Abstract
Background
Prehospital electrocardiography (PH ECG) is becoming the standard of care for patients activating emergency medical services (EMS) for symptoms of acute coronary syndrome (ACS). Little is known about the prognostic value of ischemia found on PH ECG.
Study Objectives
The purpose of this study was to determine whether manifestations of acute myocardial ischemia on PH ECG are predictive of adverse hospital outcomes.
Methods
The study was a retrospective analysis of all PH ECGs recorded in 630 patients who called “911” for symptoms of ACS and were enrolled in a prospective clinical trial. ST-segment monitoring software was added to the PH ECG device with automatic storage and transmission of ECGs to the destination emergency department (ED). Patients’ medical records were reviewed for adverse hospital outcomes.
Results
In 630 patients who called “911” for ACS symptoms, 270 (42.9%) had PH ECG evidence of ischemia. Overall, 37% of patients with PH ECG ischemia had adverse hospital outcomes compared to 27% of patients without PH ECG ischemia (p< .05). Those with PH ECG ischemia were 1.55 times more likely to have adverse hospital outcomes than those without PH ECG ischemia (CI 1.09–2.21, p<0.05), after controlling for other predictors of adverse hospital outcomes (i.e., age, gender, medical history).
Conclusions
Evidence of ischemia on PH ECG is an independent predictor of adverse hospital outcomes. ST segment monitoring in the prehospital setting may identify high-risk patients with symptoms of ACS and provide important prognostic information at presentation to the ED.
Keywords: acute coronary syndrome, emergency medical services, emergency cardiac care, electrocardiogram, prehospital electrocardiography
INTRODUCTION
The American Heart Association recommended the acquisition of a prehospital electrocardiogram (PH ECG) for patients with symptoms of acute coronary syndrome (ACS) as a Class I recommendation (supported by strong evidence) in the 2010 Advanced Cardiac Life Support guidelines.1 As a result, acquisition of a PH ECG is becoming the standard of care for patients who access emergency medical services (EMS) with suspected ACS. While past PH ECG investigations have focused on the reduction in time to reperfusion treatment in ST-elevation myocardial infarction (STEMI), little is known about its prognostic value for adverse clinical outcomes. The primary aim of this study was to determine whether manifestations of acute myocardial ischemia on the PH ECG are associated with adverse hospital outcomes.
METHODS
Data for this retrospective analysis were obtained from the ST SMART (Synthesized Twelve-lead ST Monitoring and Real-time Tele-electrocardiography) Trial, a prospective randomized clinical trial in carried out in Santa Cruz County, California from 2003–2008.2 The primary aims of the ST SMART Trial were to compare patients with and without PH ECG ST-segment monitoring in paramedic scene time, hospital time to treatment, and survival over the period of the study. 2 The primary outcome measure for the present analysis was to compare two groups (those with PH ECG ischemia and those without PH ECG ischemia) and identify the difference in the proportion of adverse hospital outcomes.
Enrollment for the study occurred 7 days a week, 24 hours a day. Paramedics were trained to identify the following inclusion criteria: all persons 30 years of age and older who called “911” with complaints of non-traumatic chest pain, anginal equivalent symptoms such as new onset shortness of breath (not due to asthma), or syncope (not due to drug overdose or intoxication). Exclusion criteria were participants who were unwilling or unable to consent.
The Institutional Review Boards at the University of California, San Francisco, and the two hospitals in Santa Cruz County approved the study with a waiver of consent in the field to avoid delays in patients reaching the hospital. Community consent was obtained by a front-page report in the county’s newspaper (Santa Cruz Sentinel, 2003) and by information posted on hospitals’ and EMS agencies’ websites. 2 Research nurses obtained written consent from all study participants once they reached the hospital.
