Abstract
G protein-coupled receptors (GPCRs) are membrane proteins that recognize molecules in the extracellular milieu and transmit signals inside cells to regulate their behaviors. Ligands for many GPCRs are hormones or neurotransmitters that direct coordinated, stereotyped adaptive responses. Ligands for other GPCRs provide information to cells about the extracellular environment. Such information facilitates context-specific decision making that may be cell autonomous. Among ligands that are important for cellular decisions are amino acids, required for continued protein synthesis, as metabolic starting materials and energy sources. Amino acids are detected by a number of class C GPCRs. One cluster of amino acid-sensing class C GPCRs includes umami and sweet taste receptors, GPRC6A, and the calcium-sensing receptor. We have recently found that the umami taste receptor heterodimer T1R1/T1R3 is a sensor of amino acid availability that regulates the activity of the mammalian target of rapamycin. This review focuses on an array of findings on sensing amino acids and sweet molecules outside of neurons by this cluster of class C GPCRs and some of the physiologic processes regulated by them.
The common feature of the class C G protein-coupled receptors (GPCRs) discussed here, T1R1/T1R3, T1R2/T1R3, GPRC6A, and the calcium-sensing receptor (CaSR), is that each can detect amino acids. We begin by pointing out some unresolved questions concerning these receptors that are elaborated upon below. Natural ligands have been identified, but it seems possible that additional ligands may be found. These receptors have large Venus flytrap ligand-binding segments that can accommodate a variety of molecules, agonists, allosteric regulators, and inhibitors. A range of evidence indicates that these receptors are dimers: 2 are thought to be obligate heterodimers (T1R1/T1R3 and T1R2/T1R3) and 2 are thought to be homodimers (GPRC6A and CaSR). Although actions of these receptors are often consistent with signaling through the Gαi family, the G protein-specific signal transduction mechanisms used by each receptor continue to be debated. The linkage of activation of these receptors to secretory responses and metabolic control is also in dispute. This review discusses roles of these receptors in pancreatic and gastrointestinal tissues. Although much is known, much also remains to be determined about the essential functions of these GPCRs.
T1R taste receptors
Identified as gustatory taste receptors and linked to a sweet-responsive locus in mice (1–3), the mammalian sweet and umami taste receptors were first characterized by Zuker and associates (4–6). T1R2 was demonstrated to be a heterodimer with T1R3 to form a receptor complex that is activated by a broad range of sweet compounds. The rodent and human T1Rs are only approximately 70% identical, which results in differences in agonist sensitivity (Table 1). For instance, in the initial studies, the human, but not rodent, T1R2/T1R3 was responsive to both aspartame and cyclamate. T1R2/T1R3 also responds to glycine and the sweet d-amino acids, but not their l-enantiomers. The T1R3 subunit was shown to heterodimerize not only with T1R2 but also with T1R1 to form the amino acid taste receptor complex (T1R1/T1R3). This heterodimer is responsive to most of the 20 l-amino acids, but not their d-enantiomers. Responses in a reconstituted system required expression of both subunits, consistent with the behavior of an obligate heterodimer. Studies looking at the purine nucleotide enhancement of the T1R1/T1R3 response to amino acids, monosodium glutamate, and the mGluR-agonist l(+)-2-amino-4-phosphonobutyric acid, led to the conclusion that T1R1/T1R3 was the umami receptor. Sequence differences between human and rodent T1R1 result in increased sensitivity of human T1R1 to glutamate (4). The connection of T1R1, T1R2, and T1R3 to both sweet and umami tastes was largely substantiated upon generation of mice in which the receptor genes were disrupted (6). Nerve activity, albeit decreased, was observed in response to sugars and umami compounds in T1R3 null mice developed in a different laboratory, suggesting that there may be additional taste mechanisms for these compounds (7). Perhaps such activity was mediated through related family members or alternative oligomeric receptor complexes (see Future Perspectives).
Table 1.
Nutrient and Pharmacological Regulatorsa
| Receptor | Agonists | Allosteric Activators | Inhibitors | References |
|---|---|---|---|---|
| T1R1/T1R3 | l-Amino acids | Purine nucleotides | Lactisole | 4, 100–103 |
| Monosodium glutamate | Cyclamate | Fibrates | ||
| S809 | Phenoxy herbicides | |||
| T1R2/T1R3 | Fructose | Cyclamate | Lactisole | 4, 100–104,114–116 |
| Galactose | S819 | Fibrates | ||
| Glucose | se2 | Phenoxy herbicides | ||
| Lactose | se3 | Gurmarin | ||
| Maltose | ||||
| Sucrose | ||||
| Gly | ||||
| d-Ala | ||||
| d-Asn | ||||
| d-Gln | ||||
| d-His | ||||
| d-Phe | ||||
|
d-Trp Artificial sweeteners (e.g. Saccharin, Sucrolose, Aspartame) |
||||
| GPRC6A | l-Amino acids | Ca2+ | Calindol | 56, 105, 106 |
| Osteocalcin | NPS2143 | |||
| Ca2+ | 2-Phenyl-indole-derived compounds 1–3 | |||
| Testosterone | ||||
| CaSR | Ca2+ | Amino acids | Calhex 231 | 31, 90, 107–110 |
| NPS R-568 | NPS2143 | |||
| Calindol | ||||
| AMG073/Cinacalcet |
Many studies have suggested that stimulation of T1R1/T1R3 and T1R2/T1R3 in gustatory neurons leads to the activation of α-gustducin, a Gαi family member associated with taste perception. Activation of α-gustducin and subsequent release of the Gβγ subunit are thought to cause an increase in phospholipase C (PLC) β-2 activity. Subsequent increases in inositol triphosphate induce intracellular Ca2+ release, leading to the gating of the transient receptor potential TRPM5 ion channel and subsequent depolarization (reviewed in Ref. 8). As early as 1996, the idea that α-gustducin might be involved in chemosenory mechanisms in nongustatory tissues was postulated after α-gustducin was found in stomach, intestine, and the pancreatic duct system (9, 10). A few years after discovery of the taste receptors, T1R2 and T1R3 were observed in mouse small intestine and human enteroendocrine l-cells and have since been evaluated as potential regulators of digestion and nutrient uptake (11). Dietary sugars increase intestinal glucose transport by increasing the expression of the gut sodium-dependent glucose co-transporter 1 (12–14). T1R3 and α-gustducin are necessary for increased sodium-dependent glucose co-transporter 1 induction by dietary carbohydrates and sucralose (15). Other studies have suggested that the activation of T1R1/T1R3 and T1R2/T1R3 by glutamate, glucose, and artificial sweeteners regulates the intestinal apical expression of a peptide transporter and the glucose transporter GLUT2 (16, 17). Thus, both heterodimers are thought to affect nutrient absorption.
