Abstract
Objectives/Hypothesis
To determine whether incorporation of intraoperative imaging via a new cone-beam computed tomography (CBCT) image-guidance system improves accuracy and facilitates resection in sinus and skull-base surgery through quantification of surgical performance.
Study Design
Landmark identification and skull base ablation tasks were performed with a CBCT intraoperative image-guidance system in the experimental group and with image-guided surgery (IGS) alone based on a pre-operative CT in the control group.
Methods
Six cadaveric heads underwent preoperative CT imaging and surgical planning identifying surgical targets. Three types of surgical tasks were planned: landmark point identification, line contour identification, and volume drill-out. Key anatomic structures (carotid artery and optic nerve) were chosen for landmark identification and line contour tasks. Complete ethmoidectomy, vidian corridor drill-out, and clival resection were performed for volume ablation tasks. The CBCT guidance system was used in the experimental group and performance was assessed by metrics of target registration error, sensitivity, and specificity of excision.
Results
Significant improvements were seen for point identification and line tracing tasks. Additional resection was performed in 67% of tasks in the CBCT group and qualitative feedback indicated unequivocal improvement in confidence for all tasks. In review of tasks in the control group, additional resection would have been performed in 35% of tasks if an intraoperative image was available.
Conclusions
An experimental prototype C-arm CBCT guidance system was shown to improve surgical precision in the identification of skull base targets and increase accuracy in the ablation of surgical target volumes in comparison to using IGS alone.
Level of Evidence
NA
Keywords: Cone-beam CT, Intraoperative Imaging, Skull Base Surgery, Image-guided Surgery, Endoscopic Sinus Surgery
Introduction
The development of advanced endoscopic techniques coupled with image-guidance in sinus and skull base surgery has sought to improve surgical outcome and reduce complications.1–3 Consequently, image-guided surgery (IGS) has become widely used for approaches to complex sinonasal and skull base lesions. Current IGS modalities do not reflect real-time changes in anatomy that occur during a surgical procedure, limiting conventional IGS to a context of rigid bony anatomy. This becomes a significant disadvantage in the setting of surgical procedures involving complex skull base lesions, which cause significant alterations of normal anatomic landmarks.
Intraoperative cone-beam CT (CBCT) is a new technology that addresses this limitation. Implemented on a mobile C-arm and integrated with a guidance system for intraoperative update of altered surgical anatomy, CBCT can provide the surgeon with valuable information on the completeness of resection and proximity to normal critical anatomy when performing complex ablation of sinonasal or skull base lesions. CBCT offers fast, low-dose, high-resolution images of intraoperative sinus and skull base anatomy. This technology, in combination with real-time navigation, has the potential to improve surgical resection without compromising safety during ablative tasks that occur in close proximity to critical structures.4–7
Several studies have assessed the potential benefits of intraoperative imaging and have shown increased accuracy and completeness of surgical resection.8,9 Intraoperative CT resulted in alteration of the surgical plan in 30% of patients9 and resulted in further surgery in 24% of cases with additional tumor resection, removal of ethmoid partitions, and frontal bone removal.10 Intraoperative CBCT has been demonstrated to result in higher conformity to surgical margins in skull base excision tasks. Chan et al., in an initial pilot study for this work, showed that intraoperative CBCT quantifiably improved surgical performance in all excision tasks and significantly increased surgical confidence in sinus and skull base surgery—most notably for challenging drill-out tasks in the clivus.4 The study detailed below builds from previous work in quantifying the extent to which a fully integrated CBCT guidance system (including not only intraoperative CBCT but also registration to preoperative images,11 availability of video endoscopy,12,13 and real-time tracking/navigation7) improves accuracy and facilitates resection in sinus and skull-base surgery through quantification of surgical performance. The current work is also distinct from the pilot study and utilized endonasal endoscopic approaches to the skull base, whereas the initial work focused on a more invasive, open approach (i.e., medial maxillectomy).
