Abstract
Keratinizing squamous metaplasia (SQM) of the ocular mucosal epithelium is a blinding corneal disease characterized by the loss of conjunctival goblet cells (GCs), pathological ocular surface keratinization and tissue recruitment of immune cells. Using the autoimmune regulator (Aire)-deficient mouse as a model for Sjögren’s syndrome (SS)-associated SQM, we identified CD4+ T lymphocytes as the main immune effectors driving SQM and uncovered a pathogenic role for interleukin-1 (IL-1). IL-1, a pleiotropic cytokine family enriched in ocular epithelia, governs tissue homeostasis and mucosal immunity. Here, we used adoptive transfer of autoreactive CD4+ T cells to dissect the mechanism whereby IL-1 promotes SQM. CD4+ T cells adoptively transferred from both Aire knockout (KO) and Aire/IL-1 receptor type 1 (IL-1R1) double KO donors conferred SQM to severe-combined immunodeficiency (scid) recipients with functional IL-1R1, but not scid recipients lacking IL-1R1. In the lacrimal gland, IL-1R1 was primarily immunolocalized to ductal epithelium surrounded by CD4+ T cells. In the eye, IL-1R1 was expressed on local mucosal epithelial and stromal cells, but not on resident antigen-presenting cells or infiltrating immune cells. In both tissues, autoreactive CD4+ T-cell infiltration was only observed in the presence of IL-1R1-postive resident cells. Moreover, persistent activation of IL-1R1 signaling led to chronic immune-mediated inflammation by retaining CD4+ T cells in the local microenvironment. Following IL-1R1-dependent infiltration of CD4+ T cells, we observed SQM hallmarks in local tissues—corneal keratinization, conjunctival GC mucin acidification and epithelial cell hyperplasia throughout the ocular surface mucosa. Proinflammatory IL-1 expression in ocular epithelial cells significantly correlated with reduced tear secretion, while CD4+ T-cell infiltration of the lacrimal gland predicted the development of ocular SQM. Collectively, data in this study indicated a central role for IL-1 in orchestrating a functional interplay between immune cells and resident cells of SS-targeted tissues in the pathogenesis of SQM.
Keywords: autoimmunity, CD4+ T cell, IL-1R1, lacrimal exocrinopathy, ocular surface mucosa, squamous metaplasia, Sjögren’s syndrome
Squamous metaplasia (SQM) of the ocular surface mucosa is a blinding disease resulting from prolonged and uncontrolled ocular inflammation. Histologically, ocular SQM is characterized by the loss of mucosal goblet cells (GCs) in the conjunctiva (early disease stage), hyperproliferation of basal and suprabasal epithelial cells in the stratified epithelium (intermediate stage) and aberrant keratinization of squamoid cells at the apical surface (terminal differentiation stage).1–3 In advanced disease, stromal fibrosis and neovascularization coupled with epithelial keratinization lead to vision-threatening corneal opacification. Although the pathogenesis of SQM is largely unknown, aqueous deficient dry eye disease is the most common etiology.4
Affecting as many as 4 million Americans, aqueous deficient dry eye disease, also known as keratoconjunctivitis sicca (KCS), is clinically categorized into two subtypes, with and without associated systemic autoimmunity. The prime example of autoimmune-mediated KCS occurs in Sjögren’s syndrome (SS-KCS), in which chronic inflammation of the lacrimal gland and ocular tissues is associated with infiltration of immune cells and dysregulated production of inflammatory cytokines.5 Phenotypic characterization of cellular infiltrates has demonstrated a heterogeneous immune cell population in the lacrimal gland, encompassing CD4+ T cells, CD8+ T cells and B cells, with CD4+ T cells serving as the main effectors.6,7 CD4+ T cells are further functionally compartmentalized into Th1, Th2, Th17 and T regulator (Treg) subsets. Although Th1 effectors have been recognized as the principal pathogenic subset in SS,8 emerging evidence suggests that Th17 and Treg cells maintain an intricate and antagonistic balance with Th1 cells to regulate auto-immunity.9–11 While impaired Treg function and pathogenic Th17 cell have been implicated in dry eye pathogenesis,12 only IFNγ-producing Th1 cells are reported to be associated with SQM.13
To better understand the pathogenesis of autoimmune-mediated SQM, we established the autoimmune regulator (Aire)-deficient mouse as a model of SQM that mimics the clinical characteristic of SS exocrinopathy and KCS.14,15 Similar to the human disease, immune cell profiling in Aire knockout (KO) mice identified CD4+ T cells as the principal immune effectors, with CD8+ T cells and B cells acting as accessory effectors. When exploring the cytokine network underpinning the immune cellular events, we found that ablation of interleukin-1 (IL-1) signaling via genetic deletion of the IL-1 receptor type 1 (IL-1R1) largely preserved epithelial integrity and mucosal phenotype in the ocular surface of Aire KO mice, indicating a link between IL-1- and CD4+ T-cell-dominated autoimmunity in the pathogenesis of SQM.15
IL-1 is a family of prototypic proinflammatory cytokines that mediates many of the local and systemic features of inflammation. The IL-1 family is comprised of two major proinflammatory ligands, IL-1α and IL-β, and an endogenous anti-inflammatory ligand IL-1R antagonist (IL-1Ra). Although in general IL-1 is primarily produced by circulating monocytes and tissue-infiltrating macrophages, it is known that the corneal epithelium and epidermis are unique in that they store abundant amounts of IL-1 for tissue homeostasis and immune defense. As such, they serve as the main producers of IL-1 locally when undergoing a stress response, stimulating inflammation in an autocrine and/or a paracrine manner by interacting with IL-1R1 expressed on their cell surface or on adjacent cells. In addition to its pro-inflammatory role in innate defense, IL-1 participates in a wide variety of non-inflammatory biological events, including growth and differentiation.16 When physiologically autoregulated, IL-1 promotes epithelial cell growth and migration during wound healing, whereas inflammation-dysregulated IL-1 contributes to several kinds of keratopathy resulting from pathological tissue remodeling, including keratoconus and pseudophakic bullous keratopathy.17–19
Previously, we and others have reported increased expression of IL-1β in impression cytology samples from human patients with SS-KCS.15,17 Both severity of SS-KCS and IL-1β were highly correlated to increased expression of SQM biomarker, small proline-rich protein 1B (SPRR1B) in the ocular surface epithelium.20 While IL-1 can directly upregulate SPRR1B expression in IL-1R1-expressing corneal epithelial cells via an autocrine mechanism, a potential paracrine mechanism also exists whereby IL-1 regulates SQM is through its interaction with IL-1R1-expressing autoreactive CD4+ T cells. A growing body of evidence suggests that IL-1 plays a critical role in modulating CD4+ T-cell functional maturation in autoimmune diseases through its interaction with IL-1R1 on T cells.21–24
In this study, we aimed to determine IL-1’s target cells in autoimmune-mediated ocular SQM. Using an adoptive transfer (AT) model selectively expressing IL-1R1 either in autoreactive CD4+ T cells or in resident cells of recipient mice, we provide evidence that autoreactive CD4+ T cells initiate local inflammation by activating IL-1R1 signaling in tissue resident cells. Reciprocally, IL-1R1-activated resident cells sustain local inflammation through the retention of infiltrating CD4+ T cells, and modulate a shift in ocular mucosal phenotype through the prolonged activation of IL-1/IL-1R1 signaling. These data suggest a functional role for IL-1 in regulating the interplay between epithelial and immune cells in the pathogenesis of SQM by facilitating effector T cells infiltration and translating chronic inflammatory stress to phenotypic changes of the ocular surface mucosa.
MATERIALS AND METHODS
All materials were purchased from Sigma (St Louis, MO, USA), except defined keratinocyte serum free medium (Gibco-BRL, Grand Island, NY, USA), Dispase II (Roche, Indianapolis, IN, USA), periodic acid Schiff (PAS) staining kit (American Master Tech Scientific, Lodi, CA, USA), Alcian blue (AB) (Fisher Scientific, Middletown, VA, USA), DAB substrate (Vector Laboratories, Burlingame, CA, USA), hematoxylin (Richard-Allan Scientific, Kalamazoo, MI, USA) and 4′,6′-diamino-2-phenylindole (Molecular Probes, Eugene, OR, USA). Lissamine green (1%) was obtained from Leiter’s Pharmacy and Compounding Center (San Jose, CA, USA). Antibodies used were as follows: anti-CD4 mouse monoclonal and anti-CD11c Armenian Hamster monoclonal (BD Pharmingen, San Diego, CA, USA), anti-IL-1R1 goat polyclonal (R&D, Minneapolis, MN, USA), anti-pan-cytokeratin (pan-CK) mouse monoclonal (Thermo Scientific, Rockford, IL, USA), anti-vimentin mouse monoclonal and anti-bromodeoxyuridine (BrdU) rat polyclonal (Abcam, Cambridge, MA, USA), anti-F4/80 rat monoclonal (AbD Serotec, Raleigh, NC, USA) and horseradish peroxidase-conjugated goat-anti-mouse secondary (Jackson Immuno-Research Laboratories, West Grove, PA, USA).
