Abstract
Arsenic is present in the environment and has become a worldwide health concern due to its toxicity and carcinogenicity. However, the specific mechanism(s) by which arsenic elicits its toxic effects has yet to be fully elucidated. The transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) has been recognized as the master regulator of a cellular defense mechanism against toxic insults. This review highlights studies demonstrating that arsenic activates the Nrf2-Keap1 antioxidant pathway by a distinct mechanism from that of natural compounds such as sulforaphane (SF) found in broccoli sprouts or tert-butylhyrdoquinone (tBHQ), a natural antioxidant commonly used as a food preservative. Evidence also suggests that arsenic prolongs Nrf2 activation and may mimic constitutive activation of Nrf2, which has been found in several human cancers due to disruption of the Nrf2-Keap1 axis. The current literature strongly suggests that activation of Nrf2 by arsenic potentially contributes to, rather than protects against, arsenic toxicity and carcinogenicity. The mechanism(s) by which known Nrf2 activators, such as the natural chemopreventive compounds SF and lipoic acid, protect against the deleterious effects caused by arsenic will also be discussed. These findings will provide insight to further understand how arsenic promotes a prolonged Nrf2 response, which will lead to the identification of novel molecular markers and development of rational therapies for the prevention or intervention of arsenic-induced diseases. The National Institute of Environmental Health Science (NIEHS) Outstanding New Environmental Scientist (ONES) award has provided the opportunity to review the progress both in the fields of arsenic toxicology and Nrf2 biology. Much of the funding has led to (1) the novel discovery that arsenic activates the Nrf2 pathway by a mechanism different to that of other Nrf2 activators, such as sulforaphane and tert-butylhydroquinone, (2) activation of Nrf2 by chemopreventive compounds protects against arsenic toxicity and carcinogenicity both in vitro and in vivo, (3) constitutive activation of Nrf2 by disrupting Keap1-mediated negative regulation contributes to cancer and chemoresistance, (4) p62-mediated sequestration of Keap1 activates the Nrf2 pathway, and (5) arsenic-mediated Nrf2 activation may be through a p62-dependent mechanism. All of these findings have been published and are discussed in this review. This award has laid the foundation for my laboratory to further investigate the molecular mechanism(s) that regulate the Nrf2 pathway and how it may play an integral role in arsenic toxicity. Moreover, understanding the biology behind arsenic toxicity and carcinogenicity will help in the discovery of potential strategies to prevent or control arsenic-mediated adverse effects.
Keywords: Nrf2, Arsenic, Keap1, Oxidative stress, p62, Autophagy, Chemoprevention
INTRODUCTION TO ARSENIC
Arsenic is a naturally occurring metalloid that exists in practically all environmental media, such as air, soil, and water. Mostly, it exists in two oxidative forms, trivalent arsenite (As(III)) and pentavalent arsenate (As(V)) [1]. Millions of people worldwide are exposed to arsenic by drinking contaminated water and inhalation of particulate matter [2, 3]. Arsenic is associated with a wide variety of adverse effects, such as skin lesions, peripheral vascular diseases, reproductive toxicity, and neurological effects [3]. In addition, several epidemiological studies have correlated arsenic exposure to various human malignancies in the skin, lung, urinary bladder, liver, and kidney [4]. Within the Past two decades, the World Health Organization (WHO), as well as the United States Environmental Protection Agency (EPA), reduced the allowable arsenic concentration in drinking water from 50 ppb to 10 ppb (WHO [5], 1993 and EPA [6], 2001). However, owing to the toxicity of arsenic, arsenic trioxide (ATO) is currently being used as a cancer chemotherapeutic for the treatment of a variety of human cancers, predominantly acute promyelocytic leukemia [7,8].
Arsenic can undergo a series of methylations and oxidative reductions to generate a number of metabolites, including monomethylarsonous acid (MMA(III)), monomethylarsonic acid (MMA(V)), dimethylarsinous acid (DMA(III)), and dimethylarsinic acid (DMA(V)), that are excreted from the bladder, making the bladder the major target organ that is susceptible to the toxic effects of arsenic [9]. Arsenic has also been shown to have multiple biological effects, including alterations in signal transduction pathways, damage to DNA, and inhibition of its repair, induction of apoptotic cell death, and effects on global DNA methylation [7]. Several studies have demonstrated that arsenic exposure results in the generation of reactive oxygen species (ROS) in various cellular systems. Moreover, addition of inhibitors of oxidative stress, such as catalase, superoxide dismutase or glutathione peroxidase, or antioxidants, such as glutathione or vitamin E, decreases the toxic effects caused by arsenic [3,10–13]. Therefore, the cytotoxic and genotoxic effects of arsenic are also attributed to its ability to be a potent inducer of ROS; however, the exact mechanism(s) by which arsenic causes its harmful effects are still under investigation.
