Abstract
A dynamic systems model was used to generate parameters describing a phenotype of Hypothalamic–Pituitary–Adrenal (HPA) behavior in a sample of 36 patients with chronic fatigue syndrome (CFS) and/ or fibromyalgia (FM) and 36 case-matched healthy controls. Altered neuroendocrine function, particularly in relation to somatic symptoms and poor sleep quality, may contribute to the pathophysiology of these disorders. Blood plasma was assayed for cortisol and ACTH every 10 min for 24 h. The dynamic model was specified with an ordinary differential equation using three parameters: (1) ACTH-adrenal signaling, (2) inhibitory feedback, and (3) non-ACTH influences. The model was ‘‘personalized’’ by estimating an individualized set of parameters from each participant’s data. Day and nighttime parameters were assessed separately. Two nocturnal parameters (ACTH-adrenal signaling and inhibitory feedback) significantly differentiated the two patient subgroups (“fatigue-predominant” patients with CFS only versus ‘‘pain-predominant’’ patients with FM and comorbid chronic fatigue) from controls (allp’s < .05), whereas daytime parameters and diurnal/nocturnal slopes did not. The same nocturnal parameters were significantly associated with somatic symptoms among patients (p’s < .05). There was a significantly different pattern of association between nocturnal non-ACTH influences and sleep quality among patients versus controls (p < .05). Although speculative, the finding that patient somatic symptoms decreased when more cortisol was produced per unit ACTH, is consistent with cortisol’s anti-inflammatory and sleep-modulatory effects. Patients’ HPA systems may compensate by promoting more rapid or sustained cortisol production. Mapping “behavioral phenotypes” of stress–arousal systems onto symptom clusters may help disentangle the pathophysiology of complex disorders with frequent comorbidity.
Keywords: Psychoneuroendocrinology, Stress–arousal, Cortisol, Glucocorticoid resistance, Feedback sensitivity, Dynamical systems, Systems medicine, Personalized medicine, Sleep quality, Somatic symptoms, Functional somatic disorders, Fibromyalgia, Chronic fatigue syndrome
1. Introduction
In medical research a fundamental shift is underway to redefine disease taxonomies, moving from descriptive categories based on ‘‘signs and symptoms’’ to a new framework that explains disease symptoms within the context of underlying molecular and environmental causes (National Research Council, 2011). At the core of this endeavor is the need for novel bioinformatic approaches that help link symptom clusters with phenotypes (arising from the interplay of genetic and environmental causes). This approach may be particularly important in understanding diseases, such as functional somatic disorders or psychosomatic conditions, in which the biological etiology is poorly understood, and psychosocial factors play a role in precipitating or perpetuating the disease process. The primary aim of this study is to conduct a ‘‘proof of concept’’ investigation of whether a phenotype of stress-arousal system behavior will provide novel insights into disease symptoms and subtypes among patients with fibromyalgia (FM) and/or chronic fatigue disorder (CFS).
Altered Hypothalamic–Pituitary–Adrenal (HPA) system function may be an important contributing mechanism in the onset or maintenance of FM, CFS and psychosomatic conditions (i.e., disorders caused or aggravated by stress and psychological distress). Existing paradigms for quantifying and understanding the role of the HPA has yielded an inconsistent body of literature (Miller et al., 2007; Tanriverdi et al., 2007), making it difficult to translate these findings into improvements in clinical care. This may reflect the fact that the HPA is a dynamic feedback-regulated system, which is often modeled with statistics poorly equipped to characterize reciprocal regulatory control as it occurs dynamically.
CFS and FM are debilitating and costly disorders characterized by “medically unexplained symptoms” (Aaron et al., 2000). They are associated with “a loss of HPA axis resiliency” (Crofford et al., 2004) and altered HPA dynamics following pharmacological challenge (Tanriverdi et al., 2007; Van Den Eede et al., 2007); however, the nature of the alterations is inconsistent across studies (Tanriverdi et al., 2007). Moreover, the extent to which HPA function differs in CFS versus FM is unclear. On the one hand, CFS and FM are highly comorbid (Aaron et al., 2000), suggesting some common pathophysiological mechanisms. On the other, genetic studies suggest that it may be useful to differentiate pain-predominant syndromes (i.e., FM with or without comorbid fatigue) from fatigue-predominant ones (i.e., CFS without FM)(Geenen and Bijlsma, 2010; Kato et al., 2009). Moreover, as pain can disturb sleep, fatigue could reflect a different etiology in FM (Geenen and Bijlsma, 2010). Negative feedback sensitivity of the HPA is generally heightened in CFS (Jerjes et al., 2007; Van Den Eede et al., 2007) but blunted in FM (Lentjes et al., 1997), which leaves the expected HPA profile associated with comorbidity uncertain. Hence, a model of HPA system behavior that can be fitted to a given individual regardless of comorbidity could potentially provide a platform for personalized treatment.
Somatic symptoms and sleep disturbance are core symptoms of CFS and FM, which are also related to HPA function and the role of cortisol as a stress-responsive and anti-inflammatory hormone. The onset of functional disorders is often preceded by psychological stress or infection (Theorell et al., 1999), and early adversity may also increase risk (Heim et al., 2009). Sleep disturbance is a core symptom of FM. For example, one US study of 500 FM patients reported that 95% of the sample reported poor sleep, which predicted pain (Bigatti et al., 2008). Both stress and poor sleep have reciprocal relations with HPA and pro-inflammatory activity (Adam et al., 2006; Irwin and Cole, 2011; Zeiders et al., 2011). One theory is that FM and CFS are associated with an immunologic disturbance (Moldofsky, 1995; Patarca-Montero et al., 2001). Cytokines can cause neuroinflammation and “sickness behaviors” that closely resemble the kinds of somatic symptoms reported by patients with functional somatic disorders, such as fatigue, concentration difficulties, enhanced pain sensitivity and mood symptoms (Dantzer and Kelley, 2007). Cytokines can also modulate glucocorticoid receptor phosphorylation, potentially leading to longer-lasting changes in the HPA signaling dynamics and function (Pace et al., 2007). Cortisol is a powerful anti-inflammatory hormone; hence, in theory, if its inhibitory feedback signaling via the glucocorticoid receptor were impaired, this could potentially act as a permissive or perpetuating factor that could amplify reciprocal effects among stress, sleep behaviors and inflammatory activity.
Allostasis, a leading theoretical model of physiological regulation, can be defined as the optimization of system dynamics to facilitate adaptive functional responses to a certain context (Boyce and Ellis, 2005; Kitano, 2007; Sterling, 2011). A canonical principle of system function is that there are crucial trade-offs between responsiveness and robustness (i.e., maintaining function despite perturbation)(Kitano, 2007), which are shaped in part by the system parameters (Kitano, 2007). An HPA system with a “high steady state gain” – e.g., characterized by heightened cortisol responses to ACTH and/or blunted feedback control – can generate more rapid or sustained cortisol responses. Henceforth, we will refer to this theoretical pattern of allostatic optimization as the “high sensitivity phenotype.” In theory, a high sensitivity phenotype would facilitate faster or more sustained cortisol responses to stressors or mitogen stimulation, which could help counter-balance excess neuroinflammatory activity. However, as optimization comes with robustness trade-offs, the highly sensitive phenotype would also be more volatile and vulnerable to intense or unanticipated psychological or immunological stressors (Carlson and Doyle, 2000).