Animal Model
Mice were handled according to UCSF animal welfare guidelines for animal care. Aire-deficient mice were generated by targeted disruption of the murine Aire gene (OMIM 240300) as described previously.15 Aire-deficient mice were backcrossed onto the non-obese diabetic (NOD) Lt/J background for more than 10 generations and then crossed with NOD mice deficient in functional IL-1R1 (point mutation in IL-1R1 loci, OMIM 147810) purchased from Jackson Laboratory (Bar Harbor, ME, USA) to create NOD.Aire−/− IL-1R1−/− mice. In addition, severe-combined immune deficiency (scid) mice (NOD.CB17-Prkdcscid/J strain; Jackson Laboratory, Sacramento, CA, USA) were crossed to NOD.IL-1R1−/− to generate IL-1R1−/− scid mice on the NOD background. Genomic DNA isolated from tail clippings was genotyped for the Aire, IL-1R1 and scid mutations by PCR with manufacturer-recommended specific primers and their optimized PCR protocols.
AT Procedure
Lymphocytes from four cervical lymph nodes and spleens of Aire wild-type (WT), Aire KO mice and Aire KO mice lacking functional IL-1R1 (all on the NOD background) were used for AT studies. The CD4+ T-cell population was enriched by magnetic bead sorting and the purity was confirmed by flow cytometry, as described previously.25 CD4+ T-cell-enriched lymphocytes in 100 μl PBS (5 × 106 cells per mouse) were injected to the tail vein of NOD.scid recipients either sufficient (IL-1R1+/+ scid) or deficient (IL-1R1−/− scid) in IL-1R1.
Tear Secretion Measurement
Mice were anesthetized with isoflurane. Pilocarpine diluted in saline was injected intraperitoneally at a dosage of 4.5 mg/kg. At 10 min following pilocarpine injection, mice were anesthetized with isoflurane and tear volume (as indicated by the length of tear-absorbed region in 15 s) was measured using Zone-Quick phenol red threads (Showa Yakuhin Kako, Tokyo, Japan).
Assessment of Corneal Hyperplasia and Central Corneal Thickness
A single dose of BrdU (Sigma-Aldrich, St Louis, MO, USA) was intraperitoneally injected into 7-week-old Aire WT and Aire KO mice (n = 5 mice per group) 90 min before euthanasia. To assess proliferative activity, BrdU-labeled cells in the S phase of the cell cycle were visualized by immuno-fluorescence. Thickness of the central corneal epithelium at the corneal apex, defined as the distance (in μm) from the epithelial surface to the basement membrane, was measured in AT recipients (n = 5–7 mice per group) by phase-contrast microscopy (× 200).
Transcriptional Profiling of IL-1 Cytokine Family and SQM Phenotypic Marker SPRR1B in Corneolimbal Epithelium Using TaqMan PCR
Corneolimbal sheets were isolated from enucleated eyes, with a modification of our previously described protocol.26 In brief, the whole corneolimbal sheet was surgically peeled off with jeweler’s forceps from the underlying stroma of enucleated eyes, after overnight enzymatic digestion of the epithelial basement membrane by Dispase II (10 mg/ml) in serum-free keratinocyte medium (Gibco) at 4°C. Total RNA was extracted from those cell sheets using an RNeasy Mini RNA isolation kit (Qiagen, Hilden, Germany). Total RNA was eluted from mini-columns with 30 μl of RNase-free water. Starting from 1 μg of total RNA, 80 μl of cDNA was synthesized by using TaqMan Reverse Transcription Reagents, which contains an oligo-d(T)16, random hexamer, RNase inhibitor, deoxy-NTP mixture and MultiScribe Reverse Transcriptase. The reaction was performed for 10 min at 25°C, 15 min at 42°C and 5 min at 95°C. cDNA was stored at −20°C until use. To compare the relative abundance of IL-1β, IL-1Ra and SPRR1B transcripts, we used a TaqMan probe fluorogenic 5′ nuclease chemistry-based gene expression assay with exon boundary-crossing primers (gene assay ID: Mm00434228_m1, Mm00434237_m1, Mm02016340_s1, respectively). Real-time PCR was performed using thermal cycling conditions at 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C for amplification in the ABI Prism 7300 Real-Time PCR System (Applied Biosystems, Fort City, CA, USA). All assays were performed in four technical replicates, and normalized with the housekeeping gene, GAPDH. Ct data were derived from 5–7 mice in each of the four groups of mice. After normalization, the fold change derived from ΔΔCt of each experimental group vs control was examined by analysis of variance (ANOVA), with a P-value<0.05 considered statistically significant. Positive and negative quality controls for reproducibility, reverse transcription and genomic DNA contamination were assessed and found to be acceptable.
Immunostaining, Immunofluorescence and Histopathology Evaluation
Histopathology of the lacrimal gland was examined by routine hematoxylin and eosin staining. DAB-based immuno-histochemistry was used to localize CD4+ T cells’ tissue distribution and quantification, in lacrimal glands and ocular tissues. Phenotype of IL-1R1-expressing resident cells in SQM ocular tissue was characterized by dual immuno-fluorescent labeling of IL-1R1 with phenotypic markers for cells known to present in SQM histopathology, including: pan-CK (epithelial cells), vimentin (fibroblasts), CD11c+ (antigen-presenting cells), CD4+ (adoptively transferred T cells) and F4/80+ (macrophages). For evaluating phenotypic/morphological tissue changes in SQM, immuno-fluorescence was used to assess SPRR1B-expressing and BrdU-labeled cells in the cornea. The acidity of mucoglycan in conjunctival GCs was evaluated in cryosections using AB-PAS staining. All staining procedures were carried out according to previously optimized protocols for Aire KO mice established in our laboratory.18 Negative controls included the omission of the primary antibody and isotype control antibody.
Quantification of CD4+ Tissue Infiltrate, GC Density and GC Acidification
Immunohistochemistry of CD4+ T-cell micrographs and GCs was photographed with a Nikon Eclipse Ti-E microscope. Image analysis was performed on those micrographs to quantify the CD4+ immune infiltration (defined by the percentage of CD4+ T-cell-infiltrated area in the total area of cross-sectioned lacrimal and ocular surface tissues) as well as the changes of GC mucophenotype (defined by the percentage of AB+ GCs in the total AB/PAS staining-identified GCs in a length-defined conjunctival GC-rich zone).15 Advanced Research Version of NIS Elements (Nikkon, v.3.2) was used to conduct image analysis. As we described previously,14 the background noise correction was first corrected by subtracting background staining intensity of the isotype antibody control image by NIS Elements before running the CD4+ T image analysis. The brown signal from DAB-chromogen-labeled CD4+ T-cell immunohistochemistry was then gated using NIS Elements, with a narrow color spectrum covering DAB’s brown color through the software color recognition function, followed by rendering the detected monochromic signals into binary data with the data output format in the ‘percentage of CD4+ T-cell signal-positive area over the total area of tissue cross-section’. The mean percentage of CD4+ T-cell infiltration was derived by averaging data from 5 non-consecutive cross-section slides for each mouse and 5 mice per group. The percentage of AB+/PAS+ GCs and goblet cell density (GCD) was counted manually in AB/PAS heterogeneously stained GCs in a length-defined GC-rich zone at × 100 power (822 μm, width of the visual field of 100 ×). Five non-consecutive sections per mouse and 7 mice per group were used to obtain the final data.
Statistics
We used unpaired t-test to compare IL-1 ligand and IL-1R expression in Aire WT and Aire KO mice. We used ANOVA or the non-parametric Kruskal–Wallis test, depending on data distribution, to compare differences in CD4+ T-cell counts, qPCR data, corneal epithelial thickness, GCD and percentage of AB+ GCs among the four AT groups. We adjusted for multiple comparisons using the Bonferroni correction. We modeled the relationships between CD4+ T-cell infiltration and readouts of SQM, SPRR1B and GCD, as well as the relationship between tear secretion and ratio of ocular surface IL-1Ra/IL-1β epithelial transcripts using linear regression. All analyses were carried out using the statistical software Stata 9.0 (College Station, TX, USA) for Macintosh.