Materials and Methods
Mobile C-arm Cone-Beam CT System
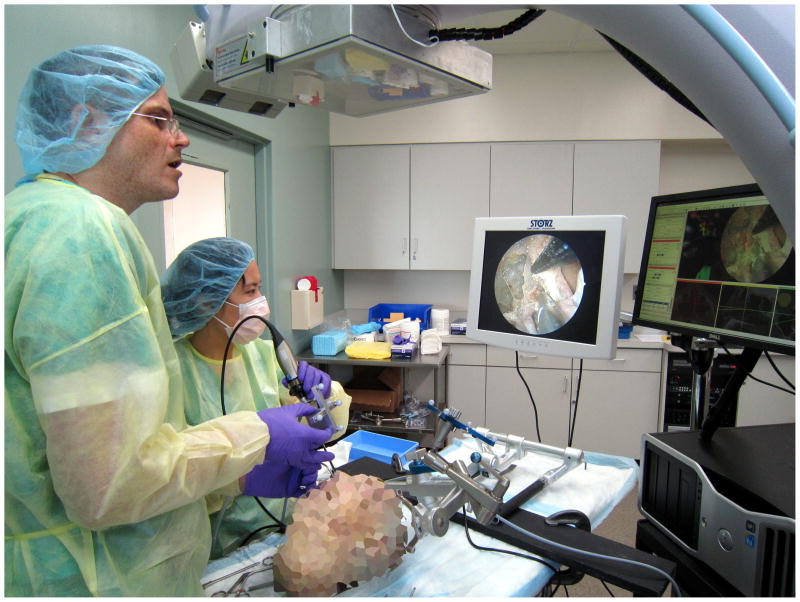
The CBCT system was based on a mobile C-arm system (Powermobil, Siemens Healthcare, Erlangen, Germany) extensively modified by replacing the image-intensifier with a flat-panel detector, motorizing the orbit, and implementing a system for computer control, geometric calibration, and 3D reconstruction.14 Application-specific acquisition protocols have been delineated for bone and soft-tissue structure visualization with scan doses of approximately 3 mGy (0.10 mSv) and 10 mGy (0.35 mSv) respectively,12 which is 1/20th-1/5th of a diagnostic head CT. Scans typically involved 200 projections acquired across ~178° orbital range in about 60 seconds. The volumetric CBCT image (512 × 512 × 512 voxel, 0.3 mm homogeneous voxel size) was reconstructed within 30 seconds, supplying the surgeon with a 15 × 15 × 15-cm field of view. A surgical tracking and navigation system developed in-house presented up-to-date CBCT image registered to preoperative images with surgical planning data, and allowed for real-time tracking of instruments and the endoscope.7 The navigation interface provided tri-planar slice navigation, overlay of planning data, and optional augmentation of the endoscopic video stream with virtual renderings of the surgical target, surrounding normal anatomy, and any other data defined in preoperative planning and registered to CT (Figure 1).
Figure 1.
Typical setup for intraoperative mobile C-arm cone-beam CT with a two-surgeon, four-handed technique for skull base surgery.
Cadaveric Study Experimental Setup
The study was performed consistent with and after obtaining required approval from institutional ethics and biosafety departments. Six cadaveric heads underwent preoperative CT imaging, registration, segmentation, and surgical planning and each side was randomized to either the “guided” group, in which the CBCT IGS system was utilized, or the “unguided” group, in which IGS alone was available with a static pre-operative CT (n=6 for each group). Manual contouring of critical anatomy and surgical ablation targets was performed. Three types of surgical tasks were planned (Table I): landmark point identification, line contour tracing, and volume drill-out. Key anatomic structures (e.g., optic nerve and carotid artery) were chosen for landmark identification and line contour tasks (Figures 2–4). Complete ethmoidectomy, vidian corridor drill-out, and clival drill-out were performed for volume ablation tasks (Figures 5,6). For the guided procedures, the surgeon was permitted to acquire as many intraoperative images as desired to perform resection of the targeted volume. Optional video augmentation with virtual renderings of the surgical target was also available to the surgeon. For unguided procedures, only the preoperative CT was available to the surgeon. Setup and acquisition time was approximately 5 minutes for each task and 1 minute to obtain an updated intraoperative CBCT image.
Table I.
Summary of Surgical Tasks
| Task | Description | Target Structures |
|---|---|---|
| Landmark Point Identification | Identify target structures with a straight image-guided pointer |
|
| Line Contour Tracing | Outline the contour of the target structure |
|
| Volume Drill-Out | Completely remove target structure |
|
Figure 2.
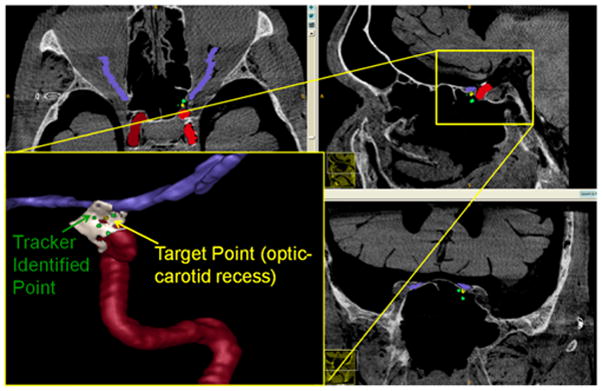
Landmark Identification Task: The true optic-carotid recess target point (yellow) and the point identified by the surgeon (green) are depicted.