Electrocardiographic procedure
All 26 paramedic-staffed emergency vehicles responding to 911 calls in the county were equipped with specially designed portable monitor-defibrillator devices (Lifepak12, Physio-Control, Redmond, Washington). The study device software enabled the following: 1) synthesis of a 12-lead ECG from five electrodes, 2) measurement of ST amplitudes (J+60 milliseconds) every 30 seconds in all 12 leads, and 3) automatic storage and transmission of an ECG to the destination ED if there was a change in ST amplitude of 0.2 mV in 1 lead or 0.1 mV in ≥ 2 contiguous leads, lasting 2.5 minutes. 2
The study device used a bandwidth of 0.05 to 150 Hz, which is the filtering recommended for diagnostic standard 12-lead ECGs. 2 A previous validation study determined a high percentage of agreement between the synthesized PH ECG and standard 12-lead ECG. 3 The portable monitor-defibrillator study device collected 20 seconds of electrocardiographic data and then selected the 10 seconds with the best signal-to-noise ratio to develop a noise-free median beat from which all 12-lead ST-segment measurements were obtained. If the initial 20-second sample was noisy, the device automatically analyzed the subsequent 20 seconds of data. 2
All county paramedics (n=80) were taught to apply the 5 electrodes and manually transmit an initial PH ECG for patients with ACS symptoms. 2 This initial manual ECG transmission activated the study ST-segment monitoring software. Any PH ECGs with subsequent ST events were transmitted automatically without paramedic decision-making. To ensure successful PH ECG transmissions, the device automatically redialed up to 3 attempts if the EMS vehicle was in a location where mobile telephone communication was unavailable.
PH ECG data were stored in the device and analyzed offline (CodeStat Suite version 8.0, Physio-Control, Redmond, Washington). PH ECGs were manually read by the investigator [JZH] using the universal criteria for the diagnosis of ACS defined by the European Society of Cardiology and the American College of Cardiology Committee. 4 An expert [CES] conducted random audits of ECG analysis to confirm the diagnosis of ACS and establish inter-rater reliability. The revised criteria were developed to improve the sensitivity and specificity of the ECG by recognizing gender and lead differences. They include: 1) ST segment elevation at the J-point with cut-off points ≥ 0.2 mV in men and ≥ 0.15 mV in women in leads V2 and V3 or ≥ 0.1mV in other leads; 2) horizontal or down-sloping ST-segment depression ≥ 0.05 mV or 3) T-wave inversion of ≥ 0.1 mV in leads with prominent R waves or R/S ratio >1. All ECG criteria had to be present in two contiguous leads. 4
Three research nurses were trained for data abstraction exclusively for the ST SMART Trial. They reviewed medical record notes and ICD-9 codes, and conducted follow-up telephone calls to obtain information about the occurrence of adverse hospital outcomes. A project director conducted random study chart audits to ensure reliable and accurate data collection. However, inter-rater reliability for the hospital outcome data abstraction was not measured. The American College of Cardiology (ACC) key data elements for measuring clinical management and outcomes of patients with ACS were used to define hospital complication variables (Table 1) and patient characteristics (demographics, cardiac history, coronary risk factors). 5 Any cardiovascular related outcome variables, as defined by the ACC, were selected for analysis.
Table 1.
Adverse hospital outcomes
| 1. Death |
| 2. AMI distinct from the admission event |
| 3. Recurrent rest angina with ECG changes |
| 4. Recurrent rest angina without ECG changes |
| 5. Heart failure that developed after hospital admission |
| 6. Cardiogenic shock |
| 7. Atrial dysrhythmia requiring intervention |
| 8. Ventricular dysrhythmia requiring intervention |
| 9. High-degree atrio-ventricular block (third degree block or second-degree block with bradycardia requiring pacing) |
AMI = acute myocardial infarction
Statistical analysis
All analyses were conducted in SPSS statistical software (Version 17, Somers, NY). ECG signs of ischemia (ST-elevation, ST-depression, T-wave inversion) were collapsed into one dichotomous independent variable (PH ECG ischemia yes/no) for analysis. The nine individual hospital complication variables were combined into one dichotomous endpoint (adverse hospital outcomes yes/no) for analysis. Descriptive statistics were used to report baseline characteristics and clinical information. χ2 statistical analyses were used to compare categorical variables. Logistic regression analysis was used to evaluate independent predictors of adverse hospital outcomes. The following independent variables were evaluated in the regression model: PH ECG evidence of ischemia, age, gender, history of myocardial infarction (MI), coronary artery disease (CAD), diabetes, hypertension, smoking, dyslipidemia, or family history of CAD. The dependent variable used in the regression model was the dichotomous endpoint of adverse hospital outcomes.
Power analysis determined that a sample size of 794 subjects would provide power of at least 80% at an alpha of .05 to detect a small effect size of h=.23 with a two-group Fisher’s exact test. Missing or incomplete data were not included for analyses.