Diverse studies have linked sweet taste receptors and α-gustducin to intestinal secretion of the amplifying hormone glucagon-like peptide 1 (GLP-1), which affects gut motility, increases satiety, and enhances insulin secretion from pancreatic β-cells in response to glucose. GLP-1 secretion induced by ingested glucose was reduced in mice null for α-gustducin; the deficit was also observed in isolated intestinal tissue. Secretion of GLP-1 induced by glucose, fructose, and sucralose from ileum explants from either T1R2 or T1R3 null mice was significantly reduced relative to that from wild type mice (18). Supported by T1R1 depletion experiments, activation of T1R1/T1R3 by phenylalanine, leucine, and glutamate in STC-1 enteroendocrine cells and primary mouse intestinal tissue induced the secretion of cholecystokinin (19). The T1R3 inhibitor lactisole inhibited glucose-induced GLP-1 secretion from human l-cells and was also reported to inhibit GLP-1 secretion in human subjects after intragastric or intraduodenal glucose infusion (20, 21). Ingestion of a diet soda sweetened with sucralose and acesulfame-K 10 minutes before an oral glucose tolerance test caused a significant increase in serum GLP-1 levels in human subjects in one study; however, no difference was seen in serum glucose or insulin concentrations between test and control groups (22). In a related study, administration of the artificial sweetener sucralose by direct infusion into the stomachs of human volunteers did not cause increased release of insulin, GLP-1, or gastric inhibitory peptide (23). A recent review provides an in-depth discussion on the effects of artificial sweeteners in the gastrointestinal tract (24). Part of the discrepancy in the studies with human subjects could be related to common polymorphisms in the T1R genes that affect the ability of these receptors to be activated by various ligands (25–28, 99). It is possible that these polymorphisms cause inconsistencies between subjects in their hormone secretion response to various artificial sweeteners. Therefore, future studies in individuals in which polymorphisms in T1R1, T1R2, and T1R3 genes are sequenced may be helpful in resolving these controversies. If these receptors enhance GLP-1 secretion, it remains to be determined whether it has a physiologic impact.
Agonist availability regulates the expression and/or membrane localization of taste receptors. Glucose or l-glutamate perfusion of rat jejunum for 20 minutes decreased the expression of T1R1, T1R2, and T1R3 at the apical membrane (16, 17). As blood glucose concentrations increased in type 2 diabetic human subjects, the expression of the receptors T1R2 and T1R3, and pathway components, TRPM5 and α-gustducin, decreased. In addition, the direct infusion of glucose into mouse jejunum resulted in a significant decrease in T1R2 expression (29). Expression of T1R1 and T1R3 was increased in the skeletal muscle of fasted mice compared with those of fed control mice (31). These findings support the idea that nutrients are relevant ligands for these receptors and suggest a feedback mechanism for receptor expression.
Although many studies have investigated the functions of T1Rs in the intestine, these GPCRs are expressed in many other tissues (31). All three T1R subunits and α-gustducin are present in mouse and human pancreatic islets and have been implicated in regulation of insulin secretion (31–33). Artificial sweeteners, sucralose, saccharin, and acesulfame-K, induce insulin secretion from rodent islets and a β-cell line (32). The activity of sweeteners was blocked by gurmarin, a peptide inhibitor of rodent T1R2/T1R3 (See Table 1) (15, 34). Fructose enhanced glucose-induced, but not basal, insulin secretion in mice and isolated mouse and human islets. This was not the case in T1R2−/− mice, islets isolated from these mice, or human islets treated with the T1R3 inhibitor lactisole. A recent study reported that l-glutamate and l-arginine stimulate T1R1/T1R3 to promote insulin secretion; however, we find that this receptor affects insulin content more than insulin secretion, as discussed further below (31, 33).
Consistent with an action of taste receptors on glucose homeostasis, T1R3−/− mice displayed delayed glucose clearance and decreased plasma insulin concentrations during an oral glucose tolerance test. However, T1R3−/− and wild-type mice displayed similar plasma insulin concentrations following an ip glucose tolerance test, suggesting that it was T1R3 expression in the gut that was important for the modulation of plasma glucose. Insulin secreted from isolated islets from T1R3−/− and wild-type mice was reported to be the same after 30 minutes of stimulation with 12.5 mM glucose; however, total internal reflection fluorescence microscopy revealed a lower rate of insulin granule fusion with secretory vesicles in islets from T1R3−/−, but not T1R2−/− mice (18). No differences in fusion kinetics were observed with 30 mM KCl as the stimulus. Still unclear is the exact role of these receptors in glucose homeostasis.