To better evaluate the utility and added insights of the proposed dynamic systems model and high sensitivity phenotype, it is important to have an appropriate comparison model. Frequently used measures are the cortisol awakening response (CAR – the rapid increase in cortisol over 30 min post-awakening) and the diurnal (or nocturnal) slope (i.e., the change from awakening to the evening nadir) (Adam et al., 2006; Sephton et al., 2000). These measures quantify circadian rhythms, which help the body meet changing demands throughout the day for neurocognitive alertness and immune cell trafficking (Sephton et al., 2000); however, the slope method does not quantify faster dynamics occurring over the time-course of minutes to hours.
This study represents a “proof of concept” intended to investigate the feasibility and utility of (1) quantifying a high-sensitivity “system behavioral phenotype” of a key stress–arousal system, the HPA axis, (2) relating this phenotype to disease symptoms, and (3) investigating whether the phenotype reveals disease subtypes within a heterogenous patient sample with chronic fatigue syndrome (CFS) and/or fibromyalgia (FM). Hence, the primary thrust of this work is not based in the specifics of the HPA axis, the mathematical model or the patient population per se, but rather in exploring a potentially important and novel method relevant to refining the taxonomy of diseases with complex biological and environmental origins. This is the first study to explore whether a personalized system behavioral phenotype of HPA activity relates to health.
2. Methods and materials
2.1. Participants
This study represents a secondary analysis of published data from a larger study (Crofford et al., 2004) focused on HPA function as the primary outcome. Forty individuals with FM, CFS, or both disorders and 40 healthy controls, individually matched for gender, menstrual status, and age within four years, were recruited from rheumatology, infectious disease or primary care outpatient clinics at the University of Michigan Medical Center. In addition, control subjects were sedentary as defined by no regular exercise for a minimum of 2 months prior to the study. ACTH data and/or blood draws could not be completed for four participants, leaving complete data available for 36 matched patient–control pairs (n = 72). All diagnoses were made using the 1990 American College of Rheumatology and the 1994 Center for Disease Control and Prevention criteria. Patients were interviewed by a single physician and final diagnoses were completed by a team of three physicians who reviewed the physical exam data and medical history. Patients with Major Depressive Disorder (MDD) were excluded. Of the 36 patients with complete ACTH and cortisol data available, 14 individuals met criteria for CFS (by both the 1998 and 1994 definitions), and did not meet criteria for FM. The remaining 22 participants were considered pain-predominant, because they all met criteria for FM, and 19 additionally met criteria for the more recent 1994 case-definition of CFS. As this study is focused on the potential use of the phenotype to help redefine disease taxonomies, we elected to examine symptoms across the whole patient sample, and then examine whether the phenotype differed between subgroups based on predominant symptom profiles, which differs from the approach taken in prior published work from this study (Crofford et al., 2004). In that study, the FM group was complicated by the fact that 7 of 12 individuals did not meet criteria for CFS by the 1988 definition but did meet criteria by the 1994 definition (which requires fewer symptoms). A primary goal of this paper is to deal with exactly these complexities by exploring an alternative to existing diagnostic categories; hence our deviation from the prior groupings was intentional and central to the current hypotheses. All participants signed informed consent forms approved by the University of Michigan Institutional Review Board.
All participants ranged in age from 18 to 59 (mean 40.35 years) and 64 were female. Inclusion criteria included being non-smoking, non-obese, and no other significant medical conditions besides CFS. All mediations, including herbal remedies, were discontinued at least ten days prior to study except stable doses of estrogen replacement and one participant receiving a stable dose of antihypertensive medication (enalapril). Female participants were matched on menstrual status with one exception (Crofford et al., 2004). The inclusion criteria were: no shift work, no travel across >3 time-zones within prior 3 months, no current major psychiatric disorders, not pregnant, a clean urine drug screen, no use of glucocorticoid medications in any form for 3 months prior, all medications discontinued within 10 days prior, and no abnormal blood chemistry results. These criteria have been described in greater detail elsewhere (Crofford et al., 2004). Median symptom duration among patients was 4.8 years (IQR = 5.1 years). Recent recommendations on minimal data reporting for this patient population were reviewed during the writing of this manuscript (Jason et al., 2012), and all available data meeting these criteria is reported.
2.2. Blood draw procedure
Participants slept at the General Clinical Research Center on the evening prior to sampling to accommodate to the environment. Activity was restricted, and meals were provided at regular times. Lights were turned out at 10 pm and on at 7 am. Standardized meals were provided at 7:30 am, 12:00 noon and 5:30 pm. Patients were not allowed to snack in between. An intravenous catheter inserted in the antecubital vein and attached to a double stopcock assembly was used to withdraw samples every 10 min for 24 h, from 9 am to 9 pm. Intravenous fluids (0.45 saline) were infused between sample withdrawals so the catheter would remain open, and heparin (1000 U/L) was added if the blood draw return diminished. Nighttime blood-draws were conducted by trained nurses with flashlights. Samples (2.7 ml) were placed into pre-chilled polypropylene tubes with 250 µl of 20 mg/ml of EDTA solution to prevent clotting, and placed on ice. Samples were spun at 2700 rpms for 10 min within 2 h of collection, and plasma was aliquotted into labeled tubes and frozen at −80 °C.
2.3. ACTH and cortisol assays
Plasma ACTH concentrations were measured in duplicate using a highly specific dual-immunoradiometric assay (Nicols Institute Diagnostics, CA). Plasma cortisol levels were quantified by radioimmunoassay (Coat-a-Count, Diagnostic Products Corporation, CA). All samples were run as case-control pairs on the same day with kits from the same lot. Intra-assay variability for ACTH and cortisol assays were 7.19% and 6.45%, and their respective sensitivities were 1 pg/ml and 0.2 u.g/dl (Crofford et al., 2004).
2.4. Nocturnal and diurnal HPA assessment
For several reasons, this study divided the neuroendocrine data into two segments roughly approximating daytime and nighttime. First, this strategy enabled us to compare the fit and utility of the dynamic model to a comparison cortisol slope model. By definition, the slope model examines the increase or decrease between the morning peak and the evening nadir. We additionally wished to differentiate the nocturnal HPA dynamics for clinical and physiological reasons. Sleep is a major modulator of pituitary-dependent hormone release (Van Cauter et al., 2000). Unrestorative sleep is one of the most common complaints in patients with functional disorders (Aaron et al., 2000; Bigatti et al., 2008), and exhaustion is a defining symptom in CFS. Some evidence suggests that cortisol may modulate deep sleep (Gronfier and Brandenberger, 1998). In addition, the sympathetic nervous system may be hyperactive at night in FM (Geenen and Bijlsma, 2010). Sympathetic innervations of the adrenal gland via the extrapituitary splanchnic nerve (Clow et al., 2010) may promote a dissociation between ACTH and cortisol, which this dynamic model can detect.
2.5. Psychological function
Frequency of somatic symptoms were assessed using the 54-item Pennebaker Inventory for Limbic Languidness (PILL) (Pennebaker, 1982). The PILL has exhibited high reliability and validity among patients with chronic fatigue syndrome (Katon and Russo, 1992) and fibromyalgia (Moshiree et al., 2007). PILL scores correlate with the number of physician visits and amount of daily activity restriction (Pennebaker, 1982). Although no subjects met criteria for current MDD, residual depressive symptoms were assessed using the Beck Depression Inventory (BDI). The BDI is a standard, well-validated measure consisting of 21 items, rated on an intensity scale of 0–3, yielding a total sum score (Beck et al., 1988). The Pittsburgh Sleep Quality Index (PSQI) is a self-report measure consisting of 19 items, which assess multiple dimensions of sleep over a 1-month period (Buysse et al., 1989). The PSQI has seven components (or subscales), which are totaled to obtain a global measure with scores ranging from 0 to 21 (higher = poorer subjective sleep quality). The internal consistency, test–retest reliability, diagnostic sensitivity, and specificity have been established in depressed, sleep-disordered, and healthy individuals (Buysse et al., 1989).
