Abstract
Bisphenol A (BPA) is a xenoestrogen that was first synthesized in 1891. Its estrogenic properties were discovered in 1930, and shortly after that chemists identified its usefulness in the production of epoxy resins. Since the 1950s BPA has been used as a synthetic monomer in the manufacturing of polycarbonate plastic, polystyrene resins, and dental sealants. Roughly 6.5 billion pounds of BPA are produced each year and it is the major estrogenic compound that leaches into nearby water and food supplies (vom Saal et al., 2007). BPA has been detected in 95% of human urine samples, which indicates that environmental exposure is widespread (Calafat et al., 2005). Moreover, BPA affects reproductive tissues and the brain. Thus many studies have focused on the effects of BPA during embryonic development. The most recent FDA update (Administration January 2010) points to “some concern about the potential effects of Bisphenol A on the brain, behavior, and prostate gland in fetuses, infants, and young children.” In light of this concern, we present an updated review of BPA’s action on the brain and behavior. We begin with a discussion of BPA’s role as both an endocrine active compound and an agent that alters DNA methylation. Next, we review publications that have reported effects of BPA on brain and behavior. We end with our interpretation of these data and suggestions for future research directions.
Keywords: Bisphenol A, Epigenetics, Social behavior, DNA methylation, Endocrine disrupting compound
BPA mechanisms of action
BPA works as an endocrine active compound and as an agent that alters DNA methylation. It is important to note that we present these topics separately but emphasize here to the reader that they may not be mutually exclusive. The epigenetic mechanisms of BPA action are very poorly understood, and at this point it is highly speculative to assume that these mechanisms are independent of endocrine activity of BPA.
Steroid-related modes of BPA action
Endocrine active compounds (EAC) are environmental agents that are either organic or man-made. While they are not produced endogenously by animals, their chemical structure is similar enough to their native counterparts that they can bind to steroid hormone receptors and either inhibit or enhance the actions of the endogenous hormones. Organic EACs have always been with us; they are present in soy, legumes, alfalfa and other plants. Humans have been consuming these in small quantities for millions of years. Man-made EACs such as pesticides, insecticides, industrial chemicals and some plasticizers and surfactants have been in use for a much shorter time but add to the EACs we interact with in our environment. By acting as steroid receptor agonists and/or antagonists, EACs can interfere with normal hormonal regulation and the proper functioning of the endocrine and neuroendocrine systems (Anway and Skinner, 2006; Gore 2008).
Estrogen receptor agonist
BPA acts as an estrogen agonist although it has a relatively low binding affinity for the nuclear estrogen receptors (ERs) α and β (Fig. 1). In a cell culture model, BPA activated an estrogen-responsive luciferase reporter at levels that were only 50% of 17-β-estradiol (E2) activation (Gould et al. 1998; Kurosawa et al. 2002). Additionally, the binding affinity of BPA to the ER-α and β is approximately 10,000 to 100,000 fold lower than that of E2 as judged by three different binding assays across four laboratories (Barkhem et al. 1998; Andersen et al. 1999).
Fig. 1.
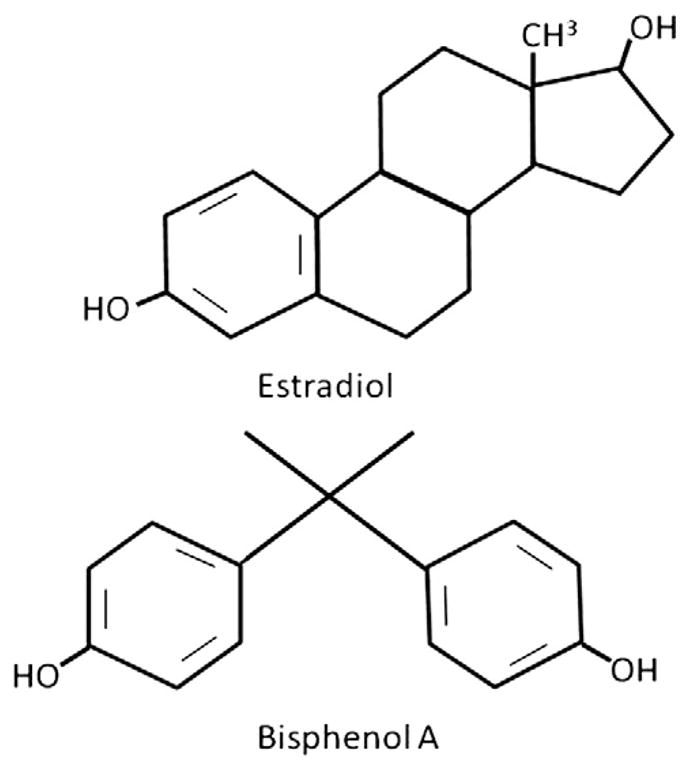
Structure of Estradiol and Bisphenol A. The chemical structures of Bisphenol A (A) and Estradiol (B) are presented. The structural similarity between the two molecules allows BPA to interact with the estrogen receptors (α and β) and other estrogen-like receptors.
Despite its low affinity, BPA is an equally strong agonist for ER-α and β (Kuiper et al. 1998). This “weak estrogenic” activity at the ERs cannot fully explain BPA’s actions on reproductive function, particularly at low doses. Specifically, exposure to doses of BPA relevant to normal human exposure during fetal development have been shown to advance puberty (Howdeshell et al. 1999), increase prostatic growth (Timms et al. 2005), alter pubertal mammary gland development (Munoz-de-Toro et al. 2005), permanently change the morphology and functionality of the female reproductive tract and ovaries in mice (Markey et al. 2005) and may also compromise sexual differentiation in the brain (Nakamura et al. 2006; Patisaul et al. 2006). It is not clear if these effects on reproductive function are mediated solely or even primarily by the classical ERs. BPA also binds membrane bound estrogen-like receptors, the membrane bound ER and GPR30, and can elicit cellular responses at picomolar to nanomolar concentrations which are below the doses required to activate nuclear ERs (Thomas and Dong, 2006; Watson et al. 2007). Recently, Takeda et al. have shown that BPA binds estrogen-related receptor gamma (ERR-γ) 80 times more potently than ER α (Takayanagi et al. 2006) and BPA potentiates ERRγ‘s high constitutive basal activity (Takayanagi et al. 2006; Liu et al. 2007; Okada et al. 2008). In addition, BPA may modulate ERs through its action on ERR-γ′. ERR-γ′ is highly expressed in the mammalian fetal brain and placenta and may account for BPA’s selective effects and accumulation in fetal tissues (Takeda et al. 2009).
Androgen receptor antagonist
The androgen receptor (AR) is the major regulatory element of androgen cell signaling and is essential for male reproductive function and development, including spermatogenesis. Several assays have shown that BPA acts as an androgen receptor antagonist (Sohoni and Sumpter, 1998; Xu et al. 2005; Bonefeld-Jorgensen et al. 2007). BPA inhibits dihydrotestosterone-induced transcriptional activity in a dose dependent fashion (Xu et al. 2005). Human AR reporter gene assays using a CAT reporter gene and anti-androgen activity measured by luciferase activity coupled to human AR in breast cancer cell lines also revealed anti-androgenic activity of BPA at the nanomolar range (IC50 = 1×10−6 M to 7×10−7 M) (Stroheker et al. 2004; Xu et al. 2005; Bonefeld-Jorgensen et al. 2007). Additionally, BPA treatment concurrent with testosterone led to a diffuse distribution of the AR between the nucleus and cytoplasm (Lee et al. 2003). Together, these results suggest that BPA not only acts as an AR antagonist, but also affects multiple steps in the activation and function of the AR, acting as a competitive inhibitor, altering nuclear localization, and subsequent trans-activation.
