Abstract
Background and Purpose
In-hospital stroke (IHS) differs from out-of-hospital stroke (OHS) in risk factors and outcomes. We compared IHS and OHS treated with thrombolysis from a large national cohort in a cross-sectional study to further clarify these differences.
Methods
The Nationwide Inpatient Sample for the years 2005 through 2010 was searched for adult acute ischemic stroke cases treated with intravenous or intra-arterial thrombolysis. Patients treated on the day of admission were classified as OHS. We compared the demographic and hospital characteristics, comorbidities, and short-term outcomes of thrombolysed IHS and OHS.
Results
IHS represented 8.7% of 11,750 thrombolysed stroke cases included in this study. IHS was associated with a higher comorbidity profile and higher rates of acute medical conditions compared to OHS. IHS had higher inpatient mortality (15.7% versus 9.6%; P<0.001) and lower rate of discharge to home/self-care (22.8% versus 30.0%; P<0.001). IHS was also associated with higher mortality among endovascular treatment group (19.3% versus 13.8%; P=0.010). The difference in the rate of all intracerebral hemorrhage (ICH) was not significant (5.3% versus 4.7%; P=0.361). In the multivariate analysis, inpatient mortality (adjusted OR, 1.59; 95% CI, 1.32–1.92; P<0.001) and favorable discharge outcome (adjusted OR, 0.79; 95% CI, 0.67–0.93; P=0.005) remained significantly worse in IHS.
Conclusions
Thrombolysed IHS is associated with worse discharge outcomes compared to thrombolysed OHS, likely due to their higher comorbidities and additional medical reasons for the index admission. Thrombolysis is not associated with a higher rate of ICH among IHS.
Keywords: in-hospital stroke, thrombolysis, ischemic stroke, intracerebral hemorrhage, nationwide inpatient sample
Introduction
Hospitalized patients are at a higher risk of stroke than the general population.1 An estimated 35,000–75,000 cases of stroke occur in patients admitted to the hospital for another reason [in-hospital stroke (IHS)] each year in the United States representing 4%–17% of all stroke cases.2 Factors contributing to the incidence of IHS include withdrawal of antiplatelet/anticoagulant agents, active cancer, cardiac diseases, cardiovascular surgeries/minimally invasive procedures, hypotension and infections.3–5 IHS differs from the stroke with onset outside of the hospital [out-of-hospital stroke (OHS)] in mechanism, severity and outcomes. IHS is more likely to be cardioembolic and have multiple territorial infarctions than OHS while small vessel occlusions are rare in IHS.6–9 Furthermore, IHS is associated with higher inpatient mortality and worse functional outcomes.6, 8, 10, 11
IHS cases are excellent candidates for time-sensitive thrombolytic treatment as they avoid the pre-hospital delays. However, decision to give thrombolytic treatment in IHS may be complicated by comorbidities, acute medical illness responsible for index hospitalization, and other medical and surgical contraindications for thrombolysis. Masjuan et al12 studied IHS and OHS treated with thrombolysis in a multi-center study and found a paradoxical trend toward higher inpatient mortality among OHSs, partly due to small sample size leading to inconclusive results. Large-scale studies comparing thrombolysis in IHS and OHS are lacking. Therefore, we sought to compare the comorbidities, medical complications, and outcomes of IHS and OHS treated with intravenous (IV) or intra-arterial thrombolysis from a national database.