2.6. Data analysis
The data analyses were organized into three stages: (1) Comparing the fit of the dynamic versus the slope model, (2) Investigating patient-control differences in HPA dynamic system parameters and examining whether diagnostic subgroups (i.e., fatigue-predominant (CFS only) versus pain-predominant (FM with or without chronic fatigue)) exhibit different patterns of system activity relative to controls, and (3) Investigating the association of the system parameters with somatic symptoms and sleep disturbance, looking at these associations separately in patients versus controls.
2.7. The descending and ascending slope models
The descending and ascending slopes for each individual were calculated as a comparison model for the dynamic model, in order to establish goodness of fit. The nadir of the circadian cortisol rhythm occurs at approximately 11 pm (p. 276) (Jacobson, 2005), and the morning peak generally occurs between 5 am and 9 am. As the time of awakening was not recorded, the slope time-periods were defined as 9 am–11 pm for the descending diurnal slope and 11 pm–9 am for the ascending nocturnal slope. Slopes for both cortisol and ACTH were calculated using within-subject linear regression of ‘Time’ with three parameters: (1) linear, (2) quadratic term, (3) the intercept. The intercept was centered at 9 am for the daytime model (to capture the approximate cortisol peak) and 1 am for the nighttime model (as the nadir lasts from approximately 11 pm–3 pm, placing the intercept of the slope model in the middle of this period (1 am) provides the optimal fit for the linear and quadratic slope parameters). Although time of awakening was not recorded, the non-linear CAR (Adam et al., 2006; Clow et al., 2010) was modeled with a fourth spline regression parameter quantifying the difference between the ascending slope and the maximal cortisol value occurring between 5 am and 8 am for each individual (approximating modeling from previously published studies as best as possible with the current available data) (Zeiders et al., 2011).
2.8. The dynamic model
The following differential equation (Eq. (1)) was used to predict the rate of change in the future level of cortisol as a function of time and the current levels of ACTH and cortisol. This mathematical specification mirrors the biological understanding that ACTH is the primary stimulator of cortisol secretion, and cortisol exerts an inhibitory effect on the central nervous system that effectively decreases cortisol secretion (Jacobson, 2005). Eq. (1) can be interpreted as following two main principles: the rate of change in the amount of cortisol over time (dC(t)/dt) will (1) increase in relation to the amount of ACTH (A) secreted and the sensitivity of the adrenal cortex to the ACTH signal (λA), and (2) decrease in relation to the amount of currently available cortisol (C), cortisol clearance rates and the sensitivity of receptors in the central nervous system to which cortisol binds (λC) (i.e., glucocorticoid and mineralcorticoid receptors (Jacobson, 2005)). The intercept (λI) captures external, or non-ACTH influences, on cortisol (e.g., hippocampal, CRH, sympathetic or basal secretion rate variation (Carlson et al., 2009)). For example, if λAand λC are held constant, but CRH increases or hippocampal inhibition of the HPA decreases, this could possibly result in an increase in cortisol, which could manifest as higher λI.
The differential model of cortisol:
| (1) |
2.9. Interpreting the system parameters
The dynamic parameter λA, represents ACTH-adrenal signaling, or the rate at which the adrenal gland produces cortisol in response to ACTH produced by the pituitary. Higher levels of λA indicate that more cortisol is produced per unit ACTH. λC represents the rate of inhibitory feedback signaling, or how effectively cortisol shuts off further HPA production of ACTH. Higher levels of λC may therefore indicate stronger inhibitory signaling in the central nervous system per unit cortisol and/or altered turn-over rates. As this analysis does not measure the signaling mechanisms directly, the system parameters serve as coarse-grained, aggregate functional measures. Henceforth, the system parameters are referred to with the following terminology: (1) λA = ACTH-adrenal signaling, (2) λC = Inhibitory feedback signaling, and (3) λI = non-ACTH influences.
2.10. Estimating the system parameters
To derive parameter estimates for each participant, we utilized the fact that a difference model constitutes an approximation of a linear differential equation (i.e., refer to the definition of the derivative (Anton and Herr, 1995)). The difference model (Eq. (2)) corresponds to a lagged regression equation predicting 10-min changes in cortisol, which models feedback relationships between ACTH and cortisol. The 10-min lag was based on published data of time-lags for HPA hormone effects, and evidence that the maximal correlation between ACTH and cortisol occurs at a 10-min lag (Sapolsky et al., 2000; Young et al., 2001); however, we cannot rule out the possibility that pulses occurring within the 10-min window were missed. Matlab and SPSS for Windows were utilized to conduct the analyses. The regression parameters derived from the difference model represent the average change in the rate of cortisol between two time-points. This change corresponds to a linear slope; however, the actual changes in cortisol are continuous. In order to model cortisol secretion continuously, a standard technique, exact discretization with a zero order hold (Seborg et al., 2004), was utilized to transform the parameters. The difference model of cortisol:
| (2) |
The steady state gain is a well-defined concept in systems biology (Alon, 2007), defined briefly here. In this context, the steady state gain – expressed as a ratio of the parameters λA/λC - can be thought of as a ‘‘volume knob,’’ such that increases in the steady state gain will yield increases in the steady state level of cortisol relative to ACTH. λI was not included in the steady state calculation because it represents effects other than ACTH on cortisol production, and primarily corresponds visually to the height of the basal floor rather than impacting the dynamics around that floor.
2.11. Statistical analyses
None of the slope variables or the system parameters exhibited distributions that significantly differed from normality per the Kolmogorov–Smirnov test (all p’s > .05). Per an a priori decision, a few data points1 exceeding three standard deviations (sd) were winsorized to 2.6 standard deviations to mitigate potential biases. Paired t-tests, which control for statistical dependencies between case-control matched pairs, were used to conduct group and subgroup comparisons on the system parameters. As patients and controls did not differ on the matching factors (all p’s > .05), these factors were only included as covariates in analyses that do not already statistically control for case-control differences (e.g., as paired t-tests do).
3. Results
3.1. Evaluating model fit
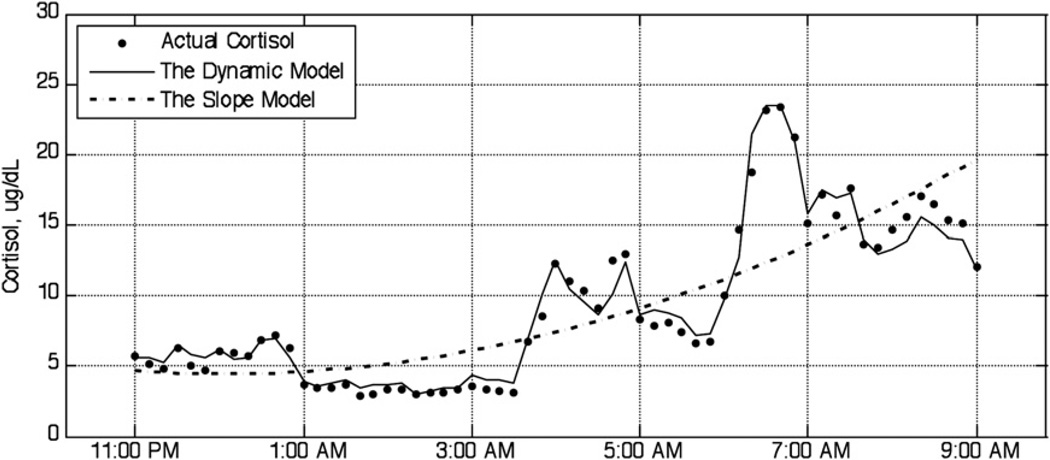
Model fit was based on Akaike’s Information Criteria (AIC), in which lower scores indicate superior fit (Akaike, 1974). The dynamic model AIC provided a better fit than the slope model 100% of the time. The AIC did not significantly differ between patients and controls. Fig. 1 illustrates fit with a representative graph of the nocturnal dynamic and slope models compared to the raw cortisol data in one representative healthy participant.