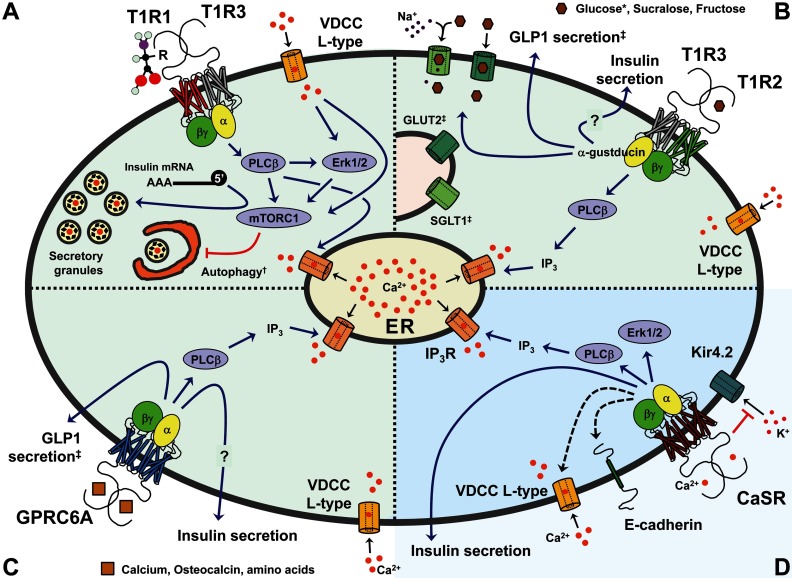
We investigated the role of T1R1/T1R3 in amino acid stimulation of the MAPKs ERK1/2 in pancreatic β-cells. ERK1/2 activation is a rapid indicator of the demand for insulin secretion in response to nutrients, hormones, and neurotransmitters. Once activated, ERK1/2 regulate nutrient-dependent insulin gene transcription (35). We found that amino acids activate ERK1/2 through T1R1/T1R3 because their activities were no longer increased by amino acids in cells depleted of one or the other receptor protomer. Amino acid-induced insulin secretion was reduced, but, as noted earlier in this paper, this was due to a significant loss of insulin content. Lowered insulin content might occur for a number of reasons. The mammalian target of rapamycin (mTOR) is controlled in most cells by amino acid availability. mTOR complex 1 (C1) inhibits autophagy and facilitates protein translation by phosphorylating and activating p70 S6 ribosomal protein kinase and phosphorylating and inhibiting eukaryotic initiation factor 4E-binding protein 1, a protein that binds and inhibits translation initiation by eukaryotic initiation factor 4E. Limiting amino acids reduces the activity of mTORC1, resulting in slowed translation, increased autophagy, and decreased cell growth. Consistent with a role for amino acid signaling through T1R1/T1R3 to mTORC1 in regulation of insulin content, depletion of T1R1 or T1R3 blocked mTORC1 activation by amino acids (Figure 1).
Figure 1.
Signaling through T1R1/T1R3, T1R2/T1R3, GPRC6A, and CaSR in β- or Gut Cells. In light green are GPCRs that can be directly activated by amino acids and use calcium as an allosteric regulator (A–C), whereas in blue is the CaSR whose main ligand is calcium and can be allosterically regulated by amino acids (D). See Table 1 for a comprehensive list of agonists, allosteric activators, and inhibitors for the reviewed GPCRs. A, T1R1/T1R3 receptor. In β, heart, and HeLa cells, the receptor activates PLCβ, ERK1/2, and mTORC1 and promotes release of calcium stores. Together, these pathways positively regulate 5′-cap-dependent transcription of the insulin message and may also promote insulin secretion. Receptor knockdown cells display reduced insulin levels and rampant autophagy. B, T1R2/T1R3 receptor. In enterocyte l-cells, the receptor activates PLCβ and promotes GLP-1 secretion and increased plasma membrane expression of the glucose transporters SGLT1 (sodium-dependent glucose co-transporter 1) and GLUT2 (glucose transporter 2). Signaling in β-cells is likely conserved and is proposed to regulate insulin secretion. C, GPRC6A receptor. In enterocyte l-cells the receptor activates PLCβ and promotes secretion of GLP-1. Signaling in β-cells is likely conserved and is proposed to regulate insulin secretion. D, CaSR receptor. In β-cells the receptor activates PLCβ and ERK1/2, induces release of ER calcium stores, stimulates expression of E-cadherin and l-type VDCC (black dotted arrows), inhibits Kir4.2 channels through protein-protein interactions, and promotes insulin secretion. The CaSR also regulates hormone secretion in other endocrine tissues. *, T1R2/T1R3 displays differential sensitivity for sweet compounds between humans and mice (4–6). †, Regulation of autophagy by the T1R1/T1R3 receptor was shown in heart and HeLa cells but not yet in β-cells. ‡, Described in enterocyte l-cells of the small intestine. See text and Table 1. ER, endoplasmic reticulum; IP3, inositol triphosphate; IP3R, IP3 receptor.
Because mTORC1 is regulated by amino acids in most or all cell types, we examined expression of T1R1 and T1R3 in mouse tissues and several cultured cell lines and found that both were expressed in all sources tested. Thus, we asked whether T1R1/T1R3 is used for amino acid activation of mTORC1 more broadly throughout the body. Depletion of either protomer from cardiomyoblasts, HeLa, and MIN6 cells reduced mTORC1 activity, consistent with the observed decreased levels of mTORC1 activity in T1R3−/− mouse tissues. In addition, receptor knockdown cells were deficient in recruitment of translation initiation complexes to 5′-capped messages (31). Autophagy, a process of degradative turnover of proteins and organelles that provides cells with nutrients to survive during conditions of nutrient limitation, is usually inhibited by mTORC1. We found that autophagy was increased as T1R1 or T1R3 expression was decreased even in the presence of amino acids. Counter to the dogma in the field which posited that mTORC1 only detects amino acids after they enter cells, we conclude that amino acid availability is first reported to mTORC1 by a T1R1/T1R3-mediated process.
Taste receptors generally signal through the Gαi protein α-gustducin, yet α-gustducin knockout mice are still able to sense sweet and umami flavors (36–39). In addition, in some tissues T1R1/T1R3 has been proposed to signal through Gαs or the Gαq family member Gα14 (32, 40, 41). In vitro reconstitution of G protein-coupled signaling using purified G proteins and purified 7-transmembrane-spanning core domains of T1R1, T1R2, and T1R3 showed that T1R1 and T1R2 activate Gαi/o family members but not Gαs or Gαq, whereas T1R3 was unable to activate any of the tested Gα-subunits (42). These in vitro data support a preference of the 7-transmembrane-spanning core domains for Gαi-subunits but fails to address how full-length heterodimeric sweet and umami receptors, as well as homodimeric T1R3 calcium-tasting receptors (43), signal in vivo to heterotrimeric G proteins.
In β-cells, Gαi family proteins inhibit insulin secretion, whereas receptors that couple to Gαs and Gαq family members generally augment glucose-stimulated insulin secretion (44–47). We find that amino acid stimulation of starved β-cells activates ERK1/2 in a cAMP-independent and pertussis toxin-insensitive manner (Wauson, E.M., M. L. Guerra, K. McGlynn, E. Ross, and M. H. Cobb, in preparation), suggesting that in β-cells T1R1/T1R3 does not signal through Gαi or Gαs. Moreover, amino acid-induced activation of ERK1/2 and mTORC1 in β-cells was reduced by PLC inhibitors, calcium chelators, and calcium channel blockers (31). Together these findings indirectly suggest that mediation of amino acid activation of mTORC1 by T1R1/T1R3 may be through Gαq but not Gαi, despite the strong connection of taste receptors to α-gustducin in other systems. Because amino acids do not inhibit insulin secretion, Gαi family members may not be coupled to amino acid receptors or inhibitory effects of Gαi may be squelched by other signaling events. Signaling through Gβγ appears to be an important and dominant component in gustatory neurons and, like Gαq but unlike Gαi, also activates PLCβ (42, 48–50). The potential synergism between Gαq and Gβγ in response to amino acid stimulation in β-cells should be explored.