Figure 4.
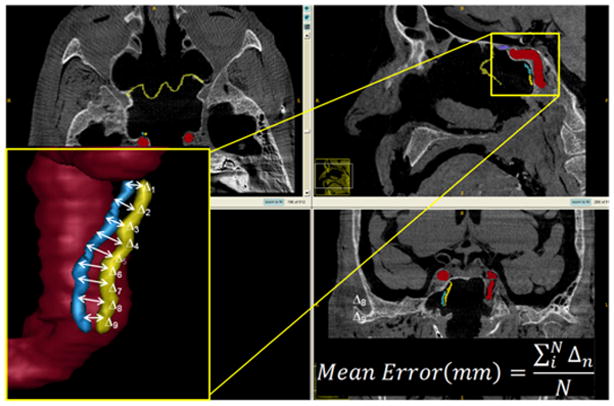
Line Contour Task Defining the Internal Carotid Artery: The distance between the true (yellow) and surgeon-delineated (blue) contours defines the mean error.
Figure 5.
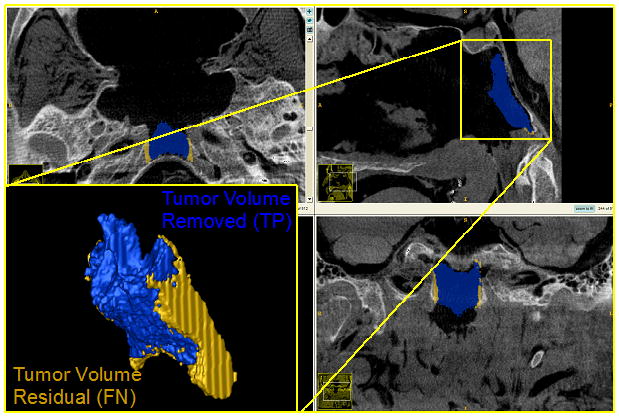
Volume Drill-Out Task: Resection of the Clivus. The amount of target volume removed (blue) represents the true-positive volume. The amount of target volume remaining after the drill-out procedure (yellow) represents the false-negative volume.
Figure 6.
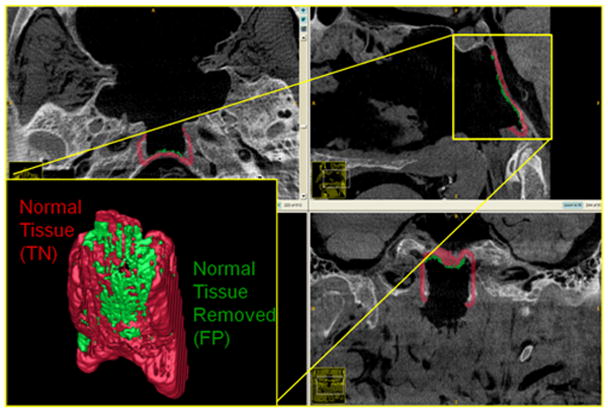
Volume Drill-Out Task: Resection of the Clivus. The amount of surrounding normal volume removed (green) represents the false-positive volume. The amount of normal volume remaining after the drill-out procedure (red) represents the true-negative volume.
Outcome Measures and Analysis
Tasks and mode of operation were randomized between groups, and performance was assessed by metrics of target registration error15 (for landmark and contour tasks) (Figure 3,4) and sensitivity and specificity of excision4 (for volumetric ablation tasks) (Figure 7). Target registration error was determined as the average spatial distance between the planned target point and the corresponding intraoperatively identified target point by the surgeon.
Figure 3.