RESULTS
PH ECGs selected for analysis
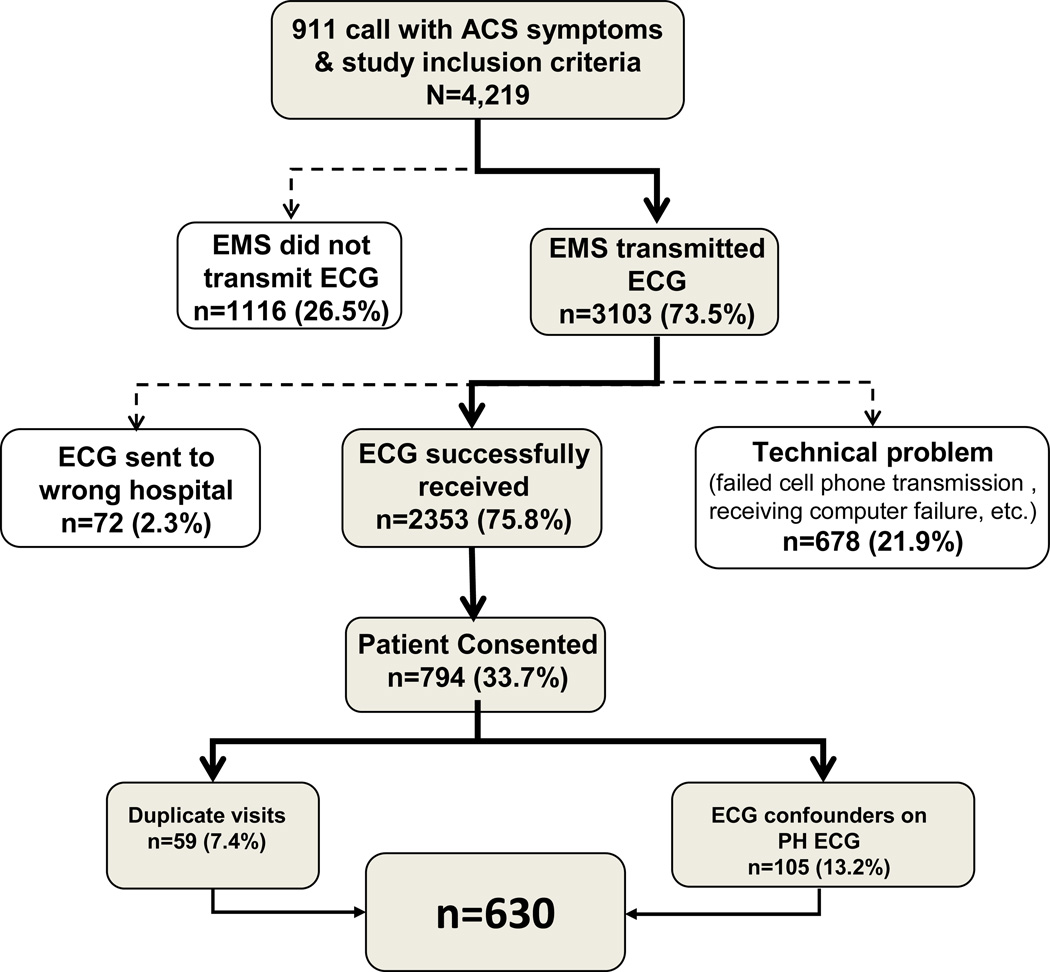
The PH ECGs analyzed included all those acquired and stored in the prehospital ECG device in the ST SMART Trial. There were a total of 794 subjects with PH ECGs enrolled in the ST SMART trial. Of these, 105 patients with left bundle branch block (LBBB), left ventricular hypertrophy (LVH), or ventricular pacing rhythm were excluded from the present analysis since universal criteria for acute MI are to be applied only in the absence of these confounding conditions.4 An additional 59 patients were excluded because they had been enrolled in the study previously, resulting in a final cohort of 630 patients for the present analysis (Figure 1).
Figure 1.
Subject enrollment summary, including total number of subjects screened for the ST SMART trial, the number of subjects excluded due to PH ECG technical problems, the number of subjects who consented, and those excluded due to duplicate visits and confounders.
Subject characteristics
The mean age of subjects was 69.77 years (SD 14.46) and 51.7% were male. Ethnicity included 91.7% white, 2.5% Latino, 0.6% black, and 5.2% mixed/unknown. Baseline sample characteristics, including coronary risk factors and cardiac history, are summarized in Table 2.
Table 2.