Methods
Data-source
The Nationwide Inpatient Sample (NIS) for years 2005 through 2010 was obtained from the Agency for Healthcare Research and Quality (AHRQ) for analysis. NIS, the largest all-payer inpatient database in the US, is a 20% stratified sample of all hospitalizations in non-federal hospitals. Approximately 1,000 hospitals are sampled each year and all the inpatient admissions from the sampled hospitals are included in NIS. It contains more than100 clinical and non-clinical discharge level variables including primary and secondary diagnoses, in-hospital procedures including the day of the procedure from the admission, demographic and hospital characteristics, and discharge outcomes. Detailed information regarding the content and the methodology of NIS is available at the AHRQ website http://www.hcup-us.ahrq.gov/nisoverview.jsp (accessed December 1, 2012).13
Case selection
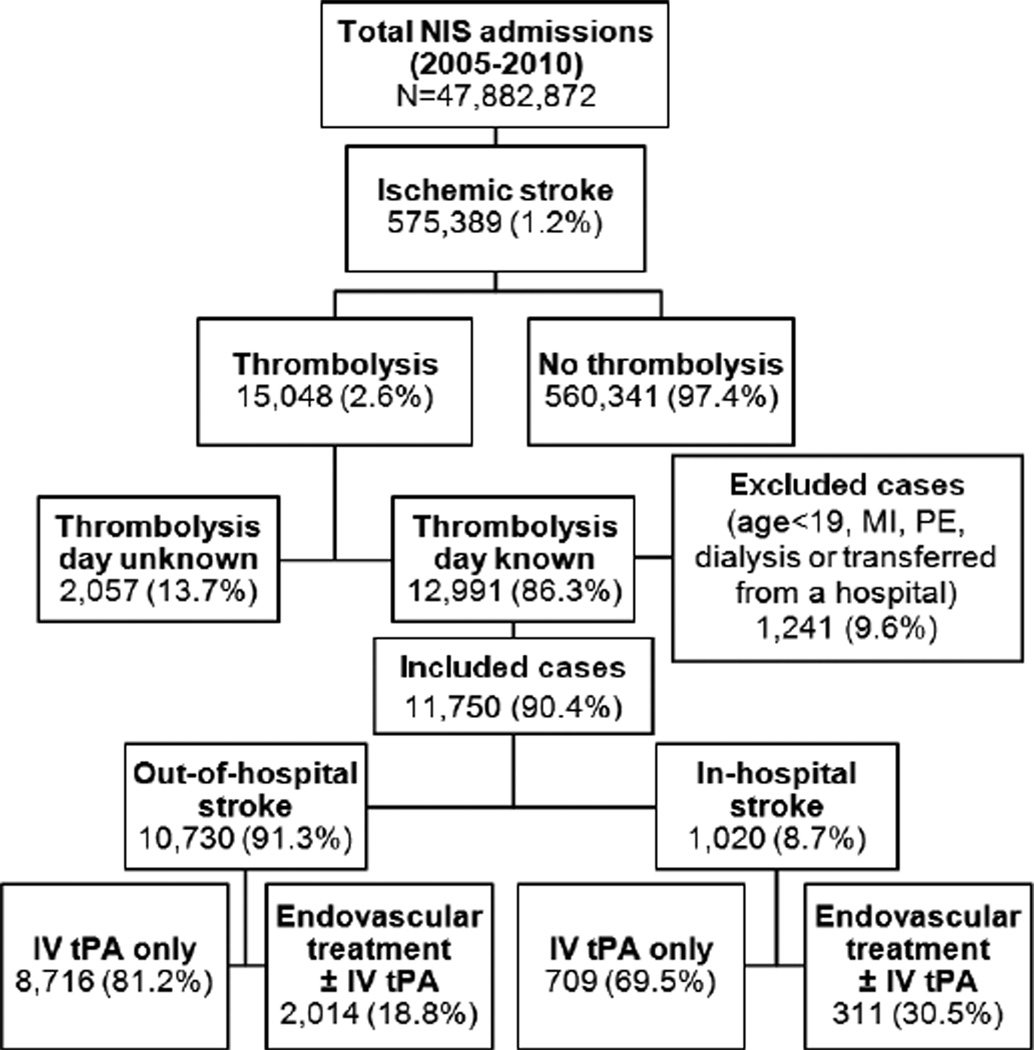
Figure 1 shows case selection flowchart of the study. Ascertainment of all diagnoses and procedures was made by using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes recorded at discharge (Supplemental Table S1; http://stroke.ahajournals.org). Acute ischemic stroke cases of age >18 years were selected using ICD-9 codes 433.×1, 434.×1, or 436,13–16 and thrombolytic infusion was ascertained by procedure code 99.10.17, 18 As NIS database lacks explicit IHS variable, cases were classified as OHS if thrombolytic treatment was administered on the day of hospitalization and as IHS if thrombolytic treatment was given on the second day of hospitalization or later. Cases with missing information regarding the thrombolysis day were excluded from the study. Patients transferred from another hospital were also excluded as they may have developed symptoms while in the previous hospital but received thrombolysis on the day of arrival to the current hospital. Additionally, the cases with acute myocardial infarction or pulmonary embolism and those on dialysis (with possibly clotted access) were excluded to avoid uncertainty of indication for thrombolytic infusion.
Figure 1.