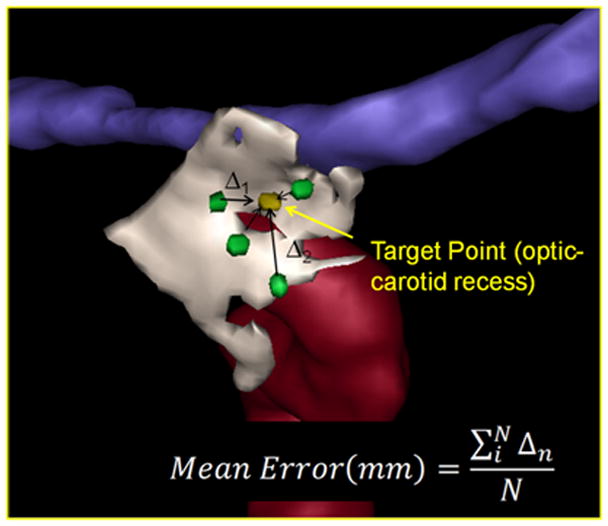
Landmark Identification Task Defining the Optic-carotid Recess: The localization error is the mean distance between the point identified by the surgeon (green) and the true location identified in planning (yellow).
Figure 7.
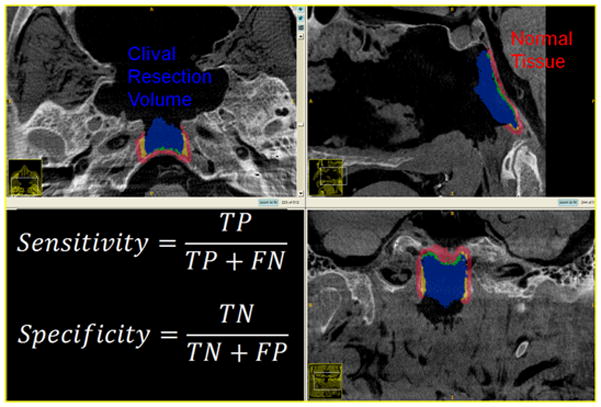
Clival Resection: Measurements of Sensitivity and Specificity.
To quantify surgical performance in drill-out tasks, the planned target volume was analyzed in terms of the amount of target tissue removed as well as the amount of residual target tissue. Correspondingly, the amount of normal tissue not intended to be resected based on preoperative planning was also analyzed. Four cardinal metrics for performance evaluation were analyzed as previously described4: true-positive fraction (i.e., fraction of the target volume excised), false-positive fraction (i.e., fraction of surrounding normal tissue excised), false-negative fraction (i.e., the fraction of the target volume remaining), and true-negative fraction (i.e., the fraction of surrounding normal tissue remaining). From these measures, the sensitivity, specificity, and predictive values were calculated for each drill-out task for each group. Sensitivity describes the fraction of lesion excised while specificity describes the fraction of normal tissue remaining (Figure 7). Such analysis was demonstrated in the pilot study of Chan et al.4 to yield quantitative measures of surgical performance and can be similarly interpreted in terms of positive predictive value (PPV – i.e., the probability that excised tissue is indeed from the target volume) and negative predictive volume (NPS – i.e., the probability that non-resected tissue is indeed normal tissue outside the target volume). Statistical significance between guided and unguided groups was assessed by a two-tailed equal-variance Student’s t-test.
A post-procedure survey was then conducted to obtain qualitative feedback within several days after the procedure. Respondents included one fellowship-trained neurosurgeon in endoscopic skull base surgery, one fellowship-trained otolaryngologist, and one otolaryngologist in the final year of fellowship training. For each case three categories of analysis was conducted: the extent of added benefit of intraoperative CBCT in performing the task; surgeon confidence; and upon review, additional steps of resection that would have been completed if the post-ablation scan was made available to the surgeon. The surgeons were queried regarding the extent to which intraoperative CBCT guidance improved the ability to perform the surgical task and improved confidence or resolved uncertainties pertaining to the task.
Results
Point identification and line tracing tasks were significantly improved (p=0.05 and 0.001 with 95% and 99% confidence intervals respectively) in the CBCT-guided group (Figure 8) in comparison to the unguided group. Volume drill-out tasks suggested improvement in both sensitivity and specificity but this was not statistically significant in the current study (Figure 9).
Figure 8.
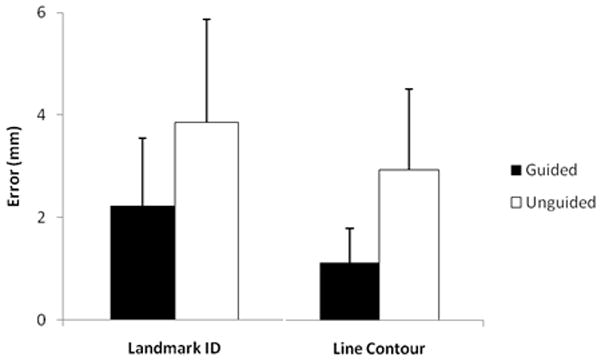
Landmark Identification (p=.05) and Line Contour Task Performance (p=.01).
