Abstract
Background
Severe ischemia-reperfusion (IR) injury leads to primary graft dysfunction following lung transplantation. Adenosine receptors modulate inflammation after IR, and the adenosine A3 receptor (A3R) is expressed in lung tissue and inflammatory cells. This study tests the hypothesis that A3R agonism attenuates lung IR injury via a neutrophil-dependent mechanism.
Methods
Wild-type and A3R knockout (A3R−/−) mice underwent 1 hr left lung ischemia followed by 2 hrs reperfusion (IR). Cl-IB-MECA, a selective A3R agonist, was administered (100 µg/kg i.v.) 5 min prior to ischemia. Study groups included sham, IR, and IR+Cl-IB-MECA (n=6/group). Lung injury was assessed by measuring lung function, wet/dry weight, histopathology, and proinflammatory cytokines and myeloperoxidase levels in bronchoalveolar lavage fluid. Parallel in vitro experiments were performed to evaluate neutrophil chemotaxis, and neutrophil activation was measured following exposure to acute hypoxia-reoxygenation.
Results
Treatment of wild-type mice with Cl-IB-MECA significantly improved lung function and decreased edema, cytokine expression, and neutrophil infiltration after IR. Cl-IB-MECA had no effects in A3R−/− mice. Cl-IB-MECA significantly decreased activation of wild-type, but not A3R−/−, neutrophils after acute hypoxia-reoxygenation and inhibited chemotaxis of wild-type neutrophils.
Conclusions
Exogenous activation of A3R by Cl-IB-MECA attenuates lung dysfunction, inflammation, and neutrophil infiltration after IR in wild-type but not A3R−/− mice. Results with isolated neutrophils suggest that the protective effects of Cl-IB-MECA are due, in part, to the prevention of neutrophil activation and chemotaxis. The use of A3R agonists may be a novel therapeutic strategy to prevent lung IR injury and primary graft dysfunction after transplantation.
Keywords: lung transplantation, inflammation
Introduction
Ischemia-reperfusion (IR) injury is the principle cause of primary graft dysfunction after lung transplantation [1, 2]. The inherent IR injury associated with transplantation contributes to significant morbidity and mortality both early in the postoperative period as primary graft dysfunction and long-term as an independent risk factor for the development of bronchiolitis obliterans, the leading cause of death after lung transplantation [1, 3]. While the pathophysiology of lung IR injury is not well understood, innate immune responses and acute inflammation are key features, and neutrophil activation and infiltration into the lung is known to propagate injury and contribute to poor lung function after transplantation [2, 4, 5].
Adenosine is an endogenous purine metabolite, and its release is augmented in the setting of inflammation and injury such as after IR [6]. Adenosine can exert potent signaling functions through four G protein-coupled adenosine receptors: A1R, A2AR, A2BR, and A3R. Adenosine receptor signaling typically acts as a cytoprotective modulator in response to stress via action through several second messenger pathways including cAMP production or the phospholipase C pathway.
Our laboratory has shown that selective A2AR activation provides significant protection from lung IR injury [7–9] and that the A2BR exerts proinflammatory effects in the setting of lung IR injury [10]. However, the role of A3R activation in lung IR injury remains unclear. The A3R is known to couple to Gi proteins that inhibit adenylyl cyclase and to Gq proteins that stimulate phospholipase C, inositol triphosphate, and intracellular calcium [11]. The A3R is widely distributed with gene expression demonstrated in many tissues and is most highly expressed in lung, liver, and inflammatory cells including neutrophils, macrophages, dendritic cells, and lymphocytes [11, 12].
To date, the role of A3R activation in inflammatory conditions such as lung IR injury is controversial. Some studies suggest that A3R activation promotes inflammation and that its deletion protects against IR injury [13, 14]. In contrast, other studies have demonstrated that A3R activation reduces IR injury in the lung and heart, and that this action could be related to the direct effects on A3R activation of neutrophils [15, 16]. In our own experience, evidence suggests that an A3R agonist attenuates lung IR injury in an isolated rabbit lung model [17]. Thus we hypothesized that specific activation of A3R via agonist provides significant protection from in vivo lung IR injury, and that this would involve a neutrophil-dependent mechanism. We tested this hypothesis using an in vivo mouse model of lung IR as well as parallel in vitro studies.