Other endocrine-related mechanisms of action
The aromatase enzyme is a key player in steroid synthesis as it catalyzes the irreversible conversion of androgens into estrogens (Simpson et al. 2002). Aromatase is found in a number of tissues including the brain, testicular Leydig cells and adipose tissue. BPA reduces aromatase enzyme activity by acting on two steroidogenic enzymes (Bonefeld-Jorgensen et al. 2007), decreasing testosterone and E2 synthesis (Akingbemi et al. 2004). Pre- and post-natal BPA exposure (from E12 to PND21) and limited exposure around the time of weaning (PND21–35) decreased testosterone levels almost two fold in male neonates and adult rats (Akingbemi et al. 2004).
The aryl hydrocarbon receptor (Ahr) is a transcription factor that mediates the effects of polyaromatic hydrocarbons. It forms a heterodimer with the Ahr-nuclear translocator (Arnt) to regulate cytochrome P450, xenobiotic metabolism and immune suppression. In utero, low dose BPA exposure increased Ahr mRNA expression in brain, testes and ovaries (Nishizawa et al. 2005b). BPA also up-regulated the mRNA level of the Ahr repressor (Ahrr) and Arnt in mid-and late-stage mouse embryos, altering the expression of Ahr and related factors and xenobiotic metabolizing enzymes (Nishizawa et al. 2005a). Thus, BPA may interfere with Ahr binding, translocation into the nucleus and activation of co-transcription factors (Jeong et al. 2000).
BPA also binds to the thyroid hormone receptor (TR) and inhibits TR-mediated transcription (Moriyama et al. 2002). BPA antagonizes thyroxin (T3) activation of the TR, possibly by displacing T3 from the TR, and can inhibit TR-mediated gene activation in culture by enhancing its interaction with nuclear receptor co-repressors.
In summary BPA has many endocrine and related targets; because of the interaction between these targets, the downstream effects of BPA are undoubtedly multiple and complex. For example, if BPA acts as an antagonist on the AR, decreased transcription of the aromatase enzyme would result, which, in turn, would reduce estradiol production. In addition, BPA may also directly decrease aromatase activity resulting in an even further reduction of cellular production of estradiol. Thus, although BPA has low affinity for the ERs, anti-estrogen actions of BPA may be enhanced by its negative effect on endogenous estradiol production.
Epigenetic modes of action and BPA
Gene expression can be heritably modified by genetic mutation, gene conversion, and by epigenetic mechanisms. Epigenetic mechanisms affect gene expression without altering the underlying DNA sequence and these heritable genomic changes are not obviously identified by traditional genetic approaches. There are several ways in which epigenetic mechanisms influence transcription. In general, these mechanisms affect the relationship between the core histones and the DNA that is wound around them. This modulation occurs directly through chemical modification to specific residues within the histone tails and cytosines within the DNA. To date, very little is known about how BPA may directly impact the activity of the histones and their tails. Zhu et al. (2009) showed that treatment with estrogenic compounds such as BPA increased the expression of histones H2A, H2B, H3 and H4 in an estrogen responsive breast derived cell line (MCF7). It is unclear whether this is a direct or indirect effect of BPA’s multiple actions on the cell and requires more detailed study. In contrast, BPA affects the genome by changing the methylation status of cytosines and increasing methyl donor availability reverses these changes (Dolinoy et al. 2007). The mechanism through which BPA modifies DNA methylation is still unclear.
DNA methylation occurs at the C5 position of cytosines in cytosine-guanine dinucleotides (CpG) which when clustered together are referred to as CpG islands. In mammalian cells, these CpG islands are frequently found near the promoter region or near the 5′ coding regions of genes, and their methylation status can regulate gene transcription (Hsieh 2000). In the classical sense, increased methylation in the CpG islands causes stable, heritable gene silencing whereas a decrease in methylation of the island results in increased gene transcription. Alterations in DNA methylation have been shown to contribute to many disease states, for example, cancer initiation and progression (Irimia et al. 2004; Sutherland et al. 2004).
Recently, a genome wide DNA methylation study was performed on both an early embryonic and fully differentiated cell line (Lister et al. 2009). This study brought to light a potential role for non-CpG methylation (mCHG and mCHH, where H=A, C or T) in transcription. Lister et al. showed that the methylation of cytosine in the context of a CHG or CHH exists in an early embryonic stem cell line, but not in a fully differentiated cell line. Of great interest is the fact that this type of DNA methylation (1) occurs frequently in the body of the gene, (2) occurs on the strand that is the template for transcription and (3) correlates with increased gene expression. Together, these findings suggest that non-CpG methylation in undifferentiated cells may be used as a way to upregulate gene expression during development. This is mechanistically in stark contrast to the above described CpG methylation, which mainly occurs in tracks at the 5′ end of genes and is associated with reduced gene activity. Much work is needed to better understand the role of non-CpG methylation in the developing cell as well as BPAs ability to alter these methylated regions. However, it is tantalizing to hypothesize that BPA may have its greatest effect in this context in utero while the organism is developing and setting up its diverse epigenome profile. During embryogenesis, sex differences in the brain are organized by hormonal secretions and sex chromosomes. Dysregulation of non-CpG methylation during these critical windows could disrupt the normal progression of brain and endocrine system development causing robust changes in the developing embryo that will persist into adulthood.
BPA as a DNA methylation agent
Research over the last several years has elucidated BPA’s ability to change DNA methylation patterns. One of the most convincing and elegant studies of BPA’s ability to alter DNA methylation was conducted by Dolinoy et al. (2006; 2007). The authors used mice with an epigenetically sensitive gene for coat color (Fig. 2). The agouti gene in the viable yellow agouti mouse (Avy) is modified by the insertion of an intracisternal A particle (IAP) retrotransposon in the 5′ region of the agouti gene. This retrotransposon contains a cryptic promoter that is sensitive to DNA methylation. Expression of the cryptic promoter leads to constitutive ectopic Agouti expression, which leads to yellow fur, obesity, diabetes, and tumorigenesis (Miltenberger et al. 1997; Morgan et al. 1999). In this experiment, dams ingested BPA and offspring coat color was used to assay BPA’s effect on DNA methylation. BPA treatment in utero shifted offspring coat color toward yellow, decreased DNA methylation at the IAP element and led to activation of the cryptic promoter. Different levels of methylation at specific CpG sites led to higher or lower expression of the Agouti gene and, therefore, variability in coat color (Fig. 2). Modulation of this phenotype and epigenotype to pseudoagouti and IAP element methylation was demonstrated with dietary supplementation including folic acid, a methyl donor, and genistein, a phytoestrogen (Dolinoy et al. 2007). Methyl donor availability controls the production of S-adenosylmethionine (SAM) which acts as the primary methyl donor for DNA methylation. Increased methionine availability in the brain increases SAM and DNA methylation (Tremolizzo et al. 2002; Guidotti et al. 2007). The mechanism through which genistein acts is still unclear. However, in both cases, both folic acid and genistein treatments increase DNA methylation at the IAP element to restore the agouti phenotype. Such increases in DNA methylation may help to correct for possible loss of DNA methylation induced by BPA. In this same study, a second gene was also tested for methylation changes to assess BPA’s impact at other loci. Site-specific DNA methylation on this gene, Cabp-IAP, was also reduced. Interestingly, it had been shown previously that the coat color of an Avy dam influences the distribution of coat color phenotype among its offspring and its grand offspring, though DNA methylation is not likely the direct epigenetic mark responsible for this transmission (Morgan et al. 1999; Blewitt et al. 2006). This suggests that BPA may alter other epigenetic mechanisms (histone tails, for example) in concert with DNA methylation and that these mechanisms may allow the inheritance of BPA altered chromatin.