Adding complexity to this system, amino acid-stimulated mTORC1 activity in C2C12 myoblasts was reduced independently both by treatment with pertussis toxin (Gαi) and by knock down of a PLCβ isoform (Gβγ and/or Gαq) (51). Together, these results indicate that the model of T1R1/T1R3 signaling, as defined in gustatory neurons, is unlikely to be generalized to all tissues. There is a gap in our understanding of the dominance and synergistic/antagonistic coupling of signals between Gα subunits and between Gα and Gβγ subunits in nongustatory tissues. With regard to β-cells, it still needs to be resolved whether signaling mechanisms activated by T1R1/T1R3 leading to different outputs are initiated by α-gustducin, Gβγ, Gαq family members, or distinct G protein-dependent or -independent events, singly or in combination.
In surveying the diverging findings on responsiveness and functionality of the taste receptors, we suggest that differences in ligand specificity, organism- and cell type-selective coupling to signal transduction pathways, and receptor-dimer partnerships may complicate interpretation of their activities (see “Future Perspectives”).
GPRC6A
GPRC6A, like the T1R1/T1R3 heterodimer, can be activated by amino acids, and some of its actions on glucose homeostasis are reminiscent of taste receptor effects. This family C-GPCR was cloned independently by 2 separate groups (52, 53). Glycine and the l-amino acids, arginine, lysine, alanine, serine, ornithine, and citrulline, activated a chimeric GPRC6A receptor expressed in Xenopus oocytes (52, 54) (Table 1). The basic amino acids are the most potent agonists of wild-type mouse GPRC6A transfected into a human embryonic kidney (HEK)293 cell line (55). In addition to amino acids, GPRC6A is activated by calcium, other cations, calcimimetics, and osteocalcin (Ocn), a protein secreted by osteoclasts (56). GPRC6A is expressed in a pancreatic β-cell line and in mouse pancreas. By activating GPRC6A, Ocn may induce insulin secretion and insulin expression in islets (56) and GLP-1 secretion in intestinal L cells (57). The effect of Ocn on serum insulin was enhanced by the dipeptidyl peptidase IV inhibitor sitagliptin and decreased by exendin-(9–39), a GLP-1 receptor agonist, suggesting that the effect of Ocn on insulin concentration was through increased GLP-1 (57). This is consistent with GLP-1 secretion induced by ornithine from an intestinal L cell line (58). Cations including calcium and magnesium either activate GPRC6A directly, or positively modulate the receptor in the presence of amino acids (55, 59). GPRC6A may also be activated by testosterone and involved in nongenomic effects of steroid hormones (60). These cell-based studies suggest a role for this receptor in glucose homeostasis but the mechanism has not yet been clarified by characterization of GPRC6A−/− mice.
GPRC6A−/− mice were independently developed in 2 laboratories that have drawn somewhat different conclusions (61, 62) (Figure 1). In one study GPRC6A−/− mice displayed the hallmarks of metabolic syndrome including insulin resistance, glucose intolerance, hyperglycemia, and hepatic steatosis, and also manifested a decrease in bone mineral density (61). These GPRC6A−/− mice secreted significantly less insulin in response to l-arginine than wild-type mice, contained less pancreatic insulin, and had reduced pancreatic mass (63). The other group reported that the GPRC6A−/− mouse neither exhibited a detectable reduction in bone mineral density, nor was there a defect in l-arginine-induced insulin secretion, either in vivo or from isolated pancreatic islets (62, 64). Additionally, no metabolic defects were observed in these mice on a normal diet. However, GPRC6A−/− mice on a high-fat diet had significantly increased body weight, and increased circulating insulin and leptin concentrations compared with wild-type mice on the same diet (65). The more obvious metabolic abnormalities appeared in mice in which exon 2 of GPRC6A had been targeted, whereas exon 6 was targeted in the other study. These differences and potential animal background differences may have contributed to the penetrance of the phenotype. GPRC6A appears to be involved in glucose metabolism; whether its primary actions are on GLP-1 secretion or directly on insulin secretion appears to be an open question.
Extracellular Ca2+ sensing receptor
The extracellular CaSR is activated by calcium and other divalent and trivalent organic and inorganic cations (66). In gastric mucosa the CaSR also senses pH and l-amino acids, aromatic > aliphatic > polar amino acids (66, 67) (Table 1). The CaSR regulates a wide variety of cellular processes, including proliferation, differentiation, gene expression, ion channel function, and hormone secretion. The most studied roles are the homeostatic maintenance of systemic calcium through regulating synthesis and secretion of PTH, and the concentration of calcium in urine by the kidney (66).
The CaSR is widely expressed, including in pancreatic acinar and ductal cells, where it is reported to regulate secretion of digestive enzymes (68–70). In islets, it is expressed predominantly in α- and β-cells (71–75). CaSR is proposed to signal through Gαi/o, Gαq/11, Gα12/13, and even Gαs (66, 68, 76, 77). Knockdown and other studies suggest a requirement for PLC/inositol triphosphate and Gq/11 in β-cells (70, 72, 78, 79). ERK1/2 activation is also important for stimulation of proliferation by CaSR (78, 80, 81). Studies in β-cells are needed to determine which heterotrimeric G proteins couple to CaSR, and how it signals to ERK1/2.
Physiologic functions of CaSR in tissues that do not control systemic calcium are poorly understood. CaSR may monitor extracellular calcium in the exocrine pancreas to prevent formation of calcium stones and pancreatitis (69, 70). It has been proposed that gastrin-secreting cells of the gastric antrum use the CaSR to sense dietary calcium; activation of CaSR by amino acids was reported to induce the secretion of cholecystokinin and GLP-1 from intestinal cells (82–84). A general model discussed later in this paper suggests that the CaSR senses Ca2+ and other divalent cations in the extracellular space to synchronize and coordinate local calcium sensing and exocytosis (85) (Figure 1). This last hypothesis seems to fit findings in islet cells. CaSR stimulates glucagon secretion from islets (78, 86). In islets and β-cell lines, CaSR activation in the presence of substimulatory glucose promotes the rapid release of calcium stores into the cytosol and a robust first-phase insulin secretion. However, prolonged incubation with high extracellular calcium inhibits second-phase secretion (30, 72, 73, 75, 78, 81).