Case-selection Flowchart. Endovascular treatment includes intra-arterial thrombolysis and/or mechanical embolectomy. IV indicates intravenous; MI, myocardial infarction; NIS, nationwide inpatient sample; PE, pulmonary embolism; tPA, tissue plasminogen activator.
The Elixhauser comorbidities,19 modified to create a weighted numeric score as recommended by van Walraven et al,20 were used to quantify patients' comorbidity profiles. The Elixhauser comorbidities have been validated for prognostication in studies using administrative datasets with ICD-9 codes.21–23 The primary outcomes of the study were favorable discharge disposition defined as discharge to home/self-care and inpatient mortality. Secondary outcomes were symptomatic or asymptomatic intracerebral hemorrhage (ICH), gastrointestinal (GI) bleeding, tracheostomy and gastrostomy tube placement. Endovascular treatment was ascertained by the performance of invasive cerebral angiogram (ICD-9 procedure code 88.41) with thrombolytic infusion (99.10), and/or mechanical thrombectomy (39.74).24, 25 We compared the outcomes of IHS and OHS among IV thrombolysis only and endovascular thrombolysis groups.
Statistical analysis
Non-parametric Elixhauser index was categorized into the following quartiles: (1) <5, (2) 5–7, (3) 8–14, and (4) >14. Missing ethnicity data (14.5%) were coded as 'missing information' without any imputation. Comparisons were made by Pearson χ2 for categorical variables. Mantel–Haenszel test was used to calculate unadjusted odds ratios. Outcomes were adjusted using multivariate logistic regression after controlling for age-group, gender, ethnicity, hospital characteristics such as bed-size, location/teaching status and region and Elixhauser index. Hosmer-Lemeshow test was used to assess goodness-of-fit of the regression models. All analyses were performed using the Statistical Package for Social Sciences version 17.0 (SPSS Inc., Chicago, Illinois) with statistical significance set at 0.05.
Results
Of the 11,750 thrombolysed ischemic strokes included in the study, 1,020 (8.7%) were IHSs. Age and gender distributions between IHS and OHS cohorts were not significantly different. IHS was more common in large sized and urban teaching hospitals and hospitals in the northeast region of the US (Table 1). Comparison of baseline characteristics and outcomes of cases with missing thrombolysis day (13.7%) to those with known thrombolysis day is shown in Supplemental Table S2. The cases with missing thrombolysis day were more likely to be from midwest and large-sized urban teaching hospitals.
Table 1.
Descriptive Summary of Baseline Demographic and Hospital Characteristics of Thrombolysed In-Hospital and Out-of-Hospital Strokes in the United States, 2005–2010.
| OHS, n (%) | IHS, n (%) | P value | |
|---|---|---|---|
| No. of cases | 10,730 (91.3) | 1,020 (8.7) | -- |
| Age-group, y | 0.726 | ||
| 19–64 | 4029 (37.5) | 371 (36.4) | |
| 65–79 | 3738 (34.8) | 366 (35.9) | |
| 80 or more | 2963 (27.6) | 283 (27.7) | |
| Female gender | 5238 (48.8) | 529 (51.9) | 0.063 |
| Ethnicity | 0.032 | ||
| Caucasian | 6889 (64.2) | 684 (67.1) | |
| African-American | 1366 (12.7) | 120 (11.8) | |
| Hispanic | 632 (5.9) | 65 (6.4) | |
| Other | 523 (4.9) | 57 (5.6) | |
| Missing information | 1320 (12.3) | 94 (9.2) | |
| Primary payer | 0.017 | ||
| Medicare | 6248 (58.2) | 603 (59.1) | |
| Medicaid | 709 (6.6) | 85 (8.3) | |
| Private insurance | 2940 (27.4) | 242 (23.7) | |
| Other | 833 (7.8) | 90 (8.8) | |
| Location/teaching status | <0.001 | ||
| Rural | 602 (5.7) | 45 (4.5) | |
| Urban, nonteaching | 4311 (40.7) | 347 (34.4) | |
| Urban, teaching | 5675 (53.6) | 616 (61.1) | |
| Hospital bed-size | 0.002 | ||
| Small | 629 (5.9) | 54 (5.4) | |
| Medium | 2484 (23.5) | 191 (18.9) | |
| Large | 7475 (70.6) | 763 (75.7) | |
| Geographic region | 0.002 | ||
| Northeast | 2435 (22.7) | 280 (27.5) | |
| Midwest | 1722 (16.0) | 140 (13.7) | |
| South | 4306 (40.1) | 378 (37.1) | |
| West | 2267 (21.1) | 222 (21.8) |
IHS indicates in-hospital stroke; OHS, out-of-hospital stroke.