Baseline sample characteristics (n=630)
| Age | 69.77 (±14.46) |
| Male Gender | 326 (51.7%) |
| Race | |
| White | 578(91.7%) |
| American Indian/Alaskan Native | 16 (2.5%) |
| Asian | 14 (2.2%) |
| Black | 4 (0.6%) |
| Unknown | 18 (3.0%) |
| Past Medical History | |
| Hypertension | 390(61.9%) |
| Dyslipidemia | 247(39.3%) |
| Smoker (current, former, or recent) | 214 (34.0%) |
| History of CAD | 197(31.3%) |
| Diabetes | 135(21.4%) |
| History of MI | 120(19%) |
| Angina pectoris | 185(29.5%) |
| PCI | 109(17.3%) |
| CABG | 76(12.1%) |
| Family History of CAD | 96(15.3%) |
CAD = coronary artery disease
MI = myocardial infarction
PCI=percutaneous coronary intervention
CABG=coronary artery bypass
Of the total of 630 patients with ACS symptoms, 270 (42.9%) had PH ECG evidence of ischemia (54 with ST-elevation, 175 with ST-depression, and 41 with isolated T-wave inversion). There were 182 (28.9%) patients who had a discharge diagnosis of ACS that included the following: STEMI (48 patients), NSTEMI (54 patients), MI of unknown origin (1 patient), and definite or probable unstable angina (79 patients).
The group with manifestations of acute myocardial ischemia on their PH ECG had a significantly higher proportion of adverse hospital outcomes compared with the group without such ECG signs. Overall, 37% (95% confidence interval [CI]: 31%-43%) of patients with PH ECG ischemia had adverse hospital outcomes compared with 27% (95% CI: 22%-32%) of the group without (p<0.05) (Table 3). Specifically, patients with PH ECG ischemia (vs. those without) had more ventricular dysrhythmia requiring intervention (9.3% vs. 3.9%, respectively, p<0.05) and more atrial dysrhythmia requiring intervention (16.3% vs. 10.0%, respectively, p<0.05) than those without PH ECG ischemia. Furthermore, having PH ECG ischemia was associated with a trend towards more cardiogenic shock (3.7 vs. 1.1%, respectively, p=0.05).
Table 3.
Adverse hospital outcome Frequencies of adverse hospital outcomes comparing patients with and without PH ECG ischemia (n=630)
| Adverse hospital outcomes | PH ECG ischemia | Without PH ECG ischemia | P value |
|---|---|---|---|
| Death | 3(1.1) | 4(1.1) | 1.000 |
| MI | 5(1.9) | 3(0.8) | 0.269 |
| Angina with ECG changes | 4(1.5) | 1(0.3) | 0.111 |
| Angina without ECG changes | 14(5.2) | 18(5.0) | 1.000 |
| New heart failure | 41(15.2) | 44(12.3) | 0.176 |
| Shock | 10(3.7) | 4(1.1) | 0.05 |
| Atrial dysrhythmia* | 44(16.3) | 36(10.0) | 0.022 |
| Ventricular dysrhythmia* | 25(9.3) | 14(3.9) | 0.005 |
| AV block | 12(4.4) | 8(2.2) | 0.222 |
| Adverse hospital outcomes | 99(36.7) | 98(27.2) | 0.007 |
requiring urgent intervention
As shown in Table 4, PH ECG ischemia was one of the strongest predictors for adverse hospital outcomes. Specifically, those with PH ECG ischemia were 1.55 times more likely to have adverse hospital outcomes than those without PH ECG ischemia, controlling for other factors in the model. The full model containing all predictors was statistically significant (χ2 = 50.66, p<0.001). The model as a whole explained between 7.8% (Cox & Snell R Square) and 10.9% (Nagelkerke R Square) of the variance of adverse hospital outcomes. Older age, hypertension, and smoking were also significant predictors of any adverse hospital outcomes.
Table 4.
Multiple logistic regression analyses on predictors of any adverse hospital outcome (n=630)
| Predictor variables | Odds ratio (95% CI) | P-value |
|---|---|---|
| Dependent variable: adverse hospital outcomes | ||
| PH ECG ischemia | 1.55(1.09–2.21) | 0.02 |
| Gender | 0.82(0.57–1.18) | 0.29 |
| Age | 1.02(1.00– 1.03) | 0.10 |
| History of MI | 0.87(0.53–1.44) | 0.60 |
| History of CAD | 1.69(1.085–2.631) | 0.02 |
| Diabetes | 1.30 (0.85– 1.97) | 0.23 |
| Hypertension | 1.64(1.10–2.44) | 0.015 |
| History of Smoking | 1.49(1.02– 2.16) | 0.04 |
| History of Dyslipidemia | 1.10(0.76– 1.60) | 0.61 |
| Family History of CAD | 0.58(0.34– 0.99) | 0.05 |
Omnibus Tests of Model Coefficients χ2 (10) = 50.663, p <.001
CI = confidence interval
MI=myocardial infarction
CAD=coronary artery disease
DISCUSSION
To our knowledge, this study is the first to provide information about the prognostic value of ST-segment monitoring in the prehospital setting. Continuous ST segment monitoring combined with clinical criteria has been described as an effective method of identifying high-risk patient subsets in the early phase of ACS after hospital admission. 6 Our study shows that data obtained from ST-segment monitoring in the prehospital setting improves the ability to identify patients earlier who are at greater risk for adverse hospital outcomes. Earlier risk stratification may help high-risk patients by allowing for the institution of more aggressive therapies earlier, without subjecting lower risk patients to unnecessary treatment.