Fig. 2.
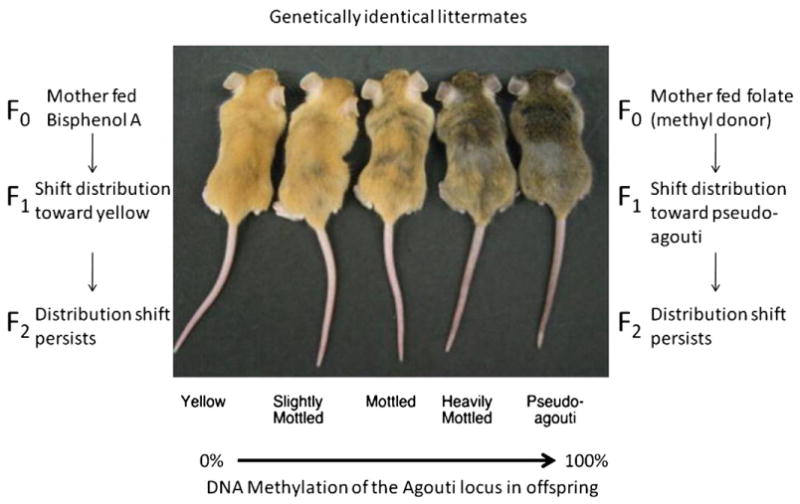
DNA methylation changes at the Agouti locus in the viable yellow agouti (Avy) mouse are phenotypically detectable and environmentally influenced. Littermates from the viable yellow agouti mouse strain are pictured. Coat color varies in these mice across a spectrum from yellow to agouti. The differences in coat color are due to a change in DNA methylation at the Agouti locus. The mice that harbor loci that exhibit a low level of Agouti methylation (for example, 0%) are yellow, while mice with a high level of DNA methylation (for example, 100%) appear agouti (brown). Treatment with BPA or folic acid can shift coat color distribution toward yellow or agouti, respectively. This coat color shift persists to the next generation indicating that the epigenetic change that occurs at the agouti locus is heritable (Morgan et al. 1999; Blewitt et al. 2006). Picture adapted from (Dolinoy et al. 2006).
Other evidence for epigenetic actions of BPA
We know from Dolinoy et al. that BPA affects the methylation of IAP elements and suspect that its actions are similar on other endogenous loci. However, relatively few studies have further characterized the DNA methylation-related effects of BPA. Ho et al. showed that developmental exposure to low doses of BPA predisposed rats to precancerous lesions and carcinogenesis of the prostate (Ho et al. 2006; Prins et al. 2008). These authors proposed that altered DNA methylation patterns in multiple signaling genes may be a potential mechanism for the early onset of prostate cancer. Specifically, they showed that the CpG island of phosphosdiesterase type 4 variant 4 (PDE4D4), an enzyme responsible for cyclic AMP metabolism, is normally gradually hypermethylated in the prostate with age (Ho et al. 2006). However, exposure to BPA caused hypomethylation of PDE4D4 and increased gene expression. Similar PDE4D4 hypomethylation and increased transcription were also observed in tumorigenic cell lines. This epigenetic dysregulation of PDE4D4 in the rat prostate may play a role in prostate epithelial cell transformation (Prins et al. 2008). Interestingly, the authors used methylation-sensitive restriction fingerprinting to show that low doses of BPA or high and low doses of E2 produce both common and unique methylation patterns in the rat prostate methylome. The fact that the methylation status of a common set of genes can be altered by different estrogenic compounds suggests that activation of the estrogen receptor pathway leads to downstream DNA methylation changes. This clearly illustrates that BPA’s role in DNA methylation is not distinct from the E2 pathway.
To date, only one report has investigated genome-wide methylation effects of BPA in the fetal brain. Using NotI restriction landmark genome scanning to assay methylation status, Yaoi et al. observed that low doses of BPA cause both hyper- and hypomethylation in the developing mouse brain (Yaoi et al. 2008). NotI sites, GC-GGCCGC (cut site at -), are widely distributed on various loci throughout the genome and can be methylated. Methylation prevents NotI from cutting at its restriction site, thus allowing for a genome-wide survey of NotI loci with altered methylation during development and following BPA exposure. Unfortunately, Yaoi et al. only further characterized the expression and methylation status of two altered loci, VPS52 and LOC72325, following maternal BPA exposure. Both genes exhibited BPA sensitivity as well as decreased methylation with age in the NotI site located within the CpG island. Increased transcription of these genes correlated well with changes in methylation status. The authors note that VPS52 codes for a protein involved in retrograde transport in the Golgi and LOC72325 has unknown function but does contain a domain that may catalyze nucleotide exchange of a molecule that regulates vesicle transport. The authors speculate that DNA methylation changes to these genes could allow for accelerated neuronal differentiation/migration. Together, these data suggest that the epigenetic actions of BPA alter gene expression, which may contribute to its effects on fetal brain development and ultimately adult behaviors.
BPA and the brain
General actions of BPA on the brain
Several studies have examined the effects of BPA either in vivo or in vitro on the developing and adult brain. Embryonic (E) exposure to BPA has been shown to disrupt normal neocortical development (Nakamura et al. 2006) and adult cortical organization (Nakamura et al. 2007). In one study, gestating pups were exposed to BPA via a single maternal subcutaneous injection at E0. BrdU was administered by intraperitoneal injection at several time points, E10.5, E12.5, E14.5 and E16.5 to evaluate cell proliferation, neuronal differentiation and migration. Brains were harvested 1 h, 2 days or 3 days after BrdU labeling. BrdU labeled brains dissected 1 h after BrdU injection were no different than controls, indicating precursor cell proliferation was not affected by BPA. BPA exposed fetal telencephalon processed from pups 2 days post-BrdU injection exhibited increased cell number in the ventricular zone and decreased cells in the cortical plate. At day 16.5 the cell number changes persisted in the ventricular zone but not the cortical plate (Nakamura et al. 2006). In a second similar study by the same group, offspring were examined at 3 weeks of age. BPA treatment did not affect total cell numbers in the cortical layers but did effect their position. BPA treated mice exposed to BrdU on E14.5 exhibited increased cell number in layers V and VI and loss of cells in layer IV when compared to controls (Nakamura et al. 2007). By 12 weeks of age the observed differences disappeared, but abnormal thalamocortical projections persisted. An in vitro study by Yokosuka et al. showed that BPA induces changes in both dendritic and synaptic development. Cultured fetal (E15) rat hypothalamic cells were treated with BPA ranging from 10 nM to 1 μM daily for 3 days. Immunocytochemistry was performed at day 10 on cells staining for microtubule associated protein-2 (MAP2) and synapsin I (SYN1) to assess both dendritic and synaptic development, respectively. The ratio of the two expression levels represents synaptic densities (Yokosuka et al. 2008). This measure of synaptic density was elevated above no treatment levels when cultures were exposed to 100nM BPA. The same effect was seen when cells were treated with 17-β estradiol. Dendritic lengths can be elaborated by E2 treatments, and BPA can have similar actions in cerebellar Purkinje cells. Rat pups (postnatal days 6–9) were injected with relatively high doses of BPA (500 μg/day) directly into the cerebrospinal fluid around the cerebellum and then sacrificed. The dendritic elaborations of the Purkinje cells were visualized with a calbindin antibody. The higher dose of BPA enhanced dendritic extensions without any effect on cell numbers. This was also noted in pups treated with estrogens and the effects, in both cases, were blocked by the anti-estrogen tamoxifen (Shikimi et al. 2004). In contrast, studies in rodent and monkey hippocampus using much lower doses of BPA (40–50 μg/kg/day) demonstrated an anti-estrogenic action on synaptogenesis (MacLusky et al. 2005; Leranth et al. 2008a; Leranth et al. 2008b). In adult female brains E2 enhanced synapse formation, and this effect was blocked by concurrent BPA treatment. Many of these studies used very high doses of BPA considering that physiological levels in the blood of the mouse and human are in nanogram per milliliter range; mouse blood 0.04 ng/ml (Wolstenholme, unpublished) and human blood 0.3 ng/ml to 18.9 ng/ml (Ikezuki et al. 2002; Schonfelder et al. 2002). Regardless, these studies suggest that BPA does not produce apoptosis, can affect cell migration, and can also affect synapse and dendrite formation. These data also highlight that BPA can have both estrogenic and anti-estrogen actions.
