist from media, and can be further modulated by changes in bulk tau expression afforded by use of inducible promoters (52). Finally, agonists are amenable to use with microfluidic strategies for isolating cell bodies from cell processes (53), and so may offer a route to spatial control over aggregation initiation. Together these characteristics may be advantageous for assessing tau aggregate toxicity and accumulation, and its temporal relationship with post-translational modifications.
AGGREGATION ANTAGONISTS
The association of aggregation-promoting missense tau mutations with neurodegenerative disease (31), combined with toxicity associated with tau aggregation in model systems (54), suggests that the accumulation of tau aggregates may contribute to neurodegeneration. If so, then direct interference with aggregation may halt disease progression in affected individuals. The strategy is attractive because tau aggregation is associated with diseased neurons but not normal biology. Early screens for tau aggregation antagonists focused on the ability of aggregation-prone tau fragments to recruit full-length tau into aggregates. These assays identified methylene blue (tetramethylthionine chloride) and other phenothiazine derivatives as inhibitors of this reaction at low or even sub-micromolar concentrations (55). Treatment of mild-to-moderate AD sufferers with methylene blue (30 – 60 mg twice daily) has been reported to halt cognitive decline, improve cerebral blood flow, and improve fluorodeoxyglucose uptake relative to placebo over a ~1 year treatment period (55). Thus at least one tau aggregation antagonist may have therapeutic utility for AD. Nonetheless, methylene blue also is a redox-active agent with affinity for the heme moieties of several proteins. In fact, this activity has been leveraged to treat methemoglobinemia in humans (i.e., to reduce heme-associated iron from the abnormal ferric state to the oxygen-carrying ferrous state) (56). Moreover, it has antioxidant activity owing to effects on the electron transport chains of mitochondria (57). Currently, it is not clear whether the therapeutic benefits of methylene blue stem from its aggregation antagonist activity, its antioxidant effects on mitochrondria, or its direct binding to and modulation of heme-containing proteins.
Additional non-phenothiazine aggregation inhibitors will help clarify this issue. Toward this end, the ability of heparin and anionic surfactants to induce the aggregation of recombinant tau or tau fragments in vitro has been leveraged to screen for novel aggregation antagonists. High throughput screening has identified over 400 candidate inhibitors composed of diverse heterocycle scaffolds (58–60). Many of these are polyalcohols, which when incubated under nonreducing conditions generate Michael acceptors capable of covalently reacting with tau protomer. For example 5,6,7-trihydroxyflavone (baicelein) oxidizes at pH 7.5 to form protein-reactive baicelein quinone with t1/2 ~15 h (61). Covalent modification effectively sequesters tau, thereby raising the apparent minimal concentration required to support the aggregation reaction (Fig. 1). Many of these compounds inhibit Aβ and α-synuclein aggregation as well, suggesting that the mechanism can be widely generalized to different aggregating proteins. Inhibition of protein aggregation through covalent modification has been shown for other scaffold classes as well (62). Further studies will be required to determine whether this mechanism can modulate tau aggregation in vivo.
Other antagonist scaffolds include phenylthiazolyl-hydrides (63), N-phenylamines (64), rhodanines (65), and thiacarbocyanines (66). The first two classes are reportedly active down to the micromolar range, whereas some rhodanines and cyanines are active at submicromolar concentrations. The mechanism of action of these compounds on tau is not established, but they may act to stabilize off-pathway aggregates, thereby depleting tau available to support the nucleation and extension reactions (Fig. 1). In fact, whether a compound acts as aggregation agonist or antagonist may depend on its ability to stabilize on- or off-aggregation pathway species (67,68). For tau, the former is generally favored by anionic compounds, whereas the latter appears to be favored by cationic compounds. Closer inspection of phenothiazine and thiacarbocyanine antagonists, representing two of the most potent antagonist scaffolds, reveals that both not only are cationic, but share push-pull electronic character (i.e., they contain an electron donating substituent separated from an electron accepting substituent by a conjugated ring system). As a result, both scaffolds are highly polarizable (i.e., charge can be localized to specific parts of the extended π system). This may facilitate π–π interactions with a complimentary set of one or more residue side chains present on tau. If so, then additional scaffolds with drug-like properties will be capable of supporting tau aggregation antagonist activity.
CONCLUSION
Tau aggregation is an established marker and potential mediator of neurodegeneration. Further development of small molecules capable of modulating tau biology will clarify the mechanisms underlying neuritic lesion formation, the feasibility of selectively detecting their presence, and the utility of antagonists for arresting their development. However, only clinical studies can validate the activity of molecules selected on the basis of biochemical or biological models. The recently demonstrated efficacy of methylene blue for treatment of Alzheimer’s disease supports further inquiry into tau-based diagnostic and therapeutic approaches.