THE Nrf2-Keap1 PATHWAY
Nrf2 is a transcription factor that is activated in response to oxidative stress. Under unstressed conditions, Nrf2 is maintained at very low levels by its negative regulator, Keap1 [Kelch-like ECH associated protein 1], which forms an E3 ubiquitin ligase complex with Cullin 3 (Cul3) and Ring-box 1 (Rbx1) and facilitates the ubiquitination of Nrf2 [14, 15]. Subsequently, Nrf2 is targeted for degradation by the 26S proteasome. When cells are exposed to stress or electrophilic compounds, pivotal cysteine residues (C273, C288, and C151) in Keap1 act as “sensors” and are S-alkylated [16–18]. It is hypothesized that modification of the critical cysteine residues in Keap1 causes a conformational change in the Keap1-Cul3-Rbx1 E3 ubiquitin ligase complex, hindering ubiquitination of Nrf2 [19]. Subsequently, Nrf2 accumulates and translocates to the nucleus, dimerizes with a small Maf protein, and binds to the antioxidant response element in the promoter region of cytoprotective genes that are responsible for the detoxification and elimination of harmful substances, including arsenic. These genes include intracellular redox-balancing proteins (e.g., heme oxygenase-1 (HO-1) and thioredoxin reductase-1 (TrxR1)), phase I and II detoxication enzymes (e.g., NAD(P)H quinone oxidoreductase-1 (NQO1), glutathione S-transferase (GST), glutamate cysteine ligase catalytic subunit, and regulatory subunit (GCLM)), xenobiotic transporters (multidrug resistance-associated proteins (MRPs)), and other stress response proteins [20–22].
ARSENIC ACTIVATES Nrf2 THROUGH A DISTINCT MECHANISM
Arsenicals have been shown to activate the Nrf2-Keap1 pathway in a variety of human cell lines including osteoblasts (MC3T3-E1) [23], keratinocytes [24], placental choriocarcinoma cells [25], HeLa [26], myeloma cells [27], bladder epithelial cells (UROtsa) [28], and breast cancer cells (MDA-MB-231) [29]. In 2008, studies conducted in our laboratory demonstrated that arsenic activates Nrf2 through a different mechanism than that of SF and tBHQ. SF- and tBHQ-mediated activation of Nrf2 is dependent upon modification of the cysteine 151 sensor in Keap1 (Keap1-C151), also known as the canonical mechanism of Nrf2 activation. However, As(III) and MMA(III) activate Nrf2 through a Keap1-C151 independent mechanism [29–31]. Recently, our group, along with three other laboratories, independently demonstrated that p62, a selective substrate adaptor protein that plays a critical role in autophagy (a bulk-lysosomal degradation pathway), directly binds to Keap1 [32–35]. Overexpression of p62 or an accumulation of p62 due to dysregulation of autophagy resulted in the sequestration of Keap1 in the autophagosomes and hindrance of the Keap1-Cul3 E3 ubiquitin ligase complex to properly ubiquitinate Nrf2 [32–35]. Furthermore, Aono et al. demonstrated that osteoblasts treated with arsenic activated Nrf2-dependent transcription of target genes, including HO-1, peroxiredoxin 1 (Prx1), and p62 [23]. An accumulation of p62 and ubiquitin-conjugated proteins was also observed [23]. p62 has also recently been confirmed to be a downstream target gene of Nrf2, creating a positive feedback loop [35]. Taken together, these studies highly suggest that autophagy and p62 may play a critical role in arsenic-mediated Nrf2 activation. Further studies are required to determine whether this p62-dependent, or noncanonical, mechanism of Nrf2 activation is the mechanism by which arsenic activates the Nrf2 pathway.