Figure 9.
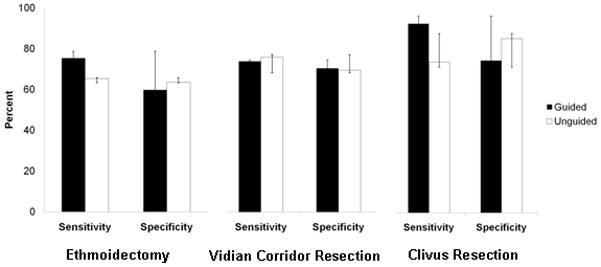
Ethmoidectomy, Vidian Corridor, and Clival Resection Performance.
For the volume drill-out tasks, additional resection was performed in 67% of tasks with a range of 1–4 scans performed for each task. Utilization of intraoperative CBCT was increased for more complex tasks such as in the resection of the clivus (average=2.3 scans) in comparison to ethmoidectomy (average=1.7 scans) and vidian corridor resection (average=1.7 scans). Qualitative feedback was obtained for each case. In all guided cases surgeon feedback indicated that the CBCT image improved confidence or resolved uncertainty pertaining to the task (Table II). Intraoperative imaging was particularly helpful in cases with a poorly pneumatized sphenoid sinus with few visible endoscopic surface landmarks and with anatomical variations of the carotid artery. When adequate pneumatization was present allowing for endoscopic visualization of skull base landmarks, complete resection of the target volume was possible without the need for repeat imaging. In these cases, for example, the carotid artery and vidian nerve were clearly visible on endoscopy obviating the need for CBCT confirmation during ablative tasks.
Table II.
Qualitative Review of Guided Tasks
| Task | Task Performance | Total # of Intraoperative CBCT Scans |
|---|---|---|
|
| ||
|
Case 1
| ||
| Left ethmoid | Further resection of ethmoid partitions along the skull base was accomplished. | 2 |
| Left vidian | The vidian nerve was completely encased in bone. Further removal of bone around the vidian canal was accomplished. | 2 |
|
| ||
|
Case 2
| ||
| Right ethmoid | Further resection of ethmoid partitions along the skull base was accomplished. | 2 |
| Right vidian | The image obtained was confirmatory. | 1 |
| Clivus | The image obtained was confirmatory. | 1 |
|
| ||
|
Case 3
| ||
| Left ethmoid | Further resection of ethmoid partitions was accomplished. Upon review and under ideal viewing conditions additional ethmoid partitions along the skull base would have been resected. | 2 |
| Right vidian | The degree of pneumatization of the vidian canal allowed for complete resection and the image was confirmatory. | 1 |
|
| ||
|
Case 4
| ||
| Left ethmoid | Upon review and under ideal viewing conditions further ethmoid partitions would have been resected. | 1 |
| Left vidian | Further removal of bone around the vidian nerve was accomplished. | 2 |
| Superior clivus | Further removal of the superior clivus was accomplished. | 4 |
| Inferior clivus | Further removal of the inferior clivus was accomplished. | 2 |
|
| ||
|
Case 5
| ||
| Right ethmoid | Further resection of ethmoid partitions was accomplished. | 2 |
| Right vidian | Further removal of bone around the vidian canal was accomplished. | 2 |
|
| ||
|
Case 6
| ||
| Right ethmoid | Total ethmoidectomy performed without need for intraoperative imaging. | 1 |
| Right vidian | Further removal of bone around the vidian canal was accomplished. The image allowed for increased resection of bone near the carotid artery. | 2 |
The post-procedure survey of unguided cases also revealed that further resection of the target volume would have been attempted in 35% of tasks had the post-ablation scan been available during the time of the procedure (Table III). This was determined by including all tasks with a grade of 3 or greater, indicating that a significant amount of additional resection would have been performed if the intraoperative image was available. Additional resection included further removal of ethmoid partitions along the skull base, additional bone removal along the inferior aspect of the pterygoid plate in the vidian corridor, and removal of bone along the inferolateral portion of the clivus. 71% of tasks would have resulted in additional surgery if all tasks with a grade 2 or greater were included in the analysis. Further fine-tuning would have resulted in removal of bone along the medial orbital wall, the lateral pterygoid plate, and lateral clivus. It is important to take into consideration, however, that anatomic boundaries of resection as well as the measure of surgical completeness were rigorously defined in the setting of normal anatomy.
Table III.