ACKNOWLEDGMENTS
The National Institutes of Health (AG14452) supported this work.
ABBREVIATIONS USED
- AD
Alzheimer’s disease
- FTLD
Frontotermporal lobar degeneration
REFERENCES
- 1.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl.) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 2.von Bergen M, Barghorn S, Muller SA, Pickhardt M, Biernat J, Mandelkow EM, Davies P, Aebi U, Mandelkow E. The core of tau-paired helical filaments studied by scanning transmission electron microscopy and limited proteolysis. Biochemistry. 2006;45:6446–6457. doi: 10.1021/bi052530j. [DOI] [PubMed] [Google Scholar]
- 3.Ksiezak-Reding H, Wall JS. Characterization of paired helical filaments by scanning transmission electron microscopy. Microsc. Res. Tech. 2005;67:126–140. doi: 10.1002/jemt.20188. [DOI] [PubMed] [Google Scholar]
- 4.Krebs MR, Bromley EH, Donald AM. The binding of thioflavin-T to amyloid fibrils: localisation and implications. J. Struct. Biol. 2005;149:30–37. doi: 10.1016/j.jsb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Gazit E. A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002;16:77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 6.Wu C, Wang Z, Lei H, Duan Y, Bowers MT, Shea JE. The Binding of Thioflavin T and Its Neutral Analog BTA-1 to Protofibrils of the Alzheimer's Disease Aβ16–22 Peptide Probed by Molecular Dynamics Simulations. J. Mol. Biol. 2008;384:718–729. doi: 10.1016/j.jmb.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Wang Z, Lei H, Zhang W, Duan Y. Dual binding modes of Congo red to amyloid protofibril surface observed in molecular dynamics simulations. J. Am. Chem. Soc. 2007;129:1225–1232. doi: 10.1021/ja0662772. [DOI] [PubMed] [Google Scholar]
- 8.Honson NS, Johnson RL, Huang W, Inglese J, Austin CP, Kuret J. Differentiating Alzheimer disease-associated aggregates with small molecules. Neurobiol. Dis. 2007;363:229–234. doi: 10.1016/j.nbd.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallet PG, Guntern R, Hof PR, Golaz J, Delacourte A, Robakis NK, Bouras C. A comparative study of histological and immunohistochemical methods for neurofibrillary tangles and senile plaques in Alzheimer's disease. Acta Neuropathol. 1992;83:170–178. doi: 10.1007/BF00308476. [DOI] [PubMed] [Google Scholar]
- 10.Lockhart A, Ye L, Judd DB, Merritt AT, Lowe PN, Morgenstern JL, Hong G, Gee AD, Brown J. Evidence for the presence of three distinct binding sites for the thioflavin T class of Alzheimer's disease PET imaging agents on beta-amyloid peptide fibrils. J. Biol. Chem. 2005;280:7677–7684. doi: 10.1074/jbc.M412056200. [DOI] [PubMed] [Google Scholar]
- 11.Ye L, Morgenstern JL, Gee AD, Hong G, Brown J, Lockhart A. Delineation of positron emission tomography imaging agent binding sites on β-amyloid peptide fibrils. J. Biol. Chem. 2005;280:23599–23604. doi: 10.1074/jbc.M501285200. [DOI] [PubMed] [Google Scholar]
- 12.Small GW, Bookheimer SY, Thompson PM, Cole GM, Huang SC, Kepe V, Barrio JR. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7:161–172. doi: 10.1016/S1474-4422(08)70019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golde TE. Disease modifying therapy for AD? J. Neurochem. 2006;99:689–707. doi: 10.1111/j.1471-4159.2006.04211.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghoshal N, Garcia-Sierra F, Wuu J, Leurgans S, Bennett DA, Berry RW, Binder LI. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer's disease. Exp. Neurol. 2002;177:475–493. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- 15.Royall DR, Palmer R, Mulroy AR, Polk MJ, Roman GC, David JP, Delacourte A. Pathological determinants of the transition to clinical dementia in Alzheimer's disease. Exp. Aging Res. 2002;28:143–162. doi: 10.1080/03610730252800166. [DOI] [PubMed] [Google Scholar]
- 16.Shin J, Lee SY, Kim SH, Kim YB, Cho ZH. Multitracer PET imaging of amyloid plaques and neurofibrillary tangles in Alzheimer's disease. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, Cole GM, Small GW, Huang SC, Barrio JR. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer's disease. J. Neurosci. 2001;21:RC189. doi: 10.1523/JNEUROSCI.21-24-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamura N, Suemoto T, Shiomitsu T, Suzuki M, Shimadzu H, Akatsu H, Yamamoto T, Arai H, Sasaki H, Yanai K, Staufenbiel M, Kudo Y, Sawada T. A novel imaging probe for in vivo detection of neuritic and diffuse amyloid plaques in the brain. J. Mol. Neurosci. 2004;24:247–255. doi: 10.1385/JMN:24:2:247. [DOI] [PubMed] [Google Scholar]