THE “DARK SIDE” OF Nrf2
Nrf2 is also beneficial for cancer cells, providing an environment conducive for cell growth and protection against oxidative stress and chemotherapeutic agents [36]. Constitutive activation of Nrf2 due to somatic mutations in Keap1 or Nrf2 that disrupt Keap1-mediated Nrf2 regulation is prominent in several types of human cancer cell lines and tumors [37–40]. More specifically, mutations in Nrf2 have been found in lung, head/neck, esophagus, skin, and larynx cancers [41, 42]. Keap1 gene mutations were initially identified in lung cancer cell lines [43] and, thereafter, several reports have identified Keap1 mutations in breast cancer [44], gall-bladder cancer [45], prostate cancer [46], and many nonsmall cell lung cancer cell lines and tumors [40]. Moreover, several studies have demonstrated a correlation between high Nrf2 protein levels in cancer cells and chemoresistance [36,46–49]. For example, the lung cancer cell line, A549, contains a mutation in the Nrf2-binding domain in Keap1 (Kelch domain) that abolishes Keap1-mediated regulation of Nrf2. As a result, A549 cells have constitutively active Nrf2 and are resistant to a variety of chemotherapeutic agents, such as cisplatin, doxorubicin, and etoposide [36, 40]. A549 cells, as well as other cancer cells, can be sensitized to chemotherapeutic-induced apoptosis through knockdown or inhibition of Nrf2 [36,50].
In addition to somatic mutations of Keap1, epigenetic mechanisms and loss of heterozygosity of Keap1 have also been found to upregulate Nrf2 in different types of cancers due to reduced levels of Keap1 [37, 47, 51]. A comprehensive genetic and epigenetic analysis of the Keap1 gene in 47 nonsmall cell lung cancer tissues and specimens was performed. Interestingly, 22 of 47 of the tumor tissue were found to be methylated at the Keap1 promoter region, which was not observed in any of the normal samples and 10 of 47 had loss of heterozygosity [51].
Nrf2: THE CULPRIT IN ARSENIC TOXICITY?
Uncovering the dual roles of Nrf2 in cancer has raised safety concerns with respect to the strategy of using natural compounds to activate Nrf2 for chemoprevention. Several studies, however, have shown that some Nrf2 chemopreventive compounds have short biological half-lives and that their ability to induce Nrf2 downstream genes ranges from hours to days [52]. In addition, although the activation of the Nrf2 pathway by these compounds is pronounced, it is transient and, therefore, intermittent dosing is suggested for chemopreventive use [53,54]. On the other hand, there is evidence suggesting that arsenic-mediated activation of Nrf2 is similar to genetic disruptions found in cancer cells, causing elevated and prolonged activation of the pathway. Human liver hepatocellular carcinoma (HepG2) cells exposed to 10 μM inorganic arsenic not only caused persistent induction of HO-1 but also prolonged Nrf2 activation for up to 60 h [55]. When keratinocytes were exposed to 100 nM arsenic for 28 weeks, Nrf2 basal activity was higher than control cells [56]. However, there is some controversy as to whether Nrf2 protein levels elevate as a protective mechanism in response to arsenic-induced ROS, or persistent activation of Nrf2 is promoting the transformation of cells.
CHEMOPREVENTIVE Nrf2 ACTIVATORS PROTECT AGAINST ARSENIC TOXICITY
Our laboratory has demonstrated the importance of Nrf2 against arsenic toxicity both in vitro and in vivo. Mouse embryonic fibroblasts (MEF) from Nrf2 wild-type mice were shown to be less susceptible to arsenic-induced toxicity compared to MEF cells from Nrf2 null mice [28]. In vivo, Nrf2 knockout mice exposed to drinking water containing 1, 10, or 100 ppm sodium arsenite for 6 weeks displayed more severe pathological changes in the bladder, liver, and lung compared to Nrf2 wild-type mice [57]. Furthermore, activation of Nrf2 by SF or tBHQ was shown to protect human bladder UROtsa cells from both arsenite and monomethylarsonous acid (MMAIII) toxicity [28]. Recently, we demonstrated that SF-mediated activation of Nrf2 protects against arsenic-mediated inflammation using a whole body arsenic-inhalation model in Nrf2 wild-type mice [58]. The SF effects, however, were abrogated in Nrf2 knockout mice [58]. The concentrations of arsenic used in these studies are environmentally and biologically relevant. Our laboratory has also shown that Nrf2 is activated by tBHQ, as well as natural compounds, such as oridonin and cinnamaldehyde in several different cell lines. These compounds also protect against arsenic-induced toxicity [28, 59–62]. Lipoic acid, a thiol-compound that is a strong antioxidant, for example, induces Nrf2 in cells and protects against ATO-induced autophagic cell death in human glioma cells [63] and protects HepG2 cells from arsenic exposure [59]. Interestingly, a study done by Shinkai et al. showed that pretreatment of mouse hepatocytes with SF not only decreases arsenic toxicity but also inhibits accumulation of arsenic in the cells due to upregulation of γ -GCS, GST isoforms, and MRP1, all of which are important for the excretion of arsenic into the extracellular space [60]. These findings may suggest a possible mechanism by which the effects of SF could predominate over those of arsenic.