Fig. 1.
Fit of the dynamic and slope models compared to measured cortisol. Note: this graph illustrates model fit for nocturnal circadian cortisol secretion in one representative healthy participant. As expected, the evening period is quiescent, while the dynamics become increasingly more agitated in the early morning period.
3.2. The slope model of cortisol
The diurnal and nocturnal slopes, with an approximated CAR, were used as comparison models to determine whether the systems model would provide novel insights. Controls exhibited a trend toward a more J-shaped quadratic slope than patients in the nocturnal period (p = .067), and higher average nocturnal levels of ACTH (p = .096), suggesting mild decrements in pituitary or sup-rapituitary drive. Neither the diurnal nor the nocturnal cortisol slopes, nor the CAR parameter, significantly differed patients or patient subgroups from controls.
3.3. Group differences in the HPA system parameters
We hypothesized that feedback signaling would be blunted among patients with pain-predominant and increased among fatigue-predominant symptom profiles based on previous literature (Jerjes et al., 2007; Lentjes et al., 1997). Hence, we expected that these opposing effects would cancel each other yielding no significant differences in individual parameters in the patient group as a whole compared to controls, which was confirmed (all p’s > .10). The steady state gain (λA/λC) provides a more global indication of the balance of ACTH-adrenal signaling relative to feedback signaling, and their net effect on system behavior. Patients had a trend toward a higher nocturnal steady state gain compared to controls (p = .086, Table 1).
Table 1.
Characteristics of patients with functional disorders relative to controls.
| Outcome (N = 72) | Patients, M (SD) | Controls, M (SD) | Paired t-test (df = 35) | p-Value |
|---|---|---|---|---|
| Self-reported symptoms | ||||
| Somatic symptoms (PILL) | 21.611 (8.247) | 3.250 (3.074) | 12.437 | <0.001** |
| Poor sleep quality (PSQI) | 9.611 (4.285) | 3.194 (2.573) | 7.912 | <0.001** |
| Depressive symptoms (BDI) | 11.278 (6.819) | 1.167 (1.935) | 8.946 | <0.001** |
| Nocturnal mean levels | ||||
| ACTH mean | 3.702 (1.552) | 4.552 (2.731) | −1.712 | 0.096† |
| Cortisol mean | 8.740 (2.633) | 8.734 (2.152) | 0.014 | 0.989 |
| Nocturnal slope model parameters (11 pm–9 am) | 0.989 | |||
| Cortisol linear slope | 0.184 (0.124) | 0.228 (0.151) | −1.580 | 0.123 |
| Cortisol quadratic slope | 0.002 (0.004) | −0.0001 (0.005) | 1.898 | 0.067† |
| Nocturnal dynamic model (11 pm–9 am) | ||||
| Steady state gain (λA/λC) | 2.356 (1.201) | 1.880 (0.944) | 1.765 | 0.086† |
| External influences | 0.066 (0.124) | 0.089 (0.092) | −1.028 | 0.311 |
Paired t-test comparisons of 36 patients and 36 matched healthy controls.
p≤ .01.
p≤ .10.
3.4. Subgroup differences in the HPA system parameters
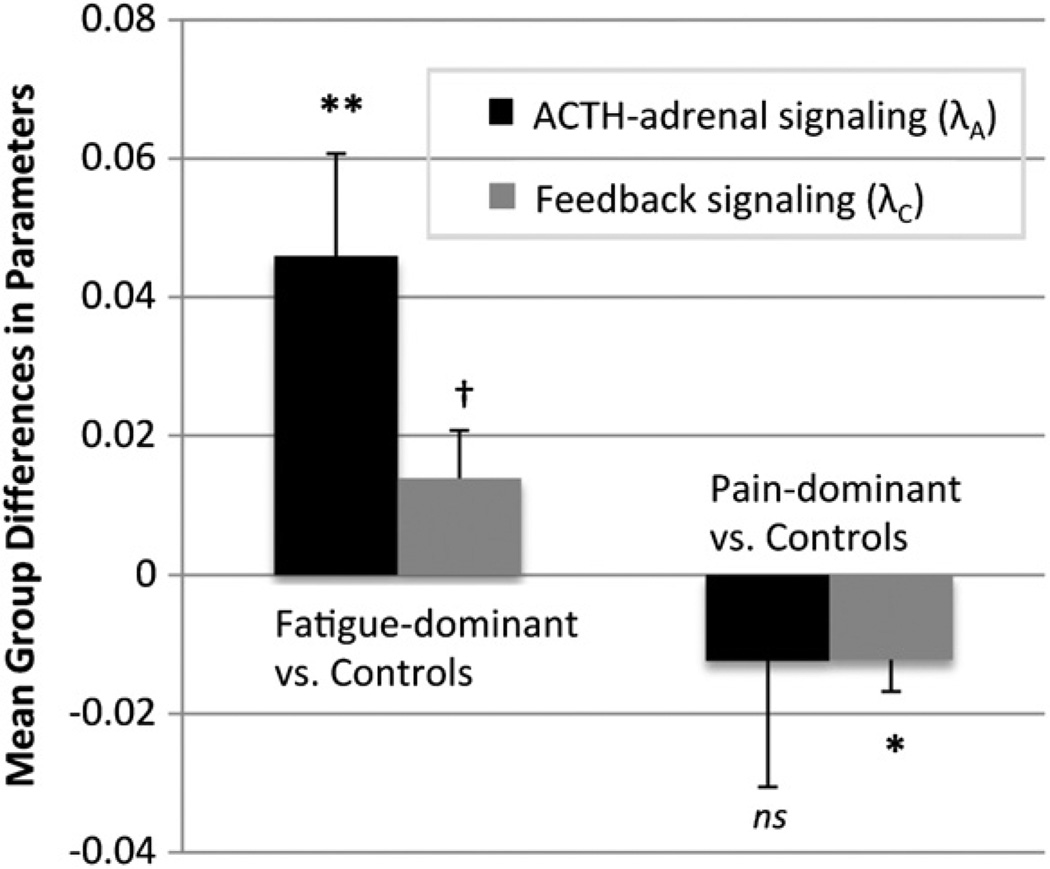
Compared to matched controls, fatigue-predominant (CFS only) patients exhibited significantly higher ACTH-adrenal signaling (λA: Mdiff)2 (SE) = .046(.015), p = 0.012), marginally significantly greater inhibitory feedback (λC: Mdiff(SE) = 0.013(.006), p = 0.054), and no differences in non-ACTH influences (p = .701) during the nocturnal period, using paired t-tests (Fig. 2). All subgroup t-tests reported herein were bootstrapped with 1000 iterations to provide more reliable estimates with small samples, and the resulting bootstrapped mean difference with its standard error and p-value are reported. In contrast, pain-predominant patients (FM with or without CFS) exhibited no significant differences in adrenal sensitivity (λA: Mdiff(SE) =−0.012(.018), p = 0.499), significantly lower non-ACTH inputs (λI: Mdiff(SE) = -.048(.021), p = 0.040), and significantly blunted inhibitory feedback (λC: Mdiff(SE) =-.012(.004), p = 0.017), which is consistent with central glucocorticoid insensitivity or resistance, although it cannot be confirmed from this data alone, as differences in cortisol clearance rates represent alternative possible explanations. When the three patients with FM who did not meet criteria for CFS were removed to create a more homogenous FM + CFS group (by the 1994 CFS criteria), inhibitory feedback remained significant (p = .031) and non-ACTH inputs became borderline significant (p = .087). The diurnal parameters did not differ when comparing patients versus controls or subgroups. No significant differences in the steady state gain were found in the subgroup comparisons. Due to non-normal distribution of symptom duration, partial spearman correlations were used to examine the relationship between symptom duration and the system parameters, controlling for age, and no significant results were found.