On univariate analysis, IHS had significantly higher Elixhauser comorbidity index compared to OHS. Dyslipidemia and hypertension were more common in OHS. IHSs were more likely to have atrial fibrillation, coronary artery disease, chronic kidney disease, congestive heart failure, coagulopathy, diabetes with chronic complications, metastatic cancer and solid tumor without metastasis. In-hospital acute medical conditions associated with IHS were acute kidney injury, acute respiratory failure, cardiac arrest, deep venous thrombosis, pneumonia, sepsis and urinary infection (Table 2).
Table 2.
Univariate Comparison of Comorbidities and Acute Medical Conditions Associated with In-Hospital Stroke and Out-of-Hospital Stroke (United States Nationwide Inpatient Sample, 2005–2010)
| OHS, n (%) | IHS, n (%) | P value | |
|---|---|---|---|
| Comorbidities | |||
| Elixhauser comorbidity quartile (index value) | <0.001 | ||
| 1st (<5) | 3013 (28.1) | 212 (20.8) | |
| 2nd (5–7) | 2951 (27.5) | 223 (21.9) | |
| 3rd (8–14) | 2824 (26.3) | 292 (28.6) | |
| 4th (>14) | 1942 (18.1) | 293 (28.7) | |
| Anemia | 1116 (10.4) | 203 (19.9) | <0.001 |
| Atrial fibrillation | 2531 (23.6) | 269 (26.4) | 0.046 |
| Coronary artery disease | 2810 (26.2) | 330 (32.4) | <0.001 |
| Chronic kidney disease | 757 (7.1) | 93 (9.1) | 0.015 |
| Coagulopathy | 254 (2.4) | 54 (5.3) | <0.001 |
| Collagen vascular disease | 219 (2.0) | 14 (1.4) | 0.143 |
| Congestive heart failure | 1352 (12.6) | 156 (15.3) | 0.014 |
| Diabetes without complications | 2456 (22.9) | 240 (23.5) | 0.642 |
| Diabetes with chronic complications | 298 (2.8) | 47 (4.6) | 0.001 |
| Dyslipidemia | 5112 (47.6) | 408 (40.0) | <0.001 |
| Hypertension | 8133 (75.8) | 721 (70.7) | <0.001 |
| Liver disease | 76 (0.7) | 11 (1.1) | 0.188 |
| Metastatic cancer | 75 (0.7) | 17 (1.7) | 0.001 |
| Solid tumor without metastasis | 132 (1.2) | 23 (2.3) | 0.006 |
| Valvular disease | 978 (9.1) | 86 (8.4) | 0.467 |
| Medical complications | |||
| Acute kidney injury | 551 (5.1) | 109 (10.7) | <0.001 |
| Acute respiratory failure | 959 (8.0) | 176 (17.3) | <0.001 |
| Cardiac arrest | 56 (0.5) | 20 (2.0) | <0.001 |
| Deep venous thrombosis | 82 (0.8) | 24 (2.4) | <0.001 |
| Pneumonia | 434 (4.0) | 112 (11.0) | <0.001 |
| Sepsis | 202 (1.9) | 77 (7.5) | <0.001 |
| Urinary infection | 1213 (11.3) | 196 (19.2) | <0.001 |
IHS indicates in-hospital stroke; OHS, out-of-hospital stroke.