RESULTS
IL-1 Signaling in Autoimmune-Mediated SQM Is Pro-Inflammatory
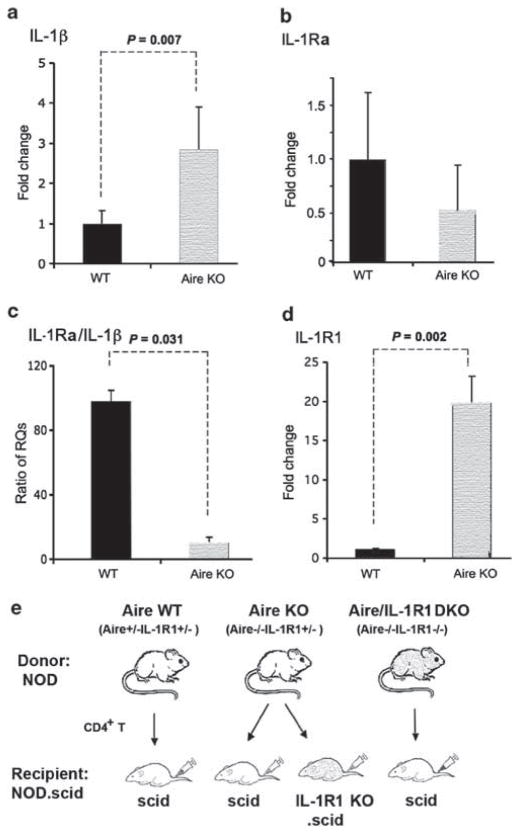
As the balance between pro- and anti-inflammatory IL-1R1 ligands is important in maintaining homeostasis of the ocular mucosal surface, we sought to determine the net effect of IL-1 signaling in Aire KO mice with autoimmune SQM. Using qPCR to examine ocular surface mRNA expression, we found a 2.82±0.91-fold increase in transcripts of pro-inflammatory IL-1β ligand in the corneolimbal epithelium of Aire KO mice in reference to 1-fold (1.00±0.32, mean±s.d.) baseline of Aire WT mice (P<0.05) (Figure 1a). The transcriptional activity of its anti-inflammatory ligand, IL-1Ra, was decreased 0.49±0.54-fold and not statistically different compared with WT control (Figure 1b). As the net immuno-modulating effect of these two antagonizing ligands is determined by their relative abundance, we used the IL-1Ra/IL-1β ratio, which shows the relative abundance of two major IL-1R1 ligands as an index of IL-1-mediated inflammatory state.17 Notably, the ratio of IL-1Ra/IL-1β in WT control mice was 97.4±11.3 (anti-inflammatory state), significantly higher than 9.7±2.1 in Aire KO mice (pro-inflammatory state) (P<0.05) (Figure 1c), confirming a proinflammatory shift in IL-1’s ligand composition in the corneal epithelia undergoing SQM. This was accompanied by a 19.2±4.7-fold increase in the expression of functional IL-1R1 in the ocular surface epithelium of Aire KO vs WT mice (baseline 1.00±0.05-fold) (P<0.05) (Figure 1d). In contrast, the decoy receptor IL-1R2 revealed no significant change in Aire KO vs WT control mice (data not shown).
Figure 1.
Transcriptional profiling of ocular surface pro- and anti-inflammatory interleukin-1 (IL-1) cytokines in autoimmune regulator (Aire) wild-type (Aire WT) and Aire knockout (Aire KO) mice. Quantitative polymerase chain reaction (PCR) for (a) IL-1β, (b) IL-1 receptor antagonist (IL-1Ra), (c) IL-1Ra/IL-1β ratio and (d) IL-1R type 1 (IL-1R1). Expression in Aire WT controls was used as the reference (designated 1-fold) to generate relative quantitation (RQ) values. Data are shown as mean RQ value±s.d. In panel c, the ratio of IL-1Ra/IL-1β was obtained by dividing glyceraldehyde 3-phosphate dehydrogenase (GAPDH)-normalized RQ value of IL-1Ra by GAPDH-normalized RQ value of IL-1β in each mouse. Ratio data were shown as mean±s.d. Seven mice were studied per group. Unpaired t-test was used to test differences between groups; P<0.05 (indicated by dotted lines) was considered statistically significant. (e) Adoptive transfer design used to dissect the cellular mechanism of IL-1R1/IL-1 signaling in CD4+ T-cell-dependent autoimmunity.
Induction of SS-Like Lacrimal Exocrinopathy by Autoreactive CD4+ T cells Is Dependent on IL-1R1 Signaling via Resident Cells Populating the Lacrimal Gland
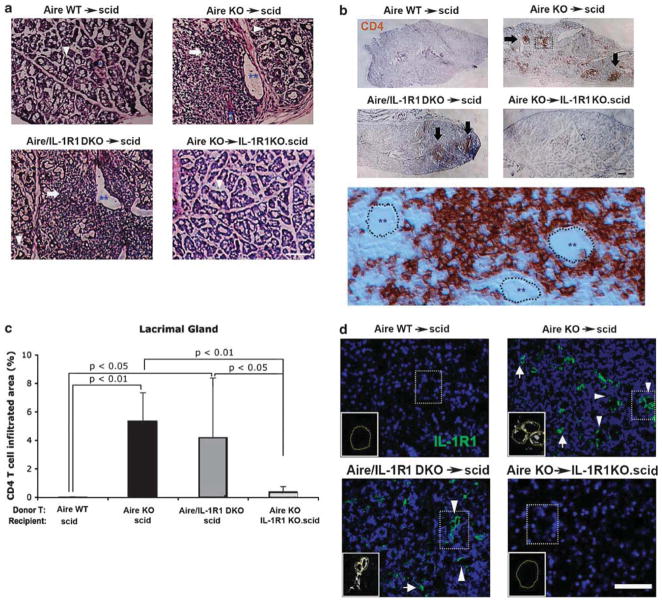
With the balance clearly tipped towards a pro-inflammatory state, we used AT to dissect the biological significance of elevated IL-1R1 signaling in the setting of CD4+ T-cell-dependent ocular autoimmunity. As summarized in Figure 1e, CD4+ T cells from three different donor phenotypes, Aire WT (Aire+/+IL-1R1+/+), Aire KO (Aire−/− IL-1R1+/+) and Aire/IL-1R1 DKO (Aire−/− IL-1R1−/−), were transferred to immunodeficient recipients either sufficient (IL-1R1+/+.scid) or deficient in IL-1R1 (IL-1R1−/−.scid). With this approach, we were able to differentiate whether the direct target of IL-1 signaling was pathogenic CD4+ T effector cells or local resident cells of the ocular surface and lacrimal tissues. Immunodeficient recipients of CD4+ T cells from Aire WT (Aire WT→scid) and Aire KO mice (Aire KO→scid) served as negative and positive controls, respectively. As summarized in Figure 2, SS-like lacrimal exocrinopathy was transferred to scid recipients in a CD4+ T-cell-dependent manner. Histological characteristics of the normal gland, including compact acinar units, interlobular ducts and septum, were well preserved in scid recipients of Aire WT, non-pathogenic CD4+ T cells (Figure 2a, upper left panel). In contrast, recipients of Aire KO, pathogenic CD4+ T cells demonstrated SS-like exocrinopathy, characterized by pronounced, inflammatory multifocal infiltrates, severe acinar tissue destruction and dilated intralobular lacrimal ducts (Figure 2a, upper right panel). Interestingly, exocrinopathy persisted even after functional IL-1 signaling was genetically deleted in pathogenic CD4+ T cells from Aire-deficient donors (Figure 2a, lower left panel). In contrast, exocrinopathy was abolished when IL-1R1 was genetically deleted in scid recipients (Figure 2a, lower right panel). Immuno-localization studies identified the presence of focal, CD4+ T-cell infiltrates in the inflamed gland with predominantly periductal localization (Figure 2b, middle panel). In Figure 2c, densitometry of CD4+ T-cell immunohistochemistry confirmed that the percentage of infiltrating cells in recipients with functional IL-1R1 (Aire KO→scid (5.32±2.02%) and Aire/IL-1R1 DKO→scid (4.15±4.21%)) significantly outnumbered those of recipients without IL-1R1 (Aire KO→IL-1R1KO.scid (0.35±0.40%)) or the nearly T-cell-free WT control mice (Aire WT→scid (0.01±0.01%)). Immuno-fluorescent localization studies revealed IL-1R1 expression in epithelial cells lining the interlobular ducts, with particularly high expression levels in ducts surrounded by CD4+ T-cell infiltrates (Figure 2d). A minor population of single, IL-1R1-expressing cells was sparsely distributed throughout the interstitium of the inflamed gland. A similar distribution was noted in both Aire KO→scid (Figure 2d, upper right) and Aire/IL-1R1 DKO→scid mice (Figure 2d, lower left), suggesting that the identified IL-1R1 was expressed by host structural cells rather than the infiltrating CD4+ T cells. In comparison, IL-1R1 immunosignals were not detectable in non-inflamed glands free of immune infiltrates (Figure 2d, upper left and lower right). Thus, autoimmune CD4+ T-cell infiltration appeared to induce IL-1R1 expression in resident cells of the lacrimal gland and signaling via these IL-1R1-expressing target cells (rather than CD4+ T cells) elicited pathological changes in the lacrimal tissue.
Figure 2.
Histopathology, CD4+ T-cell lymphocytic infiltration and IL-1 receptor type 1 (IL-1R1) expression in lacrimal glands of autoimmune regulator knockout (Aire KO) mice. (a) Hematoxylin and eosin (H&E) staining of cryosectioned lacrimal glands to assess immune cell infiltration and tissue destruction. Arrowheads indicate healthy functional acinar units, while arrows indicate focal infiltration of inflammatory cells frequently observed in the periductal interstitium. Blue asterisks denote secretory ducts in lacrimal lobules. Increased duct diameter was noted in inflamed glands (double asterisks) compared with healthy tissue (a single asterisk). Scale bar =50 μm. (b) Immunohistochemistry was used to profile the pattern and intensity of CD4+ T cells in the lacrimal gland. Clusters of infiltrating CD4+ T-cell effectors (brown) formed multiple foci as indicated by black arrows. The middle panel displays a high magnification view of the area boxed with dot lines in Aire KO→scid. Ductal epithelia are outlined by dotted lines with double blue asterisks within the ductal lumen. Scale bar = 100 μm. (c) Quantitative comparison of CD4+ T-cell infiltration between adoptive transfer groups. Data are shown as mean percentage of CD4+-infiltrated area±s.d., with P<0.05 considered statistically significant. Micrographs are representative of 7 mice per group. (d) Immunofluorescent localization of IL-1R1 in the lacrimal gland. Arrowheads indicate intralobular or interlobular ducts, while arrows point to sporadic IL-1R1-expressing cells dispersed within periductal infiltrates. Insets denote circular expression pattern of IL-1R1 within ductal epithelial cells. Scid, severe-combined immunodeficiency.