Fig. 2.
HPA system parameter differences in patient subgroups versus matched healthy controls. **p≤ .01, *p≤ .05, †p≤ .10. Paired t-test comparisons of patients and individually-matched healthy controls on systems-derived neuroendocrine outcomes. Fatigue-predominant patients (n = 14) met criteria for CFS and not FM, whereas pain-predominant patients (n = 22) met criteria for FM with or without chronic fatigue. These subtypes are associated with significant differences in neuroendocrine system activity. The y-axis represents the mean differences (from case-control paired t-tests) of two system parameters (ACTH-adrenal signaling and inhibitory cortisol feedback signaling). Positive values indicate that patients exhibited amplified signaling relative to controls, whereas negative values indicate decreased signaling of patients relative to controls.
3.5. Association of somatic symptoms with HPA system parameters (Table 2)
Table 2.
The nocturnal dynamic system parameters and steady state gain are associated with somatic symptoms in patients with functional disorders.
| Outcome = somatic symptoms | Standardized β | t-Test (df = 32) | p-Value |
|---|---|---|---|
| Unadjusted model | |||
| ACTH-adrenal signaling (λA) | −0.553 | −3.105 | 0.004** |
| Inhibitory feedback (λC) | 0.425 | 2.309 | 0.028* |
| External influences (λI) | −0.284 | −1.789 | 0.083† |
| Adjusted final model | (df = 31) | ||
| Age | −0.136 | −0.808 | 0.425 |
| Gender | 0.013 | 0.076 | 0.940 |
| Depressive symptoms | −0.158 | −0.970 | 0.339 |
| Steady state gain (λA/λC) | −0.401 | −2.416 | 0.022* |
The nocturnal parameters were calculated between 11 pm and 9 am. Higher levels of somatic symptoms were associated with: ↓λA = less cortisol per unit ACTH, ↑λC = greater inhibition/more efficient removal of cortisol from circulation, ↓λI = lower cortisol per unit of the external influences, and ↓λA/λC= steady state gain decreases indicate that overall there is a lower level of cortisol activation for a given amount of ACTH secretion.
p < .01.
p < .05.
p ≤ .10.
As expected, patients reported significantly higher levels of somatic and depressive symptoms, and greater sleep disturbance compared to controls (Table 1). We examined whether the HPA dynamic system model parameters would be associated with somatic symptoms among patients, and found significant relations with the nocturnal (Table 2), but not diurnal, dynamic model parameters (λA: p = .004, λC: p = .028, λI: p = .083) and the steady state gain reflecting the overall relative balance of cortisol to ACTH (λA/λC). The data show that somatic symptoms increase when, during the nocturnal period, cortisol is leaving the system at a faster rate than it is being produced. This relationship remained significant controlling for age, gender and depressive symptoms. No significant associations were found involving diurnal/nocturnal slope parameters.
3.6. Association of sleep disturbance with HPA system parameters (Table 3 and Fig. 3)
Table 3.
Loss of Regulatory Association between Sleep Quality and Nocturnal Cortisol Secretion Among Patients but not Controls.
| Outcome = λI Nocturnal Non-ACTH Influences | Standardized β | t-Test (df = 32) | p-Value |
|---|---|---|---|
| Healthy Controls (n = 36) | |||
| Age | .304 | 2.068 | 0.047* |
| Gender | .124 | 0.843 | 0.405 |
| Poor sleep quality (PSQI) | −.547 | −3.105 | 0.001** |
| Patients (n = 36) | |||
| Age | .378 | 2.201 | 0.035* |
| Gender | .072 | 0.417 | 0.679 |
| Poor sleep quality (PSQI) | .004 | 0.022 | 0.983 |
The Pittsburgh Sleep Quality Index (PSQI) global score (higher = worse) reflects sleep disturbance over the previous month. Separate multiple regression analyses were conducted in controls and patients examining the association between PSQI and nocturnal Non-ACTH influences (λI) on cortisol, controlling for demographics.
p < .01.
p < .05.
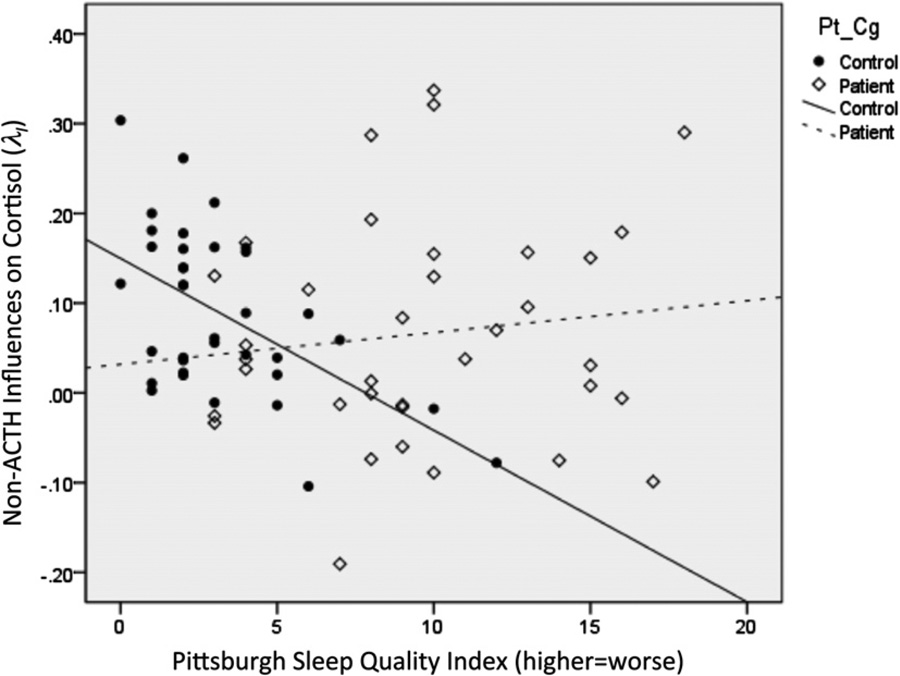
Fig. 3.
Loss of regulatory association between sleep quality and nocturnal cortisol secretion among patients with chronic fatigue syndrome and/or fibromyalgia compared to healthy controls. PSQI, Pittsburgh Sleep Quality Index global score, in which higher scores = worse sleep quality over the previous month. Among healthy controls, worse sleep quality is associated with significantly lower levels of the parameter reflecting the intercept in the dynamic model. This parameter reflects non-ACTH influences on cortisol and is primarily correlated with the level of cortisol during the circadian nadir in the early evening. See Tables 3 and 4.