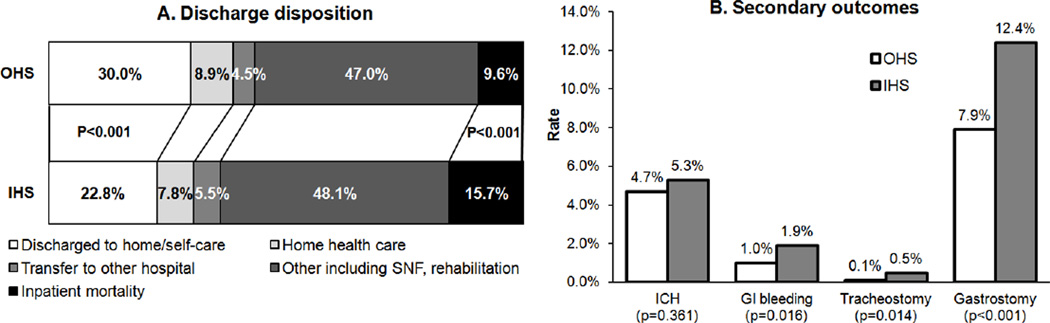
IHS had higher unadjusted inpatient mortality (15.7% versus 9.6%; odds ratio [OR], 1.76; 95% confidence interval [CI], 1.47–2.11, P<0.001) and lower favorable discharge disposition rate (22.8% versus 30.0%; OR, 0.69; 95% CI, 0.59–0.81, P<0.001) compared to OHS. The unadjusted rate of all ICH did not differ significantly between the two groups (5.3% versus 4.7%; OR, 1.14; 95% CI, 0.86–1.53; P=0.361) (Figure 2). Univariate outcomes by endovascular treatment showed higher inpatient mortality and lower rate of favorable discharge among IHS treated with IV thrombolysis only as well as among IHS treated with endovascular treatment (Table 3). In the multivariate analysis, IHS was associated with lower rate of discharge to home/self-care (adjusted OR, 0.79; 95% CI, 0.67–0.93; P=0.005) and higher inpatient mortality (adjusted OR, 1.59; 95% CI, 1.32–1.92; P<0.001) (Table 4).
Figure 2.
Comparison of Outcomes between Thrombolysed In-Hospital Stroke and Out-of-Hospital Stroke. GI indicates gastrointestinal; ICH, intracerebral hemorrhage; IHS, in-hospital stroke; OHS, out-of-hospital stroke; and SNF, skilled nursing facility.
Table 3.
Univariate Comparison of Primary and Secondary Outcomes between In-Hospital Stroke and Out-of-Hospital Stroke by Endovascular Treatment Group
| IV thrombolysis only | Endovascular treatment ± IV thrombolysis |
|||||
|---|---|---|---|---|---|---|
| OHS | IHS | P value | OHS | IHS | P value | |
| Inpatient mortality | 8.6% | 14.1% | <0.001 | 13.8% | 19.3% | 0.010 |
| Discharge to home/self-care | 30.9% | 25.1% | 0.001 | 26.0% | 17.7% | 0.002 |
| All ICH | 4.3% | 5.2% | 0.238 | 6.3% | 5.5% | 0.568 |
| GI bleeding | 1.1% | 1.7% | 0.136 | 0.8% | 2.3% | 0.022 |
| Tracheostomy | 0.1% | 0.7% | <0.001 | 0.2% | 0.0% | 0.379 |
| Gastrostomy | 7.6% | 12.4% | <0.001 | 8.8% | 12.2% | 0.056 |
GI indicates gastrointestinal; ICH, intracerebral hemorrhage; IHS, in-hospital stroke; and OHS, out-of-hospital stroke.
Table 4.
Multivariate Analysis: In-Hospital Stroke as a Predictor of Discharge to Home/Self-care and Inpatient Mortality in Ischemic Strokes Treated with Thrombolysis.
