Abstract
Enterotoxigenic Escherichia coli are associated with noninflammatory diarrhea and stimulate adenylate cyclase activity of mammalian cells, thereby increasing intracellular cyclic adenosine 3′,5′-monophosphate (cyclic AMP). Increased concentrations of cyclic AMP in polymorphonuclear neutrophils (PMN) inhibit phagocytosis, candidacidal activity, granule discharge, and chemotactic responsiveness. We examined the effect of enterotoxin on the interaction of human PMN with E. coli. Enterotoxigenic and nonenterotoxigenic strains, including serotypes of E. coli identical except for the presence or absence of the plasmid coding for enterotoxin production, were utilized. Enterotoxigenic and nonenterotoxigenic E. coli, tumbled with PMN, were phagocytized and killed (>97%) equally well, and these strains stimulated PMN hexose monophosphate shunt activity equivalently.
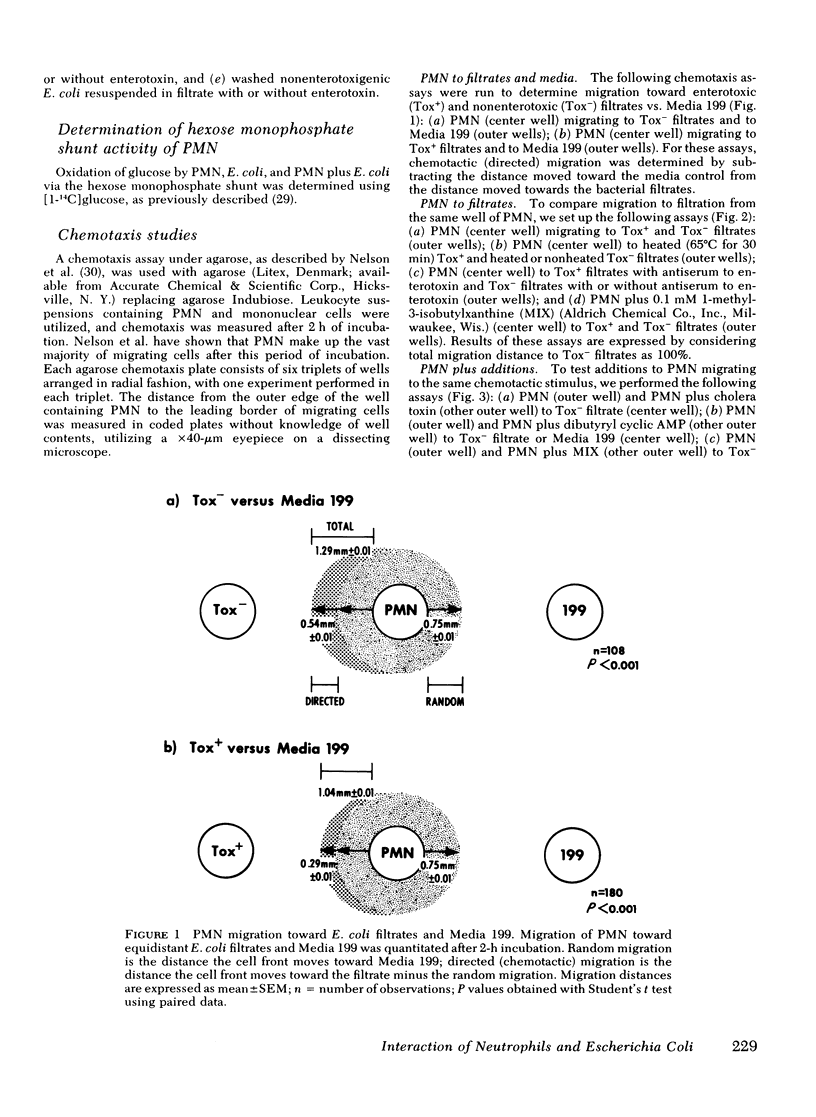
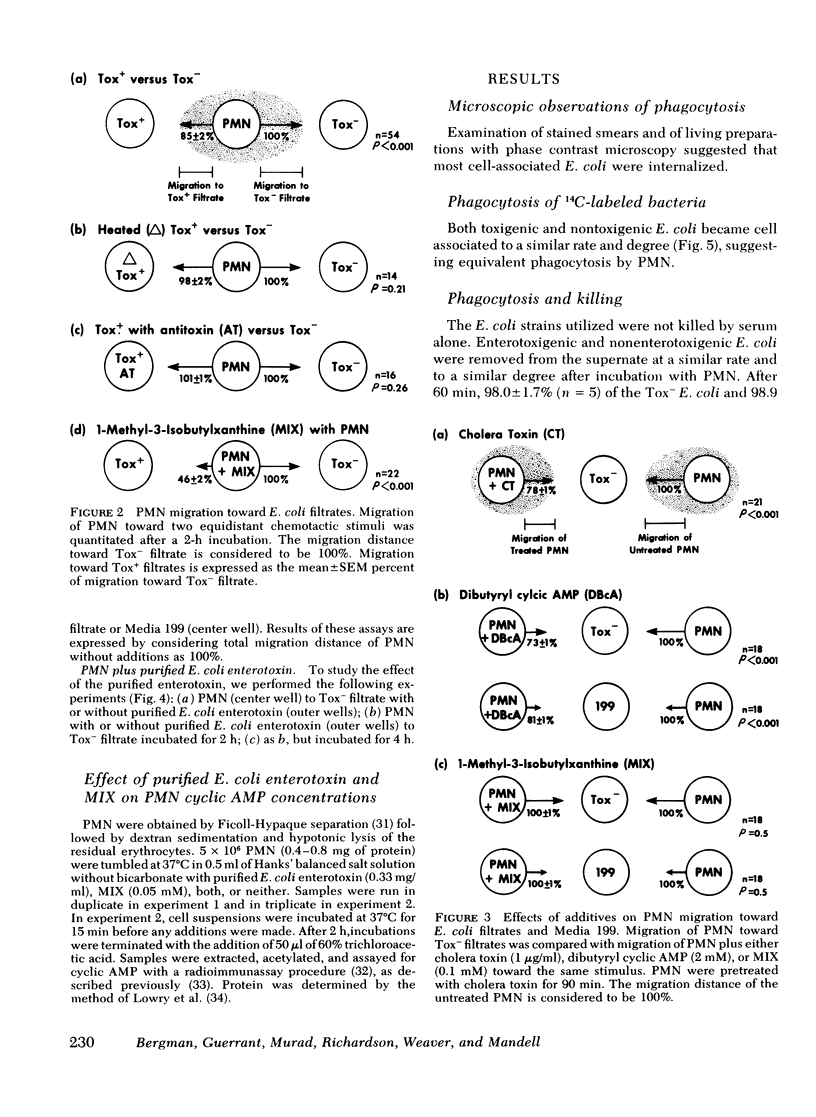
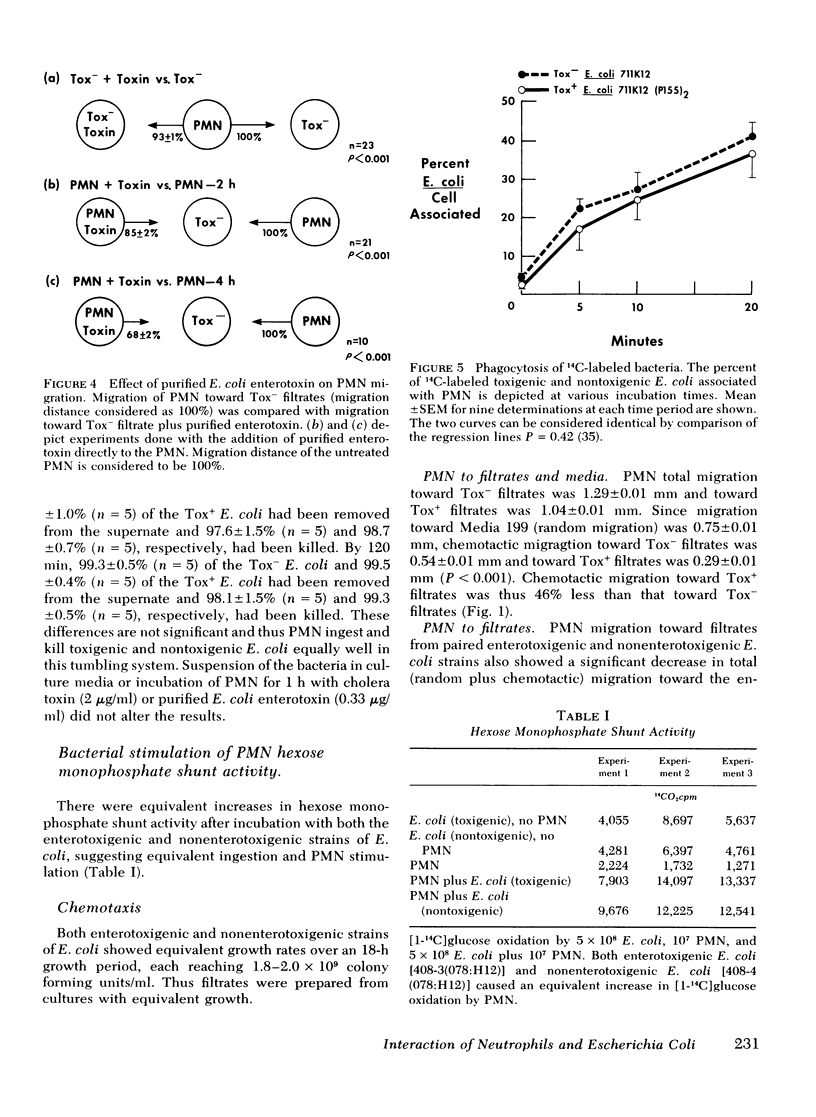
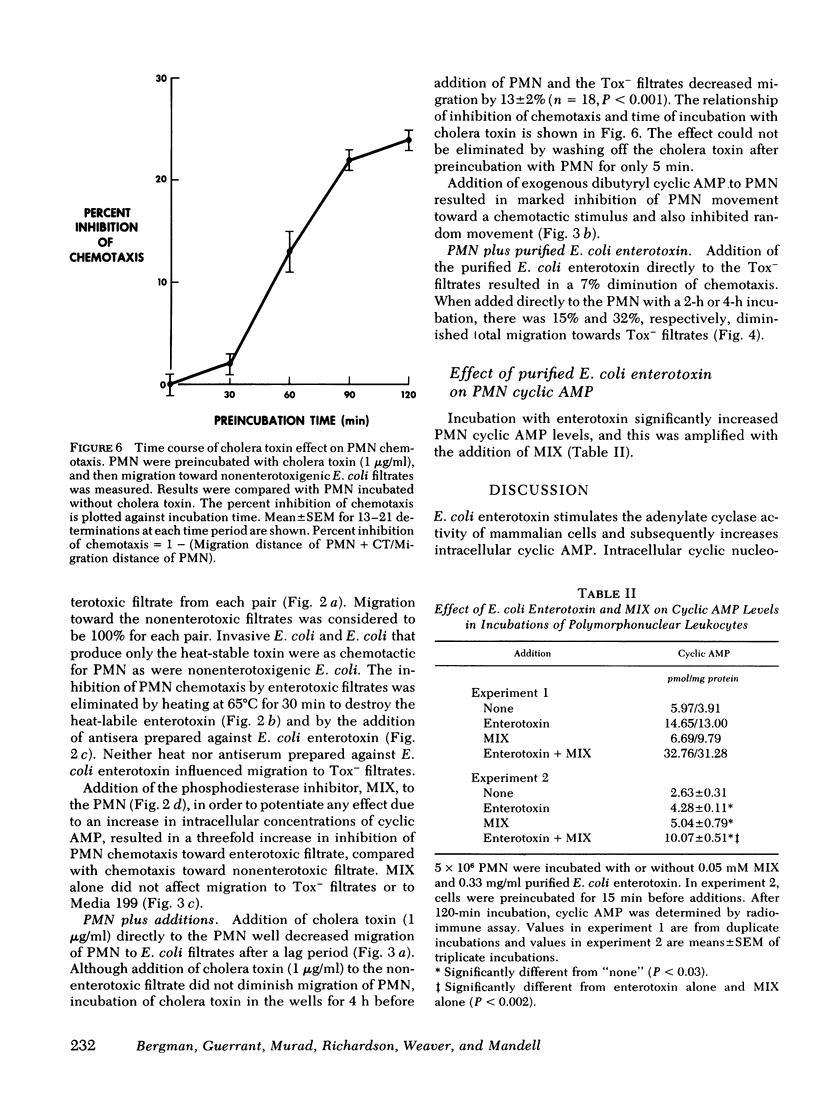
However, a chemotaxis assay under agarose demonstrated that filtrates of 10 enterotoxigenic strains were less chemotactic for PMN by 15±2% total migration or 46±1% directed migration, when compared with 6 non-enterotoxigenic strains (P < 0.001). Inactivation of the enterotoxin by heat (65°C for 30 min) or antibodies formed to E. coli enterotoxin eliminated the inhibitory effect of the enterotoxic filtrates for PMN chemotaxis. Addition of purified E. coli enterotoxin directly to the PMN decreased chemotaxis to E. coli filtrates by 32±2% (P < 0.001). These data suggest that the effect was due to the heat-labile enterotoxin. The phosphodiesterase inhibitor, 1-methyl-3-isobutylxanthine (0.1 mM), which potentiates effects due to an increase in intracellular cyclic AMP, further decreased total PMN migration (random plus directed) toward enterotoxic filtrates to 46% of that to nonenterotoxic filtrates (P < 0.001). Addition of cholera toxin (1 μg/ml), which is similar to E. coli enterotoxin, to the PMN inhibited total migration toward nonenterotoxic filtrates by 16±2% (P < 0.001). Exogenous dibutyryl cyclic AMP (2 mM) inhibited total PMN migration toward E. coli filtrates by 32% (P < 0.001). PMN intracellular cyclic AMP levels increased by 220% after 2 h of incubation with purified E. coli enterotoxin. The decreased chemotactic attractiveness of enterotoxic E. coli filtrates appears to be related to the ability of enterotoxin to increase cyclic AMP in PMN. Enterotoxin production by E. coli may be advantageous to the microbe by decreasing its chemotactic appeal for PMN.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Glover A., Koornhof H. J., Rabson A. R. In vitro stimulation of neutrophil motility by levamisole: maintenance of cgmp levels in chemotactically stimulated levamisole-treated neutrophils. J Immunol. 1976 Aug;117(2):428–432. [PubMed] [Google Scholar]
- Bourne H. R., Lehrer R. I., Cline M. J., Melmon K. L. Cyclic 3',5'-adenosine monophosphate in the human lukocyte: synthesis, degradation, andeffects n neutrophil candidacidal activity. J Clin Invest. 1971 Apr;50(4):920–929. doi: 10.1172/JCI106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cox J. P., Karnovsky M. L. The depression of phagocytosis by exogenous cyclic nucleotides, prostaglandins, and theophylline. J Cell Biol. 1973 Nov;59(2 Pt 1):480–490. doi: 10.1083/jcb.59.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth J. A., Hendley J. O., Mandell G. L. Attachment and ingestion of gonococci human neutrophils. Infect Immun. 1975 Mar;11(3):512–516. doi: 10.1128/iai.11.3.512-516.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta S. T., Moon H. W., Whipp S. C. Detection of heat-labile Escherichia coli enterotoxin with the use of adrenal cells in tissue culture. Science. 1974 Jan 25;183(4122):334–336. doi: 10.1126/science.183.4122.334. [DOI] [PubMed] [Google Scholar]