Epithelial Hyperplasia Is Associated with CD4+ T Cell-Dependent, Local IL-1R1-Mediated Inflammation of the Ocular Surface
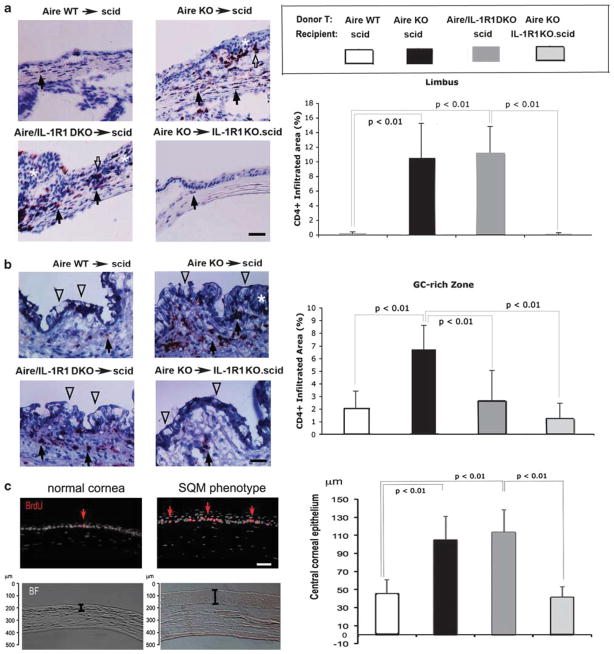
In addition to the lacrimal gland, the ocular surface mucosa is a primary target of SS-like Aire-mediated autoimmunity.14,27 Similar to lacrimal immune infiltration, CD4+ T-cell tissue infiltration was observed in the ocular surface mucosa of recipients expressing IL-1R1. Immunohistochemical staining demonstrated CD4+ lymphocytes dispersedly infiltrated throughout the entire ocular mucosa with dense focal aggregates at two effector sites, namely the corneo-conjunctival demarcating limbus (Figure 3a, upper right and lower left panels) and the GC-rich zone of the conjunctiva (Figure 3b, upper right and lower left panels). Epithelial hyperplasia was also observed in these two lymphocytic-infiltrating foci (white asterisks, Figure 3a and b) with a concomitant decreased number of GCs (Figure 3b, open arrowheads). Densitometry shown in Figure 3a (bar graph) confirmed that the limbal infiltration (defined as the percentage of CD4+-infiltrated tissue area) was significantly higher in IL-1R1-sufficient scids receiving autoreactive CD4+ T cells, including Aire KO→scid (10.5±4.7%) and Aire/IL-1R1 DKO→scid (11.2±3.6%) compared with IL-1R1-deficient scids receiving autoreactive T cells or non-autoreactive T cells, Aire KO→IL-1R1KO.scid (0.1±0.1%) and Aire WT→scid (0.2±0.2%), respectively. In contrast to the limbal effector site, infiltration differences in the GC-rich effector site were slightly less pronounced (Figure 3b, bar graph). CD4+ T cells were present in low amounts in the conjunctiva of Aire WT→scid control mice (1.9±1.7%). This may reflect a subset of resident-type of CD4+ T cells populating the scid’s conjunctival lymphoid tissues that is known to represent an important segment of the mucosa-associated lymphoid tissue.38 Nonetheless, CD4+ T-cell infiltration in Aire KO→scid (6.6±2.3%) was still significantly higher than controls, whereas Aire/IL-1R1 DKO→scid (2.6±2.4%) and Aire KO→IL-1R1KO.scid mice (1.1±0.9%) did not differ from controls. To further characterize the hyperplastic feature of ocular epithelial SQM at the cellular level, we conducted BrdU pulse therapy to detect cells in active proliferation. To evaluate the overall tissue architectural change, we measured the thickness of the central cornea. A dramatic increase in BrdU-labeled basal epithelial cells found in Aire KO mice indicated increased cellular proliferative activity in corneas undergoing SQM (Figure 3c, upper panels). Quantitation of the central corneal epithelial thickness (defined as the length from epithelial surface to basement membrane) revealed a significant increase in inflamed eyes of Aire KO→scid (105.4±25.7 μm) and Aire/IL-1R1 DKO→scid (113.5±24.6 μm) mice, while the epithelial thickness of Aire KO CD4+ T-cell recipients without IL-1R1 (Aire KO→IL-1R1KO.scid, 41.6±11.5 μm) were indistinguishable from Aire WT→scid controls (46.1±14.6 μm) (Figure 3c, lower panels and bar graph). Taken together, these results suggested that CD4+ T-cell-mediated chronic inflammation induced corneal epithelial hyperplasia in a host-IL-1R1-dependent manner.
Figure 3.
Characterization of CD4+ T-cell infiltration and tissue morphological changes in squamous metaplasia (SQM). Micrographs demonstrate representative infiltration of CD4+ T cells in the (a) limbus and (b) goblet cell (GC)-rich zone of the conjunctival epithelium. Cells infiltrating the stromal and epithelial compartments are indicated by closed and open arrows, respectively. Pronounced stratification of the corneal epithelium and GC-rich zone, histopathological features characteristic of squamous metaplasia, are indicted by white asterisks. Clusters of GCs are denoted by open arrowheads. Bar graphs show densitometric comparison of CD4+ T-cell infiltration between adoptive transfer groups. Data are shown as mean percentage of CD4+-infiltrated area±s.d., with P<0.05 considered statistically significant. Results are representative of 7 mice per group. (c) Images representative of epithelial hyperplasia in SQM. Proliferating basal epithelial cells were labeled by pulse bromodeoxyuridine (BrdU) (stained red, upper panels). Epithelial over-stratification of corneas undergoing SQM (SQM phenotype) was shown in bright-field (BF) images in the bottom panel. A bar graph was used to compare central corneal epithelial thickness among the four adoptive transfer groups. Data are plotted as the mean±s.d. thickness (in μm), with P<0.05 considered statistically significant. Results are representative of 7 mice per group.
With the presence of functional IL-1R1 in recipients acting as a major determinant for CD4+ T-cell-dependent local inflammation and tissue structural change (Figures 2 and 3), we investigated the localization and cellular identity of IL-1R1-expressing cells within the ocular surface tissues. Immunolocalization revealed low, yet detectable expression in stromal cells of Aire WT→scid controls (Figure 4a, upper leftmost panel, open arrowhead), while the overlying epithelial cells were IL-1R1−. Conversely, in Aire KO→scid and Aire/IL-1R1 DKO→scid groups, where CD4+ T-cell-mediated ocular inflammation was present and tissue architecture disrupted, de novo IL-1R1 expression was detected throughout the overstratified ocular surface epithelium (Figure 4a, upper middle and lower left panels, closed arrowheads), with expression in the stromal counterpart also markedly increased (open arrowheads). Aberrant expression of IL-1R1 occurred across the entire ocular surface tissue, extending from the central cornea to the limbus and throughout the conjunctiva. Cells with positive immunoreactivity to IL-1R1 demonstrated squamoid morphology in the epithelial compartment and adopted a spindle shape in the stromal compartment of corneas undergoing SQM (Figure 4a, insets displaying oversaturated monochromatic (white) signaling from IL-1R1-expressing stromal cells (open arrowheads) with associated nuclei (blue)). Notably, IL-1R1 immunosignals adopting characteristic cellular morphology could be discerned from background autofluorescence in isotype antibody controls and IL-1R1KO.scids that was characterized by an acellular, linear staining pattern (Figure 4a, autofluorescence delineated with a caret and shown in oversaturated magnified lower right panels). To ascertain whether IL-1R1+ cells shown in Figure 4a were ocular structural cells, we profiled their phenotype using double immunofluorescent labeling to colocalize IL-1R1 and phenotypic markers specific for different cell types known to be present in SQM histopathology. Markers included pan-CK for ocular surface epithelial cells, vimentin for stromal fibroblasts, CD11c for resident Langerhan’s cells (LCs), stromal dendritic cells and circulation-derived infiltrating antigen-presenting cells, CD4 for effector T cells and F4/80 for macrophages (MØ). Results indicated that in SQM tissue, the non-immune resident population, including epithelial cells and stromal fibroblasts, were IL-1R1+, whereas immune cells, including resident and infiltrating CD11c+ APCs, CD4+ effectors and F4/80+ macrophages, were IL-1R1− (Figure 4b). With tissue structural cells serving as the primary targets of IL-1 in auto-immune-mediated SQM disease, we asked whether IL-1/IL-1R1 signaling may occur in an autocrine manner following the release of IL-1 ligand from the epithelium. We examined the transcriptional activity of IL-1β in each group of AT mice using quantitative TaqMan gene expression assay with molecular weight verification for PCR specificity in semiquantitative PCR (Figure 4c). IL-1β transcripts were markedly increased in the two AT groups where epithelial expression of IL-1R1 was 17.8±2.5 times upregulated in Aire KO→scid and 23.4±5.9 times in Aire/IL-1R1 DKO→scid, in reference to the 1-fold control group Aire WT→scid (P<0.05). Of particular interest, IL-1β ligand expression in the Aire KO→IL-1R1KO.scid recipients was not significantly different from the control group, despite a mild 4.2±1.9-fold increase. In Figure 4d, we confirmed complete knock down of functional IL-1R1 in IL-1R1 KO.scid mice. Genotype analysis using manufacturer-recommended primers clearly distinguished WT, heterozygous and IL-1R1 KO mice.