| Discharge to home/self-care | Inpatient mortality | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| IHS versus OHS | 0.79 (0.67–0.93) | 0.005 | 1.59 (1.32–1.92) | <0.001 |
| Age, y (19–64) | Reference | Reference | ||
| 65–79 | 0.57 (0.51–0.62) | <0.001 | 1.49 (1.27–1.76) | <0.001 |
| ≥80 | 0.23 (0.20–0.27) | <0.001 | 2.16 (1.83–2.56) | <0.001 |
| Female versus male | 0.89 (0.81–0.97) | 0.009 | 0.91 (0.80–1.03) | 0.121 |
| Ethnicity (Caucasian) | Reference | Reference | ||
| African-American | 0.94 (0.82–1.08) | 0.365 | 0.89 (0.72–1.10) | 0.290 |
| Hispanic | 0.91 (0.76–1.10) | 0.334 | 0.99 (0.75–1.29) | 0.929 |
| Other | 1.06 (0.87–1.30) | 0.563 | 1.12 (0.85–1.48) | 0.417 |
| Missing information | 0.99 (0.86–1.13) | 0.870 | 1.15 (0.95–1.40) | 0.149 |
| Bed-size (small) | Reference | Reference | ||
| Medium | 1.11 (0.90–1.36) | 0.334 | 1.18 (0.86–1.61) | 0.305 |
| Large | 1.14 (0.94–1.39) | 0.168 | 1.48 (1.10–1.98) | 0.009 |
| Location/teaching status (rural) | Reference | Reference | ||
| Urban, non-teaching | 0.89 (0.74–1.08) | 0.256 | 1.09 (0.80–1.47) | 0.586 |
| Urban, teaching | 0.98 (0.81–1.19) | 0.827 | 1.38 (1.02–1.85) | 0.036 |
| Region (Northeast) | Reference | Reference | ||
| Midwest | 1.56 (1.34–1.80) | <0.001 | 0.71 (0.57–0.88) | 0.002 |
| South | 1.46 (1.30–1.65) | <0.001 | 0.91 (0.78–1.07) | 0.263 |
| West | 1.39 (1.21–1.60) | <0.001 | 0.95 (0.79–1.14) | 0.554 |
| Elixhauser index quartile (1st) | Reference | Reference | ||
| 2nd | 0.44 (0.39–0.49) | <0.001 | 1.48 (1.22–1.81) | <0.001 |
| 3rd | 0.33 (0.29–0.36) | <0.001 | 1.93 (1.60–2.34) | <0.001 |
| 4th | 0.18 (0.15–0.21) | <0.001 | 2.63 (2.17–3.20) | <0.001 |
IHS indicates in-hospital stroke; and OHS, out-of-hospital stroke.
Discussion
Our data suggest that inpatient mortality is higher and favorable discharge disposition is lower in thrombolysed IHS compared to thrombolysed OHS. Previous studies have shown that IHSs are more likely to be embolic resulting in more severe deficits at onset.3, 7, 25, 26 Kimura et al8 reported higher median National Institutes of Health Stroke Scale (NIHSS) in IHS compared to OHS. These studies indicate that IHS represents more severe stroke cases with poorer expected outcomes with or without thrombolytic treatment. Additionally, evaluation of the IHS patients may be delayed for various reasons such as the use of sedative or paralytic medications, delirium, and complexities of hospital practice leading to longer in-hospital delays among IHS, further contributing to the poor outcomes.11, 12
While we did not find difference in age distribution between the two groups, Kimura et al8 found that IHS patients were older than OHS. As our study included only the patients treated with thrombolysis, this finding might suggest that elderly IHS patients were preferentially excluded from thrombolytic treatment by the treating clinicians. We could not find previous reports comparing hospital characteristics between IHS and OHS. We found that the rate of thrombolysed IHS was higher in large sized, urban teaching hospitals, a finding potentially indicative of greater adherence of academic institutions to evidence based use of thrombolytic treatment irrespective of the in-hospital onset of the stroke. Vera et al11 found higher comorbidities in patients with IHS. Similarly, in this study, IHS had significantly higher Elixhauser comorbidity index which is associated with worse outcomes after stroke.23 Similar to prior reports,6, 8, 9, 12 IHS had higher rate of atrial fibrillation and lower rates of dyslipidemia and hypertension in our study. Of note, several comparisons in this study may have reached statistical significance with small absolute differences due to large sample size.
Despite the presumed higher use of antiplatelet and/or antithrombotic treatment12 and higher incidence of embolic stroke with more severe deficits and larger infarct size among IHS, the rate of the most feared complication of thrombolysis (i.e., ICH) was not significantly different between the two groups, potentially implying relative safety of thrombolysis in IHS. The rate of all ICH in this study was lower than that in previous studies reporting symptomatic ICH27, 28 likely due to under-ascertainment of hemorrhagic conversion of ischemic stroke using the only available ICD-9 code for intracerebral hemorrhage.
Higher use of endovascular treatment in IHS may be suggestive of more number of patients not eligible for systemic thrombolysis due to recent surgery or bleeding, or higher clot burden and therefore greater resistance to recanalization by IV thrombolysis alone in IHS.29 The worse outcomes in the endovascular group may be due to selective endovascular treatment of patients with more severe deficits, delayed recognition of stroke, or poor response to systemic thrombolysis.