In considering the model suggested above, secretory granules are estimated to contain 60–120 mM calcium (87); recent measurements of calcium release into pseudoislet intercellular spaces ([Ca2+]inter) indicate that within 15 minutes after stimulation with glucose, [Ca2+]inter increases by about 1.31 mM (86). Such a concentration of calcium may be high enough to activate CaSR signaling. In support of this model, in fibroblasts cocultured with HEK293 cells, the local change in extracellular calcium following fibroblast exocytosis is sufficient to stimulate the CaSR on neighboring HEK293 cells (88). A recent study in MIN6 cells reports that calcium promotes expression of the epithelial adhesion protein E-cadherin and stimulates cell-cell tethering (80). CaSR expression appears to be regulated by its 3-dimensional distribution in the tissue; although MIN6 pseudoislets express one-third of the CaSR as MIN6 monolayers, pseudoislets respond more robustly to glucose stimulation compared with monolayers, and this response is decreased in CaSR-depleted pseudoislets (80, 81, 89). These findings suggest that islets and pseudoislets should be more sensitive to calcium than monolayers, that there should be CaSR-dependent crosstalk between α- and β-cells, and that CaSR signaling should be important for pancreatic islet formation.
The CaSR also modulates β-cell granule secretion by proximity to 2 different types of cation channels: l-type voltage-dependent calcium channel (VDCC) and potassium inward rectifying (Kir) channels. Calcium stimulation was reported to promote expression of the l-type VDCC (80), and 2 recent studies showed that both glucose and l-histidine promote colocalization of the CaSR with the l-type VDCC (90, 91). Although the physical details are lacking, this putative interaction is highly relevant because the l-type VDCC is an important component of insulin secretion. With regard to Kir channels, in kidney cells, stimulation of CaSR promotes its association with Kir4.1 and Kir4.2, and this association inactivates both potassium channels (92). A recent study in INS1 cells shows that the Kir4.2 channel is a negative regulator of glucose-stimulated insulin secretion, and that it interacts with the CaSR (93). Similar to the case in kidney cells, association with the CaSR may inhibit Kir4.2 function, which may be required for efficient glucose-stimulated insulin secretion. The potential regulation of l-type VDCC and Kir4.1/Kir4.2 channels by CaSR signaling in β-cells should be further explored. As with the GPCRs discussed earlier in this paper, CaSR appears to be intimately involved with glucose homeostasis.
Future Perspectives
The heterotrimeric G protein signaling downstream of the umami, sweet, CaSR, and GPRC6A receptors still needs to be defined in greater detail. It seems clear that extrapolation from characterized systems (eg, gustatory neurons) will be inadequate to explain signaling in all systems. It is likely that complex relations exist between Gα subunits as well as between Gα and Gβγ subunits that need to be explored.
Although much is known about CaSR desensitization and membrane trafficking between the plasma membrane and endolysosomal compartments (reviewed in Refs. 94 and 95), little is known about how umami, sweet, and GPRC6A receptors are desensitized, or how they are internalized and recycled/degraded in response to agonist stimulation.
These receptors are structurally similar and have a large common set of ligands and allosteric regulators (eg, amino acids and calcium; see Table 1). A recent study showed that T1R3, a protomer thought to be in obligate heterodimers, can homodimerize and act as an unexpected calcium taste receptor (43). This suggests that homo-, hetero-, and higher order oligomeric interactions among different family members should be explored (96, 97); such interactions introduce potential for unanticipated ligand regulation and novel outputs. Crosstalk between other members of the class C family of GPCRs has been reported, some of which involves formation of higher order oligomers (reviewed in Ref. 98).
Finally, it is self-evident that these class C receptors are promiscuous in their ligand selection (or, at the least, we have not recognized essential specificity determinants); rather they seem to respond to common ligands with a different order of selectivity (Table 1). Screens to determine what other nutrient arrays activate or modulate the activity of these combinatorial receptor states should provide a better understanding of how cells integrate nutritional cues to make appropriate decisions.
Acknowledgments
We thank Michael Kalwat and Hashem Dbouk for critically reviewing the manuscript and Dionne Ware for administrative support. All are active members of the Cobb laboratory.
This work was supported by NIH Grants R01 DK55310 and R37 DK34128 and a grant from the Cancer Prevention and Research Institute of Texas (to M.H.C.) and NIH training grant 2T32CA124334–06 (to A.L.R.).
Disclosure Summary: The authors declare no conflicts of interest.
Footnotes
- CaSR
- calcium-sensing receptor
- GLP-1
- glucagon-like peptide 1
- GPCR
- G protein-coupled receptor
- HEK
- human embryonic kidney
- Kir
- potassium inward rectifying
- mTOR
- mammalian target of rapamycin
- Ocn
- osteocalcin
- PLC
- phospholipase C
- VDCC
- voltage-dependent calcium channel.