Figure 4.
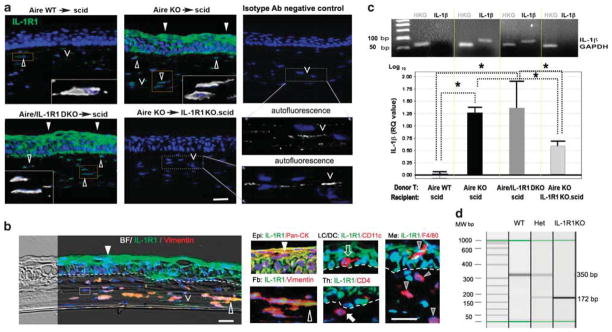
Characterization and localization of IL-1 receptor type 1 (IL-1R1)-expressing cells in CD4+ T-cell-mediated squamous metaplasia (SQM). (a) Immunolocalization of IL-1R1-expressing cells (shown in green) in the corneas of adoptive transfer recipients. Nuclei were counterstained with 4′,6′-diamino-2-phenylindole (DAPI) (blue). Closed arrowheads denote IL-1R1-expressing cells in the epithelial compartment, while open arrowheads indicate IL-1R1-expressing cells in the stromal compartment. Boxed areas within each image are shown in high power insets with oversaturated Alexa 488 signal (shown in white color). Autofluorescence observed in IL-1R1 KO.scid recipients and isotype controls (denoted with carets ‘V’) are provided in high-power subfigures in the right bottom panel of (a). Autofluorescence appears as linear, nonspecific staining associated with collagen lamella that is distinctly different from IL-1R1+ cells in the stroma (open arrowheads) that are fusiform in shape and possess a cell nucleus. Bar = 50 μm. Pictures are representative of 5 mice per group. (b) Dual-labeling of IL-1R1 (green) with cell type-specific markers (red) to profile cells populating the SQM microenvironment. In the left panel, a bright-filed (BF) image is merged with an immunofluorescent picture to provide an overview of IL-1R1+ and IL-1R1−cells in the SQM cornea. In the right panel, high-powered micrographs show distinct cell types constituting the mixed-cell population. Markers for tissue structure cells included vimentin (open arrowhead) for stromal fibroblasts (Fb) and pan-cytokeratin (pan-CK, closed arrowhead) for epithelial (Epi) cells. Immune cell markers included CD4 (closed arrow) and F4/80 (gray arrowhead) for infiltrating T cells and macrophages (MØ), respectively. CD11c (open arrow) was used to identify resident antigen-presenting cells (APCs), including epithelial Langerhan’s cells (LC), stromal dendritic cells (DCs) and infiltrating APCs. Yellow signal indicates colocalization of IL-1R1 with cell-specific marker, while red signal indicates that a given cell type does not express IL-1R1. Dotted line indicates epithelial basement membrane. Boxed areas above and below the dotted line (left panel) represent IL-1R1− immune cells phenotypically characterized in the high-power subfigures (right panel). Nuclei stained blue with DAPI. Scale bar =50 μm. (c) Upper panel shows confirmation of IL-1β transcript expression by traditional semiquantitative polymerase chain reaction (PCR). Gel image was obtained following electrophoresis of PCR products amplified from equivalent amounts of cDNA for 22 cycles. Expected molecular weight of IL-1β amplicons and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) amplicons are 90 and 70 bp, respectively. The lower panel shows TaqMan quantitative PCR analysis of IL-1β transcripts in the corneolimbal epithelium. The control group, Aire WT→scid, was used as the reference (designated 1-fold) to generate relative quantitation (RQ) values of GAPDH-normalized IL-1β expression for the other three adoptive transfer groups. Data are shown as mean RQ value±s.d. (in log10 unit, where the baseline 1-fold = 0). Statistically significant differences between groups, P<0.05, are indicated by dotted lines with overlying asterisk. (d) Genotyping image of IL-1R1 in scid recipients with or without IL-1R1. Using specific primers targeting the mutated gene loci, three genotypes of scid mice are easily discernable by a 172 and 350 bp bands in the electrophoresis image of PCR products. IL-1R1 WTs (IL-1R1+/+) uniformly expresses a 350 bp band, IL-1R1 knockouts (IL-1R1−/−) uniformly expresses a 172 bp band, while IL-1R1 heterozygotes (IL-1R+/−) have both bands. Aire WT, autoimmune regulator wild type; scid, severe-combined immunodeficiency.
Keratinizing SQM and GC Loss are Induced by CD4+ T-Cell-Dependent, Local IL-1R1-Mediated Inflammation of the Ocular Surface
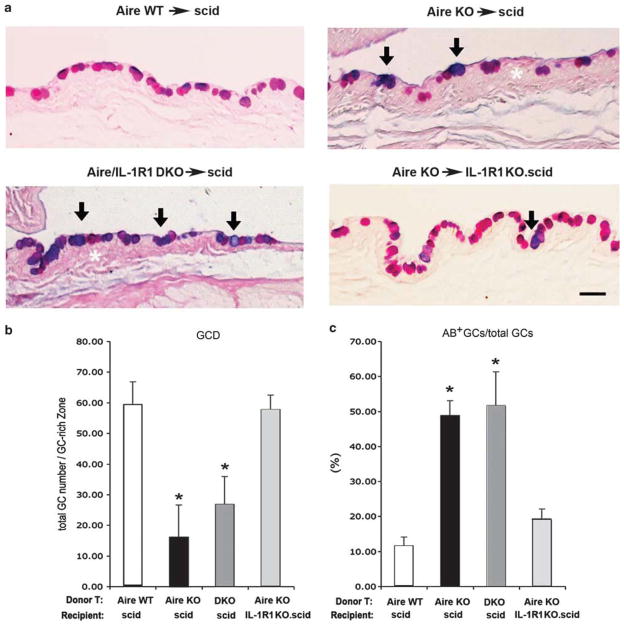
We used SPRR1B as a biomarker for keratinization to evaluate mucosal epithelial phenotype and identified changes in conjunctival GCs by histology. The expression of cornified envelope precursor protein, SPRR1B (Figure 5a) and its mRNA transcripts (Figure 5b and c) was significantly upregulated in autoreactive CD4+ T-cell recipients when ocular structural cells expressed IL-1R1. Compared with Aire WT→scid control mice, Aire KO→scid and Aire/IL-1R1 DKO→scid had significantly increased SPRR1B transcription at 43.8±3.8- and 112.8±7.19-fold, respectively (Figure 5b). On the contrary, autoreactive T-cell recipients lacking IL-1R1 (Aire KO→IL-1R1KO.scid) had a 4.7±9.7-fold decrease in SPRR1B transcripts that was not significantly different than controls (Aire WT→scid, baseline 1.0±1.5-fold). Metaplastic changes in the cornea occurred in parallel with changes in GC phenotype. GCs in the early stage of SQM demonstrated an AB-positive acidification of its mucoglycoconjugates, followed by a decline in GC number (Figure 6a). GCD in inflamed eyes of Aire KO→scid and Aire/IL-1R1 DKO→scid mice (16.0±12.3 and 27.6±10.5 cells, respectively) was significantly lower than that of Aire KO→IL-1R1KO.scid and Aire WT→scid (59.8±7.6 and 57.3±5.1 cells, respectively). As GCD decreased, there was a corresponding increase in the percentage of AB+ GC/total GCs. The percentage of AB+ cells was 47±4% in Aire KO→scid and 52±11% in Aire/IL-1R1 DKO→scid. Both were statistically higher than the 19.3±2.5% in Aire KO→IL-1R1KO.scid and 11.8±2.2% in Aire WT→scid mice (P<0.05). These data indicated a critical role for IL-1R1 in CD4+ T-cell-dependent pathological keratinization of the corneal epithelium and loss/acidification of conjunctival GCs.
Figure 5.