Our data showed that 42.9% of patients activating “911” for symptoms of ACS without confounders, including LBBB, LVH, and ventricular pacing rhythm, had evidence of ischemia in the prehospital setting, and these patients were at greater risk for adverse events during the course of their hospitalization than those without such PH ECG signs. Specifically, those with evidence of PH ECG ischemia had a higher prevalence of adverse hospital outcomes compared to those without (37% vs. 27%, respectively, p<0.05). Moreover, we found that ischemia in the prehospital setting is an independent predictor of adverse hospital outcomes.
Patients presenting to the ED with ECG evidence of ischemia are at particular risk for cardiac death, non-fatal ischemic events, dysrhythmias, heart failure, stroke, and major bleeding.7 ST-T-wave changes indicative of ischemia can predict myocardial infarction and subsequent development of life-threatening complications. 8–10 Our findings are in general agreement with previous studies that have found electrocardiographic evidence of ischemia on the initial hospital admission ECG to predict these adverse hospital outcomes. 8,10
An early study by Brush and colleagues studied the admission ECG to identify patients who could be safely hospitalized in an intermediate care unit. 8 Those with evidence of ischemia had significantly higher incidences of life-threatening complications (ventricular fibrillation, sustained ventricular tachycardia, or heart block) than those without evidence of ischemia. Other complications (unsustained ventricular tachycardia, heart failure, third heart sound, jugular venous distension, cardiogenic shock, conduction disturbances, atrial dysrhythmias, or recurrent chest pain) were 3–10 times more likely in those with ischemia versus those without (p<0.01).8 Some differences from our study were that Brush et al.’s inclusion criteria were less stringent and included patients with LVH, LBBB, and paced rhythm. They also applied less sensitive ECG criteria and evaluated a single snapshot 10-second admission ECG. We applied more recent universal criteria for ECG interpretation, which considers gender differences. 4
Goldman et al. conducted a large study to identify clinical factors that predicted which patients presenting with chest pain would have complications requiring intensive care.10 They analyzed the admission hospital ECG and found electrocardiographic abnormalities to be the most important predictor of major complications within the first 24 hours after hospital presentation.10 Those with ischemia had significantly more dysrhythmia, pump failure, and recurring ischemia than those without ischemia. This study, like the prior one by Brush et al., examined the prognostic value of a single admission ECG to determine patients at risk of complications, versus PH ECG obtained with ST segment monitoring. 8 The sample populations of these studies were similar since they evaluated consecutive patients presenting to the ED with chest pain, although differed from ours in that all of our patients arrived by ambulance. 8,10
A more recent study by Pelter et al. examined the prognostic value of continuous ST-segment monitoring for patients admitted with ACS in determining adverse hospital outcomes.11 Similar to our findings, investigators found that transient myocardial ischemia was an independent predictor for adverse hospital outcomes. Pelter et al. reported that 46% of patients with ischemia had adverse hospital outcomes that included dysrhythmias, shock, pulmonary edema, acute MI after admission, abrupt closure after percutaneous coronary intervention, transfer to intensive care unit, or death.11 A difference from our study was that these patients were all admitted and treated for a confirmed ACS diagnosis whereas our sample population included all those with symptoms of ACS who activated EMS.
Limitations
Our study has potential limitations that should be considered. First, our study was not powered to reliably examine the association of PH ECG ischemia with all-cause mortality. Second, PH ECGs were recorded using a 5-electrode reduced lead configuration that was specifically developed for the ST SMART study. It is important to consider that different methods of ECG acquisition can result in different ST or T-wave morphologies, and therefore should be interpreted with caution. Finally, our study population was not very diverse, which limits the generalizability of our findings.