Given that the nocturnal (but not diurnal) dynamic model parameters were altered in patient subgroups and were associated with symptoms, we investigated whether sleep disturbance per the PSQI played a role. Neither nocturnal ACTH-adrenal signaling (λA) nor inhibitory feedback (λC) were associated with the PSQI. Among controls but not patients, PSQI scores were strongly associated with the intercept parameter representing external, non-ACTH influences (λI) (e.g., hippocampal HPA influences, CRH or sympathetic influences) (Fig. 3), suggesting the loss of a regulatory sleep-neuroendocrine association among patients. When controlling for age and gender effects, PSQI scores (higher = worse sleep) remained significantly associated with lower λI among controls (β = −.547, p = .001), while age was associated with a significantly increased λI among both patients and controls (Table 3). Furthermore, among controls but not patients, λI was significantly associated with four of the seven PSQI component scales (all p’s < .05; Table 4). Assisting in its interpretation, λI was positively associated with the level of the 1 am nadir, as well as a steeper linear and more J-shaped ascending slope leading up to the sleep-to-wake transition (all p’s < .05; Table 4).
Table 4.
Correlations between nocturnal non-ACTH influences (λI), cortisol slopes, and sleep quality components among healthy controls.
| PSQI components (higher = worse) | R-value | p-Value |
|---|---|---|
| Pearson correlations with λI: external, non-ACTH influences on cortisol secretion | ||
| Shorter duration | −.432 | .008** |
| Poorer quality | −.409 | .013* |
| Lower habitual efficacy | −.365 | .029* |
| Longer latency | −.341 | .042* |
| Greater disturbance | −.281 | .097† |
| Greater daytime dysfunction | −.227 | .183 |
| Greater use of sleep meds | −.086 | .617 |
| Nocturnal slope parameters | ||
| 1am intercept | .689 | <.001** |
| Linear slope | .315 | .007** |
| Quadratic slope | −.385 | .001** |
| CAR discontinuity | −.055 | .647 |
In healthy controls, but not patients (see Table 3), worse sleep quality across four of the seven PSQI components (i.e., higher scores) was associated with a lower value for λI, the parameter corresponding to the dynamic model intercept and representing the external, non-ACTH influences on cortisol. If all other parameters are held constant, increasing λI will move all of the cortisol values predicted by the dynamic model higher along the y-axis by a constant amount.
p≤ .01.
p≤ .05.
p≤ .10.
4. Discussion
Many complex systems models have been developed, but few have been integrated into standard clinical research practices. This study aims to help bridge the gap. This is the first study to utilize a personalized systems model (modeled at the individual-level with time-series data) to investigate the role of cortisol and HPA dynamics in the pathophysiology of FM and/or CFS. This study presents a novel strategy for mapping symptom profiles and patient subgroups onto behavioral phenotypes of neuroendocrine system activity. Such strategies pave the way to determine a “new taxonomy of disease” (National Research Council, 2011) through the integration of symptom reports, diagnostic assessments, and biological system behavior.
Patients with CFS and/or FM exhibit a “high sensitivity phenotype” of HPA system behavior, characterized by rapid or sustained release cortisol during the nocturnal period, although the specific mechanisms differed by subgroup (pain versus fatigue predominant). A more sensitive phenotype was associated with fewer somatic symptoms among patients. Hence, this phenotype may represent an adaptive allostatic optimization to short-term demands (i.e., quick mobilization of energy in response to stressors and/or inflammatory stimuli), which could have adverse health effects in the long-term (Sapolsky, 2000).
If somatic symptoms among patients are a manifestation of cytokine-induced “sickness behavior” (Dantzer, 2001), then more rapid/sustained cortisol secretion (i.e., the high sensitivity phenotype) could provide beneficial anti-inflammatory effects. The defining symptoms of CFS and FM – e.g., fatigue, enhanced pain, and impaired cognition – are consistent with the effects of excess inflammatory cytokines combined with insufficient counter-regulatory HPA activity (Irwin, 2008; Krueger, 2008). Unfortunately, no cytokine data was available; however, future extensions of the HPA model that integrate time series cytokine measures could investigate these hypotheses (Kapsimalis et al., 2008; Krueger, 2008).
Differences in patient subgroups identified by the systems model can be partially understood by comparison to the pharmacological challenge test literature. However, it should be clarified that these challenge tests have not yielded consistent results among CFS and FM patients. For example, two pharmacologic challenge studies found evidence of increased ACTH sensitivity (Gaab et al., 2002; Scott et al., 1998a) among CFS patients, which is consistent with this study’s findings in the fatigue-predominant subgroup. However, others have failed to find differences (Gaab et al., 2003; Hudson and Cleare, 1999), and one study reported blunted ACTH sensitivity (Scott et al., 1998b). These studies have used varying pharmacological agents and dosages, often in supraphysiological quantities. As even the “low dose” ACTH test is greater than the peak physiologic levels in this sample, it is possible that the behavior of the HPA system following high or supraphysiological doses may not provide insight into naturalistic system behavior during the nocturnal nadir, which this study suggests is critical to assess.
Pain-predominant patients (FM with or without concomitant chronic fatigue) exhibited significantly blunted feedback signaling, and no differences in ACTH-adrenal signaling. When inhibitory feedback is decreased, cortisol production is not shut off as quickly and tissues may become insensitive to GR-mediated signaling. Several previous studies of FM have found evidence of blunted inhibitory feedback in GR, both centrally (McCain and Tilbe, 1989) and peripherally in leukocytes (Lentjes et al., 1997). Whereas the current study excluded all patients with MDD, another previous study found reduced feedback sensitivity only among FM patients with comorbid MDD (Wingenfeld et al., 2010). Nonetheless, it is possible that a loss of HPA regulatory control in the brain and immune system may serve as a maintaining factor in FM. This dynamic model’s feedback sensitivity parameter may provide an indirect measure of central GR insensitivity; however, this cannot be confirmed without future validation tests assessing cortisol clearance rates and pharmacologic challenge measures of central sensitivity.
Increasing age was also associated with higher λI in both controls and patients. Though speculative, it is possible that λI could be a marker of the inhibitory influence of the hippocampus on the HPA axis via activation of its mineralcorticoid (MR) receptors. Some evidence suggests that cortisol peak and nadir activity may be mediated by MR expression in the hippocampus (Jacobson, 2005) and glucocorticoid levels are elevated in MR knockout mice (Gass et al., 2000; Jacobson, 2005). Aging is associated with hippocampal atrophy and an increased cortisol nadir (Lupien et al., 1998). Hence the positive association of age and λI (which largely determines the early evening nadir of the dynamic model) suggests that increases in this parameter may reflect, in part, decreases in MR-mediated hippocampal inhibition.
Sleep disruption is a core symptom of both CFS and FM. This study found that the normal bidirectional regulatory association between nocturnal HPA function and sleep appeared to be dysregulated in patients with functional disorders. Although seemingly quiescent, sleep is a “dynamic behavioral state” (Irwin, 2008) that regulates and is regulated by neuroendocrine activity (Zeiders et al., 2011). Among healthy participants but not patients, greater sleep disturbance in the previous month was associated with significantly lower nocturnal levels of the dynamic model parameter (λI) reflecting external or non-ACTH influences on cortisol production. Within the context of the model, this parameter defines the early evening nadir of the cortisol circadian rhythm and is associated with a steeper ascending cortisol slope across the nighttime. Hence, our finding of better sleep quality overall, and longer duration specifically, being associated with higher λI is consistent with previous literature showing that longer sleep duration was associated with a steeper cortisol slope (Zeiders et al., 2011), while greater sleep disruption (PSQI) was negatively associated with cortisol levels upon awakening (Backhaus et al., 2004).