BPA and sex differences in brain
Several well-documented sex differences reside in the hypothalamus. These differences typically emerge during the end of gestation and shortly after birth and are caused by androgen’s direct or indirect actions in males. Indirect actions of androgens are in fact mediated by estrogen receptors after testosterone is aromatized to E2 in the hypothalamus. If BPA acts as an estrogen or an anti-estrogen in this region it is logical to hypothesize that some sexually dimorphic areas could be affected.
One well-characterized sexually dimorphic area is the anteroventral periventricular nucleus of the hypothalamus (AVPV). This area is sexually dimorphic both in the number of total neurons and also the numbers of tyrosine hydroxylase (TH) containing neurons, which, in this area of the brain, are producing dopamine. One function of the AVPV TH cells is to regulate gonadotropin releasing hormone (GnRH) neurons in the medial preoptic area (Terasawa et al. 1980). There is no suggestion that the cells have direct behavioral functions, but GnRH secretion is sexually dimorphic. In both dimensions, total cell number in the AVPV and total number of TH-cells, females have more cells than do males. In rats, injections of BPA for the first 2 days after birth increases AVPV cell numbers in males, making them nearly equal to the numbers of TH cells in the normal female. Estradiol injections have no effect on TH cell numbers in males. These data suggest an anti-estrogen action of BPA in this neural cell population (Patisaul et al. 2006). A similar study was conducted in pregnant mice implanted with subcutaneous Alzet pumps to deliver low tonic levels of BPA to embryos and suckling offspring. The offspring in the control group had the expected sex difference in TH cells in the AVPV (females>males). Both the 25 ng and 250 ng doses decreased cell numbers in females to the levels of control males (Rubin et al. 2006). These data suggest that in the mouse, unlike the rat, BPA may have an estrogenic action on this developing cell population.
An additional sexually dimorphic cell group, also in the hypothalamus, is the sexually dimorphic nucleus (SDN). This cluster of neurons is smaller in females than in males and can be visualized with either general neuronal staining or using antibodies to calbindin. Using the same paradigm as described for the AVPV rat study both the volume of the SDN and the numbers of calbindin labeled cells were examined (Patisaul et al. 2007). A sex difference between normal males and females was found (male>females) and females treated as neonates with BPA had more calbindin stained cells than control females. The result suggests that BPA has an estrogen-like action in the developing rat SDN.
Because some of BPA’s behavioral effects are related to anxiety, attention and processing of stimuli, the dopaminergic cell population in the midbrain is worth discussion. Two studies conducted in mice examined the effects of neonatal BPA on midbrain dopamine (quantified with TH antibodies) neurons. Mice were exposed in utero from day E8–17 and also after birth from P3–7. BPA (5 mg/kg/day) was administered orally. Males that received this treatment had fewer TH neurons in the A9 and A10 populations of dopamine cells at 2, 4 and 6 weeks of age, compared to controls (Tanida et al. 2009). In a second study mice were exposed to BPA in chow during pregnancy and lactation (Tando et al. 2007). TH cell density dropped in females only in the substantia nigra. Taken together the two studies show that the midbrain dopamine neurons are susceptible to perturbations caused by neonatal BPA exposure.
The data on neuronal sex differences and BPA are not consistent with a model in which BPA acts simply as a weak estrogen. In general these data suggest both estrogen-like as well as anti-estrogen actions of this compound. However not all neural sex differences are caused by steroid hormones - some have been attributed to sex chromosome complement such as number of vasopressin fibers in the lateral septum (Arnold and Chen, 2009). Prior to gonadal secretions or plasma differences in testosterone, the number of TH positive, dopaminergic neurons was sexually dimorphic (males>females) in cell cultures developed from E14 mesencephalon, and was affected by sex chromosome complement (XY>XX) (Carruth et al. 2002). Thus, TH neurons in the midbrain may well be differentiated by sex chromosome genes. It is possible that via this pathway BPA might affect neuronal development, perhaps by epigenetic actions.
BPA effects on behavior
Acting as an estrogen agonist BPA could have substantial effects on behaviors particularly if exposure occurred during the neonatal period. There are several adult behaviors that are sexually dimorphic and differentiated by neonatal exposure to estrogens. According to the theory of sexual differentiation exposure to estrogen agonists, including BPA, during the critical developmental period should have a larger effect on female than male behavior. Most of these data are summarized in Table 1.
Table 1.
Brain and behavioral effects of BPA on rodent models.
| Behavioral measurement | BPA effect | Species | Reference |
|---|---|---|---|
| Brain effects | |||
| AVPV, TH-immunoreactive cell number | increased in males with BPA injections in PND 1 & 2 | Sprague–Dawley rats | Patisaul et al., 2006 |
| AVPV, TH-immunoreactive cell number | Exposure during gestation and lactation, BPA decreases cell numbers in females | CD-1 mice | Rubin et al. 2006 |
| Midbrain, TH immunostaining | decreased in males after in utero exposure | ICR mice | Tanida et al. 2009 |
| SDN, volume and cell number | number of calbinbin cells increased in males after neonatal BPA treatment | Sprague–Dawley rats | Patisaul et al. 2007 |
| Substantia nigra, TH immunostaining | decreased density of neurons in females exposed during pregnancy and lactation | ddY strain mice | Tando et al. 2007 |
| Sexual behavior | |||
| Female Lordosis | no change | Long–Evans and Wistar rats | Adewale et al. 2009; Monje et al. 2009; Ryan et al. 2010 |
| Female behavior in paced mating test | Decreased exit latencies and increased lordosis frequency in females exposed prior to or after birth | Sprague–Dawley rats | Farabollini et al. 2002 |
| Proceptive hopping and darting | Decreased in females exposed to BPA | Wistar rats | Monje et al. 2009 |
| Male sexual behavior | post natal treatment increased intromissions, prenatal treatment increased latency to intromissions | Sprague–Dawley rats | Farabollini et al. 2002 |
| Social Behavior | |||
| Social preference | unaffected in adult males or females | Sprague–Dawley rats | Farabollini et al. 2002 |
| Social preference | decreased in juvenile males treated in utero | C57BL/6J mice | Cox et al., 2010 |
| Juvenile play in rats | BPA during gestation and 1 week after birth increased play directed toward females | Sprague–Dawley rats | Dessi-Fulgheri et al. 2002 |
| Juvenile play in rats | social play decreased in juvenile females exposed to BPA during gestation and lactation | Sprague–Dawley rats | Porrini et al. 2005 |
| Learning and memory | |||
| Morris Water Maze | life-long exposure to BPA reduced memory for escape platform in females | Fischer 344 rats | Carr et al. 2003 |
| Morris Water Maze | males exposed to BPA during gestation and until PN21 took longer to escape and had reduced memory for escape platform | ICR mice | Xu et al., 2010 |
| Passive avoidance task | impaired learning in males | ICR mice | Xu et al., 2010 |
| Active avoidance task | males exposed during gestation and lactation were slower to learn | F344/N rats | Negishi et al. 2004 |
| Barnes Maze, Radial Arm Maze, Passive Avoidance | no effect | C57/BL-6 mice, F344/N rats | Ryan and Vandenbergh 2006; Negishi et al 2004 |
| Anxiety and novelty | |||
| Elevated Plus Maze | loss of sexual dimorphism in time in center and number of rears | CD-1 mice | Rubin et al. 2006 |
