Abstract
Kisspeptin signaling through its receptor, Kiss1R, is crucial for many reproductive functions including puberty, sex steroid feedback, and overall fertility. Although the importance of Kiss1R in the brain is firmly established, its role in regulating reproduction at the level of the pituitary is not well understood. This study presents molecular analysis of the role of kisspeptin and Kiss1R signaling in the transcriptional regulation of the gonadotropin gene β-subunits, LHβ and FSHβ, using LβT2 gonadotrope cells and murine primary pituitary cells. We show that kisspeptin induces LHβ and FSHβ gene expression, and this induction is protein kinase C dependent and mediated by the immediate early genes, early growth response factor 1 and cFos, respectively. Additionally, kisspeptin induces transcription of the early growth response factor 1 and cFos promoters in LβT2 cells. Kisspeptin also increases gonadotropin gene expression in mouse primary pituitary cells in culture. Furthermore, we find that Kiss1r expression is enhanced in the pituitary of female mice during the estradiol-induced LH surge, a critical component of the reproductive cycle. Overall, our findings indicate that kisspeptin regulates gonadotropin gene expression through the activation of Kiss1R signaling through protein kinase C, inducing immediate early genes in vitro, and responds to physiologically relevant cues in vivo, suggesting that kisspeptin affects pituitary gene expression to regulate reproductive function.
The reproductive neuroendocrine axis is centrally regulated by inputs from kisspeptin neurons to GnRH neurons, which in turn regulate the release of LH and FSH from the pituitary gonadotropes. LH and FSH exert their actions on the gonads, regulating estradiol and testosterone production, which have feedback effects on the pituitary and hypothalamus (including kisspeptin neurons). Kisspeptin, the product of the Kiss1 gene, and its Gq/11-coupled receptor Kiss1R (formerly termed GPR54) were first discovered to be important for reproduction in 2003, when 3 studies reported profound deficits in fertility, puberty, cyclicity, and sex steroid and gonadotropin levels in humans and mice carrying mutations in the Kiss1R gene (1–3). The role of the kisspeptin system in reproductive function has since been further characterized with the use of Kiss1R- and Kiss1-null mice (4–8). It is now well established that kisspeptin signaling through Kiss1R is necessary for normal functioning of the reproductive axis.
Abundant evidence in a variety of mammals demonstrate the direct and potent actions of hypothalamic kisspeptins acting at the level of the GnRH neurons: kisspeptin induces LH and FSH secretion, which is prevented by a GnRH receptor antagonist, kisspeptin induces GnRH neuronal firing and release of GnRH in hypothalamic explants, and GnRH neurons express Kiss1R (9–11). Although hypothalamic kisspeptin signaling has been firmly substantiated, less is known of the role of kisspeptin and Kiss1R in the pituitary. In a number of mammalian species, Kiss1r mRNA is expressed in the pituitary, along with several other peripheral tissues, including kidney, testis, ovary, pancreas, adipocytes, and placenta (9, 12).
Previous studies demonstrated that kisspeptin treatment could increase LH in numerous mammalian species, presumably due to kisspeptin acting on GnRH neurons (9, 13). Importantly, several studies have shown that treatment of primary pituitary cultures from rats and baboons with the bioactive form of kisspeptin, kisspeptin-10, can induce LH secretion (14, 15). These findings, that kisspeptin can stimulate pituitary cultures to secrete LH, suggest that kisspeptin may act at various levels of the hypothalamic-pituitary-gonadal axis, as illustrated by its capacity to increase both GnRH and LH release. Moreover, the findings that kisspeptin is present in the portal bloodstream of the median eminence in sheep and primates, and that Kiss1R colocalizes with LHβ in rat primary pituitary cultures (12, 16), further support a possible role for kisspeptin signaling outside of the hypothalamus.
Although it is well known that Kiss1 expression is strongly regulated by sex steroid feedback (9, 17, 18), hormonal regulation of Kiss1r is less well understood. In ovariectomized (OVX) rats, pituitary Kiss1r was decreased under negative feedback by 17β-estradiol (19). This suggests a physiologically relevant role of pituitary Kiss1R, but this has not been investigated in other animal models or under other sex steroid feedback conditions, such as the estradiol-induced LH surge. Further clarification of sex steroid-dependent regulation of Kiss1r expression will provide a useful context for interpreting the studies of kisspeptin-induced LH secretion.