This study has several important limitations related to the administrative nature of the database. NIS database lacks information regarding symptom onset. Therefore, we used the day of thrombolysis in relation to the day of admission to define IHS indirectly. Though this definition is expected to correctly identify a vast majority of IHS cases, misclassification is possible. For instance, IHS patients that developed the symptoms on the day of hospitalization and subsequently were given treatment on the same day are incorrectly classified as OHS. Similarly, OHS cases admitted before midnight and treated after midnight would be misclassified as IHS. NIS also lacks stroke severity measure such as NIHSS, a strong predictor of the outcome,30 thus limiting the adjusted analyses. NIS does not contain standard outcome measure such as 3-month modified Rankin Scale (mRS) or etiologic classification such as Trial of Org 10172 in Acute Stroke Treatment (TOAST) subtype. However, discharge destination as a surrogate for functional status has been shown to have high predictive value for 3- and 12-month post-stroke mRS.31 Coding error is another potential source of bias. However, the ICD-9 codes used to select acute ischemic stroke have high specificity and positive predictive value.14–16, 32 The ICD-9 procedure code 99.10 has the sensitivity of 55–70% and the specificity of 98% for thrombolytic treatment in stroke.33–35 Therefore, under-ascertainment is possible but case identification is likely to be accurate. We were not able to differentiate symptomatic from asymptomatic ICH due to lack of clinical data in NIS. Finally, the differences in the geographic distribution and hospital characteristics of included and excluded cases might potentially have introduced bias. Despite the limitations, inclusion of large number of patients from various demographic backgrounds and from academic and non-academic institutions makes the results highly generalizable.
Conclusions
In conclusion, IHS comprises of a significant subgroup of stroke with greater potential for thrombolytic treatment benefit as they avoid pre-hospital delays. However, IHS results in worse short-term outcomes when compared to OHS due to their coexistent medical illnesses and comorbidities. Despite IHS being a high risk group for complications of thrombolytic treatment, the rate of ICH in IHS was comparable to that in OHS in our study, potentially indicating relative safety of thrombolysis in IHS. Prospective studies of thrombolytic therapy for IHS from clinical data-source are needed to confirm our findings.
Supplementary Material
Acknowledgments
Sources of Funding
Supported in part by NIH grants: NS044364, HL096944, NS52220, AG040039, NS077378, and NS079211
Dr Levine has previously served on the Advisory Board of Genentech, Inc (honorarium donated to Stroke Research), is Associate Editor of MEDLINK, receives research funding from the National Institutes of Health, and has served as an expert witness in acute stroke cases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Moradiya reports no disclosures.
References
- 1.Azzimondi G, Nonino F, Fiorani L, Vignatelli L, Stracciari A, Pazzaglia P, et al. Incidence of stroke among inpatients in a large italian hospital. Stroke. 1994;25:1752–1754. doi: 10.1161/01.str.25.9.1752. [DOI] [PubMed] [Google Scholar]
- 2.Alberts MJ, Brass LM, Perry A, Webb D, Dawson DV. Evaluation times for patients with in-hospital strokes. Stroke. 1993;24:1817–1822. doi: 10.1161/01.str.24.12.1817. [DOI] [PubMed] [Google Scholar]
- 3.Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2:741–746. doi: 10.1016/s1474-4422(03)00586-6. [DOI] [PubMed] [Google Scholar]
- 4.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34:2518–2532. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 5.Selim M. Perioperative stroke. N Engl J Med. 2007;356:706–713. doi: 10.1056/NEJMra062668. [DOI] [PubMed] [Google Scholar]
- 6.Dulli D, Samaniego EA. Inpatient and community ischemic strokes in a university hospital. Neuroepidemiology. 2007;28:86–92. doi: 10.1159/000098551. [DOI] [PubMed] [Google Scholar]
- 7.Kelley RE, Kovacs AG. Mechanism of in-hospital cerebral ischemia. Stroke. 1986;17:430–433. doi: 10.1161/01.str.17.3.430. [DOI] [PubMed] [Google Scholar]
- 8.Kimura K, Minematsu K, Yamaguchi T. Characteristics of in-hospital onset ischemic stroke. Eur Neurol. 2006;55:155–159. doi: 10.1159/000093574. [DOI] [PubMed] [Google Scholar]
- 9.Park HJ, Cho HJ, Kim YD, Lee DW, Choi HY, Kim SM, et al. Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol. 2009;16:582–588. doi: 10.1111/j.1468-1331.2009.02538.x. [DOI] [PubMed] [Google Scholar]
- 10.Farooq MU, Reeves MJ, Gargano J, Wehner S, Hickenbottom S, Majid A. In-hospital stroke in a statewide stroke registry. Cerebrovasc Dis. 2008;25:12–20. doi: 10.1159/000111494. [DOI] [PubMed] [Google Scholar]
- 11.Vera R, Lago A, Fuentes B, Gallego J, Tejada J, Casado I, et al. In-hospital stroke: a multi-centre prospective registry. Eur J Neurol. 2011;18:170–176. doi: 10.1111/j.1468-1331.2010.03105.x. [DOI] [PubMed] [Google Scholar]
- 12.Masjuan J, Simal P, Fuentes B, Egido JA, Diaz-Otero F, Gil-Nunez A, et al. In-hospital stroke treated with intravenous tissue plasminogen activator. Stroke. 2008;39:2614–2616. doi: 10.1161/STROKEAHA.107.512848. [DOI] [PubMed] [Google Scholar]
- 13.Healthcare Cost and Utilization Project (HCUP): Overview of the Nationwide Inpatient Sample (NIS) [Accessed December 1, 2012];Agency for Healthcare Research and Quality web site. 2012 Jun; www.hcup-us.ahrq.gov/nisoverview.jsp.