Material and Methods
Animals
Male C57BL/6 wild-type (WT) mice (Jackson Laboratory, Bar Harbor, ME) and A3R knockout (A3R−/−) mice of 8–12 weeks of age were used. The A3R−/− mice [18], congenic to C57BL/6, were a gift from Dr. Marlene Jacobson (Merck Research Labs, West Point, PA). Mice were randomly assigned to seven groups (n=6/group) that underwent sham surgery (left thoracotomy + 2 hrs perfusion) or IR (1 hr left lung ischemia + 2 hrs reperfusion) in the presence of either Cl-IB-MECA (Sigma-Aldrich, St. Louis, MO; 100 µg/kg), a specific A3R agonist that is 2500- and 1400-fold selective for A3R vs. A1R and A2AR, respectively [19], or equivalent vehicle (0.1 mL 0.1% DMSO) administered via an external jugular vein injection 5 min prior to ischemia. The dose of Cl-IB-MECA used was based upon previous studies in mice [15, 20]. All experiments were approved by the University of Virginia’s Institutional Animal Care and Use Committee and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication No. 85-23, revised 1985).
Lung Ischemia-Reperfusion
Mice underwent 1 hr of lung ischemia (via left hilar occlusion) followed by 2 hrs reperfusion using an established model as previously described [21, 22]. Sham animals underwent left thoracotomy followed by 2 hrs of perfusion. Sham animals underwent 2 hours of perfusion because all experimental groups underwent 2 hours of reperfusion after ischemia. Comparison of lung function in sham animals after 2 hours versus 3 hours of perfusion results in no differences in lung function, thus 2 hours of perfusion is routinely performed in sham animals.
Pulmonary Function
Upon completion of reperfusion, pulmonary function was evaluated by measuring pulmonary compliance, pulmonary artery pressure, and mean airway pressure using an isolated mouse lung system (Hugo Sachs Elektronik, March-Hugstetten, Germany) as previously described [10, 23].
Lung Wet/Dry Weight
Using separate groups of mice (n=6/group), the left lung was harvested and weighed immediately after the 2-hour reperfusion period and placed in a desiccation oven until a stable dry weight was achieved. Lung wet/dry weight was then calculated as an indication of pulmonary edema.
Bronchoalveolar Lavage (BAL)
After measurement of pulmonary function, BAL of the left lung was performed using 0.4 mL normal saline. BAL fluid was centrifuged and the supernatant was stored at −80°C.
Cytokine and Myeloperoxidase Measurements
Levels of proinflammatory cytokines (IL-6, CXCL1 and CCL2) in BAL fluid and cell culture supernatant was performed using a murine Bio-Plex Cytokine Assay (Bio-Rad Laboratories, Hercules, CA) as previously described [24]. The samples were analyzed as instructed using a Bio-Plex array reader and a bead-based multiplex technology. Myeloperoxidase levels in BAL fluid and in cell culture supernatant were measured using a murine myeloperoxidase ELISA kit (Cell Sciences, Canton, MA) as instructed by the manufacturer.
Pulmonary Neutrophil Counts
Lungs (n=6/group) were flushed via the pulmonary artery with 3 mL normal saline followed by 5 mL of 10% buffered formalin and submersion in formalin for 15 hours. Lungs were embedded in paraffin and sectioned. Immunohistochemistry was performed using anti-mouse neutrophil primary antibody (GR1.1, Santa Cruz, Biotechnology) and alkaline phosphatase-conjugated secondary antibody. Signals were detected using Fast-Red (Sigma Aldrich, St. Louis, MO). Sections were counterstained with hematoxylin. Neutrophils were counted from three different high-powered fields per lung at 400× magnification and averaged.
Neutrophil Isolation
Neutrophils were purified from mouse bone marrow by immuno-magnetic selection using an antibody directed against mouse Ly-6G (Miltenyi Biotech, Inc., Auburn, CA). Mouse tibias and femurs were flushed with buffer (phosphate buffered saline with 2% EDTA and 0.5% bovine serum albumin), and cells were passed through a 40 µm filter, exposed to red blood cell lysis buffer, and washed to create a single cell suspension. Ly-6G-positive cells were then isolated using immuno-magnetic selection according the manufacturer’s directions. Purity of the isolated cells was confirmed by flow-cytometry to be greater than 92% Ly-6G-positive neutrophils.
In Vitro Hypoxia-Reoxygenation (HR)
Isolated neutrophils were cultured in RPMI media (1×106 cells/well) and exposed to either 1 µM Cl-IB-MECA or equivalent vehicle (0.01% DMSO). Cells then underwent 3 hours of hypoxia (5% O2 in media) followed by 1 hour of reoxygenation (21% O2 in media) using a hypoxia chamber as previously described [25]. Supernatant was then centrifuged at 4°C and stored at −80°C.
Neutrophil Migration
Neutrophil migration was assessed using a standard 24-well microchemotaxis chamber (Millipore, Billerica, MA). Neutrophils (5×105 cells/well) were exposed to either 1 µM Cl-IB-MECA or equivalent vehicle (0.01% DMSO) and were added to the upper wells of the chamber, which were separated from the lower wells by a polycarbonate membrane (5 µm pores). Serum-free RPMI media or RPMI media with 10% fetal bovine serum (FBS), a known neutrophil chemoattractant [26], was added to the lower wells, and the cells were incubated at 37°C for 4 hrs. Migrated cells were quantified by colorimetric absorbance according to the manufacturer’s instructions. The chemotaxis index was calculated as follows: absorbance of cells migrated in the presence of FBS divided by absorbance of cells migrated in serum-free media.