- 19.Styren SD, Hamilton RL, Styren GC, Klunk WE. X-34, a fluorescent derivative of Congo red: a novel histochemical stain for Alzheimer's disease pathology. J. Histochem. Cytochem. 2000;48:1223–1232. doi: 10.1177/002215540004800906. [DOI] [PubMed] [Google Scholar]
- 20.Ikonomovic MD, Abrahamson EE, Isanski BA, Debnath ML, Mathis CA, Dekosky ST, Klunk WE. X-34 labeling of abnormal protein aggregates during the progression of Alzheimer's disease. Methods Enzymol. 2006;412:123–144. doi: 10.1016/S0076-6879(06)12009-1. [DOI] [PubMed] [Google Scholar]
- 21.Klunk WE, Lopresti BJ, Ikonomovic MD, Lefterov IM, Koldamova RP, Abrahamson EE, Debnath ML, Holt DP, Huang GF, Shao L, DeKosky ST, Price JC, Mathis CA. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer's disease brain but not in transgenic mouse brain. J. Neurosci. 2005;25:10598–10606. doi: 10.1523/JNEUROSCI.2990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamura N, Suemoto T, Furumoto S, Suzuki M, Shimadzu H, Akatsu H, Yamamoto T, Fujiwara H, Nemoto M, Maruyama M, Arai H, Yanai K, Sawada T, Kudo Y. Quinoline and benzimidazole derivatives: candidate probes for in vivo imaging of tau pathology in Alzheimer's disease. J. Neurosci. 2005;25:10857–10862. doi: 10.1523/JNEUROSCI.1738-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatoon S, Grundke-Iqbal I, Iqbal K. Brain levels of microtubule-associated protein tau are elevated in Alzheimer's disease: a radioimmuno-slot-blot assay for nanograms of the protein. J. Neurochem. 1992;59:750–753. doi: 10.1111/j.1471-4159.1992.tb09432.x. [DOI] [PubMed] [Google Scholar]
- 24.Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H, Wong P, Price D, Shen Y. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer's disease patients. Proc Natl Acad Sci U S A. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp. Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 26.Ye L, Velasco A, Fraser G, Beach TG, Sue L, Osredkar T, Libri V, Spillantini MG, Goedert M, Lockhart A. In vitro high affinity α-synuclein binding sites for the amyloid imaging agent PIB are not matched by binding to Lewy bodies in postmortem human brain. J. Neurochem. 2008;105:1428–1437. doi: 10.1111/j.1471-4159.2008.05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J. Med. Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 28.Lockhart A. Imaging Alzheimer's disease pathology: one target, many ligands. Drug Discov. Today. 2006;11:1093–1099. doi: 10.1016/j.drudis.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Ye L, Morgenstern JL, Lamb JR, Lockhart A. Characterisation of the binding of amyloid imaging tracers to rodent Aβ fibrils and rodent-human Aβ co-polymers. Biochem. Biophys. Res. Commun. 2006;347:669–677. doi: 10.1016/j.bbrc.2006.06.126. [DOI] [PubMed] [Google Scholar]
- 30.Novak M, Kabat J, Wischik CM. Molecular characterization of the minimal protease resistant tau unit of the Alzheimer's disease paired helical filament. EMBO J. 1993;12:365–370. doi: 10.1002/j.1460-2075.1993.tb05665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rademakers R, Cruts M, van Broeckhoven C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum. Mutat. 2004;24:277–295. doi: 10.1002/humu.20086. [DOI] [PubMed] [Google Scholar]
- 32.Chirita CN, Congdon EE, Yin H, Kuret J. Triggers of full-length tau aggregation: a role for partially folded intermediates. Biochemistry. 2005;44:5862–5872. doi: 10.1021/bi0500123. [DOI] [PubMed] [Google Scholar]
- 33.Gotz J, Probst A, Spillantini MG, Schafer T, Jakes R, Burki K, Goedert M. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J. 1995;14:1304–1313. doi: 10.1002/j.1460-2075.1995.tb07116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duff K, Knight H, Refolo LM, Sanders S, Yu X, Picciano M, Malester B, Hutton M, Adamson J, Goedert M, Burki K, Davies P. Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiology of disease. 2000;7:87–98. doi: 10.1006/nbdi.1999.0279. [DOI] [PubMed] [Google Scholar]