- Downey R. J., Diedrich B. F. A new method for assessing particle ingestion by phagocytic cells. Exp Cell Res. 1968 Jun;50(3):483–489. doi: 10.1016/0014-4827(68)90411-4. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Formal S. B., Hornick R. B., Snyder M. J., Libonati J. P., Sheahan D. G., LaBrec E. H., Kalas J. P. Pathogenesis of Escherichia coli diarrhea. N Engl J Med. 1971 Jul 1;285(1):1–9. doi: 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- Estensen R. D., Hill H. R., Quie P. G., Gogan N., Goldberg N. D. Cyclic GMP and cell movement. Nature. 1973 Oct 26;245(5426):458–460. doi: 10.1038/245458a0. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Evans D. G., Gorbach S. L. Polymyxin B-Induced Release of Low-Molecular-Weight, Heat-Labile Enterotoxin from Escherichia coli. Infect Immun. 1974 Nov;10(5):1010–1017. doi: 10.1128/iai.10.5.1010-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- Giannella R. A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976 Jul;14(1):95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Banwell J. G., Chatterjee B. D., Jacobs B., Sack R. B. Acute undifferentiated human diarrhea in the tropics. I. Alterations in intestinal micrflora. J Clin Invest. 1971 Apr;50(4):881–889. doi: 10.1172/JCI106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Kean B. H., Evans D. G., Evans D. J., Jr, Bessudo D. Travelers' diarrhea and toxigenic Escherichia coli. N Engl J Med. 1975 May 1;292(18):933–936. doi: 10.1056/NEJM197505012921801. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L. Characterization of the Chinese hamster ovary cell assay for the enterotoxins of Vibrio cholerae and Escherichia coli and for specific antisera, and toxoid. J Infect Dis. 1977 May;135(5):720–728. doi: 10.1093/infdis/135.5.720. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Chen L. C., Sharp G. W. Intestinal adenyl-cyclase activity in canine cholera: correlation with fluid accumulation. J Infect Dis. 1972 Apr;125(4):377–381. doi: 10.1093/infdis/125.4.377. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Ganguly U., Casper A. G., Moore E. J., Pierce N. F., Carpenter C. C. Effect of Escherichia coli on fluid transport across canine small bowel. Mechanism and time-course with enterotoxin and whole bacterial cells. J Clin Invest. 1973 Jul;52(7):1707–1714. doi: 10.1172/JCI107352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Moore R. A., Kirschenfeld P. M., Sande M. A. Role of toxigenic and invasive bacteria in acute diarrhea of childhood. N Engl J Med. 1975 Sep 18;293(12):567–572. doi: 10.1056/NEJM197509182931201. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor H. S., Tao P., Wisdom C. Action of Escherichia coli enterotoxin: adenylate cyclase behavior of intestinal epithelial cells in culture. Infect Immun. 1974 Jun;9(6):1003–1010. doi: 10.1128/iai.9.6.1003-1010.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S., Murad F. Regulation of adenosine cyclic 3',5'-monophosphate and guanosine cyclic 3',5'-monophosphate levels and contractility in bovine tracheal smooth muscle. Mol Pharmacol. 1977 Mar;13(2):330–341. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandell G. L., Hook E. W. Leukocyte function in chronic granulomatous disease of childhood. Studies on a seventeen year old boy. Am J Med. 1969 Sep;47(3):473–486. doi: 10.1016/0002-9343(69)90231-9. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Staphylococcal infection and leukocyte bactericidal defects in a 22-year-old woman. Arch Intern Med. 1972 Nov;130(5):754–757. [PubMed] [Google Scholar]