Statistical Analysis
Independent, pairwise group comparisons of differences in measured results were performed using ANOVA followed by the Students t test for unpaired data. Significance was defined as p<0.05. All results are presented as mean ± standard error of the mean.
Results
Pulmonary Dysfunction after IR is Attenuated by A3R Agonist
To determine the impact of selective A3R activation on lung function after IR, pulmonary function and edema were assessed in mice after sham surgery or IR in the presence of vehicle or Cl-IB-MECA (100 µg/kg), a selective and potent A3R agonist. As expected, IR resulted in significant pulmonary dysfunction in WT mice that received vehicle as measured by decreased compliance, increased pulmonary artery pressure, increased mean airway pressure, and increased edema (wet/dry weight) (Table 1). Cl-IB-MECA treatment significantly improved pulmonary function after IR in WT mice as demonstrated by increased compliance, decreased pulmonary artery pressure, decreased mean airway pressure, and decreased edema. Lung dysfunction after IR in A3R−/− mice was similar to WT mice after IR, and Cl-IB-MECA had no protective effects in A3R−/− mice after IR (Table 1). WT and A3R−/− sham animals demonstrated no significant differences in lung function or wet/dry weight (data not shown).
Table 1.
Summary of Lung Function, Edema and Cytokine Expression after Ischemia-Reperfusion
| P values | |||||||
|---|---|---|---|---|---|---|---|
| WT Sham |
WT IR | WT IR + MECA |
A3R−/− IR |
A3R−/− IR + MECA |
WT IR vs. WT Sham |
WT IR + MECA vs. WT IR |
|
| Lung compliance | 7.07 ア | 3.09 ア | 5.10 ア | 2.81 ア | 3.01 ア | *P=0.001 | #P=0.001 |
| (µl/cm H20) | 0.52 | 0.23* | 0.30# | 0.40 | 0.41 | ||
| Pulmonary artery | 5.30 ア | 11.47 ア | 5.77 ア | 10.05 ア | 9.77 ア | *P=0.005 | #P=0.006 |
| pressure (cm H20) | 0.17 | 1.06* | 0.30# | 0.92 | 0.83 | ||
| Airway resistance | 0.98 ア | 1.69 ア | 0.89 ア | 1.18 ア | 1.39 ア | *P=0.04 | #P=0.02 |
| (cm H20/µl/sec) | 0.03 | 0.20* | 0.02# | 0.07 | 0.21 | ||
| Wet/dry weight | 4.29 ア | 5.85 ア | 4.51 ア | 5.24 ア | 5.74 ア | *P=0.006 | #P=0.01 |
| 0.16 | 0.28* | 0.13# | 0.17 | 0.22 | |||
| IL-6 (pg/ml) | 971 ア | 2749 ア | 1843 ア | 2837 ア | 2567 ア | *P=0.001 | #P=0.02 |
| 93.1 | 309.9* | 148.6# | 220 | 213 | |||
| CCL2 (pg/ml) | 2066 ア | 5650 ア | 2992 ア | 6070 ア | 4779 ア | *P=0.008 | #P=0.03 |
| 78.5 | 917* | 278# | 764 | 371 | |||
| CXCL1 (pg/ml) | 2478 ア | 6224 ア | 4565 ア | 7691 ア | 6793 ア | *P=0.0001 | #P=0.02 |
| 121 | 599.9* | 229# | 520 | 600 | |||
| MPO (ng/ml) | 7.24 ア | 23.12 ア | 8.84 ア | 26.87 ア | 26.36 ア | *P=0.01 | #P=0.03 |
| 1.97 | 5.77* | 2.36# | 5.34 | 3.41 | |||
Values are presented as mean ア SEM. WT = wild-type, A3R−/− = A3R knockout, MECA = 100 µg/kg Cl-IB-MECA, MPO = myeloperoxidase. n = 6/group.
A3R Agonist Decreases Inflammation after IR
To determine the degree of inflammation after IR, proinflammatory cytokines (IL-6, CXCL1 and CCL2) and myeloperoxidase in BAL fluid were measured in mice after sham surgery or IR in the presence of Cl-IB-MECA (100 µg/kg) or vehicle. Myeloperoxidase was measured in BAL fluid as an indicator of neutrophil infiltration into alveolar airspaces. The levels of IL-6, CXCL1, CCL2 and myeloperoxidase were all significantly increased in WT lungs after IR compared to sham (Table 1). Cl-IB-MECA treatment resulted in significantly reduced levels of proinflammatory cytokines and myeloperoxidase in WT but not A3R−/−mice (Table 1). WT and A3R−/− sham animals demonstrated no significant differences in proinflammatory cytokine and myeloperoxidase levels (data not shown).