References
- 1. Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551 [DOI] [PubMed] [Google Scholar]
- 2. Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604 [DOI] [PubMed] [Google Scholar]
- 3. Max M, Shanker YG, Huang L, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63 [DOI] [PubMed] [Google Scholar]
- 4. Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416:199–202 [DOI] [PubMed] [Google Scholar]
- 5. Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390 [DOI] [PubMed] [Google Scholar]
- 6. Zhao GQ, Zhang Y, Hoon MA, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266 [DOI] [PubMed] [Google Scholar]
- 7. Damak S, Rong M, Yasumatsu K, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853 [DOI] [PubMed] [Google Scholar]
- 8. Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294 [DOI] [PubMed] [Google Scholar]
- 9. Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of α-gustducin. Proc Natl Acad Sci USA. 1996;93:6631–6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Höfer D, Drenckhahn D. Identification of the taste cell G-protein, α-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol. 1998;110:303–309 [DOI] [PubMed] [Google Scholar]
- 11. Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305 [DOI] [PubMed] [Google Scholar]
- 12. Freeman TC, Wood IS, Sirinathsinghji DJ, Beechey RB, Dyer J, Shirazi-Beechey SP. The expression of the Na+/glucose cotransporter (SGLT1) gene in lamb small intestine during postnatal development. Biochim Biophys Acta. 1993;1146:203–212 [DOI] [PubMed] [Google Scholar]
- 13. Miyamoto K, Hase K, Takagi T, et al. Differential responses of intestinal glucose transporter mRNA transcripts to levels of dietary sugars. Biochem J. 1993;295(Pt 1):211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shirazi-Beechey SP, Hirayama BA, Wang Y, Scott D, Smith MW, Wright EM. Ontogenic development of lamb intestinal sodium-glucose co-transporter is regulated by diet. J Physiol. 1991;437:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104:15075–15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mace OJ, Lister N, Morgan E, et al. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J Physiol. 2009;587:195–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geraedts MC, Takahashi T, Vigues S, et al. Transformation of postingestive glucose responses after deletion of sweet taste receptor subunits or gastric bypass surgery. Am J Physiol Endocrinol Metab. 2012;303:E464–E474 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Daly K, Al-Rammahi M, Moran A, Marcello M, Ninomiya Y, Shirazi-Beechey SP. Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am J Physiol Gastrointest Liver Physiol. 2013;304:G271–G282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 2007;104:15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerspach AC, Steinert RE, Schönenberger L, Graber-Maier A, Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab. 2011;301:E317–E325 [DOI] [PubMed] [Google Scholar]
- 22. Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care. 2009;32:2184–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma J, Bellon M, Wishart JM, et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G735–G739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown RJ, Rother KI. Non-nutritive sweeteners and their role in the gastrointestinal tract. J Clin Endocrinol Metab. 2012;97:2597–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shigemura N, Shirosaki S, Ohkuri T, et al. Variation in umami perception and in candidate genes for the umami receptor in mice and humans. Am J Clin Nutr. 2009;90:764S–769S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inoue M, Glendinning JI, Theodorides ML, et al. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics. 2007;32:82–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fushan AA, Simons CT, Slack JP, Manichaikul A, Drayna D. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr Biol. 2009;19:1288–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raliou M, Grauso M, Hoffmann B, et al. Human genetic polymorphisms in T1R1 and T1R3 taste receptor subunits affect their function. Chem Senses. 2011;36:527–537 [DOI] [PubMed] [Google Scholar]
- 29. Young RL, Sutherland K, Pezos N, et al. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2009;58:337–346 [DOI] [PubMed] [Google Scholar]
- 30. Straub SG, Kornreich B, Oswald RE, Nemeth EF, Sharp GW. The calcimimetic R-467 potentiates insulin secretion in pancreatic β cells by activation of a nonspecific cation channel. J Biol Chem. 2000;275:18777–18784 [DOI] [PubMed] [Google Scholar]
- 31. Wauson EM, Zaganjor E, Lee A, et al. The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy. Mol Cell. 2012;47:851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakagawa Y, Nagasawa M, Yamada S, et al. Sweet taste receptor expressed in pancreatic β-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE. 2009;4:e5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oya M, Suzuki H, Watanabe Y, Sato M, Tsuboi T. Amino acid taste receptor regulates insulin secretion in pancreatic β-cell line MIN6 cells. Genes Cells. 2011;16:608–616 [DOI] [PubMed] [Google Scholar]
- 34. Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R960–R971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khoo S, Griffen SC, Xia Y, Baer RJ, German MS, Cobb MH. Regulation of insulin gene transcription by extracellular-signal regulated protein kinases (ERK) 1 and 2 in pancreatic β cells. J Biol Chem. 2003;278:32969–32977 [DOI] [PubMed] [Google Scholar]
- 36. McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569 [DOI] [PubMed] [Google Scholar]
- 37. Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800 [DOI] [PubMed] [Google Scholar]
- 38. Ruiz CJ, Wray K, Delay E, Margolskee RF, Kinnamon SC. Behavioral evidence for a role of α-gustducin in glutamate taste. Chem Senses. 2003;28:573–579 [DOI] [PubMed] [Google Scholar]
- 39. He W, Yasumatsu K, Varadarajan V, et al. Umami taste responses are mediated by α-transducin and α-gustducin. J Neurosci. 2004;24:7674–7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muroi Y, Ishii T. Umami taste receptor functions as an amino acid sensor via Gαs subunit in N1E-115 neuroblastoma cells. J Cell Biochem. 2012;113:1654–1662 [DOI] [PubMed] [Google Scholar]
- 41. Shindo Y, Miura H, Carninci P, et al. G α14 is a candidate mediator of sweet/umami signal transduction in the posterior region of the mouse tongue. Biochem Biophys Res Commun. 2008;376:504–508 [DOI] [PubMed] [Google Scholar]
- 42. Sainz E, Cavenagh MM, LopezJimenez ND, et al. The G-protein coupling properties of the human sweet and amino acid taste receptors. Dev Neurobiol. 2007;67:948–959 [DOI] [PubMed] [Google Scholar]