| Elevated Plus Maze | 4 daily injections starting at birth lead to decreased number of entries into and time spent in open arms in males | Long–Evans rats | Patisaul and Bateman 2008 |
| Elevated Plus Maze | reduced the time spent in the distal end of the open arms | C57BL/6J mice | Cox et al., 2010 |
| Elevated Plus Maze | males and females from dams ingesting BPA from gestational day 11 until PN8 did not display sex differences but controls did | CD-1 mice | Gioiosa et al. 2007 |
| Elevated Plus Maze | females exposed from gestation to weaning had reduced the time spent in the open arms | C57/BL-6 mice | Ryan and Vandenbergh 2006 |
| Novelty preference | females whose dams were given daily BPA treatment from gestation until weaning had less interest in a novel arena | Sprague Dawley rats | Adriani et al. 2003 |
| Novelty preference | males and females quicker to enter novel arena | CD-1 mice | Gioiosa et al. 2007 |
| Impulsivity task | BPA given to dams throughout gestation and until weaning males displayed more nose poking | Sprague–Dawley rats | Adriani et al. 2003 |
| Exploratory activity | Dams treated during gestation and lactation offspring less exploration | Wistar rats | Poimenova et al. 2010 |
| Corticosterone levels | basal levels higher in females and in both sexes exposed to novel environment | Wistar rats | Poimenova et al. 2010 |
| Mother–infant interactions | |||
| Maternal behavior | BPA given to dams throughout pregnancy and lactation reduced duration of licking and grooming of pups | Sprague–Dawley rats | Della Seta et al. 2005 |
| Maternal behavior | Dams received BPA on gestational days 14–18 and showed a number of changes in maternal behavior | CD-1 mice | Palanza et al. 2002 |
| Maternal behavior | BPA exposed moms treat males offspring similar to female offspring | Cynomolgus monkey | Nakagami et al. 2009 |
Social behaviors
One behavior that is exquisitely sensitive to these early effects is the female’s ability to display sexual receptivity. When female pups are treated with E2 shortly before or after birth it decreases their ability to perform receptivity as adults. Several studies have examined the receptive behavior of adult female rats exposed during some point (s) in the neonatal period to BPA. The majority of the studies concluded that female lordosis, the receptivity posture, was unaffected by neonatal BPA treatment (Adewale et al. 2009; Monje et al. 2009; Ryan et al. 2010). One study using a paced mating paradigm instead of a standard pair test reported an enhancement in lordosis in females treated with BPA either prior to, or after birth (Farabollini et al. 2002). Proceptive hopping and darting, which accompany lordosis, were slightly lower in females treated with BPA for a week starting on the day of birth as compared with control females (Monje et al. 2009). Another behavior that should be affected by BPA is the capacity of females to display male sexual behavior. Mounting and thrusting should be enhanced in adult females exposed to BPA as neonates. As far as we can tell, this experiment has not been done. The current sexual behavior data do not support exclusively estrogenic action of BPA on neurobehavioral development.
A few other sexually dimorphic behaviors have been examined in animals exposed to BPA. Adult rats and mice prefer to interact with members of the opposite sex when given a choice. One study observed that social preference behavior in adult male and female rats treated either pre- or post-birth with BPA were unaffected (Farabollini et al. 2002). Recently we have assessed behaviors in juvenile mice that were gestated in the presence or absence of BPA and raised by a foster dam, to limit exposure to BPA after gestation (Cox et al. 2010). In a similar social preference task, juveniles were given either a confined adult male or an empty container. Control male juveniles spent more time investigating a confined adult male than did the control females or the males that were exposed to BPA during gestation. Play is another behavior that is sexually dimorphic (males>females) in juvenile rats. Female rats exposed to BPA both during gestation and prior to weaning engaged in more anogenital sniffing and social investigation during play with females than did control females. The opposite trend was noted in males (Dessi-Fulgheri et al. 2002). In a study with juvenile females, grooming and social play with males was reduced in female rats that were exposed to BPA pre- and post-birth (Porrini et al. 2005). Preliminary data from our laboratory suggests that juvenile female mice exposed to BPA, only during gestation, display less social investigation during play than controls (Wolsten-holme et al., unpublished). In rats there are data supporting both roles for androgen and estrogen receptors in organization of play behavior (Meaney 1988; Olesen et al. 2005). In mice the responsible agents are not known, and play is less dimorphic in this species. Because BPA can also have anti-androgen actions (Sohoni and Sumpter, 1998; Xu et al. 2005; Bonefeld-Jorgensen et al. 2007), if play is differentiated by actions of both ER and AR it may be difficult to assess the effects of BPA on this behavior. In fact the data to date suggest more of an effect of BPA on social interactions than “play” per se.
In summary, BPA does not appear to have classic estrogen-like effects on the “gold-standard” sexually differentiated lordosis behavior. Its actions on social preference and play depend on the species, dose and sex. Some of the examined behaviors may also be affected by second order actions of BPA on motivation and/or anxiety.
Learning and anxiety
Spatial learning tasks are sexually dimorphic in rodents (Fugger et al. 1998). Learning in a Morris water maze was examined in juvenile rats previously gavaged daily (PN0–14) with BPA or vehicle. There were small sex differences in the control animals in the task. A high dose of BPA had more effect on females than males, which was demonstrated by a reduced memory for the location of the escape platform on the probe trials (Carr et al. 2003). Male rats exposed to BPA from day 3 of gestation until weaning did not perform differently from controls in passive avoidance but were a bit slower to learn an active avoidance task (Negishi et al. 2004). Finally, BPA had no effect on spatial memory in the Barnes maze in mice exposed both pre- and post-birth to BPA (Ryan and Vandenbergh, 2006).
Recently a study with ICR male mice used four doses of BPA, three of which were within the range of normal human exposure (Xu et al. 2010). Pregnant dams were given BPA orally from gestational day 7 until postnatal day 21 (weaning). At 21 and 56 days of age males were tested in two learning tasks. At both ages males exposed to the two highest doses of BPA exhibited phenotypic changes from controls. In general, BPA treated males took longer to escape from the water maze. Specifically, in the probe trial, males exposed to the highest doses spent less time than controls (and males given the lowest BPA dose) in the quadrant that formerly contained the escape platform. In the passive avoidance step down task, the younger males treated with the three highest doses of BPA were impaired. These are very exciting findings since they demonstrate impairments in two types of memory tasks in animals raised on very low doses of BPA. In addition, the authors performed western blots to show that ERβ protein along with three NMDA, N-methyl-D-aspartic acid, receptor subtypes were down regulated in the hippocampus of BPA exposed males. While earlier work did not reveal any effects of BPA on learning, the recent paper by Xu et al. suggests the opposite. Xu et al. explain their results with the following hypothesis: BPA may have estrogenic activity that could induce down-regulation of ERβ expression in the hippocampus, which could in turn lead to a decrease in the expression of NMDA receptor subunits at the transcriptional level.