A3R Agonist Decreases Neutrophil Infiltration after IR
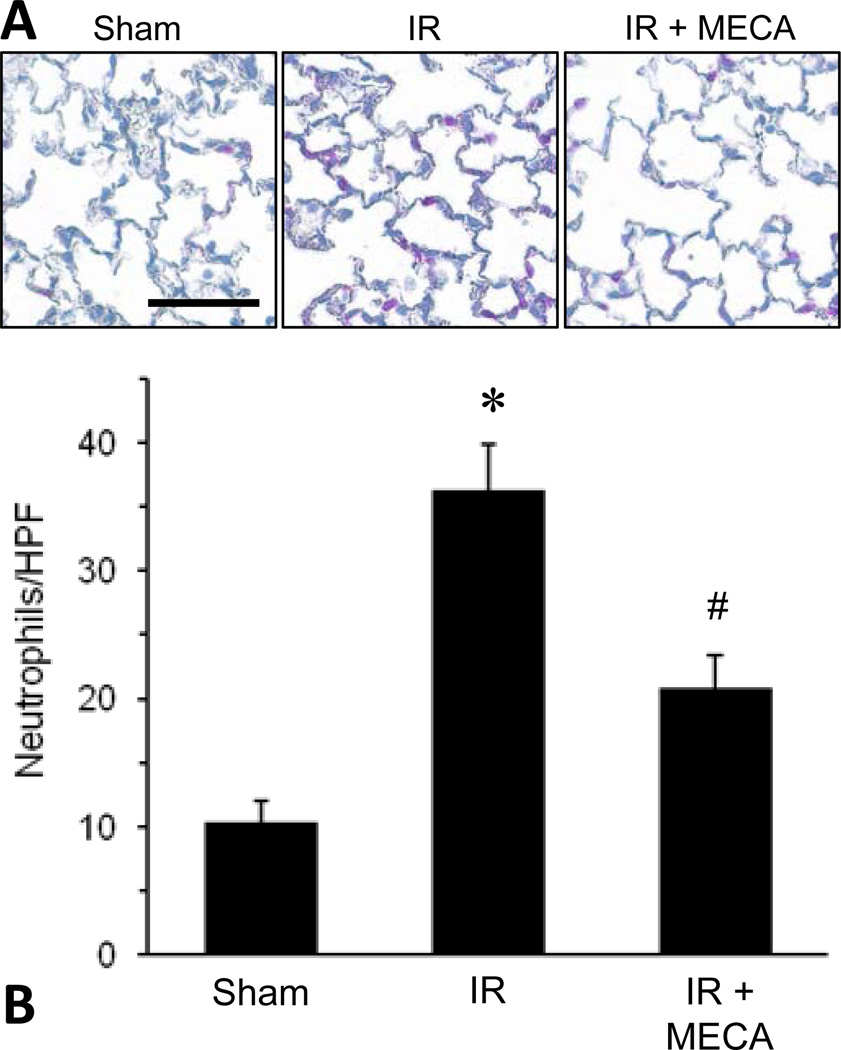
To directly measure pulmonary neutrophil infiltration, neutrophils were quantified, via immunohistochemistry, in lung sections from WT mice after sham surgery or IR and treated with Cl-IB-MECA (100 µg/kg) or vehicle. As expected, neutrophil infiltration (mean number of neutrophils per high-powered field) was significantly increased in WT mice after IR compared to sham. Cl-IB-MECA treatment significantly decreased neutrophil infiltration after IR (Figure 1). This suggests that the protection from lung IR injury by Cl-IB-MECA may involve a neutrophil-dependent mechanism.
Figure 1.
Pulmonary neutrophil infiltration after IR is attenuated by A3R agonism. WT lungs that underwent sham surgery or IR in the presence or absence of Cl-IB-MECA (MECA, 100 µg/kg) were fixed and sectioned. Immunostaining for neutrophils (pink color in representative images in panel A) was performed, and the average number of neutrophils per high-powered field (neutrophils/HPF) is shown in panel B. *p<0.05 vs. Sham, #p<0.05 vs. IR (n=4–6/group). Scale bar = 100 µm.