Qualitative Review of Unguided Tasks
| Case ID | Task Performance |
|---|---|
| To what extent would the post-operative image have affected your surgical approach or decision-making in subsequent steps of the procedure? 1=Not at all 2=A small degree 3=Somewhat 4=Quite a bit |
|
|
| |
|
Case 1
| |
| Right ethmoid | 2 A partition adjacent to the orbit would have been removed. |
| Right vidian | 1 |
| Superior clivus | 1 |
| Inferior clivus | 3 Further bone along the infererolateral clivus would have been removed. |
|
| |
|
Case 2
| |
| Left ethmoid | 1 |
| Left vidian | 2 Additional bone along the lateral anterior portion of the pterygoid plate would have been removed. |
|
| |
|
Case 3
| |
| Right ethmoid | 3 Additional ethmoid partitions would have been further resected. |
| Left vidian | 2 Additional bone along the pterygoid plate would have been removed. |
| Clivus | 2 Additional bone along the lateral clivus would have been removed. |
|
| |
|
Case 4
| |
| Right ethmoid | 3 Additional ethmoid partitions would have been further resected. |
| Right vidian | 2 Additional bone along the inferior medial sphenoid bone would have been removed. |
|
| |
|
Case 5
| |
| Left ethmoid | 2 A partition adjacent to the orbit would have been removed. |
| Left vidian | 3 The inferior posterior pterygoid bone would have been further removed. |
| Clivus | 1 |
|
| |
|
Case 6
| |
| Left ethmoid | 4 Additional ethmoid partitions would have been further resected. |
| Left vidian | 1 |
| Clivus | 3 Additional bone along the right lateral and inferior aspects of the clivus would have been resected. |
Discussion
The adoption of IGS is now widespread among skull base surgeons, and its use is supported by existing literature,1,16–18 however, conventional IGS systems are limited by the inability to update the preoperative image with dynamic changes that occur during surgery. In the setting of complex sinonasal or skull base pathology, this can be problematic. Although there have been few reports on the use of fluoroscopy and intraoperative MRI, there are significant drawbacks limiting their use. In comparison to MRI, intraoperative CBCT is faster, less expensive, and provides high-resolution images of bony anatomy.19 The radiation dose of an intraoperative CBCT measures ~3 mGy or (~0.10 mSv), which is 5–10% of the dose of a conventional head CT scan.20 The mobile C-arm CBCT technology used in this study provided a platform with a relatively open geometry with reasonable patient access and the ability to provide accurate, near real-time updates to landmark localization, spatial orientation, and registration of planning data in a manner that accounts for intraoperative changes to tissue. The system also incorporated preoperative planning data with contouring of critical anatomic structures and resection volumes, allowing for modifications to the surgical plan and increased completeness of surgery (Figures 5–7). The current embodiment of the mobile C-arm provides superior CBCT image quality than image intensifier based mobile C-arms and better image quality at a comparable dose to other flat panel based mobile scanners, i.e., the O-arm (Medtronic, Minneapolis, MN) and xCAT (Xoran Technologies, Ann Arbor, MI).5 Direct technical comparisons however have not yet been performed and would be pertinent for future study. Comparison with stationary CT is difficult due to the vast difference in design of the modalities but one can argue that an open C-arm design can provide superior patient access. Further technical details outside the scope of this paper can be referenced in previous publications.5,12
Our results show that such a CBCT guidance system has the potential to improve surgical precision in the localization of skull base targets and surrounding normal, critical anatomy. Through integration with preoperative imaging and planning data, CBCT has the potential to facilitate resection of skull base targets while preserving surrounding structures.
The indications for intraoperative imaging are currently being more clearly defined. The American Academy of Otolaryngology—Head and Neck Surgery has endorsed the use of intraoperative imaging in select circumstances to assist the surgeon in providing localization of anatomic structures.21 Intraoperative CT has been demonstrated to be useful in frontal sinus surgery22,23 and in ensuring completeness of ethmoidectomy.9 Increased surgical accuracy and resection can be facilitated in the setting of extensive polypoid inflammation or fibro-osseous lesions. Applications in skull base surgery are especially pertinent. Qualitative surgical feedback indicates that intraoperative CBCT may be particularly useful in accurately delineating boundaries of surgical resection, allowing the surgeon to fine-tune intraoperative decision-making. This can be indispensable with poorly-defined endoscopic landmarks and necessity for high-volume bone removal adjacent to critical structures such as the orbit and neurovasculature. In the context of anatomical distortion due to extensive disease, intraoperative CBCT can also be particularly useful to differentiate surgical landmarks and provide further insight into the progression of surgery with increased confidence and safety.