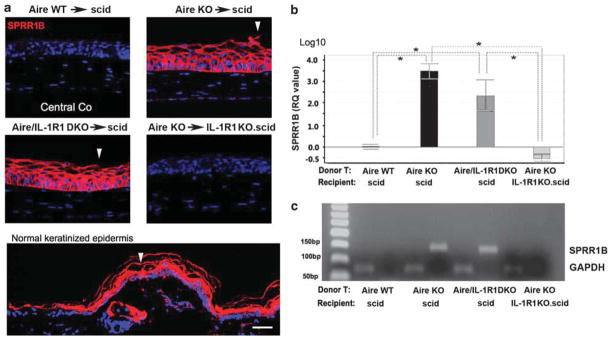
Localization and quantitation of ocular surface keratinization protein, small proline-rich protein 1B (SPRR1B). (a) Immunofluorescence of central corneal (Co) SPRR1B in each adoptive transfer group. Dorsal epidermis of control severe-combined immunodeficiency (scid) mouse was used as positive control for keratinization (bottom panel). Closed arrowheads indicate areas of desquamation in the denucleated surface layer of the hypertrophic corneal epithelium. Scale bar =50 μm. (b) Quantitative polymerase chain reaction (qPCR) of corneal epithelial SPRR1B expression. Expression in the control group, Aire WT→scid, was used as the reference (designated 1-fold) to generate relative quantitation (RQ) values. Data are shown as mean RQ value±s.d. (in log10 unit, where the baseline 1-fold =0). Statistically significant differences between groups, P<0.05, are indicated by dotted lines with overlying asterisk, n = 5 mice per group. (c) Conventional semiquantitation PCR was used to confirm TaqMan qPCR as in Figure 4d. Expected molecular weight of SPRR1B (135 bp) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (70 bp) amplicons are shown. Aire WT, autoimmune regulator wild type.
Figure 6.
Histopathological evaluation of goblet cell density (GCD) and acidification by Alcian blue-periodic acid Schiff (AB-PAS) staining. (a) AB-PAS staining of the conjunctival GC-rich zone in each adoptive transfer group. GCs stained with both AB+ and PAS+ appeared dark blue/purple (arrows), in contrast to pink cells stained with PAS alone. White asterisks denote conjunctival epithelial hyperplasia in the GC-rich zone. Scale bar = 100 μm.
(b) Quantitative analysis of GCD as determined by the total GC number (sum of all AB- and PAS-stained cells) in a defined area of the GC-rich zone using × 100 magnification. Data are presented as mean±s.d. of total GC number/GC-rich area. (c) Percentage of AB+ cells in the total GC pool. Data are expressed as mean±s.d. of the percentage of AB+ GCs/total GCs. Asterisks indicate statistically significant differences vs the control group, Aire WT→scid, P<0.05. Aire WT, autoimmune regulator wild type; scid, severe-combined immunodeficiency.
With SPRR1B expression and GC changes clearly dependent on the local activation of IL-1R1, we examined their association with infiltrating local effector cells using regression analysis. CD4+ T-cell infiltration was quantified in three anatomical target locations: the lacrimal gland, limbus and GC-rich zone of the conjunctiva (Figure 7a–c). In each case, CD4+ T-cell infiltration was a significant predictor of SQM. Corneal SPRR1B was significantly correlated to infiltration of the lacrimal gland (Figure 7a; R2 = 0.54, P<0.001) and limbus (Figure 7b; R2 = 0.60, P<0.001), whereas GCD was correlated to CD4+ T-cell infiltration of the GC-rich zone (Figure 7c; R2 = 0.42, P<0.01). We also examined whether IL-1β might modulate ocular autoimmunity beyond the local autocrine/paracrine mechanism by assessing the relationship between lacrimal gland secretory function and ocular surface expression of proinflammatory IL-1 using the IL-1Ra/IL-1β ratio as an index. Consistent with previous reports, we found significantly reduced tear secretion in Aire KO vs Aire WT mice (7.3±3.5 vs 18.7±4.0 mm/15 s, respectively) was highly correlated to a decrease in the IL-1Ra/IL-1β ratio (Figure 7d; R2 = 0.62, P<0.01). These data suggested that the IL-1 cytokine system potentially functions as a molecular link between ocular CD4+ T-cell-mediated inflammation and lacrimal gland exocrine function in SS-associated autoimmunity.
Figure 7.
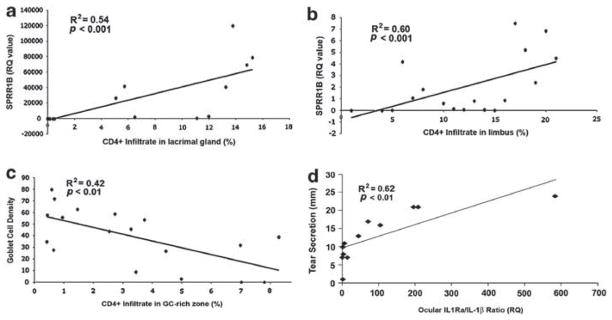
The local inflammatory response predicts clinical characteristics associated with autoimmune squamous metaplasia (SQM). Linear regression analysis was performed to examine relationships between CD4+ T-cell infiltration, SQM phenotypic marker small proline-rich protein 1B (SPRR1B), goblet cell density, ocular IL-1 receptor antagonist (IL-1Ra)/IL-1β ratio and tear secretion. CD4+ T-cell infiltration in the (a) lacrimal gland and (b) limbus was highly correlated to the ectopic expression of keratinization biomarker, SPRR1B, in the cornea, while CD4+ T-cell infiltration in the conjunctival GC-rich zone (c) was inversely correlated to GCD. (d) The ocular inflammatory state indicated by low IL-1Ra/IL-1β ratio was positively correlated to reduced tear secretion. Tear secretion is expressed in millimeters (mm) of wetting by cotton thread test. Squared correlation coefficients (R2) and corresponding P-values for each regression analysis are provided in individual graphs. Raw data are plotted with the best-fit regression line to demonstrate statistically significant correlations, P<0.01.
DISCUSSION
SQM is induced when dysregulated local immunity promotes altered growth and differentiation of the ocular mucosal epithelium. As an essential cytokine component of innate immunity, IL-1 orchestrates the release of a wide variety of secondary cytokines that exert pleiotropic effects on multiple cell types to promote both inflammation and phenotypic alterations of the underlying epithelium.15 With this inherent versatility, IL-1 possessed specific characteristics essential to induce SQM in the setting of autoimmunity. To define its specific contributions, it was important to consider that IL-1’s bioactivity is universally determined at three major levels: (1) synthesis and release from immune and non-immune cells; (2) interaction with membrane receptors; and (3) activation of intracellular signal-transduction pathways to regulate target gene expression. Focusing on the first step of this process, previous studies provided evidence of increased synthesis and release of IL-1β and IL-1Ra in tears of SS-KCS patients and in the peripheral circulation of SS patients with germinal center formation in the exocrine glands.17,28 In both SS patients and Aire KO mice, we established a pathological link between IL-1β expression and severity of keratinization.15 Recently, the ratio of IL-1Ra/IL-1β has been advocated as a more relevant disease readout than evaluating IL-1β expression alone in IL-1-mediated autoimmune diseases, such as Alzheimer disease, multiple sclerosis, inflammatory bowel disease and Graves’ ophthalmopathy.29,30 Here, we provided the first in vivo evidence demonstrating a reduced IL-1Ra/IL-1β ratio (pro-inflammatory state) in the ocular surface epithelium of Aire KO mice during autoimmune KCS, with a 9.5-fold decrease (P<0.05) compared with control mice (Figure 1c). Taken together with an upregulated expression of IL-1’s functional receptor, IL-1R1, in ocular surface epithelium (Figure 1d), pro-inflammatory-shifted IL-1/IL-1R signaling axis links innate immunity to adaptive immunity in ocular autoimmune diseases.31 Data provided here also suggest the IL-1Ra/IL-1β ratio as a potential inflammatory cytokine index to complement current clinical immune infiltrates scoring method for assessing ocular inflammation in SS.
Having previously addressed the first level of IL-1 regulation—synthesis and release—we moved to examining IL-1 signaling at the receptor level by investigating what type(s) of cells are the main target(s) of IL-1, and how IL-1R1-mediated signaling via a given cell type contributed to phenotypic alteration of the ocular surface mucosa. Through the AT of pathogenic CD4+ T cells from Aire KO mice to scid recipients with intact IL-1R1 signaling, we induced ectopic expression of IL-1R1 in epithelial cells lining the lacrimal ducts and across the ocular surface. Ectopic expression of IL-1R1 was accompanied by an intense influx of CD4+ effectors to the surrounding tissue, as well as structural changes consistent with ocular SQM (ie, epithelial keratinization and hyperplasia) and lacrimal gland exocrinopathy (ie, acinar atrophy, tissue disorganization and ductal dilatation). Each of these pathological characteristics was absent when scid recipients lacked functional IL-1R1, suggesting an essential interplay between of CD4+ T cell and the local expression of IL-1R1 in the pathogenesis of SQM. While these data supported the conventional view that target tissues in autoimmune disease are passively damaged by infiltrating, autoantigen-primed effector cells, they also provided strong evidence that tissue cells actively participate in augmenting and propagating both inflammation and local tissue damage by modulating immune cell infiltration.