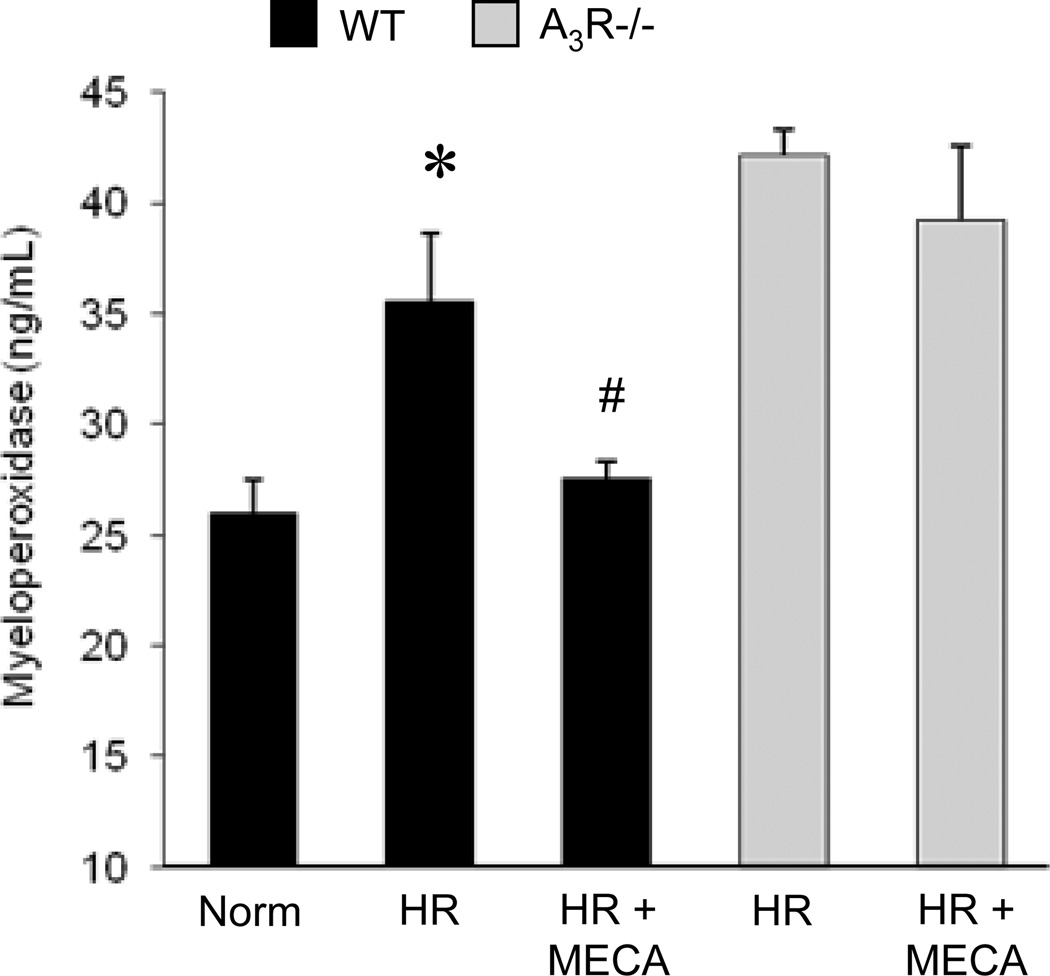
A3R Agonist Attenuates Neutrophil Activation after Hypoxia-Reoxygenation (HR)
To determine the effect of A3R agonism specifically on neutrophil activation, isolated neutrophils were incubated for 30 min in the presence of Cl-IB-MECA (1 µM) or vehicle and then exposed to either normoxia (4 hrs) or HR (3 hrs-1 hr). HR is used as an in vitro model of pulmonary IR as previously described [25]. Measurement of myeloperoxidase, a peroxidase enzyme abundantly present in neutrophil granules, in the culture supernatant was used as an indication of neutrophil activation [27]. As expected, release of myeloperoxidase was significantly increased after HR (Figure 2) indicating activation. Cl-IB-MECA treatment prevented myeloperoxidase release by WT neutrophils, but not A3R−/− neutrophils, after HR (Figure 2). This finding suggests that A3R agonism has a direct effect on neutrophils and limits their activation in response to HR.
Figure 2.
A3R agonism decreased activation of neutrophils from WT, but not A3R−/−, mice after hypoxia-reoxygenation. Myeloperoxidase in cell culture supernatant was measured after primary neutrophils from WT or A3R−/− mice were incubated in the presence of Cl-IB-MECA (MECA, 1 µM) or vehicle, and underwent 4 hrs of normoxia (Norm) or 3 hrs hypoxia and 1 hr reoxygenation (HR). *p<0.05 vs. Norm, #p<0.05 vs. HR (n=8/group).