- 35.Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 36.Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, Andorfer C, Rosenberry TL, Lewis J, Hutton M, Janus C. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J. Neurosci. 2007;27:3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeTure M, Ko LW, Yen S, Nacharaju P, Easson C, Lewis J, van Slegtenhorst M, Hutton M, Yen SH. Missense tau mutations identified in FTDP-17 have a small effect on tau-microtubule interactions. Brain Res. 2000;853:5–14. doi: 10.1016/s0006-8993(99)02124-1. [DOI] [PubMed] [Google Scholar]
- 38.Gotz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J. Biol. Chem. 2001;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- 39.Lim F, Hernandez F, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J. FTDP-17 mutations in tau transgenic mice provoke lysosomal abnormalities and Tau filaments in forebrain. Molecular and cellular neurosciences. 2001;18:702–714. doi: 10.1006/mcne.2001.1051. [DOI] [PubMed] [Google Scholar]
- 40.Tanemura K, Akagi T, Murayama M, Kikuchi N, Murayama O, Hashikawa T, Yoshiike Y, Park JM, Matsuda K, Nakao S, Sun X, Sato S, Yamaguchi H, Takashima A. Formation of filamentous tau aggregations in transgenic mice expressing V337M human tau. Neurobiology of disease. 2001;8:1036–1045. doi: 10.1006/nbdi.2001.0439. [DOI] [PubMed] [Google Scholar]
- 41.Sato S, Tatebayashi Y, Akagi T, Chui DH, Murayama M, Miyasaka T, Planel E, Tanemura K, Sun X, Hashikawa T, Yoshioka K, Ishiguro K, Takashima A. Aberrant tau phosphorylation by glycogen synthase kinase-3β and JNK3 induces oligomeric tau fibrils in COS-7 cells. J. Biol. Chem. 2002;277:42060–42065. doi: 10.1074/jbc.M202241200. [DOI] [PubMed] [Google Scholar]
- 42.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenmann H, Grigoriadis N, Eldar-Levy H, Avital A, Rozenstein L, Touloumi O, Behar L, Ben-Hur T, Avraham Y, Berry E, Segal M, Ginzburg I, Abramsky O. A novel transgenic mouse expressing double mutant tau driven by its natural promoter exhibits tauopathy characteristics. Experimental neurology. 2008;212:71–84. doi: 10.1016/j.expneurol.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Sahara N, Murayama M, Lee B, Park JM, Lagalwar S, Binder LI, Takashima A. Active c-jun N-terminal kinase induces caspase cleavage of tau and additional phosphorylation by GSK-3β is required for tau aggregation. Eur. J. Neurosci. 2008;27:2897–2906. doi: 10.1111/j.1460-9568.2008.06258.x. [DOI] [PubMed] [Google Scholar]
- 45.Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 46.Perez M, Valpuesta JM, Medina M, Montejo de Garcini E, Avila J. Polymerization of tau into filaments in the presence of heparin: the minimal sequence required for tau-tau interaction. Journal of neurochemistry. 1996;67:1183–1190. doi: 10.1046/j.1471-4159.1996.67031183.x. [DOI] [PubMed] [Google Scholar]
- 47.Chirita CN, Necula M, Kuret J. Anionic Micelles and Vesicles Induce tau Fibrillization in vitro. J. Biol. Chem. 2003;278:25644–25650. doi: 10.1074/jbc.M301663200. [DOI] [PubMed] [Google Scholar]
- 48.Shirahama K, Tsujii K, Takagi T. Free-boundary electrophoresis of sodium dodecyl sulfate-protein polypeptide complexes with special reference to SDS-polyacrylamide gel electrophoresis. J. Biochem. 1974;75:309–319. doi: 10.1093/oxfordjournals.jbchem.a130398. [DOI] [PubMed] [Google Scholar]
- 49.Congdon EE, Kim S, Bonchak J, Songrug T, Matzavinos A, Kuret J. Nucleation-dependent tau filament formation: the importance of dimerization and an estimation of elementary rate constants. J. Biol. Chem. 2008;283:13806–13816. doi: 10.1074/jbc.M800247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim YS, Randolph TW, Manning MC, Stevens FJ, Carpenter JF. Congo red populates partially unfolded states of an amyloidogenic protein to enhance aggregation and amyloid fibril formation. J. Biol. Chem. 2003;278:10842–10850. doi: 10.1074/jbc.M212540200. [DOI] [PubMed] [Google Scholar]