How does signaling via IL-1R1 expressed on local resident cells facilitate the tissue recruitment of autoreactive CD4+ T cells? As a potent proinflammatory cytokine, IL-1β could modulate CD4+ T-cell response through at least two processes, afferent activation of APCs and/or efferent activation of autoreactive CD4+ T cells. In our previous work, we demonstrated a 22-fold increase in cornea-infiltrating APCs in Aire KO mice relative to WT controls.15 Interestingly, the number of corneal APCs in Aire/IL-1R1 DKO mice was comparable to that of Aire KO mice. The phenotypic characterization of APC in this study suggested that neither tissue resident LCs nor infiltrating dendritic cells expressed IL-1R1 in the setting of SQM (Figure 4b). Taken together, these findings suggested that APC activation in Aire KO mice occurred through cytokines other than IL-1, such as IFNγ.32 In a recent microarray screening of inflammatory molecules in corneal epithelial cells from Aire KO mice, we found that CD74, which encodes the MHC II molecule to activate CD4+ T cells, was the most significantly upregulated gene (44.8-fold increase) compared with Aire WT controls. Upregulation of CD74 was significantly reduced in the quiescent ocular surface epithelium of Aire KO mice lacking IL-1R1, suggesting that ocular epithelial cells may function as local APCs via IL-1R1 signaling (unpublished data). Our data are in agreement with previous reports of MHC II upregulation in conjunctival cells during chronic conjunctivitis.32 As part of the efferent loop of lymphocyte activation, IL-1 has been reported to facilitate tissue recruitment of activated CD4+ T cells from the lymph node through IL-1R1-mediated upregulation of cell adhesion molecule, ICAM-1, on ocular epithelial cells.33–35 Thus, epithelial cell expressing ICAM-1 may facilitate T-cell homing and retention, thereby perpetuating chronic inflammation of the ocular surface in Aire KO mice. The detailed molecular mechanism whereby IL-1R1-expressing epithelial cells sustain CD4+ T-cell infiltration warrants further study.
Yet, in the pathogenesis of autoimmune-mediated SQM, pro-inflammatory IL-1 does not work alone. IFNγ-producing Th1 cells dominate the effector CD4+ T-cell population in target tissues of Aire KO mice.27 Thus, IFNγ likely cooperates with IL-1 to induce inflammation and both of these pleiotropic cytokines have been implicated in the multistep process of ocular SQM.13,15,20 Indeed, both IL-1β and IFNγ upregulate cornified envelope protein, SPRR1B, in primary corneal epithelial cells via signaling pathways that intersect at the level of p38 MAPK.20 Moreover, crosslinking of cornified envelope proteins has been linked to IFNγ-dependent upregulation of2,36 transglutaminase I. The interplay between local IL-1/IL-R1 signaling and IFNγ-producing CD4+ T cells may also direct SQM development via a temporal mechanism. For example, IFNγ exacerbates desiccating stress-induced epithelial apoptosis within the first 2 weeks of dry eye development following the AT of pathogenic CD4+ T cells,37–39 while we observed a dramatic increase in IL-1R1-mediated proliferation of corneal epithelial cells 6 weeks after the AT of autoreactive CD4+ T cells (Figure 3c). Whether a temporal shift from IFNγ-mediated apoptosis to IL-1-mediated cellular hyperproliferation and tissue hyperplasia represents ocular pathology progression from the early KCS stage to the later SQM stage in SS ocular autoimmunity remain to be determined. Cooperation of IL-1/IFNγ cytokines may also occur in the modulation of conjunctival GC phenotype. We showed a qualitative, IL-1-dependent switch from neutral-to-acidic GC mucins in both germline Aire KO mice40 and CD4+ T-cell-transferred scid recipients (Figure 6). Literature suggests that mucin acidification predicts SQM change of the esophageal mucosa.41 On the other hand, GC pathology caused by IFNγ has been show to consist of a quantitative decrease in GCD,13 which has been the gold standard for clinical diagnosis of SQM since 1965.1,3,42 Thus, IL-1 and IFNγ appear to synergistically foster SQM at both the immunoregulatory and cellular phenotypic modulatory levels.
SQM may represent a failure in wound healing characterized by chronic stromal inflammation, basal epithelial hyperplasia and epidermal-like keratinization of the surface epithelium, following the desiccating and/or immune cytokine injury. As an active cytokine participant in maintaining mucosal phenotype, IL-1 facilitates tissue regeneration followed by tissue remodeling via the dynamic autoregulation of IL-1Ra/IL-1β. In the current work, we provided evidence of an autoreactive CD4+ T-cell-skewed IL-1Ra/IL-1β balance that led to defective tissue regeneration/remodeling (Figure 3c) and aberrant phenotypic restoration (Figures 5 and 6). The effect of IL-1/IL-1R1-mediated inflammation was not limited to dysregulated ocular surface tissue repair with metaplastic phenotype, but also associated with the functional impairment of the lacrimal gland (Figure 7). Our regression analysis data not only underscore the essential link between ocular IL-1/IL-1R1 signaling, CD4+ T-mediated ocular inflammation and SQM, but also provide evidence that ocular surface inflammation is largely predictive of lacrimal exocrinopathy in the setting of SS auto-immune disease.
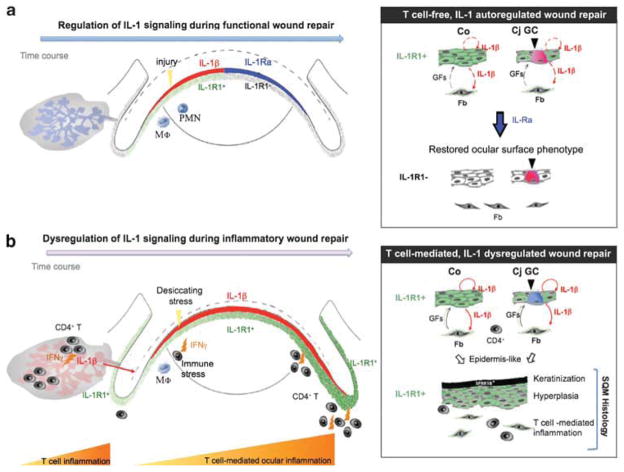
On the basis of our recent publications and major findings from the current work, we provide an integral view of IL-1’s diverse pathogenic roles in SS-associated SQM (Figure 8). The immune pathogenesis of IL-1/IL-1R1 signaling on the ocular surface and in the lacrimal gland depends on tissue-specific CD4+ T cells and activation of IL-1R1-expressing epithelial and stromal cells (Figure 4). Persistent activation of IL-1 signaling sets off an aberrant wound healing response, characterized by uncontrolled growth and epidermal-like differentiation, which ultimately links chronic inflammation to SQM in autoimmune diseases like SS.
Figure 8.
Schematic model proposing an integral role for interleukin (IL)-1/IL-1 receptor type 1 (IL-1R1) signaling in the molecular events of CD4+ T-cell-mediated inflammation and dysregulated homeostatic tissue repair in ocular squamous metaplasia (SQM). (a) Epithelial IL-1/IL-1R1 signaling is an essential, cytokine-mediated mechanism for tissue repair. Upon wounding, pre-stored IL-1β (shown in red) is released by the ocular epithelium to rapidly recruit innate immune cells (eg, neutrophils (PMN), macrophages (Mφ)) to the injury site where they elicit transient local inflammation. IL-1β induces IL-1R1 expression on epithelial and stromal cells of the ocular surface. Through its interactions with IL-1R1-expressing stromal cells, IL-1β activates limbal and corneal fibroblasts (Fb) to secrete growth- and migration-promoting factors, such as keratinocyte and hepatocyte growth factors (GFs), respectively.43,44 In the later stages of re-epithelialization, regenerated ocular epithelial cells shut down the IL-1-mediated repair machinery by upregulating key inhibitory factors, such as IL-1Ra (shown in blue).45,46 The ocular mucosal phenotype is restored, consisting of a transparent, non-keratinized corneal (Co) epithelium and neutral mucin-secreting goblet cells (GCs) in the conjunctiva (Cj). (b) With chronic infiltration of IFNγ-producing autoreactive CD4+ effector T cells, IL-1’s counterbalancing system is disrupted, as evidenced by the ongoing production of IL-1β (shown in red), the persistent expression of IL-1R1 throughout the ocular surface (shown in green) and the decreased IL-1Ra/IL-1β ratio. Unopposed IL-1/IL-1R1 signaling promotes defective cell growth and differentiation, leading to the characteristic SQM phenotype with the upregulation of cornified envelope proteins (eg, small proline-rich protein 1B (SPRR1B)), epithelial hyperplasia, as well as the depletion and acidification of mucin-secreting GCs. Collectively, this model places the IL-1/IL-1R cytokine system in a central role during the development of autoimmune-mediated SQM.