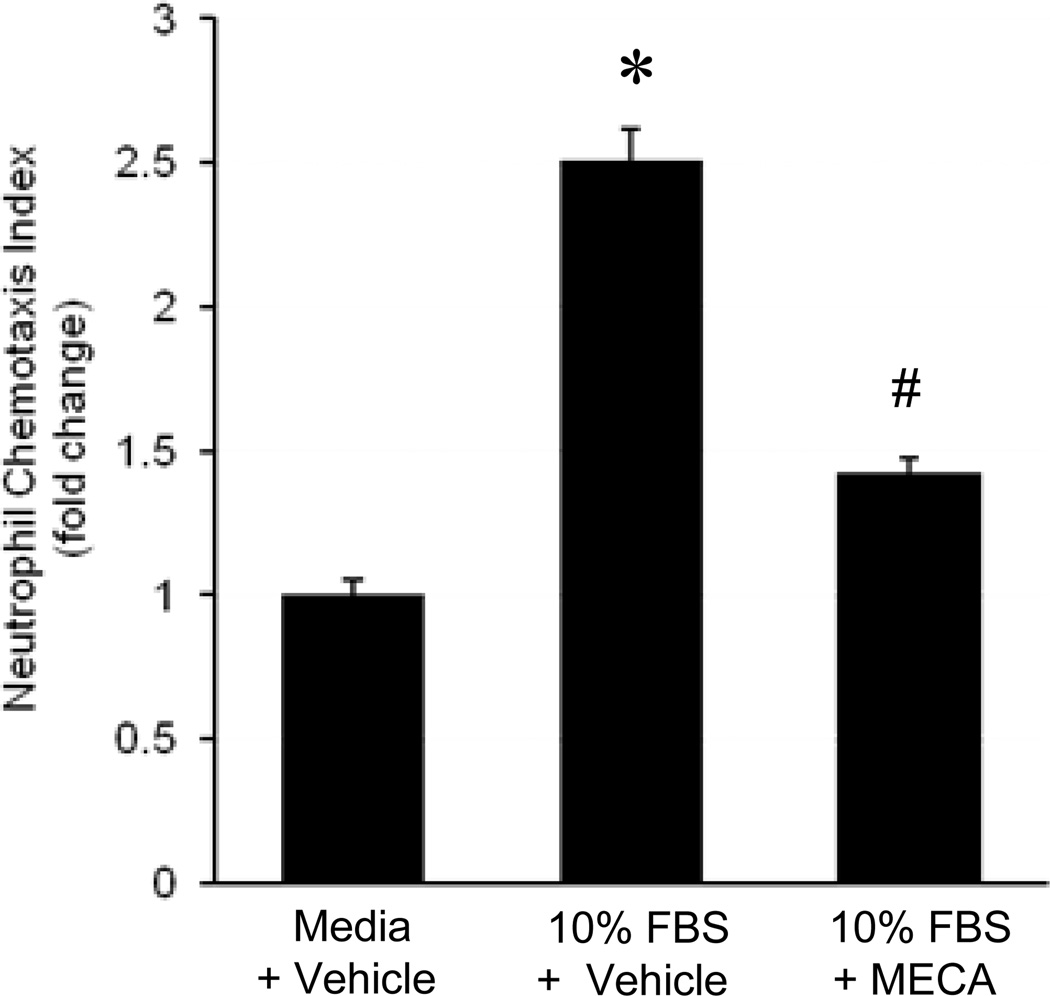
A3R Agonist Attenuates Neutrophil Chemotaxis
To determine the effect of A3R agonism on neutrophil chemotaxis, a trans-well migration assay was performed on isolated neutrophils from WT mice. Neutrophils were incubated for 30 min in the presence of Cl-IB-MECA (1 µM) or equivalent vehicle and were then exposed to either media or media containing 10% FBS (a known neutrophil chemoattractant) in the lower well and incubated for an additional 4 hrs. Migration (chemotaxis) was then quantified and expressed as a chemotaxis index as described in the Materials and Methods. As expected, FBS exposure increased neutrophil chemotaxis over 2-fold, which was significantly attenuated by Cl-IB-MECA (Figure 3). This demonstrates a direct effect of A3R agonism on neutrophil chemotaxis.
Figure 3.
A3R agonism attenuated neutrophil chemotaxis in vitro. Neutrophils from WT mice were added to the upper wells of a trans-well migration assay, incubated with Cl-IB-MECA (MECA, 1 µM) or vehicle, and then exposed to media or media with 10% fetal bovine serum (FBS) in the lower wells. After incubating for 4 hrs at 37°C, trans-migrated cells were quantified and presented as a chemotaxis index. *p<0.05 vs. media + vehicle, #p<0.05 vs. 10% FBS + vehicle (n=8/group).
Comment
This study used both an in vivo model of mouse lung IR injury and in vitro experiments with purified mouse neutrophils to demonstrate that selective activation of A3R, via Cl-IB-MECA, decreases lung dysfunction and inflammation after IR. The results further suggest that the anti-inflammatory actions of Cl-IB-MECA are mediated, at least in part, through direct effects on neutrophils. Lung IR injury involves multiple pathways such as innate immune responses, generation of reactive oxygen species, cytokines, neutrophil infiltration, and cell death [2]. Neutrophils are known to infiltrate the transplanted lung after reperfusion and directly contribute to injury [1]. Several studies, including our own, suggest that lung IR injury involves innate immune responses such as early activation of invariant NKT cells and macrophages, which contribute to neutrophil infiltration and subsequent neutrophil-dependent injury [24, 28, 29]. Neutrophils are also known to mediate changes in endothelial and epithelial permeability in the lung after IR, and their infiltration into the lung correlates with poor lung oxygenation and lung function [4].