There are a few important differences to note among these studies. First, the concentrations of arsenic and/or chemopreventive compounds varied from the nanomolar to micromolar range and, second, the duration of the exposure to arsenic and/or chemopreventive compounds also varied, ranging from 2 to 48 h. Further studies are needed to determine whether dose and time of exposure by arsenic and/or chemopreventive compounds are determinants of Nrf2 activation being beneficial or detrimental to cell health and survival. Aside from the aforementioned differences, these studies seem to support the notion that activation of Nrf2 by the so-called beneficial compounds, such as tBHQ and SF, is through a Keap1-C151-dependent mechanism and is distinct from the p62-dependent mechanism that has been identified for arsenic. These studies suggest that activation through the Keap1-C151-dependent mechanism may elicit a positive chemopreventive Nrf2 response, whereas the p62-dependent mechanism may mimic the constitutive activation observed in certain cancers, deemed the dark side of Nrf2.
THE ROLE OF Nrf2 WHEN ARSENIC IS USED AS A CANCER THERAPEUTIC
Not only is arsenic present in the environment, but the metalloid, in the form of ATO, is also used in the treatment of several human malignancies (for a full review refer to [64]). ATO has also been shown to induce Nrf2 and its downstream cytoprotective genes, NQO1 and HO-1, in human oral squamous cell lines, multiple myeloma cell lines, rat cardiac myocytes, and liver epithelial cells [27, 65, 66]. cDNA microarray analysis revealed that Nrf2, along with HO-1, GCLM, NQO1, epoxide hydrolase 1, and thioredoxin reductase, were elevated in an ATO-resistant ovarian cancer cell line when compared to parental cells, which have low Nrf2 protein levels and are not resistant to ATO [67]. The cells resistant to ATO had continuous cancer cell growth, cell survival, tumor metastasis, and aggressiveness and were also resistant to cisplatin and paclitaxel [67]. In another study, microarray analysis of 59 cell lines from the NCI-60 tumor cell line panel that were resistant to ATO also revealed an enrichment of Nrf2 mRNA [68]. Supporting a role for Nrf2 in chemoresistance, knockdown of Nrf2 by shRNA was shown to sensitize A549 cells to ATO [68]. In addition, Nrf2 knockdown in glioma cells potentiated ATO-induced oxidative damage and cell death [69]. Morales et al. also demonstrated that ATO induces Nrf2 in multiple myeloma cell lines [70]. In the same study, inhibition of ATO-induced ROS with butylated hydroxyanisole did not affect Nrf2 activation or cell death, demonstrating that ATO-mediated induction of Nrf2 or cell death is not mediated through ROS [70]. More work is needed to determine the specific mechanisms of how ATO activates Nrf2 and how cancer cells become resistant to ATO. However, the results of these studies on ATO highly suggest that Nrf2 protects cells from the cytotoxic effects of chemotherapeutic arsenic. Therefore, inhibition of Nrf2 may sensitize cancer cells to chemotherapeutics, including ATO, and induce cell death. Taken together, the evidence supports the notion that arsenic-mediated activation of Nrf2 not only may cause toxicity and promote carcinogenicity but also contribute to chemoresistance.
THE FUTURE OF ARSENIC AND Nrf2-Keap1 RESEARCH
Although much progress has been made in elucidating the role of Nrf2 in arsenic exposure within the past decade, a great deal still remains unknown. It is clear that arsenicals at environmentally relevant doses induce the Nrf2-Keap1 pathway as supported by much of the current literature. However, whether arsenic-mediated activation of Nrf2 protects or contributes to arsenic toxicity and carcinogenicity has not yet been clarified. Both long-term in vitro and in vivo arsenic studies are needed to determine whether arsenic-mediated autophagy and/or prolonged Nrf2 activation contributes to arsenic toxicity and carcinogenicity.