Acknowledgments
We appreciate the generosity of Dr Mark Anderson (Diabetes Center, University of California at San Francisco) for providing Aire-deficient mice and suggestions in experimental design. We also thank Dr Walter Finkbeiner (Department of Pathology, University California at San Francisco), Dr Victoria Monterroso (Department of Pathology, University of Costa Rica) and Dr Driss Zoukhri (Tufts University, School of Dental Medicine, Boston) for their insightful comments on our manuscript. This work was supported by National Eye Institute Grants R01-EY016203, 3R01-EY016203-04S1 and EY02162.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Nelson JD, Wright JC. Conjunctival goblet cell densities in ocular surface disease. Arch Ophthalmol. 1984;102:1049–1051. doi: 10.1001/archopht.1984.01040030851031. [DOI] [PubMed] [Google Scholar]
- 2.Nishida K, Yamanishi K, Yamada K, et al. Epithelial hyperproliferation and transglutaminase 1 gene expression in Stevens–Johnson syndrome conjunctiva. Am J Pathol. 1999;154:331–336. doi: 10.1016/S0002-9440(10)65279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng SC. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985;92:728–733. doi: 10.1016/s0161-6420(85)33967-2. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder SC, Tseng SC, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56. doi: 10.1097/00003226-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Research in dry eye. Report of the research subcommittee of the international dry eye WorkShop (2007) Ocul Surf. 2007;5:179–193. doi: 10.1016/s1542-0124(12)70086-1. [DOI] [PubMed] [Google Scholar]
- 6.Adamson TC, 3rd, Fox RI, Frisman DM, et al. Immunohistologic analysis of lymphoid infiltrates in primary Sjögren’s syndrome using monoclonal antibodies. J Immunol. 1983;130:203–208. [PubMed] [Google Scholar]
- 7.Skopouli FN, Fox PC, Galanopoulou V, et al. T cell subpopulations in the labial minor salivary gland histopathologic lesion of Sjögren’s syndrome. J Rheumatol. 1991;18:210–214. [PubMed] [Google Scholar]
- 8.El Annan J, Chauhan SK, Ecoiffier T, et al. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009;50:3802–3807. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjögren’s syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 10.Siemasko KF, Gao J, Calder VL, et al. In vitro expanded CD4+CD25+Foxp3+ regulatory T cells maintain a normal phenotype and suppress immune-mediated ocular surface inflammation. Invest Ophthalmol Vis Sci. 2008;49:5434–5440. doi: 10.1167/iovs.08-2075. [DOI] [PubMed] [Google Scholar]
- 11.Zheng X, de Paiva CS, Li DQ, et al. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest Ophthalmol Vis Sci. 2010;51:3083–3091. doi: 10.1167/iovs.09-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan SK, El Annan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to treg suppression. J Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 14.Chen YT, Li S, Nikulina K, et al. Immune profile of squamous metaplasia development in autoimmune regulator-deficient dry eye. Mol Vis. 2009;15:563–576. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YT, Nikulina K, Lazarev S, et al. Interleukin-1 as a phenotypic immunomodulator in keratinizing squamous metaplasia of the ocular surface in Sjögren’s syndrome. Am J Pathol. 2010;177:1333–1343. doi: 10.2353/ajpath.2010.100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eller MS, Yaar M, Ostrom K, et al. A role for interleukin-1 in epidermal differentiation: regulation by expression of functional versus decoy receptors. J Cell Sci. 1995;108(Part 8):2741–2746. doi: 10.1242/jcs.108.8.2741. [DOI] [PubMed] [Google Scholar]
- 17.Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 18.Fabre EJ, Bureau J, Pouliquen Y, et al. Binding sites for human interleukin 1 alpha, gamma interferon and tumor necrosis factor on cultured fibroblasts of normal cornea and keratoconus. Curr Eye Res. 1991;10:585–592. doi: 10.3109/02713689109013850. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum JT, Planck ST, Huang XN, et al. Detection of mRNA for the cytokines, interleukin-1 alpha and interleukin-8, in corneas from patients with pseudophakic bullous keratopathy. Invest Ophthalmol Vis Sci. 1995;36:2151–2155. [PubMed] [Google Scholar]
- 20.Li S, Gallup M, Chen YT, et al. Molecular mechanism of proinflammatory cytokine-mediated squamous metaplasia in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:2466–2475. doi: 10.1167/iovs.09-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deknuydt F, Bioley G, Valmori D, et al. IL-1beta and IL-2 convert human treg into T(H)17 cells. Clin Immunol. 2009;131:298–307. doi: 10.1016/j.clim.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Matsuki T, Nakae S, Sudo K, et al. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 23.Sutton C, Brereton C, Keogh B, et al. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Sasson SZ, Hu-Li J, Quiel J, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci USA. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Nikulina K, DeVoss J, et al. Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Invest Ophthalmol Vis Sci. 2008;49:34–41. doi: 10.1167/iovs.07-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YT, Li W, Hayashida Y, et al. Human amniotic epithelial cells as novel feeder layers for promoting ex vivo expansion of limbal epithelial progenitor cells. Stem Cells. 2007;25:1995–2005. doi: 10.1634/stemcells.2006-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devoss JJ, Shum AK, Johannes KP, et al. Effector mechanisms of the autoimmune syndrome in the murine model of autoimmune polyglandular syndrome type 1. J Immunol. 2008;181:4072–4079. doi: 10.4049/jimmunol.181.6.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reksten TR, Jonsson MV, Szyszko EA, et al. Cytokine and autoantibody profiling related to histopathological features in primary Sjögren’s syndrome. Rheumatology (Oxford) 2009;48:1102–1106. doi: 10.1093/rheumatology/kep149. [DOI] [PubMed] [Google Scholar]
- 29.Arend WP, Malyak M, Guthridge CJ, et al. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 30.van Exel E, Eikelenboom P, Comijs H, et al. Vascular factors and markers of inflammation in offspring with a parental history of late-onset Alzheimer disease. Arch Gen Psychiatry. 2009;66:1263–1270. doi: 10.1001/archgenpsychiatry.2009.146. [DOI] [PubMed] [Google Scholar]
- 31.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawasaki S, Kawamoto S, Yokoi N, et al. Up-regulated gene expression in the conjunctival epithelium of patients with Sjö gren’s syndrome. Exp Eye Res. 2003;77:17–26. doi: 10.1016/s0014-4835(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 33.Byeseda SE, Burns AR, Dieffenbaugher S, et al. ICAM-1 is necessary for epithelial recruitment of gammadelta T cells and efficient corneal wound healing. Am J Pathol. 2009;175:571–579. doi: 10.2353/ajpath.2009.090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen WS, Lin KC, Chen CH, et al. Autoantibody and biopsy grading are associated with expression of ICAM-1, MMP-3, and TRAIL in salivary gland mononuclear cells of Chinese patients with Sjögren’s syndrome. J Rheumatol. 2009;36:989–996. doi: 10.3899/jrheum.080733. [DOI] [PubMed] [Google Scholar]
- 35.Gao J, Morgan G, Tieu D, et al. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjögren’s syndrome-like MRL/lpr mice. Exp Eye Res. 2004;78:823–835. doi: 10.1016/j.exer.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Nishida K, Dota A, et al. Elevated expression of transglutaminase 1 and keratinization-related proteins in conjunctiva in severe ocular surface disease. Invest Ophthalmol Vis Sci. 2001;42:549–556. [PubMed] [Google Scholar]
- 37.Zhang X, Chen W, De Paiva CS, et al. Interferon-{gamma} exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Invest Ophthalmol Vis Sci. 2011;52:6279–6285. doi: 10.1167/iovs.10-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Chen W, De Paiva CS, et al. Desiccating stress induces CD4(+) T-cell-mediated Sjögren’s syndrome-like corneal epithelial apoptosis via activation of the extrinsic apoptotic pathway by interferon-gamma. Am J Pathol. 2011;179:1807–1814. doi: 10.1016/j.ajpath.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaumburg CS, Siemasko KF, De Paiva CS, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental auto-immune lacrimal keratoconjunctivitis. J Immunol. 2011;187:3653–3662. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 40.Chen YT, Lazarev S, Gallup M, et al. Interleukin-1 is a determinant for ocular mucin alteration in autoimmune regulator-mediated keratinizing squamous metaplasia. ARVO Meet Abstr. 2010;51:1561. [Google Scholar]
- 41.Chen YY, Wang HH, Antonioli DA, et al. Significance of acid-mucin-positive nongoblet columnar cells in the distal esophagus and gastroesophageal junction. Hum Pathol. 1999;30:1488–1495. doi: 10.1016/s0046-8177(99)90172-7. [DOI] [PubMed] [Google Scholar]
- 42.Pflugfelder SC, Huang AJ, Feuer W, et al. Conjunctival cytologic features of primary Sjögren’s syndrome. Ophthalmology. 1990;97:985–991. doi: 10.1016/s0161-6420(90)32478-8. [DOI] [PubMed] [Google Scholar]
- 43.Ishida Y, Kondo T, Kimura A, et al. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-beta signal pathway. J Immunol. 2006;176:5598–5606. doi: 10.4049/jimmunol.176.9.5598. [DOI] [PubMed] [Google Scholar]
- 44.Li DQ, Tseng SC. Differential regulation of keratinocyte growth factor and hepatocyte growth factor/scatter factor by different cytokines in human corneal and limbal fibroblasts. J Cell Physiol. 1997;172:361–372. doi: 10.1002/(SICI)1097-4652(199709)172:3<361::AID-JCP10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Ishida Y, Kondo T, Kimura A, et al. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-beta signal pathway. J Immunol. 2006;176:5598–5606. doi: 10.4049/jimmunol.176.9.5598. [DOI] [PubMed] [Google Scholar]
- 46.Moore JE, McMullen TC, Campbell IL, et al. The inflammatory milieu associated with conjunctivalized cornea and its alteration with IL-1 RA gene therapy. Invest Ophthalmol Vis Sci. 2002;43:2905–2915. [PubMed] [Google Scholar]