- Mashiter K., Mashiter G. D., Hauger R. L., Field J. B. Effects of cholera and E. coli enterotoxins on cyclic adenosine 3',5'-monophosphate levels and intermediary metabolism in the thyroid. Endocrinology. 1973 Feb;92(2):541–549. doi: 10.1210/endo-92-2-541. [DOI] [PubMed] [Google Scholar]
- Merson M. H., Morris G. K., Sack D. A., Wells J. G., Feeley J. C., Sack R. B., Creech W. B., Kapikian A. Z., Gangarosa E. J. Travelers' diarrhea in Mexico. A prospective study of physicians and family members attending a congress. N Engl J Med. 1976 Jun 10;294(24):1299–1305. doi: 10.1056/NEJM197606102942401. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Griffith D. P., Templeton G. B. Further observations on the potentiation of the antibacterial effect of methenamine by acetohydroxamic acid. J Infect Dis. 1976 May;133(5):564–567. doi: 10.1093/infdis/133.5.564. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Rivkin I., Rosenblatt J., Becker E. L. The role of cyclic AMP in the chemotactic responsiveness and spontaneous motility of rabbit peritoneal neutrophils. The inhibition of neutrophil movement and the elevation of cyclic AMP levels by catecholamines, prostaglandins, theophylline and cholera toxin. J Immunol. 1975 Oct;115(4):1126–1134. [PubMed] [Google Scholar]
- Sack R. B., Gorbach S. L., Banwell J. G., Jacobs B., Chatterjee B. D., Mitra R. C. Enterotoxigenic Escherichia coli isolated from patients with severe cholera-like disease. J Infect Dis. 1971 Apr;123(4):378–385. doi: 10.1093/infdis/123.4.378. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Hoffstein S. Leukocytic proteases and the immunologic release of lysosomal enzymes. Am J Pathol. 1972 Sep;68(3):539–564. [PMC free article] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]