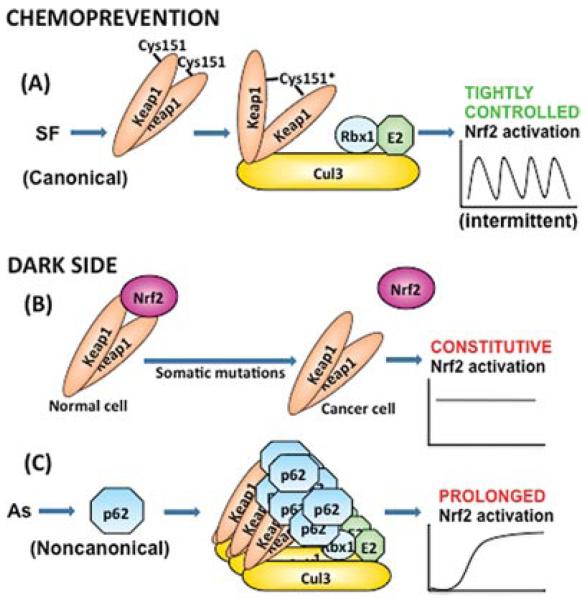
Our studies demonstrate that arsenic activates the Nrf2-Keap1 antioxidant pathway by a distinct mechanism from that of natural compounds such as SF or tBHQ. As shown in Figure 1, SF and tBHQ attenuate the deleterious effects of arsenic by activating the canonical Keap1-C151 Nrf2 pathway. This mechanism is dynamically regulated and provides intermittent increases in Nrf2. On the other hand, the mechanism by which arsenic activates Nrf2 is Keap1-C151 independent; instead, arsenic may activate Nrf2 through a p62-dependent mechanism. Unlike the Keap1-C151 dependent mechanism, this pathway results in prolonged activation of Nrf2.
FIGURE 1.
The chemopreventive and dark side of Nrf2. (A) Nrf2 activation through the Keap1-C151 canonical pathway by chemopreventive compounds, such as sulforaphane (SF), is intermittent. (B) Somatic mutations in pro-carNrf2 or Keap1 found in human cancers and tumors have constitutive Nrf2 activation. (C) Arsenic-mediated Nrf2 activation is prolonged due to p62-Keap1 sequestration in autophagosomes.
Further investigation is required to determine whether p62 is indeed the dominant arsenic-mediated mechanism and whether this arsenic activation in fact mimics the dark side of Nrf2, as found in chemoresistant human cancer cell lines and tumors. Furthermore, there is a lack of sufficient evidence to suggest that the differentiation between the protective and the dark side effects of Nrf2 activators are determined by the mechanism of activation. Additional research may also reveal whether activation by different Nrf2 inducers may result in upregulation of differential downstream genes. If the two differentiated modes of Nrf2 activation determine whether or not Nrf2 is protective or harmful, then canonical Nrf2 activators (Keap1-C151 dependent) have the opportunity to be developed into therapeutics for the prevention or intervention of arsenic toxicity.
ACKNOWLEDGMENTS
The authors would like to thank A. S. McElhinny and N. F. Villeneuve for constructive criticism of the manuscript. The authors are grateful to be invited by A.L. Slitt to contribute to this special issue ofJournal of Biochemical and Molecular Toxicology. This review was funded by the NIEHS grant (ES015010) and NCI grant (CA154377) awarded to D. D. Zhang, the Novartis Graduate Student Fellowship (Society of Toxicology) awarded to A. Lau, and ES006694, a center grant.