Some of the stronger effects of BPA are on anxiety-related behaviors. In several studies, sex differences are reportedly eliminated in BPA treated animals. For example, in the elevated plus maze (EPM), time spent in the center, time stopped, and rears were dimorphic (in different directions for different behaviors) in CD-1 mice and these sex differences were lost in the BPA treated animals (Rubin et al. 2006). In a study with adult male rats, BPA treatment during development reduced the number of entries into and time spent in the open arms of the EPM (Patisaul and Bateman, 2008). In juvenile C57BL/6J mice, we have noted that BPA treatment during gestation also reduced the time spent in the open arms of the EPM (Cox et al. 2010). Adriani et al. examined a set of “emotional” behaviors in rats that had received BPA during gestation through weaning (Adriani et al. 2003). When juveniles were tested for responses to a novel area, BPA treated mice of both sexes were more active; in addition, females treated with BPA showed less interest in a new area than did control females. As adults, males treated with BPA during development engaged in more nose poking in an impulsivity test and were less sensitive to amphetamines than control males. In sum, BPA may increase anxiety in a number of different tasks in both rats and mice.
In a study using a low dose of BPA, given to pregnant mouse dams in oil, novelty seeking behavior was tested in juveniles (Gioiosa et al. 2007). The novelty item was a new type of cage bedding introduced in a novel compartment. In general, males were a bit faster to enter and to rear in the novel environment if they had received BPA, while females displayed more self grooming if they had been exposed to BPA. In a recent study pregnant and lactating rats received a medium level dose of oral BPA and starting at 46 days of age the offspring were tested in a Y maze for spatial recognition (Poimenova et al., 2010). The BPA-treated offspring exhibited less exploratory behavior. The most interesting finding was that basal corticosterone levels in plasma were elevated in females exposed to BPA and after a mild stress (exposure to the Y maze) corticosterone was elevated in both sexes of BPA exposed rats. Taken together BPA has effects on these types of behaviors. The fact that none of these behaviors has been shown to be sexually differentiated by hormones developmentally suggests that BPA may affect these tasks via a non-estrogen action, perhaps via alterations in the adrenal–hypothalamic–pituitary axis.
Mother–infant interactions
Another avenue for BPA effects in development is through an action on infant-maternal interactions, which may have an epigenetic impact on the behavior of the offspring. In rats, dams that ingested BPA during gestation directed less care toward their pups than did control females (Della Seta et al. 2005). In a trans-generational study, mice were exposed to BPA either as infants or as pregnant adults, from gestational days 14 to 18 (Palanza et al. 2002). After the birth of their pups dams were tested for a number of maternal behaviors. In general, females that either received BPA when they were infants or during their gestation spent less time in the nest with their pups than controls. Dams that were exposed to BPA both during their own development and as adults during gestation behaved in a manner similar to controls. In a recent study in cynomolgus monkeys, pregnant females received continual infusions of BPA for the last 28 days of gestation (Nakagami et al. 2009). Infant and maternal behaviors were monitored. Male control infants spent more time clinging to their mothers and less time looking outward than BPA-treated males or females in either group. Mothers behaved in a similar fashion: those with control male infants looked outward less than mothers with female or BPA male infants. These data indicate that infant monkeys and their mothers behave in a sex specific manner and that BPA exposed mothers treat their male infants the same as females. While our lab has not directly examined maternal behavior, we have recently shown that the effects of BPA on juvenile mouse behaviors are more dramatic when the pups are reared by foster dams (Cox et al. 2010). Because maternal licking and grooming in rats has been shown to change DNA methylation status of glucocorticoid receptor and ERα, BPA induced maternal behavioral changes may influence BPA’s effects on offspring (Weaver et al. 2004; Champagne et al. 2006).
Conclusions and future directions
As we show here, BPA’s effects on the brain, genome and behavior are wide-ranging; including, but not limited to, changes in the structural development of the brain, disruption of estrogen regulation at a number of levels, alterations in DNA methylation of the genome, and effects on social behavior, anxiety, and maternal behavior. These diverse phenotypes are most likely linked by a complex regulatory network in the brain during and after development. BPA may impact this hypothetical network in several ways (Fig. 3). First, as a low potency estrogenic compound, it could weakly activate ERα, ERβ, ERR-γ and/or inhibit AR, altering the expression of genes responsive to these receptors. At the same time, it may also act as an agent that affects DNA methylation, changing gene expression patterns during development in primarily hormone-sensitive cells. Finally, it may be a combination of these two pathways (or others not mentioned) where BPA affects the transcription of an estrogen responsive gene and the methylation level of that same gene. In fact, recently, in utero BPA exposure has been shown to decrease methylation at two sites in the Hoxa10 homeobox gene (Bromer et al. 2010). This decreased methylation results in increased binding of ERα to the Hoxa10 estrogen response element (ERE) and increased ERE-mediated gene expression. The authors have proposed that methylation of the promoter and ERE region of Hoxa10 could be the mechanism through which BPA alters expression of estrogen sensitive genes. Intriguingly, we do not know whether BPA acts to change the methylation status of specific regions of DNA or if its actions are global, such as decreasing genomic methylation by reducing the activity of DNA methyltrans-ferases (DNMTs). In addition, the existence of a DNA demethylase still remains controversial (reviewed in (Wu and Zhang, 2010); however, there is an increasing body of evidence suggesting the existence of active DNA demethylation in neurons (Weaver et al. 2007; Nelson et al. 2008; Ma et al. 2009). If BPA was exerting its activity through disruption of these enzymes, then BPA would be acting in a manner unrelated to its estrogenic activity. This could affect multiple signaling pathways and may explain the discrepancies in the reported behavioral phenotypes. Direct investigation of BPA’s actions on the enzymes involved in DNA methylation is needed to clarify such a role.
Fig. 3.
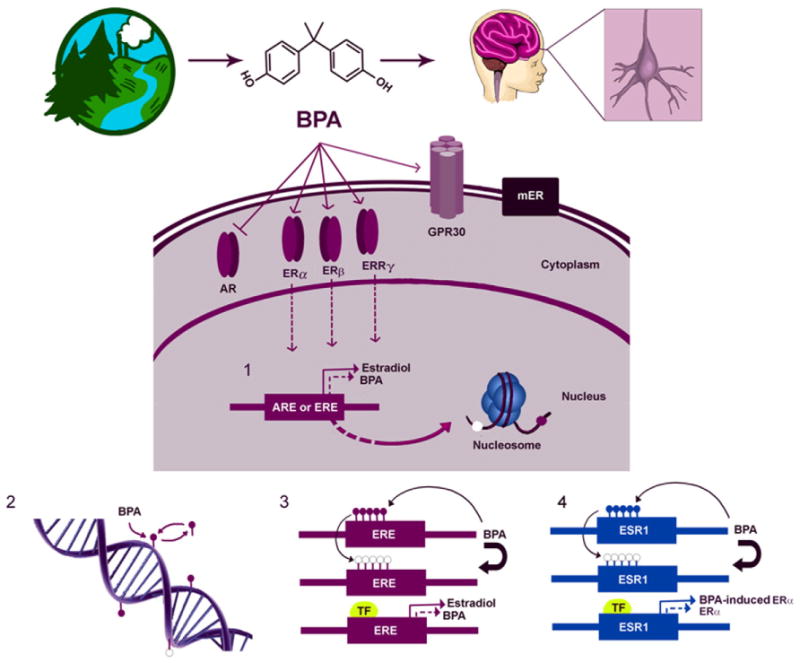
A model of BPA’s potential modes of action. Environmental exposure to BPA affects the developing brain and behavior. Acting as an endocrine activating compound, BPA can weakly activate several estrogen (ER) and estrogen-related receptors such as ERα, ERβ, ERR-γ, membrane ER (mER) and GPR30 and/or antagonize the androgen receptor (AR). (1) BPA is a low potency activator of these receptors and may change the expression of genes containing estrogen (ERE) or androgen (ARE) response elements in comparison to the endogenous ligand. This gene activation may in turn lead to downstream epigenetic changes like DNA methylation or histone modifications which could further impact transcription. (2) At the chromatin level, BPA may act to change DNA methylation at specific loci, although the specificity and exact mechanism has not been determined (Gilbert and Liu, 2010). (3) A combined action of BPA is also possible where BPA changes the methylation state (● → ○) of specific regions of an estrogen responsive gene or (4) the genes that code for the receptors themselves, ESR1 for example, and alters its expression by changing chromatin structure and allowing the binding of transcription factors (TF). Note: There are multiple ways that each of these modes of action may act separately or in conjunction.