It is well established that adenosine potently inhibits neutrophil superoxide production, adhesion and chemotaxis, and production of proinflammatory mediators [6, 30, 31]. To date, most research on the role of adenosine signaling in inflammation has focused on the protective role of the A2AR activation. However, the role of A3R in inflammatory states such as IR is not well understood. Proinflammatory properties of the A3R have been demonstrated in a sepsis model that showed decreased neutrophil migration in A3R−/− mice [13]. In addition, a 2006 study reported that neutrophils rapidly hydrolyze released ATP to adenosine that subsequently acts via A3R recruited to the leading edge of the cell to promote neutrophil migration [32]. In contrast, the present study demonstrates that specific A3R activation decreases neutrophil activation and chemotaxis both in vivo and in vitro.
Our results show that activation of A3R via Cl-IB-MECA significantly attenuates lung dysfunction, pulmonary edema and production of key pro-inflammatory cytokines (IL-6, CCL2 and CXCL1) after IR. In addition, neutrophil activation and infiltration in lungs were attenuated by Cl-IB-MECA after IR as shown by reduced myeloperoxidase levels as well as direct numbers of neutrophils by immunohistochemistry. Finally, in vitro experiments demonstrated that Cl-IB-MECA attenuated the release of myeloperoxidase by primary neutrophils after hypoxia-reoxygenation and attenuated neutrophil chemotaxis. Several recent studies support our results. Using a lipopolysaccharide model of lung inflammation, Wagner et al. demonstrated conserved lung histology and reduced neutrophil infiltration in mice pretreated with Cl-IB-MECA [20]. Similarly, in a feline model of lung IR injury, Rivo et al. have shown conserved lung function with selective A3R activation [20]. In addition, van der Hoeven et al. found that A3R activation suppressed superoxide production and chemotaxis of mouse neutrophils [16]. Therefore, while our results have precedent in the literature, we know of no previous study that has combined both in vivo and in vitro experiments to demonstrate conserved lung function and decreased neutrophil activation and chemotaxis in an IR model. These results help clarify the enigmatic role of A3R in inflammatory states and suggest that A3R agonism may be a novel therapeutic strategy to prevent lung IR injury after transplantation.
The fact that the A3R−/− mice did not have a more severe degree of lung IR injury than WT mice, as might be expected, could be due to several reasons. First, adenosine can act on all four adenosine receptors which could have synergistic or antagonistic effects. The pharmacology of adenosine receptor signaling is complex, and the role of these individual receptors can be pro-inflammatory or anti-inflammatory. Hence, in A3R−/− mice, adenosine could still exert an overall anti-inflammatory effect via binding to other adenosine receptors such as A2AR, thereby preventing an enhancement of lung injury after IR. Second, the downstream adenosine receptor signaling cascades that occur in A3R−/− mice remain unknown. It is possible that the crosstalk between the various adenosine receptor signaling pathways have been altered in A3R−/− mice. Importantly, however, the present study demonstrated that the specific activation of A3R by Cl-IB-MECA in WT mice, but not A3R−/− mice, resulted in significant protection from lung injury and dysfunction after IR, which demonstrates the specificity of the agonist and the lack of non-specific effects of Cl-IB-MECA.
One limitation of the current study is that, although the in vivo quantification of neutrophil numbers and myeloperoxidase levels accurately represent neutrophil infiltration after IR, we cannot conclude based solely on these results that the action of Cl-IB-MECA is directly on neutrophils. However, the results from the in vitro experiments suggest that the protective effects of Cl-IB-MECA are due, at least in part, to direct effects on neutrophils. Regarding the A3R−/− mice, the effects of genetic knockout of A3R on the expression of other adenosine receptors must be considered. However, the density of A1R and A2AR subtypes was previously shown to be unchanged in the A3R−/− mice compared to WT mice [18].
In conclusion, the present study demonstrates that selective activation of A3R by Cl-IB-MECA attenuates lung dysfunction, inflammation, and neutrophil infiltration after IR in WT but not A3R−/− mice. Furthermore, in vivo and in vitro results suggest that the protective effects of Cl-IB-MECA are due, at least in part, to the direct prevention of neutrophil activation and chemotaxis. Therefore, the use of A3R agonists to target neutrophils may be a novel therapeutic strategy to prevent IR injury and primary graft dysfunction after lung transplantation.