REFERENCES
- 1.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133(1):1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 2.Lantz RC, Hays AM. Role of oxidative stress in arsenic-induced toxicity. Drug Metab Rev. 2006;38(4):791–804. doi: 10.1080/03602530600980108. [DOI] [PubMed] [Google Scholar]
- 3.Schuhmacher-Wolz U, Dieter HH, Klein D, Schneider K. Oral exposure to inorganic arsenic: evaluation of its carcinogenic and non-carcinogenic effects. Crit Rev Toxicol. 2009;39(4):271–298. doi: 10.1080/10408440802291505. [DOI] [PubMed] [Google Scholar]
- 4.Platanias LC. Biological responses to arsenic compounds. J Biol Chem. 2009;284(28):18583–18587. doi: 10.1074/jbc.R900003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidelines for Drinking-Water Quality. 2nd edition. WHO; Geneva: 1993. ISBN 924 154460. [Google Scholar]
- 6.Natural Primary Drinking Water Regulations; Arsenic and Clarifications to Compliance and New Source Contaminants Monitoring; Proposed Rule. Federal Register. 2001;66(78):20580. [Google Scholar]
- 7.Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011;123(2):305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evens AM, Tallman MS, Gartenhaus RB. The potential of arsenic trioxide in the treatment of malignant disease: past, present, and future. Leuk Res. 2004;28(9):891–900. doi: 10.1016/j.leukres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Vahter M, Concha G. Role of metabolism in arsenic toxicity. Pharmacol Toxicol. 2001;89(1):1–5. doi: 10.1034/j.1600-0773.2001.d01-128.x. [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem. 2004;255(1–2):67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- 11.Nesnow S, Roop BC, Lambert G, Kadiiska M, Mason RP, Cullen WR, Mass MJ. DNA damage induced by methylated trivalent arsenicals is mediated by reactive oxygen species. Chem Res Toxicol. 2002;15(12):1627–1634. doi: 10.1021/tx025598y. [DOI] [PubMed] [Google Scholar]
- 12.Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res. 2003;533(1–2):37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Schoen A, Beck B, Sharma R, Dube E. Arsenic toxicity at low doses: epidemiological and mode of action considerations. Toxicol Appl Pharmacol. 2004;198(3):253–267. doi: 10.1016/j.taap.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24(24):10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99(18):11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23(22):8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X, Ma Q. Nrf2 cysteine residues are critical for oxidant/electrophile-sensing, Keap1-dependent ubiquitination-proteasomal degradation, and transcription activation. Mol Pharmacol. 2009;76(6):1265–1278. doi: 10.1124/mol.109.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A. 2004;101(7):2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 21.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58(5–6):262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48(2):91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aono J, Yanagawa T, Itoh K, Li B, Yoshida H, Kumagai Y, Yamamoto M, Ishii T. Activation of Nrf2 and accumulation of ubiquitinated A170 by arsenic in osteoblasts. Biochem Biophys Res Commun. 2003;305(2):271–277. doi: 10.1016/s0006-291x(03)00728-9. [DOI] [PubMed] [Google Scholar]
- 24.Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: involvement of hydrogen peroxide. Exp Cell Res. 2003;290(2):234–245. doi: 10.1016/s0014-4827(03)00341-0. [DOI] [PubMed] [Google Scholar]
- 25.Massrieh W, Derjuga A, Blank V. Induction of endogenous Nrf2/small maf heterodimers by arsenic-mediated stress in placental choriocarcinoma cells. Antioxid Redox Signal. 2006;8(1–2):53–59. doi: 10.1089/ars.2006.8.53. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki T, Blank V, Sesay JS, Crawford DR. Maf genes are involved in multiple stress response in human. Biochem Biophys Res Commun. 2001;280(1):4–8. doi: 10.1006/bbrc.2000.4064. [DOI] [PubMed] [Google Scholar]