Another aspect further complicating a clear understanding of BPA’s effects and mechanism of action is the high degree of variability in the dosing regimen in published reports. As reviewed above, BPA’s behavioral effects are specific to both timing and dose of exposure. Critical windows of vulnerability to environmental agents appear during embryogenesis during the organizing phases of development. Tissue specific responses may be due to the availability of co-activators and the presence of downstream signaling cascades in the examined tissue. It has been hypothesized that BPA’s actions are most potent in hormone-sensitive organs and in brain regions when endogenous steroids are particularly low. In order to more fully understand the behavioral effects of BPA and potential risks to humans, BPA’s mechanisms of action must be better characterized. Thus, the downstream molecular targets need to be identified following BPA exposure in order to understand both estrogenic and the potential non-estrogenic actions of BPA.
A final area of BPA action that needs to be characterized is whether its impact on developing offspring is epigenetically heritable. The best characterized example of an EAC with germline heritable actions on behavior is elegant work done on rats treated with vinclozolin, an anti-fungal agent known for its anti-androgen actions. When gestating females are exposed to vinclozolin their male offspring have a rapid decline in fertility shortly after reaching puberty (Anway et al. 2005; Skinner et al. 2008). Males sired by these exposed males have the same condition. This work has been extended out many generations beyond the lifetime of EAC loading effects. In addition there are behavioral ramifications on subsequent generations of males and females (Crews et al. 2007). This effect of vinclozolin is produced by germline transmission of an epigenetic alteration (see D. Crews review in this issue). This is one of the earliest and still exciting demonstrations of EACs actions on epigenetic transgenerational inheritance. We know BPA has heritable affects on sperm function, through at least the third generation (Salian et al. 2009). However, transgenerational actions on brain and behavior have yet to be reported. Given the ubiquitous presence of BPA in our environment the potential transgenerational impacts of this EAC are large and important.
Acknowledgments
We would like to thank Jessica Johnson for her artistic help in creating Fig. 3, Julia Taylor at the University of Missouri for providing us with unpublished serum BPA levels from the mouse, Sarah Nelson and Jim Cronk for critical reading of the manuscript and the NIH for support, R01 MH086711 (ER).
References
- Adewale HB, Jefferson WN, et al. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod. 2009;81 (4):690–699. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Administration, U. S. F. a. D. Update on Bisphenol A for Use in Food Contact Applications. Jan, 2010. [Google Scholar]
- Adriani W, Seta DD, et al. Altered profiles of spontaneous novelty seeking, impulsive behavior, and response to D-amphetamine in rats perinatally exposed to bisphenol A. Environ Health Perspect. 2003;111 (4):395–401. doi: 10.1289/ehp.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akingbemi BT, Sottas CM, et al. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145 (2):592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- Andersen HR, Andersson AM, et al. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect. 1999;107 (Suppl 1):89–108. doi: 10.1289/ehp.99107s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147 (6 Suppl):S43–S49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, et al. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308 (5727):1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30 (1):1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, et al. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54 (1):105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- Blewitt ME, Vickaryous NK, et al. Dynamic reprogramming of DNA methylation at an epigenetically sensitive allele in mice. PLoS Genet. 2006;2 (4):e49. doi: 10.1371/journal.pgen.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC, Long M, et al. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect. 2007;115 (Suppl 1):69–76. doi: 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer JG, Zhou Y, et al. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010 doi: 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, et al. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113 (4):391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr R, Bertasi F, et al. Effect of neonatal rat bisphenol a exposure on performance in the Morris water maze. J Toxicol Environ Health A. 2003;66 (21):2077–2088. doi: 10.1080/713853983. [DOI] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, et al. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002;5 (10):933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, et al. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147 (6):2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Cox KH, Gatewood JD, et al. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC, et al. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA. 2007;104 (14):5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Seta D, Minder I, et al. Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain Res Bull. 2005;65 (3):255–260. doi: 10.1016/j.brainresbull.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Dessi-Fulgheri F, Porrini S, et al. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect. 2002;110 (Suppl 3):403–407. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Weidman JR, et al. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114 (4):567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, et al. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104 (32):13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabollini F, Porrini S, et al. Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ Health Perspect. 2002;110 (Suppl 3):409–414. doi: 10.1289/ehp.02110s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugger HN, Cunningham SG, et al. Sex differences in the activational effect of ERalpha on spatial learning. Horm Behav. 1998;34 (2):163–170. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- Gilbert ER, Liu D. Flavonoids influence epigenetic-modifying enzyme activity: structure–function relationships and the therapeutic potential for cancer. Curr Med. 2010 doi: 10.2174/092986710791111161. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Fissore E, et al. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav. 2007;52 (3):307–316. doi: 10.1016/j.yhbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Gore AC. Neuroendocrine systems as targets for environmental endocrine-disrupting chemicals. Fertil Steril. 2008;89 (2 Suppl):e101–e102. doi: 10.1016/j.fertnstert.2007.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould JC, Leonard LS, et al. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142 (1–2):203–214. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Ruzicka W, et al. S-adenosyl methionine and DNA methyltransferase-1 mRNA overexpression in psychosis. NeuroReport. 2007;18 (1):57–60. doi: 10.1097/WNR.0b013e32800fefd7. [DOI] [PubMed] [Google Scholar]
- Ho SM, Tang WY, et al. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66 (11):5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, et al. Exposure to bisphenol A advances puberty. Nature. 1999;401 (6755):763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- Hsieh CL. Dynamics of DNA methylation pattern. Curr Opin Genet Dev. 2000;10 (2):224–228. doi: 10.1016/s0959-437x(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, et al. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17 (11):2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Irimia M, Fraga MF, et al. CpG island promoter hypermethylation of the Ras-effector gene NORE1A occurs in the context of a wild-type K-ras in lung cancer. Oncogene. 2004;23 (53):8695–8699. doi: 10.1038/sj.onc.1207914. [DOI] [PubMed] [Google Scholar]
- Jeong HG, Kimand JY, et al. Down-regulation of murine Cyp1a-1 in mouse hepatoma Hepa-1c1c7 cells by bisphenol A. Biochem Biophys Res Commun. 2000;277 (3):594–598. doi: 10.1006/bbrc.2000.3717. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139 (10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kurosawa T, Hiroi H, et al. The activity of bisphenol A depends on both the estrogen receptor subtype and the cell type. Endocr J. 2002;49 (4):465–471. doi: 10.1507/endocrj.49.465. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Chattopadhyay S, et al. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75 (1):40–46. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, et al. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci USA. 2008a;105 (37):14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Szigeti-Buck K, et al. Bisphenol A prevents the synaptogenic response to testosterone in the brain of adult male rats. Endocrinology. 2008b;149 (3):988–994. doi: 10.1210/en.2007-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462 (7271):315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Matsushima A, et al. Receptor binding characteristics of the endocrine disruptor bisphenol A for the human nuclear estrogen-related receptor gamma. Chief and corroborative hydrogen bonds of the bisphenol A phenol-hydroxyl group with Arg316 and Glu275 residues. FEBS J. 2007;274 (24):6340–6351. doi: 10.1111/j.1742-4658.2007.06152.x. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323 (5917):1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, et al. The environmental estrogen bisphenol a inhibits estradiol-induced hippocampal synaptogenesis. Environ Health Perspect. 2005;113 (6):675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey CM, Wadia PR, et al. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72 (6):1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. The sexual differentiation of social play. Trends Neurosci. 1988;11 (2):54–58. doi: 10.1016/0166-2236(88)90164-6. [DOI] [PubMed] [Google Scholar]