- 43. Tordoff MG, Alarcón LK, Valmeki S, Jiang P. T1R3: a human calcium taste receptor. Sci Rep. 2012;2:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gibson TB, Lawrence MC, Gibson C, et al. Inhibition of glucose-stimulated activation of extracellular signal-regulated protein kinases 1 and 2 by epinephrine in pancreatic β-cells. Diabetes. 2006;55:1066–1073 [DOI] [PubMed] [Google Scholar]
- 45. Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA. 1987;84:3434–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fukudo S, Virnelli S, Kuhn CM, Cochrane C, Feinglos MN, Surwit RS. Muscarinic stimulation and antagonism and glucoregulation in nondiabetic and obese hyperglycemic mice. Diabetes. 1989;38:1433–1438 [DOI] [PubMed] [Google Scholar]
- 47. Duttaroy A, Zimliki CL, Gautam D, Cui Y, Mears D, Wess J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes. 2004;53:1714–1720 [DOI] [PubMed] [Google Scholar]
- 48. Rössler P, Kroner C, Freitag J, Noè J, Breer H. Identification of a phospholipase C β subtype in rat taste cells. Eur J Cell Biol. 1998;77:253–261 [DOI] [PubMed] [Google Scholar]
- 49. Zhang Y, Hoon MA, Chandrashekar J, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301 [DOI] [PubMed] [Google Scholar]
- 50. Huang L, Shanker YG, Dubauskaite J, et al. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062 [DOI] [PubMed] [Google Scholar]
- 51. Mercan F, Lee H, Kolli S, Bennett AM. Novel role for SHP-2 in nutrient-responsive control of S6 kinase 1 signaling. Mol Cell Biol. 2013;33:293–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuang D, Yao Y, Lam J, Tsushima RG, Hampson DR. Cloning and characterization of a family C orphan G-protein coupled receptor. J Neurochem. 2005;93:383–391 [DOI] [PubMed] [Google Scholar]
- 53. Wellendorph P, Bräuner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene. 2004;335:37–46 [DOI] [PubMed] [Google Scholar]
- 54. Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Bräuner-Osborne H. Deorphanization of GPRC6A: a promiscuous L-α-amino acid receptor with preference for basic amino acids. Mol Pharmacol. 2005;67:589–597 [DOI] [PubMed] [Google Scholar]
- 55. Christiansen B, Hansen KB, Wellendorph P, Bräuner-Osborne H. Pharmacological characterization of mouse GPRC6A, an L-α-amino-acid receptor modulated by divalent cations. Br J Pharmacol. 2007;150:798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26:1680–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mizokami A, Yasutake Y, Gao J, et al. Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS ONE. 2013;8:e57375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oya M, Kitaguchi T, Pais R, Reimann F, Gribble F, Tsuboi T. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J Biol Chem. 2013;288:4513–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pi M, Faber P, Ekema G, et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280:40201–40209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pi M, Parrill AL, Quarles LD. GPRC6A mediates the non-genomic effects of steroids. J Biol Chem. 2010;285:39953–39964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pi M, Chen L, Huang MZ, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS ONE. 2008;3:e3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wellendorph P, Johansen LD, Jensen AA, et al. No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J Mol Endocrinol. 2009;42:215–223 [DOI] [PubMed] [Google Scholar]
- 63. Pi M, Wu Y, Lenchik NI, Gerling I, Quarles LD. GPRC6A mediates the effects of L-arginine on insulin secretion in mouse pancreatic islets. Endocrinology. 2012;153:4608–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smajilovic S, Clemmensen C, Johansen LD, et al. The L-α-amino acid receptor GPRC6A is expressed in the islets of Langerhans but is not involved in L-arginine-induced insulin release. Amino Acids. 2013;44:383–390 [DOI] [PubMed] [Google Scholar]
- 65. Clemmensen C, Smajilovic S, Madsen AN, Klein AB, Holst B, Brauner-Osborne H. Increased susceptibility to diet-induced obesity in GPRC6A receptor knockout mice. J Endocrinol. 2013;217:151–160 [DOI] [PubMed] [Google Scholar]
- 66. Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297 [DOI] [PubMed] [Google Scholar]
- 67. Wellendorph P, Bräuner-Osborne H. Molecular basis for amino acid sensing by family C G-protein-coupled receptors. Br J Pharmacol. 2009;156:869–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Magno AL, Ward BK, Ratajczak T. The calcium-sensing receptor: a molecular perspective. Endocr Rev. 2011;32:3–30 [DOI] [PubMed] [Google Scholar]
- 69. Bruce JI, Yang X, Ferguson CJ, et al. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J Biol Chem. 1999;274:20561–20568 [DOI] [PubMed] [Google Scholar]
- 70. Rácz GZ, Kittel A, Riccardi D, Case RM, Elliott AC, Varga G. Extracellular calcium sensing receptor in human pancreatic cells. Gut. 2002;51:705–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rasschaert J, Malaisse WJ. Expression of the calcium-sensing receptor in pancreatic islet B-cells. Biochem Biophys Res Commun. 1999;264:615–618 [DOI] [PubMed] [Google Scholar]
- 72. Kato M, Doi R, Imamura M, Furutani M, Hosotani R, Shimada Y. Calcium-evoked insulin release from insulinoma cells is mediated via calcium-sensing receptor. Surgery. 1997;122:1203–1211 [DOI] [PubMed] [Google Scholar]
- 73. Squires PE, Persaud SJ, Jones PM, Buchan AM. The extracellular calcium-sensing receptor in PHHI β cells: does reduced auto-inhibitory input contribute to hypersecretion of insulin? Diabetologia. 2000;43:1078–1080 [DOI] [PubMed] [Google Scholar]
- 74. Squires PE, Harris TE, Persaud SJ, Curtis SB, Buchan AM, Jones PM. The extracellular calcium-sensing receptor on human β-cells negatively modulates insulin secretion. Diabetes. 2000;49:409–417 [DOI] [PubMed] [Google Scholar]
- 75. Komoto I, Kato M, Itami A, et al. Expression and function of the calcium-sensing receptor in pancreatic islets and insulinoma cells. Pancreas. 2003;26:178–184 [DOI] [PubMed] [Google Scholar]
- 76. Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580 [DOI] [PubMed] [Google Scholar]
- 77. Sharp GW. Mechanisms of inhibition of insulin release. Am J Physiol. 1996;271:C1781–C1799 [DOI] [PubMed] [Google Scholar]
- 78. Gray E, Muller D, Squires PE, et al. Activation of the extracellular calcium-sensing receptor initiates insulin secretion from human islets of Langerhans: involvement of protein kinases. J Endocrinol. 2006;190:703–710 [DOI] [PubMed] [Google Scholar]
- 79. Sassmann A, Gier B, Gröne HJ, Drews G, Offermanns S, Wettschureck N. The Gq/G11-mediated signaling pathway is critical for autocrine potentiation of insulin secretion in mice. J Clin Invest. 2010;120:2184–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hills CE, Younis MY, Bennett J, Siamantouras E, Liu KK, Squires PE. Calcium-sensing receptor activation increases cell-cell adhesion and β-cell function. Cell Physiol Biochem. 2012;30:575–586 [DOI] [PubMed] [Google Scholar]