- 27.Matulis SM, Morales AA, Yehiayan L, Croutch C, Gutman D, Cai Y, Lee KP, Boise LH. Darinaparsin induces a unique cellular response and is active in an arsenic trioxide-resistant myeloma cell line. Mol Cancer Ther. 2009;8(5):1197–206. doi: 10.1158/1535-7163.MCT-08-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang XJ, Sun Z, Chen W, Eblin KE, Gandolfi JA, Zhang DD. Nrf2 protects human bladder urothelial cells from arsenite and monomethylarsonous acid toxicity. Toxicol Appl Pharmacol. 2007;225(2):206–213. doi: 10.1016/j.taap.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang XJ, Sun Z, Chen W, Li Y, Villeneuve NF, Zhang DD. Activation of Nrf2 by arsenite and monomethylarsonous acid is independent of Keap1-C151: enhanced Keap1-Cul3 interaction. Toxicol Appl Pharmacol. 2008;230(3):383–389. doi: 10.1016/j.taap.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci U S A. 2010;107(44):18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He X, Ma Q. Critical cysteine residues of Kelch-like ECH-associated protein 1 in arsenic sensing and suppression of nuclear factor erythroid 2-related factor 2. J Pharmacol Exp Ther. 2010;332(1):66–75. doi: 10.1124/jpet.109.160465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30(13):3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12(3):213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 34.Riley BE, Kaiser SE, Shaler TA, Ng AC, Hara T, Hipp MS, Lage K, Xavier RJ, Ryu KY, Taguchi K, Yamamoto M, Tanaka K, Mizushima N, Komatsu M, Kopito RR. Ubiquitin accumulation in autophagy-deficient mice is dependent on the Nrf2-mediated stress response pathway: a potential role for protein aggregation in autophagic substrate selection. J Cell Biol. 2010;191(3):537–552. doi: 10.1083/jcb.201005012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain A, Lamark T, Sjottem E, Bowitz Larsen K, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285(29):22576–91. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, Zhang DD. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29(6):1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, Shibata T, Yamamoto M, Hirohashi S. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68(5):1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 38.Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26(8):2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J Biol Chem. 2006;281(34):24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- 40.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3(10):e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci U S A. 2008;105(36):13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, Eom HS, Yoo NJ, Lee SH. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220(4):446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 43.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21(5):689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Nioi P, Nguyen T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochem Biophys Res Commun. 2007;362(4):816–821. doi: 10.1016/j.bbrc.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 45.Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, Hirohashi S. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135(4):1358–1368. 1368, e1–4. doi: 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- 46.Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, Wu H, Bova SG, Biswal S. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol Cancer Ther. 2010;9(2):336–346. doi: 10.1158/1535-7163.MCT-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang T, Chen N, Zhao F, Wang XJ, Kong B, Zheng W, Zhang DD. High levels of Nrf2 determine chemoresistance in type II endometrial cancer. Cancer Res. 2010;70(13):5486–5496. doi: 10.1158/0008-5472.CAN-10-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shim GS, Manandhar S, Shin DH, Kim TH, Kwak MK. Acquisition of doxorubicin resistance in ovarian carcinoma cells accompanies activation of the NRF2 pathway. Free Radic Biol Med. 2009;47(11):1619–1631. doi: 10.1016/j.freeradbiomed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Hu L, Miao W, Loignon M, Kandouz M, Batist G. Putative chemopreventive molecules can increase Nrf2-regulated cell defense in some human cancer cell lines, resulting in resistance to common cytotoxic therapies. Cancer Chemother Pharmacol. 2009;66(3):467–74. doi: 10.1007/s00280-009-1182-7. [DOI] [PubMed] [Google Scholar]
- 50.Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, Zhang DD. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci U S A. 2011;108(4):1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muscarella LA, Parrella P, D'Alessandro V, la Torre A, Barbano R, Fontana A, Tancredi A, Guarnieri V, Balsamo T, Coco M, et al. Frequent epigenetics inactivation of KEAP1 gene in non-small cell lung cancer. Epigenetics. 2011;6(6):710–719. doi: 10.4161/epi.6.6.15773. [DOI] [PubMed] [Google Scholar]
- 52.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31(1):90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Primiano T, Egner PA, Sutter TR, Kelloff GJ, Roebuck BD, Kensler TW. Intermittent dosing with oltipraz: relationship between chemoprevention of aflatoxin-induced tumorigenesis and induction of glutathione S-transferases. Cancer Res. 1995;55(19):4319–4324. [PubMed] [Google Scholar]