Acknowledgments
This work was supported by NIH grants R01HL092953 (VEL) and T32HL007849 (ILK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 2.den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010;299:H1283–H1299. doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 3.Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–1047. doi: 10.1016/s0003-4975(01)03606-2. [DOI] [PubMed] [Google Scholar]
- 4.Ross SD, Tribble CG, Gaughen JR, Jr, Shockey KS, Parrino PE, Kron IL. Reduced neutrophil infiltration protects against lung reperfusion injury after transplantation. Ann Thorac Surg. 1999;67:1428–1433. doi: 10.1016/s0003-4975(99)00248-9. [DOI] [PubMed] [Google Scholar]
- 5.Sharma AK, Laubach VE, Ramos SI, et al. Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;139:474–482. doi: 10.1016/j.jtcvs.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32:856–864. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazoni LM, Laubach VE, Mulloy DP, et al. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. J Thorac Cardiovasc Surg. 2008;135:156–165. doi: 10.1016/j.jtcvs.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 8.LaPar DJ, Laubach VE, Emaminia A, et al. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg. 2011;142:887–894. doi: 10.1016/j.jtcvs.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma AK, Linden J, Kron IL, Laubach VE. Protection from pulmonary ischemia-reperfusion injury by adenosine A2A receptor activation. Respir Res. 2009;10:58. doi: 10.1186/1465-9921-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anvari F, Sharma AK, Fernandez LG, et al. Tissue-derived proinflammatory effect of adenosine A2B receptor in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;140:871–877. doi: 10.1016/j.jtcvs.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther. 2008;117:123–140. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci U S A. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue Y, Chen Y, Pauzenberger R, Hirsh MI, Junger WG. Hypertonic saline up-regulates A3 adenosine receptor expression of activated neutrophils and increases acute lung injury after sepsis. Crit Care Med. 2008;36:2569–2575. doi: 10.1097/CCM.0b013e3181841a91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HT, Ota-Setlik A, Xu H, D'Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am J Physiol Renal Physiol. 2003;284:F267–F273. doi: 10.1152/ajprenal.00271.2002. [DOI] [PubMed] [Google Scholar]
- 15.Ge ZD, Peart JN, Kreckler LM, et al. Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5'-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;319:1200–1210. doi: 10.1124/jpet.106.111351. [DOI] [PubMed] [Google Scholar]
- 16.van der Hoeven D, Wan TC, Auchampach JA. Activation of the A(3) adenosine receptor suppresses superoxide production and chemotaxis of mouse bone marrow neutrophils. Mol Pharmacol. 2008;74:685–696. doi: 10.1124/mol.108.048066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazoni LM, Walters DM, Unger EB, Linden J, Kron IL, Laubach VE. Activation of A1, A2A, or A3 adenosine receptors attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;140:440–446. doi: 10.1016/j.jtcvs.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem. 2000;275:4429–4434. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson KA. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol Sci. 1998;19:184–191. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner R, Ngamsri KC, Stark S, Vollmer I, Reutershan J. Adenosine receptor A3 is a critical mediator in LPS-induced pulmonary inflammation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L502–L512. doi: 10.1152/ajplung.00083.2010. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Sharma AK, Linden J, Kron IL, Laubach VE. CD4+ T lymphocytes mediate acute pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2009;137:695–702. doi: 10.1016/j.jtcvs.2008.10.044. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z, Sharma AK, Marshall M, Kron IL, Laubach VE. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. Am J Respir Cell Mol Biol. 2009;40:375–381. doi: 10.1165/rcmb.2008-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao M, Fernandez LG, Doctor A, et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1018–L1026. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 24.Sharma AK, LaPar DJ, Zhao Y, et al. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med. 2011;183:1539–1549. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-alpha during pulmonary ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2007;293:L105–L113. doi: 10.1152/ajplung.00470.2006. [DOI] [PubMed] [Google Scholar]
- 26.Struyf S, Proost P, Lenaerts JP, Stoops G, Wuyts A, Van Damme J. Identification of a blood-derived chemoattractant for neutrophils and lymphocytes as a novel CC chemokine, Regakine-1. Blood. 2001;97:2197–2204. doi: 10.1182/blood.v97.8.2197. [DOI] [PubMed] [Google Scholar]
- 27.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 28.Eppinger MJ, Jones ML, Deeb GM, Bolling SF, Ward PA. Pattern of injury and the role of neutrophils in reperfusion injury of rat lung. J Surg Res. 1995;58:713–718. doi: 10.1006/jsre.1995.1112. [DOI] [PubMed] [Google Scholar]
- 29.Fiser SM, Tribble CG, Long SM, et al. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121:1069–1075. doi: 10.1067/mtc.2001.113603. [DOI] [PubMed] [Google Scholar]
- 30.Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983;158:1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linden J. Regulation of leukocyte function by adenosine receptors. Adv Pharmacol. 2011;61:95–114. doi: 10.1016/B978-0-12-385526-8.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]