- 14.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29:1602–1604. doi: 10.1161/01.str.29.8.1602. [DOI] [PubMed] [Google Scholar]
- 15.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiol Drug Saf. 2008;17:20–26. doi: 10.1002/pds.1518. [DOI] [PubMed] [Google Scholar]
- 16.Williams GR. Incidence and characteristics of total stroke in the United States. BMC Neurol. 2001;1:2. doi: 10.1186/1471-2377-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateman BT, Schumacher HC, Boden-Albala B, Berman MF, Mohr JP, Sacco RL, et al. Factors associated with in-hospital mortality after administration of thrombolysis in acute ischemic stroke patients: an analysis of the nationwide inpatient sample 1999 to 2002. Stroke. 2006;37:440–446. doi: 10.1161/01.STR.0000199851.24668.f1. [DOI] [PubMed] [Google Scholar]
- 18.Dubinsky R, Lai SM. Mortality of stroke patients treated with thrombolysis: analysis of nationwide inpatient sample. Neurology. 2006;66:1742–1744. doi: 10.1212/01.wnl.0000218306.35681.38. [DOI] [PubMed] [Google Scholar]
- 19.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 21.Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50:1109–1118. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 22.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42:355–360. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, Hill MD. Stroke: The Elixhauser index for comorbidity adjustment of in-hospital case fatality. Neurology. 2008;71:283–287. doi: 10.1212/01.wnl.0000318278.41347.94. [DOI] [PubMed] [Google Scholar]
- 24.Brinjikji W, Rabinstein AA, Kallmes DF, Cloft HJ. Patient outcomes with endovascular embolectomy therapy for acute ischemic stroke: a study of the national inpatient sample: 2006 to 2008. Stroke. 2011;42:1648–1652. doi: 10.1161/STROKEAHA.110.607952. [DOI] [PubMed] [Google Scholar]
- 25.Choi JH, Bateman BT, Mangla S, Marshall RS, Prabhakaran S, Chong J, et al. Endovascular recanalization therapy in acute ischemic stroke. Stroke. 2006;37:419–424. doi: 10.1161/01.STR.0000198808.90579.65. [DOI] [PubMed] [Google Scholar]
- 26.Levine SR. Acute cerebral ischemia in a critical care unit. A review of diagnosis and management. Arch Intern Med. 1989;149:90–98. [PubMed] [Google Scholar]
- 27.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-PA stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 28.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 29.Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–531. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muir KW, Weir CJ, Murray GD, Povey C, Lees KR. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke. 1996;27:1817–1820. doi: 10.1161/01.str.27.10.1817. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi AI, Chaudhry SA, Sapkota BL, Rodriguez GJ, Suri MF. Discharge destination as a surrogate for modified Rankin scale defined outcomes at 3- and 12-months poststroke among stroke survivors. Arch Phys Med Rehabil. 2012;93:1408–1413. doi: 10.1016/j.apmr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 33.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke. 2008;39:924–928. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi AI, Harris-Lane P, Siddiqi F, Kirmani JF. International classification of diseases and current procedural terminology codes underestimated thrombolytic use for ischemic stroke. J Clin Epidemiol. 2006;59:856–858. doi: 10.1016/j.jclinepi.2006.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.