- 54.Baer-Dubowska W. Cancer chemopreventive agents-drugs for the 21st century? Acta Pol Pharm. 2006;63(5):369–373. [PubMed] [Google Scholar]
- 55.Abiko Y, Shinkai Y, Sumi D, Kumagai Y. Reduction of arsenic-induced cytotoxicity through Nrf2/HO-1 signaling in HepG2 cells. J Toxicol Sci. 2010;35(3):419–423. doi: 10.2131/jts.35.419. [DOI] [PubMed] [Google Scholar]
- 56.Pi J, Diwan BA, Sun Y, Liu J, Qu W, He Y, Styblo M, Waalkes MP. Arsenic-induced malignant transformation of human keratinocytes: involvement of Nrf2. Free Radic Biol Med. 2008;45(5):651–658. doi: 10.1016/j.freeradbiomed.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang T, Huang Z, Chan JY, Zhang DD. Nrf2 protects against As(III)-induced damage in mouse liver and bladder. Toxicol Appl Pharmacol. 2009;240(1):8–14. doi: 10.1016/j.taap.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng Y, Tao S, Lian F, Chau BT, Chen J, Sun G, Fang D, Lantz RC, Zhang DD. Sulforaphane prevents pulmonary damage in response to inhaled arsenic by activating the Nrf2-defense response. Toxicol Appl Pharmacol. doi: 10.1016/j.taap.2012.08.028. (in press). doi: 10.1016/j.taap.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huerta-Olvera SG, Macias-Barragan J, Ramos-Marquez ME, Armendariz-Borunda J, Diaz-Barriga F, Siller-Lopez F. Alpha-lipoic acid regulates heme oxygenase gene expression and nuclear Nrf2 activation as a mechanism of protection against arsenic exposure in HepG2 cells. Environ Toxicol Pharmacol. 2010;29(2):144–149. doi: 10.1016/j.etap.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Shinkai Y, Sumi D, Fukami I, Ishii T, Kumagai Y. Sulforaphane, an activator of Nrf2, suppresses cellular accu mulation of arsenic and its cytotoxicity in primary mouse hepatocytes. FEBS Lett. 2006;580(7):1771–1774. doi: 10.1016/j.febslet.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 61.Du Y, Villeneuve NF, Wang XJ, Sun Z, Chen W, Li J, Lou H, Wong PK, Zhang DD. Oridonin confers protection against arsenic-induced toxicity through activation of the Nrf2-mediated defensive response. Environ Health Perspect. 2008;116(9):1154–1161. doi: 10.1289/ehp.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wondrak GT, Villeneuve NF, Lamore SD, Bause AS, Jiang T, Zhang DD. The cinnamon-derived dietary factor cinnamic aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells. Molecules. 2010;15(5):3338–3355. doi: 10.3390/molecules15053338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng TJ, Wang YJ, Kao WW, Chen RJ, Ho YS. Protection against arsenic trioxide-induced autophagic cell death in U118 human glioma cells by use of lipoic acid. Food Chem Toxicol. 2007;45(6):1027–1038. doi: 10.1016/j.fct.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 64.Emadi A, Gore SD. Arsenic trioxide - An old drug redis-covered. Blood Rev. 2010;24(4–5):191–199. doi: 10.1016/j.blre.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X, Su Y, Zhang M, Sun Z. Opposite effects of arsenic trioxide on the Nrf2 pathway in oral squamous cell carcinoma in vitro and in vivo. Cancer Lett. 2012;318(1):93–98. doi: 10.1016/j.canlet.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Sumi D, Sasaki T, Miyataka H, Himeno S. Rat H9c2 cardiac myocytes are sensitive to arsenite due to a modest activation of transcription factor Nrf2. Arch Toxicol. 2011;85(12):1509–1516. doi: 10.1007/s00204-011-0700-7. [DOI] [PubMed] [Google Scholar]
- 67.Ong PS, Chan SY, Ho PC. Microarray analysis revealed dysregulation of multiple genes associated with chemoresistance to As(2)O(3) and increased tumor aggressiveness in a newly established arsenic-resistant ovarian cancer cell line, OVCAR-3/AsR. Eur J Pharm Sci. 2012;45(3):367–378. doi: 10.1016/j.ejps.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Liu Q, Zhang H, Smeester L, Zou F, Kesic M, Jaspers I, Pi J, Fry RC. The NRF2-mediated oxidative stress response pathway is associated with tumor cell resistance to arsenic trioxide across the NCI-60 panel. BMC Med Genomics. 2010;3:37. doi: 10.1186/1755-8794-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Liang Y, Zheng T, Yang G, Zhang X, Sun Z, Shi C, Zhao S. Inhibition of heme oxygenase-1 enhances anti-cancer effects of arsenic trioxide on glioma cells. J Neurooncol. 2011;104(2):449–458. doi: 10.1007/s11060-010-0513-1. [DOI] [PubMed] [Google Scholar]
- 70.Morales AA, Gutman D, Cejas PJ, Lee KP, Boise LH. Reactive oxygen species are not required for an arsenic trioxide-induced antioxidant response or apoptosis. J Biol Chem. 2009;284(19):12886–12895. doi: 10.1074/jbc.M806546200. [DOI] [PMC free article] [PubMed] [Google Scholar]