CONCLUSION
ST-segment monitoring used in the prehospital setting may provide better risk stratification than the initial snapshot ECG conducted in the ED in patients with cardiac symptoms activating “911.” Future research is needed to determine whether earlier diagnosis of acute myocardial ischemia in the prehospital phase of ACS will result in improved patient survival and quality of life.
ARTICLE SUMMARY.
Why is this topic important?
Prehospital electrocardiography (PH ECG) is becoming the standard of care for patients activating emergency medical services (EMS) for symptoms of acute coronary syndrome (ACS). However, little is known about the prognostic value of myocardial ischemia identified in the prehospital setting.
What does this study attempt to show?
The purpose of this study was to determine whether manifestations of acute myocardial ischemia on the PH ECG are predictive of adverse hospital outcomes.
What are the key findings?
Our data showed that 42.9% of patients activating “911” for symptoms of ACS had evidence of ischemia in the prehospital setting, and these patients were at a greater risk for adverse events during the course of their hospitalization than were those without such PH ECG signs (37% vs. 27%, respectively, p<0.05). In addition, we found that ischemia in the prehospital setting is an independent predictor of adverse hospital outcomes.
How is patient care impacted?
This information may enable clinicians to better identify high-risk patients earlier and institute aggressive therapy without subjecting lower-risk patients to unnecessary treatment. Patients may benefit from earlier risk stratification and more timely intervention by the identification of myocardial ischemia in the prehospital setting.
Acknowledgments
Grant support: NINR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S640. doi: 10.1161/CIRCULATIONAHA.110.970889. [DOI] [PubMed] [Google Scholar]
- 2.Drew BJ, Sommargren CE, Schindler DM, Benedict K, Zegre-Hemsey J, Glancy JP. A Simple Strategy Improves Prehospital Electrocardiogram Utilization and Hospital Treatment for Patients with Acute Coronary Syndrome (from the ST SMART Study) American Journal of Cardiology. 2011;107(3):347–352. doi: 10.1016/j.amjcard.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drew BJ, Dempsey ED, Joo TH, Sommargren CE, Glancy JP, Benedict K, Krucoff MW. Prehospital synthesized 12-lead ECG ischemia monitoring with trans-telephonic transmission in acute coronary syndromes: pilot study results of the ST SMART trial. Journal of Electrocardiology. 2004;37(Suppl):214–221. doi: 10.1016/j.jelectrocard.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, et al. Universal definition of myocardial infarction: Kristian Thygesen, Joseph S. Alpert and Harvey D. White on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. European Heart Journal. 2007;28(20):2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 5.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) Journal of the American College of Cardiology. 2001;38(7):2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 6.Patel DJ, Holdright DR, Knight CJ, Mulcahy D, Thakrar B, Wright C, Sparrow J, Wicks M, Hubbard W, Thomas R, et al. Early continuous ST segment monitoring in unstable angina: prognostic value additional to the clinical characteristics and the admission electrocardiogram. Heart. 1996;75(3):222–228. doi: 10.1136/hrt.75.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brieger D, Fox KA, Fitzgerald G, Eagle KA, Budaj A, Avezum A, Granger CB, Costa B, Anderson FA, Jr, Steg PG. Predicting freedom from clinical events in non-ST-elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009;95(11):888–894. doi: 10.1136/hrt.2008.153387. [DOI] [PubMed] [Google Scholar]
- 8.Brush JE, Jr, Brand DA, Acampora D, Chalmer B, Wackers FJ. Use of the initial electrocardiogram to predict in-hospital complications of acute myocardial infarction. N Engl J Med. 1985;312(18):1137–1141. doi: 10.1056/NEJM198505023121801. [DOI] [PubMed] [Google Scholar]
- 9.Stark ME, Vacek JL. The initial electrocardiogram during admission for myocardial infarction. Use as a predictor of clinical course and facility utilization. Arch Intern Med. 1987;147(5):843–846. [PubMed] [Google Scholar]
- 10.Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to the emergency departments with acute chest pain. N Engl J Med. 1996;334(23):1498–1504. doi: 10.1056/NEJM199606063342303. [DOI] [PubMed] [Google Scholar]
- 11.Pelter MM, Adams MG, Drew BJ. Transient myocardial ischemia is an independent predictor of adverse in-hospital outcomes in patients with acute coronary syndromes treated in the telemetry unit. Heart Lung. 2003;32(2):71–78. doi: 10.1067/mhl.2003.11. [DOI] [PubMed] [Google Scholar]