An unexpected but highly intriguing finding is the fact that the relationship between cortisol and λI was strong in controls, but completely absent in patients with CFS and/or FM. This suggests the possibility that CFS and FM patients may have a pathophysiological alteration of the normal neuroendocrine regulatory association with sleep. We speculated that higher λI may reflect reduced MR-mediated inhibition on the HPA axis. Mineralcorticoid antagonists appear to improve sleep quality in certain patient samples (e.g., resistant hypertension) (Gaddam et al., 2010), which is directionally consistent with the finding that higher λI was associated with better sleep among controls. While mineralcorticoid therapy did not have beneficial effects on wellness in one study of CFS patients (Rowe et al., 2001), that study did not conduct in-depth assessments of sleep. Hence, the regulatory relations among MR, sleep and blood pressure, particularly during the nadir of the circadian rhythm, may merit further study.
This study is an initial investigation of a new approach to translational applications of systems modeling and therefore has a number of important limitations. Recent recommendations on minimal measures to be included in CFS studies reveal constructs that were not collected by the original study (e.g., body mass index, functional impairment, etcetera) (Jason et al., 2012). Multiple comparison corrections were not utilized because the analysis was partially exploratory. The parameters are “coarse-grained” functional aggregates of multiple molecular-level events. Other potentially important system components could be additionally included. In this case, pulsatility analyses conducted in a prior analysis of this sample did not reveal significant findings (Crofford et al., 2004); however, other mathematical models of HPA activity could provide additional valuable insights (Sriram et al., 2012; Walker et al., 2010). However, these models typically require substantially more parameters than the model herein, and each additional parameter estimated requires more data. Current limitations to gathering time-series data include restrictions on the amount of blood drawn, the costs of multiple assays, the lengthy use of an indwelling catheter, and difficulty accessing concurrent markers of central nervous system activity in vivo. Nonetheless, rapid developments in less-invasive technologies, ranging from microfluidics chips to oral assays, may help resolve these barriers (Weston and Hood, 2004). Other dynamic models such as homeostatic model assessment of insulin resistance (Bonora et al., 2000; Matthews et al., 1985) obtained quantitative approximations of model parameters based on fasting samples. Hence, it is possible that with future validation studies using pharmacologic probes, HPA dynamic indices might be approximated with far fewer measures.
Patients with functional somatic disorders tend to have nocturnal HPA system dynamics consistent with a high sensitivity phenotype, in which ACTH-stimulated cortisol secretion is more rapid and/or sustained. It is unknown whether these changes preceded or followed from disease onset, but based on their inverse relationship with somatic symptoms, they appear to serve an adaptive function. As a whole, the results suggest that a larger system involving regulatory associations among sleep, neuroendocrine and immune function may be involved in the pathophysiology of CFS and FM. The ability to construct a personalized ‘‘system behavioral phenotype’’ of the HPA that maps onto symptom clusters may be invaluable in identifying the biological basis of disorders of unclear etiology.
Acknowledgments
We thank C.B. Brucksch, E.A. Young, E.P. Somers, L. Masterson, K. Spindler, E. Dawson, and K. Dalbec for project support, and N. Adler, H. El Samad, and M. Aschbacher for intellectual input.
Funding sources
The original study, which provided the data for this secondary analysis, was supported by the NIH R01AR43138 and the University of Michigan General Clinical Research Center NIH M01-RR00042. This research was supported in part by funding to K. Aschbacher from the NIH Ruth L. Kirschstein National Service Award MH019391–20, the Samueli Institute, Alexandria, VA and from the Institute for Integrative Health, Baltimore, MD.
L.J.C. has a grant from Bioenergy, but this grant is unrelated to this study. LIC provides consultation to Glenmark, which is also unrelated to this study. MD is the Vice President and Chief Medical Officer for Neuronetics, Inc., but the work on this study is unrelated to his activities with that company.
Footnotes
This included two points for ACTH-adrenal signaling, one for feedback signaling, one for the steady state ratio, and one for the CAR discontinuity parameter.
Bootstrapped mean differences between case–control pairs with the corresponding standard errors and p values are reported.
Disclosure statement
All authors report nothing to disclose. No authors have any other disclosures.
References
- Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch. Intern. Med. 2000;160:221–227. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc. Natl. Acad. Sci. USA. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. New look at statistical-model identification. IEEE Trans. Autom. Control AC. 1974;19:716–723. [Google Scholar]
- Alon U. An introduction to systems biology: design principles of biological circuits. Boca Raton, FL: Chapman & Hall/CRC; 2007. [Google Scholar]
- Anton H, Herr A. Calculus with Analytic Geometry. New York: Wiley; 1995. [Google Scholar]
- Backhaus J, Junghanns K, Hohagen F. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrino. 2004;29:1184–1191. doi: 10.1016/j.psyneuen.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the beck depression inventory – 25 years of evaluation. Clin. Psychol. Rev. 1988;8:77–100. [Google Scholar]
- Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum. 2008;59:961–967. doi: 10.1002/art.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Doyle J. Highly optimized tolerance: robustness and design in complex systems. Phys. Rev. Lett. 2000;84:2529–2532. doi: 10.1103/PhysRevLett.84.2529. [DOI] [PubMed] [Google Scholar]
- Carlson NE, Johnson TD, Brown MB. A Bayesian approach to modeling associations between pulsatile hormones. Biometrics. 2009;65:650–659. doi: 10.1111/j.1541-0420.2008.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Crofford LJ, Young EA, Engleberg NC, Korszun A, Brucksch CB, McClure LA, Brown MB, Demitrack MA. Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain Behav. Immun. 2004;18:314–325. doi: 10.1016/j.bbi.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann. NY Acad. Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab J, Huster D, Peisen R, Engert V, Heitz V, Schad T, Schurmeyer T, Ehlert U. Assessment of cortisol response with low-dose and high-dose ACTH in patients with chronic fatigue syndrome and healthy comparison subjects. Psychosomatics. 2003;44:113–119. doi: 10.1176/appi.psy.44.2.113. [DOI] [PubMed] [Google Scholar]
- Gaab J, Huster D, Peisen R, Engert V, Heitz V, Schad T, Schurmeyer TH, Ehlert U. Hypothalamic–pituitary–adrenal axis reactivity in chronic fatigue syndrome and health under psychological, physiological, and pharmacological stimulation. Psychosom. Med. 2002;64:951–962. doi: 10.1097/01.psy.0000038937.67401.61. [DOI] [PubMed] [Google Scholar]
- Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM, Calhoun DA. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J. Hum. Hypertens. 2010;24:532–537. doi: 10.1038/jhh.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass P, Kretz O, Wolfer DP, Berger S, Tronche F, Reichardt HM, Kellendonk C, Lipp HP, Schmid W, Schutz G. Genetic disruption of mineralocorticoid receptor leads to impaired neurogenesis and granule cell degeneration in the hippocampus of adult mice. EMBO Rep. 2000;1:447–451. doi: 10.1093/embo-reports/kvd088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geenen R, Bijlsma JW. Deviations in the endocrine system and brain of patients with fibromyalgia: cause or consequence of pain and associated features? Ann. NY Acad. Sci. 2010;1193:98–110. doi: 10.1111/j.1749-6632.2009.05290.x. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Brandenberger G. Ultradian rhythms in pituitary and adrenal hormones: their relations to sleep. Sleep Med. Rev. 1998;2:17–29. doi: 10.1016/s1087-0792(98)90051-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Nater UM, Maloney E, Boneva R, Jones JF, Reeves WC. Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Arch. Gen. Psychiatry. 2009;66:72–80. doi: 10.1001/archgenpsychiatry.2008.508. [DOI] [PubMed] [Google Scholar]
- Hudson M, Cleare AJ. The 1microg short Synacthen test in chronic fatigue syndrome. Clin. Endocrinol. (Oxf.) 1999;51:625–630. doi: 10.1046/j.1365-2265.1999.00856.x. [DOI] [PubMed] [Google Scholar]
- Irwin MR. Human psychoneuroimmunology: 20 years of discovery. Brain Behav. Immun. 2008;22:129–139. doi: 10.1016/j.bbi.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L. Hypothalamic–pituitary–adrenocortical axis regulation. Endocrinol. Metab. Clin. North Am. 2005;34:271–292. doi: 10.1016/j.ecl.2005.01.003. vii. [DOI] [PubMed] [Google Scholar]
- Jason LA, Unger ER, Dimitrakoff JD, Fagin AP, Houghton M, Cook DB, Marshall GD, Jr, Klimas N, Snell C. Minimum data elements for research reports on CFS. Brain Behav. Immun. 2012;26:401–406. doi: 10.1016/j.bbi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerjes WK, Taylor NF, Wood PJ, Cleare AJ. Enhanced feedback sensitivity to prednisolone in chronic fatigue syndrome. Psychoneuroendocrinology. 2007;32:192–198. doi: 10.1016/j.psyneuen.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Kapsimalis F, Basta M, Varouchakis G, Gourgoulianis K, Vgontzas A, Kryger M. Cytokines and pathological sleep. Sleep Med. 2008;9:603–614. doi: 10.1016/j.sleep.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengard B, Pedersen NL. A population-based twin study of functional somatic syndromes. Psychol. Med. 2009;39:497–505. doi: 10.1017/S0033291708003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon W, Russo J. Chronic fatigue syndrome criteria. A critique of the requirement for multiple physical complaints. Arch. Intern. Med. 1992;152:1604–1609. doi: 10.1001/archinte.152.8.1604. [DOI] [PubMed] [Google Scholar]
- Kitano H. Towards a theory of biological robustness. Mol. Syst. Biol. 2007:3. doi: 10.1038/msb4100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM. The role of cytokines in sleep regulation. Curr. Pharm. Des. 2008;14:3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentjes EG, Griep EN, Boersma JW, Romijn FP, de Kloet ER. Glucocorticoid receptors, fibromyalgia and low back pain. Psychoneuroendocrino. 1997;22:603–614. doi: 10.1016/s0306-4530(97)00061-9. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McCain GA, Tilbe KS. Diurnal hormone variation in fibromyalgia syndrome: a comparison with rheumatoid arthritis. J. Rheumatol. Suppl. 1989;19:154–157. [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychol. Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Moldofsky H. Sleep, neuroimmune and neuroendocrine functions in fibromyalgia and chronic fatigue syndrome. Adv. Neuroimmunol. 1995;5:39–56. doi: 10.1016/0960-5428(94)00048-s. [DOI] [PubMed] [Google Scholar]
- Moshiree B, Price DD, Robinson ME, Gaible R, Verne GN. Thermal and visceral hypersensitivity in irritable bowel syndrome patients with and without fibromyalgia. Clin. J. Pain. 2007;23:323–330. doi: 10.1097/AJP.0b013e318032e496. [DOI] [PubMed] [Google Scholar]
- National Research Council. Washington, DC: National Academies Press; 2011. Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease. [PubMed] [Google Scholar]
- Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patarca-Montero R, Antoni M, Fletcher MA, Klimas NG. Cytokine and other immunologic markers in chronic fatigue syndrome and their relation to neuropsychological factors. Appl. Neuropsychol. 2001;8:51–64. doi: 10.1207/S15324826AN0801_7. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW. The Psychology of Physical Symptoms. New York: Springer; 1982. [Google Scholar]
- Rowe PC, Calkins H, DeBusk K, McKenzie R, Anand R, Sharma G, Cuccherini BA, Soto N, Hohman P, Snader S, Lucas KE, Wolff M, Straus SE. Fludrocortisone acetate to treat neurally mediated hypotension in chronic fatigue syndrome: a randomized controlled trial. JAMA. 2001;285:52–59. doi: 10.1001/jama.285.1.52. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Scott LV, Medbak S, Dinan TG. Blunted adrenocorticotropin and cortisol responses to corticotropin-releasing hormone stimulation in chronic fatigue syndrome. Acta Psychiatr. Scand. 1998a;97:450–457. doi: 10.1111/j.1600-0447.1998.tb10030.x. [DOI] [PubMed] [Google Scholar]
- Scott LV, Medbak S, Dinan TG. The low dose ACTH test in chronic fatigue syndrome and in health. Clin. Endocrinol. (Oxf.) 1998b;48:733–737. doi: 10.1046/j.1365-2265.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- Seborg DE, Edgar TF, Mellichamp D. Process Dynamics and Control. Springer; 2004. [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer I. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Sriram K, Rodriguez-Fernandez M, Doyle FJ., 3rd Modeling Cortisol Dynamics in the Neuro-endocrine Axis Distinguishes Normal, Depression, and Post-traumatic Stress Disorder (PTSD) in Humans, PLoS Comput. Biol. 2012;8:e1002379. doi: 10.1371/journal.pcbi.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P. Allostasis: a model of predictive regulation. Physiol Behav. 2011 doi: 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Tanriverdi F, Karaca Z, Unluhizarci K, Kelestimur F. The hypothalamo–pituitary–adrenal axis in chronic fatigue syndrome and fibromyalgia syndrome. Stress. 2007;10:13–25. doi: 10.1080/10253890601130823. [DOI] [PubMed] [Google Scholar]
- Theorell T, Blomkvist V, Lindh G, Evengard B. Critical life events, infections, and symptoms during the year preceding chronic fatigue syndrome (CFS): an examination of CFS patients and subjects with a nonspecific life crisis. Psychosom. Med. 1999;61:304–310. doi: 10.1097/00006842-199905000-00009. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- Van Den Eede F, Moorkens G, Van Houdenhove B, Cosyns P, Claes SJ. Hypothalamic–pituitary–adrenal axis function in chronic fatigue syndrome. Neuropsychobiology. 2007;55:112–120. doi: 10.1159/000104468. [DOI] [PubMed] [Google Scholar]
- Walker JJ, Terry JR, Lightman SL. Origin of ultradian pulsatility in the hypothalamic–pituitary–adrenal axis. Proc Biol Sci. 2010;277:1627–1633. doi: 10.1098/rspb.2009.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston AD, Hood L. Systems biology, proteomics, and the future of health care: toward predictive, preventative, and personalized medicine. J. Proteome Res. 2004;3:179–196. doi: 10.1021/pr0499693. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Nutzinger D, Kauth J, Hellhammer DH, Lautenbacher S. Salivary cortisol release and hypothalamic pituitary adrenal axis feedback sensitivity in fibromyalgia is associated with depression but not with pain. J. Pain. 2010;11:1195–1202. doi: 10.1016/j.jpain.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Young EA, Carlson NE, Brown MB. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology. 2001;25:267–276. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]
- Zeiders KH, Doane LD, Adam EK. Reciprocal relations between objectively measured sleep patterns and diurnal cortisol rhythms in late adolescence. J. Adolesc. Health. 2011;48:566–571. doi: 10.1016/j.jadohealth.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]