- 81. Kitsou-Mylona I, Burns CJ, Squires PE, Persaud SJ, Jones PM. A role for the extracellular calcium-sensing receptor in cell-cell communication in pancreatic islets of langerhans. Cell Physiol Biochem. 2008;22:557–566 [DOI] [PubMed] [Google Scholar]
- 82. Buchan AM, Squires PE, Ring M, Meloche RM. Mechanism of action of the calcium-sensing receptor in human antral gastrin cells. Gastroenterology. 2001;120:1128–1139 [DOI] [PubMed] [Google Scholar]
- 83. Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012;590:2917–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang Y, Chandra R, Samsa LA, et al. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol. 2011;300:G528–G537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hodgkin MN, Hills CE, Squires PE. The calcium-sensing receptor and insulin secretion: a role outside systemic control 15 years on. J Endocrinol. 2008;199:1–4 [DOI] [PubMed] [Google Scholar]
- 86. Gerbino A, Maiellaro I, Carmone C, et al. Glucose increases extracellular [Ca2+] in rat insulinoma (INS-1E) pseudoislets as measured with Ca2+-sensitive microelectrodes. Cell Calcium. 2012;51:393–401 [DOI] [PubMed] [Google Scholar]
- 87. Hutton JC, Penn EJ, Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983;210:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hofer AM, Curci S, Doble MA, Brown EM, Soybel DI. Intercellular communication mediated by the extracellular calcium-sensing receptor. Nat Cell Biol. 2000;2:392–398 [DOI] [PubMed] [Google Scholar]
- 89. Jones PM, Kitsou-Mylona I, Gray E, Squires PE, Persaud SJ. Expression and function of the extracellular calcium-sensing receptor in pancreatic β-cells. Arch Physiol Biochem. 2007;113:98–103 [DOI] [PubMed] [Google Scholar]
- 90. Parkash J. Glucose-mediated spatial interactions of voltage dependent calcium channels and calcium sensing receptor in insulin producing β-cells. Life Sci. 2011;88:257–264 [DOI] [PubMed] [Google Scholar]
- 91. Parkash J, Asotra K. L-histidine sensing by calcium sensing receptor inhibits voltage-dependent calcium channel activity and insulin secretion in β-cells. Life Sci. 2011;88:440–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Huang C, Sindic A, Hill CE, et al. Interaction of the Ca2+-sensing receptor with the inwardly rectifying potassium channels Kir4.1 and Kir4.2 results in inhibition of channel function. Am J Physiol Renal Physiol. 2007;292:F1073–F1081 [DOI] [PubMed] [Google Scholar]
- 93. Okamoto K, Iwasaki N, Doi K, et al. Inhibition of glucose-stimulated insulin secretion by KCNJ15, a newly identified susceptibility gene for type 2 diabetes. Diabetes. 2012;61:1734–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Breitwieser GE. Minireview: the intimate link between calcium sensing receptor trafficking and signaling: implications for disorders of calcium homeostasis. Mol Endocrinol. 2012;26:1482–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Magalhaes AC, Dunn H, Ferguson SS. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol. 2012;165:1717–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hlavackova V, Zabel U, Frankova D, et al. 2012 Sequential inter- and intrasubunit rearrangements during activation of dimeric metabotropic glutamate receptor 1. Sci Signal 5:ra59. [DOI] [PubMed] [Google Scholar]
- 97. Albizu L, Cottet M, Kralikova M, et al. Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat Chem Biol. 2010;6:587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Prezeau L, Rives ML, Comps-Agrar L, Maurel D, Kniazeff J, Pin JP. Functional crosstalk between GPCRs: with or without oligomerization. Curr Opin Pharmacol. 2010;10:6–13 [DOI] [PubMed] [Google Scholar]
- 99. Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci USA. 2004;101:14258–14263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99:4692–4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Maillet EL, Margolskee RF, Mosinger B. Phenoxy herbicides and fibrates potently inhibit the human chemosensory receptor subunit T1R3. J Med Chem. 2009;52:6931–6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang F, Klebansky B, Fine RM, et al. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci USA. 2008;105:20930–20934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Servant G, Tachdjian C, Tang XQ, et al. Positive allosteric modulators of the human sweet taste receptor enhance sweet taste. Proc Natl Acad Sci USA. 2010;107:4746–4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Faure H, Gorojankina T, Rice N, et al. Molecular determinants of non-competitive antagonist binding to the mouse GPRC6A receptor. Cell Calcium. 2009;46:323–332 [DOI] [PubMed] [Google Scholar]
- 105. Gloriam DE, Wellendorph P, Johansen LD, et al. Chemogenomic discovery of allosteric antagonists at the GPRC6A receptor. Chem Biol. 2011;18:1489–1498 [DOI] [PubMed] [Google Scholar]
- 106. Nemeth EF, Steffey ME, Hammerland LG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA. 1998;95:4040–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gowen M, Stroup GB, Dodds RA, et al. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest. 2000;105:1595–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Petrel C, Kessler A, Maslah F, et al. Modeling and mutagenesis of the binding site of Calhex 231, a novel negative allosteric modulator of the extracellular Ca(2+)-sensing receptor. J Biol Chem. 2003;278:49487–49494 [DOI] [PubMed] [Google Scholar]
- 109. Bräuner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets. 2007;8:169–184 [DOI] [PubMed] [Google Scholar]
- 110. Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov. 2012;11:603–619 [DOI] [PubMed] [Google Scholar]
- 111. Conigrave AD, Hampson DR. Broad-spectrum L-amino acid sensing by class 3 G-protein-coupled receptors. Trends Endocrinol Metab. 2006;17:398–407 [DOI] [PubMed] [Google Scholar]
- 112. Wellendorph P, Johansen LD, Bräuner-Osborne H. Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol Pharmacol. 2009;76:453–465 [DOI] [PubMed] [Google Scholar]
- 113. Yasumatsu K, Ohkuri T, Sanematsu K, et al. Genetically-increased taste cell population with G(α)-gustducin-coupled sweet receptors is associated with increase of gurmarin-sensitive taste nerve fibers in mice. BMC Neurosci. 2009;10:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sigoillot M, Brockhoff A, Meyerhof W, Briand L. Sweet-taste-suppressing compounds: current knowledge and perspectives of application. Appl Microbiol Biotechnol. 2012;96:619–630 [DOI] [PubMed] [Google Scholar]
- 115. Imoto T, Miyasaka A, Ishima R, Akasaka K. A novel peptide isolated from the leaves of Gymnema sylvestre–I. Characterization and its suppressive effect on the neural responses to sweet taste stimuli in the rat. Comp Biochem Physiol A Comp Physiol. 1991;100:309–314 [DOI] [PubMed] [Google Scholar]