The measurement of surgical performance with objective feedback is also critical for training purposes. Measures of sensitivity and specificity of surgical resection can provide important feedback to the trainee including information regarding their accuracy and proficiency in endoscopic skull base resections.
This study was limited to a cadaveric model which is perhaps oversimplified in comparison to more challenging clinical use, and therefore the utility in the operating room is being more fully defined in ongoing clinical feasibility studies and trials. Furthermore, resection was carried out in the context of targets defined in normal anatomy, which is unlikely in the patient with complex skull base pathology. In this study, the planned target volumes were abstract rather than based on resection of pathologic lesions. As a result, anatomic boundaries of resection were rigorously defined and the experimental evaluation of completeness in this study would differ from completeness defined by a resection of real pathology. The qualitative analysis was incorporated into this study to assess surgeon feedback as to the utility of this IGS system in improving surgeon confidence and completeness of surgical resection and is subject to participant as well as recall bias. The quantitative analysis shows a statistically significant improvement in accuracy of point identification and line contouring tasks as defined by degree of error in the guided group. The fairly small number of cadaver dissections in this study may have limited determination of a statistically significant improvement in the volume drill-out task. Additionally, the volume drill-out tasks were performed by a surgeon with extensive experience in endoscopic skull base resections, and it is therefore possible that the CBCT system may have provided less utility than it would for a less experienced surgeon. Future work will include clinical trials with advanced C-arm prototypes, which could potentially improve patient safety and surgical accuracy in the resection of complex skull base lesions.
Conclusion
A prototype C-arm CBCT guidance system was shown to improve surgical precision in the identification of skull base targets and increase accuracy in the ablation of surgical target volumes in comparison to the use of IGS alone. Through integration with real-time tracking and registration to preoperative imaging, planning, and continued development of video endoscopy, CBCT has the potential to facilitate precise surgical resection of skull base targets and increase surgeon confidence while preserving critical surrounding structures.
Acknowledgments
Funding: NIH R01-CA-127444
There were no commercial interests in the technologies described in this paper. The research in this study was supported by NIH grant R01-CA-127444 and in collaboration with Siemens Health Care (Erlangen, Germany). The authors extend thanks to Dr. Michael Marohn at the Minimally Invasive Surgery Training center at Johns Hopkins University for the use of their facility.
Footnotes
Conflict of Interest: None
This manuscript was presented at:
The 21st North American Skull Base Society Meeting
Scottsdale, Arizona, USA
References
- 1.Citardi MJ, Batra PS. Intraoperative surgical navigation for endoscopic sinus surgery: rationale and indications. Curr Opin Otolaryngol Head Neck Surg. 2007;15:23–7. doi: 10.1097/MOO.0b013e3280123130. [DOI] [PubMed] [Google Scholar]
- 2.Metson R. Image-guided sinus surgery: lessons learned from the first 1000 cases. Otolaryngol Head Neck Surg. 2003;128:8–13. doi: 10.1067/mhn.2003.40. [DOI] [PubMed] [Google Scholar]
- 3.Sindwani R, Metson R. Impact of image guidance on complications during osteoplastic frontal sinus surgery. Otolaryngol Head Neck Surg. 2004;131:150–5. doi: 10.1016/j.otohns.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Chan Y, Siewerdsen JH, Rafferty MA, et al. Cone-beam computed tomography on a mobile C-arm: novel intraoperative imaging technology for guidance of head and neck surgery. J Otolaryngol Head Neck Surg. 2008;37:81–90. [PubMed] [Google Scholar]