- Miltenberger RJ, Mynatt RL, et al. The role of the agouti gene in the yellow obese syndrome. J Nutr. 1997;127 (9):1902S–1907S. doi: 10.1093/jn/127.9.1902S. [DOI] [PubMed] [Google Scholar]
- Monje L, Varayoud J, et al. Neonatal exposure to bisphenol A alters estrogen-dependent mechanisms governing sexual behavior in the adult female rat. Reprod Toxicol. 2009;28 (4):435–442. doi: 10.1016/j.reprotox.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Morgan HD, Sutherland HG, et al. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23 (3):314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87 (11):5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- Munoz-de-Toro M, Markey CM, et al. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146 (9):4138–4147. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami A, Negishi T, et al. Alterations in male infant behaviors towards its mother by prenatal exposure to bisphenol A in cynomolgus monkeys (Macaca fascicularis) during early suckling period. Psychoneuroendocrinology. 2009;34 (8):1189–1197. doi: 10.1016/j.psyneuen.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Itoh K, et al. Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of Bisphenol A. J Neurosci Res. 2006;84 (6):1197–1205. doi: 10.1002/jnr.21020. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Itoh K, et al. Prenatal exposure to bisphenol A affects adult murine neocortical structure. Neurosci Lett. 2007;420 (2):100–105. doi: 10.1016/j.neulet.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Negishi T, Kawasaki K, et al. Behavioral alterations in response to fear-provoking stimuli and tranylcypromine induced by perinatal exposure to bisphenol A and nonylphenol in male rats. Environ Health Perspect. 2004;112 (11):1159–1164. doi: 10.1289/ehp.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ED, Kavalali ET, et al. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28 (2):395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa H, Imanishi S, et al. Effects of exposure in utero to bisphenol a on the expression of aryl hydrocarbon receptor, related factors, and xenobiotic metabolizing enzymes in murine embryos. J Reprod Dev. 2005a;51 (5):593–605. doi: 10.1262/jrd.17026. [DOI] [PubMed] [Google Scholar]
- Nishizawa H, Morita M, et al. Effects of in utero exposure to bisphenol A on mRNA expression of arylhydrocarbon and retinoid receptors in murine embryos. J Reprod Dev. 2005b;51 (3):315–324. doi: 10.1262/jrd.16008. [DOI] [PubMed] [Google Scholar]
- Okada H, Tokunaga T, et al. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ Health Perspect. 2008;116 (1):32–38. doi: 10.1289/ehp.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen KM, Jessen HM, et al. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146 (9):3705–3712. doi: 10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- Palanza PL, Howdeshell KL, et al. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110 (Suppl 3):415–422. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm Behav. 2008;53 (4):580–588. doi: 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, et al. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28 (1):111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, et al. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28(1):1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Poimenova A, Markaki E, et al. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- Porrini S, Belloni V, et al. Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res Bull. 2005;65 (3):261–266. doi: 10.1016/j.brainresbull.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Prins GS, Tang WY, et al. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin Pharmacol Toxicol. 2008;102 (2):134–138. doi: 10.1111/j.1742-7843.2007.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, et al. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147(8):3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav. 2006;50 (1):85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Hotchkiss AK, et al. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol Sci. 2010;114 (1):133–148. doi: 10.1093/toxsci/kfp266. [DOI] [PubMed] [Google Scholar]
- Salian S, Doshi T, et al. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009;85 (21–22):742–752. doi: 10.1016/j.lfs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, et al. Parent bisphenol A accumulation in the human maternal–fetal–placental unit. Environ Health Perspect. 2002;110 (11):A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikimi H, Sakamoto H, et al. Dendritic growth in response to environmental estrogens in the developing Purkinje cell in rats. Neurosci Lett. 2004;364 (2):114–118. doi: 10.1016/j.neulet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, et al. Aromatase—a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, et al. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS ONE. 2008;3 (11):e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158 (3):327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Stroheker T, Picard K, et al. Steroid activities comparison of natural and food wrap compounds in human breast cancer cell lines. Food Chem Toxicol. 2004;42 (6):887–897. doi: 10.1016/j.fct.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Sutherland KD, Lindeman GJ, et al. Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene. 2004;23 (46):7726–7733. doi: 10.1038/sj.onc.1207787. [DOI] [PubMed] [Google Scholar]
- Takayanagi S, Tokunaga T, et al. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRgamma) with high constitutive activity. Toxicol Lett. 2006;167 (2):95–105. doi: 10.1016/j.toxlet.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Liu X, et al. Placenta expressing the greatest quantity of bisphenol A receptor ERR{gamma} among the human reproductive tissues: predominant expression of type-1 ERRgamma isoform. J Biochem. 2009;146 (1):113–122. doi: 10.1093/jb/mvp049. [DOI] [PubMed] [Google Scholar]
- Tando S, Itoh K, et al. Effects of pre- and neonatal exposure to bisphenol A on murine brain development. Brain Dev. 2007;29 (6):352–356. doi: 10.1016/j.braindev.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Tanida T, Warita K, et al. Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: mixed exposure to phenol, phthalate, and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei. Toxicol Lett. 2009;189 (1):40–47. doi: 10.1016/j.toxlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Wiegand SJ, et al. A role for medial preoptic nucleus on afternoon of proestrus in female rats. Am J Physiol. 1980;238 (6):E533–E539. doi: 10.1152/ajpendo.1980.238.6.E533. [DOI] [PubMed] [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102 (1–5):175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Timms BG, Howdeshell KL, et al. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci USA. 2005;102 (19):7014–7019. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolizzo L, Carboni G, et al. An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc Natl Acad Sci USA. 2002;99 (26):17095–17100. doi: 10.1073/pnas.262658999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24 (2):131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, et al. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids. 2007;72 (2):124–134. doi: 10.1016/j.steroids.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7 (8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, D’Alessio AC, et al. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27 (7):1756–1768. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SC, Zhang Y. Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol. 2010 doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LC, Sun H, et al. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology. 2005;216 (2–3):197–203. doi: 10.1016/j.tox.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Xu XH, Zhang J, et al. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-d-aspartate receptors of hippocampus in male offspring mice. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Yaoi T, Itoh K, et al. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Commun. 2008;376 (3):563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Yokosuka M, Ohtani-Kaneko R, et al. Estrogen and environmental estrogenic chemicals exert developmental effects on rat hypothalamic neurons and glias. Toxicol In Vitro. 2008;22 (1):1–9. doi: 10.1016/j.tiv.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Edwards RJ, et al. Increased expression of histone proteins during estrogen-mediated cell proliferation. Environ Health Perspect. 2009;117 (6):928–934. doi: 10.1289/ehp.0800109. [DOI] [PMC free article] [PubMed] [Google Scholar]