- 51.Chang E, Kim S, Yin H, Nagaraja HN, Kuret J. Pathogenic missense MAPT mutations differentially modulate tau aggregation propensity at nucleation and extension steps. Journal of neurochemistry. 2008 doi: 10.1111/j.1471-4159.2008.05692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bandyopadhyay B, Li G, Yin H, Kuret J. Tau aggregation and toxicity in a cell culture model of tauopathy. J. Biol. Chem. 2007;282:16454–16464. doi: 10.1074/jbc.M700192200. [DOI] [PubMed] [Google Scholar]
- 53.Gross PG, Kartalov EP, Scherer A, Weiner LP. Applications of microfluidics for neuronal studies. J. Neurol. Sci. 2007;252:135–143. doi: 10.1016/j.jns.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Mocanu MM, Nissen A, Eckermann K, Khlistunova I, Biernat J, Drexler D, Petrova O, Schonig K, Bujard H, Mandelkow E, Zhou L, Rune G, Mandelkow EM. The potential for β-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous Tau in inducible mouse models of tauopathy. J. Neurosci. 2008;28:737–748. doi: 10.1523/JNEUROSCI.2824-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wischik CM, Bentham P, Wischik DJ, Seng KM. 11th International Conference on Alzheimer's Disease. Chicago, IL: 2008. Tau aggregation inhibitor (TAI) therapy with rember(tm) arrests disease progression in mild and moderate Alzheimer's disease over 50 weeks. [Google Scholar]
- 56.Wendel WB. The Control of Methemoglobinemia with Methylene Blue. The Journal of clinical investigation. 1939;18:179–185. doi: 10.1172/JCI101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. Faseb J. 2008;22:703–712. doi: 10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- 58.Larbig G, Pickhardt M, Lloyd DG, Schmidt B, Mandelkow E. Screening for inhibitors of tau protein aggregation into Alzheimer paired helical filaments: a ligand based approach results in successful scaffold hopping. Curr. Alzheimer Res. 2007;4:315–323. doi: 10.2174/156720507781077250. [DOI] [PubMed] [Google Scholar]
- 59.Mandelkow E, Mandelkow E-M, Biernat J, Bergen MV, Pickhardt M. Treating neurodegenerative conditions. PCT International Application. 2006 [Google Scholar]
- 60.Taniguchi S, Suzuki N, Masuda M, Hisanaga S, Iwatsubo T, Goedert M, Hasegawa M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J. Biol. Chem. 2005;280:7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 61.Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of α-synuclein and disaggregates existing fibrils. J. Biol. Chem. 2004;279:26846–26857. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
- 62.Crowe A, Ballatore C, Hyde E, Trojanowski JQ, Lee VM. High throughput screening for small molecule inhibitors of heparin-induced tau fibril formation. Biochem. Biophys. Res. Comm. 2007;358:1–6. doi: 10.1016/j.bbrc.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pickhardt M, Larbig G, Khlistunova I, Coksezen A, Meyer B, Mandelkow EM, Schmidt B, Mandelkow E. Phenylthiazolyl-hydrazide and its derivatives are potent inhibitors of tau aggregation and toxicity in vitro and in cells. Biochemistry. 2007;46:10016–10023. doi: 10.1021/bi700878g. [DOI] [PubMed] [Google Scholar]
- 64.Pickhardt M, Biernat J, Khlistunova I, Wang YP, Gazova Z, Mandelkow EM, Mandelkow E. N-phenylamine derivatives as aggregation inhibitors in cell models of tauopathy. Current Alzheimer research. 2007;4:397–402. doi: 10.2174/156720507781788765. [DOI] [PubMed] [Google Scholar]
- 65.Bulic B, Pickhardt M, Khlistunova I, Biernat J, Mandelkow EM, Mandelkow E, Waldmann H. Rhodanine-based tau aggregation inhibitors in cell models of tauopathy. Angewandte Chemie (International ed. 2007;46:9215–9219. doi: 10.1002/anie.200704051. [DOI] [PubMed] [Google Scholar]
- 66.Chirita C, Necula M, Kuret J. Ligand-dependent inhibition and reversal of tau filament formation. Biochemistry. 2004;43:2879–2887. doi: 10.1021/bi036094h. [DOI] [PubMed] [Google Scholar]
- 67.Necula M, Breydo L, Milton S, Kayed R, van der Veer WE, Tone P, Glabe CG. Methylene blue inhibits amyloid Abeta oligomerization by promoting fibrillization. Biochemistry. 2007;46:8850–8860. doi: 10.1021/bi700411k. [DOI] [PubMed] [Google Scholar]
- 68.Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J. Biol. Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]