- 5.Schafer S, Nithiananthan S, Mirota DJ, et al. Mobile C-arm cone-beam CT for guidance of spine surgery: Image quality, radiation dose, and integration with interventional guidance. Medical Physics. 2011;38:4563–74. doi: 10.1118/1.3597566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siewerdsen JH. Cone-beam CT with a flat-panel detector: From image science to image-guided surgery. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2011;648:S241–S50. doi: 10.1016/j.nima.2010.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uneri A, Schafer S, Mirota D, et al. TREK: an integrated system architecture for intraoperative cone-beam CT-guided surgery. International Journal of Computer Assisted Radiology and Surgery. 2011:1–15. doi: 10.1007/s11548-011-0636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das S, Maeso PA, Figueroa RE, et al. The use of portable intraoperative computed tomography scanning for real-time image guidance: a pilot cadaver study. Am J Rhinol. 2008;22:166–9. doi: 10.2500/ajr.2008.22.3152. [DOI] [PubMed] [Google Scholar]
- 9.Jackman AH, Palmer JN, Chiu AG, et al. Use of intraoperative CT scanning in endoscopic sinus surgery: a preliminary report. Am J Rhinol. 2008;22:170–4. doi: 10.2500/ajr.2008.22.3153. [DOI] [PubMed] [Google Scholar]
- 10.Batra PS, Kanowitz SJ, Citardi MJ. Clinical utility of intraoperative volume computed tomography scanner for endoscopic sinonasal and skull base procedures. Am J Rhinol. 2008;22:511–5. doi: 10.2500/ajr.2008.22.3216. [DOI] [PubMed] [Google Scholar]
- 11.Nithiananthan S, Brock KK, Daly MJ, et al. Demons deformable registration for CBCT-guided procedures in the head and neck: convergence and accuracy. Med Phys. 2009;36:4755–64. doi: 10.1118/1.3223631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daly MJ, Siewerdsen JH, Moseley DJ, et al. Intraoperative cone-beam CT for guidance of head and neck surgery: Assessment of dose and image quality using a C-arm prototype. Medical Physics. 2006;33:3767–80. doi: 10.1118/1.2349687. [DOI] [PubMed] [Google Scholar]
- 13.Mirota D, Wang H, Taylor R, et al. Toward Video-Based Navigation for Endoscopic Endonasal Skull Base Surgery. In: Yang GZ, Hawkes D, Rueckert D, Noble A, Taylor C, editors. Medical Image Computing and Computer-Assisted Intervention – MICCAI. Springer; Berlin/Heidelberg: 2009. pp. 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachar G, Barker E, Nithiananthan S, et al. Three-Dimensional Tomosynthesis and Cone-Beam Computed Tomography: An Experimental Study for Fast, Low-Dose Intraoperative Imaging Technology for Guidance of Sinus and Skull Base Surgery. Laryngoscope. 2009;119:434–41. doi: 10.1002/lary.20089. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick JM, West JB. The distribution of target registration error in rigid-body point-based registration. Medical Imaging, IEEE Transactions on. 2001;20:917–27. doi: 10.1109/42.952729. [DOI] [PubMed] [Google Scholar]
- 16.Fried MP, Parikh SR, Sadoughi B. Image-guidance for endoscopic sinus surgery. Laryngoscope. 2008;118:1287–92. doi: 10.1097/MLG.0b013e31816bce76. [DOI] [PubMed] [Google Scholar]
- 17.Kingdom TT, Orlandi RR. Image-guided surgery of the sinuses: current technology and applications. Otolaryngol Clin North Am. 2004;37:381–400. doi: 10.1016/S0030-6665(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 18.Smith TL, Stewart MG, Orlandi RR, et al. Indications for image-guided sinus surgery: the current evidence. Am J Rhinol. 2007;21:80–3. doi: 10.2500/ajr.2007.21.2962. [DOI] [PubMed] [Google Scholar]
- 19.Bartling SH, Leinung M, Graute J, et al. Increase of accuracy in intraoperative navigation through high-resolution flat-panel volume computed tomography: experimental comparison with multislice computed tomography-based navigation. Otol Neurotol. 2007;28:129–34. doi: 10.1097/01.mao.0000244364.16826.09. [DOI] [PubMed] [Google Scholar]
- 20.Daly MJ, Siewerdsen JH, Moseley DJ, et al. Intraoperative cone-beam CT for guidance of head and neck surgery: Assessment of dose and image quality using a C-arm prototype. Medical physics. 2006;33:3767–80. doi: 10.1118/1.2349687. [DOI] [PubMed] [Google Scholar]
- 21.Intra-Operative Use of Computer Aided Surgery. American Academy of Otolaryngology-Head and Neck Surgery. 2011 http://www.entnet.org/Practice/policyIntraOperativeSurgery.cfm.
- 22.Rafferty MA, Siewerdsen JH, Chan Y, et al. Investigation of C-arm cone-beam CT-guided surgery of the frontal recess. Laryngoscope. 2005;115:2138–43. doi: 10.1097/01.mlg.0000180759.52082.45. [DOI] [PubMed] [Google Scholar]
- 23.Chennupati SK, Woodworth BA, Palmer JN, et al. Intraoperative IGS/CT updates for complex endoscopic frontal sinus surgery. ORL J Otorhinolaryngol Relat Spec. 2008;70:268–70. doi: 10.1159/000133653. [DOI] [PubMed] [Google Scholar]