Although the studies of kisspeptin-induced LH secretion from primary pituitary cultures have been informative, it is currently unknown whether kisspeptin signaling can regulate gonadotropins at the transcriptional level. The observation that kisspeptin can act directly on the pituitary to cause LH secretion does not necessarily provide information about whether kisspeptin activation of Kiss1R is influencing LH (or FSH) gene expression, and if so, by what mechanism. The LβT2 pituitary gonadotrope cell line is a useful and appropriate in vitro model with which to test the transcriptional effects of Kiss1R signaling. LβT2 gonadotropes express the gonadotropin subunits LHβ and FSHβ, as well as the common α glycoprotein subunit, and can respond to GnRH, activin, and sex steroid treatments (20). The mechanisms of the transcriptional regulation of LHβ and FSHβ in LβT2 gonadotropes have been extensively studied (20), but whether Kiss1R signaling modulates expression of these genes is unknown.
Materials and Methods
Hormones and treatments
Human kisspeptin-10 (Metastin (45–54) amide) and GnRH were purchased from Sigma-Aldrich (St Louis, Missouri). The protein kinase C inhibitor (PKCi), Go 6983, was purchased from Tocris Bioscience (Minneapolis, Minnesota).
Plasmid constructs
The −1.7-kb rat LHβ, −1-kb murine FSHβ, and −1.1-kb GnRH receptor luciferase (luc) reporters were described previously (20–22). The −1-kb cFos and FSHβ containing an activator protein-1 (AP-1) mutation have been previously described (23). The 1-kb murine early growth response factor 1 (Egr-1) promoter was cloned by PCR from genomic DNA and linked to the luciferase reporter in pGL3 basic plasmid using KpnI and HindIII sites. Mutagenesis of the c-Fos and Egr1 promoter luciferase plasmids was performed using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, California) following the manufacturer's instructions, and mutations were confirmed by dideoxyribonucleotide sequencing. The Egr-1 multimer, consisting of 4 tandem copies of the consensus Egr-1 site with 2-bp spacers, was cloned in the multiple cloning site in front of the thymidine kinase minimal promoter in the pGL3 basic luciferase reporter. LHβ containing SF-1 and Egr-1 mutations was provided by Dr. Varykina Thackray (24). The SF-1 mutations were at −128 bp and −59 bp, and the Egr-1 mutations were at −111 bp and −50 bp, all of which have been previously described (25–27). The AP-1 multimer was purchased from Agilent Technologies (Santa Clara, California), and the AFos expression vector has been previously described (23). The Kiss1R expression vector was purchased from Thermo Scientific (Logan, Utah). The following mutations in the Egr-1 promoter (serum response factor [SRF]; E-twenty six (ETS); cAMP response element [CRE]) were designed using the primers listed in Supplemental Table 1 published on The Endocrine Society's Journals Online web site: −83 bp SRF, −105 bp SRF, −353 bp SRF, −368 bp SRF, −91 bp ETS, and −138 bp CRE. Mutations in 2 Hox sites on the cFos promoter at −313 bp and −59 bp were designed using the primers listed in Supplemental Table 1. The AP-1 mutation at −295 bp, the signal transducer and activator of transcription (STAT) mutation at −345 bp, and the SRF mutation at −310 bp in the cFos promoter have been previously described (23).
Cell culture and transient transfections
Transient transfections were performed in the LβT2 pituitary gonadotrope cell line (28). Cells were maintained on 10-cm dishes (at 37°C and 5% CO2) in DMEM (Mediatech Inc., Herndon, Virginia) containing 10% fetal bovine serum (Omega Scientific, Inc., Tarzana, California) and penicillin/streptomycin antibiotics (Invitrogen/Life Technologies, Grand Island, New York) as previously described (29). Trypsin-EDTA (1×, Sigma-Aldrich) was used to dissociate the cells. Cells were plated in 12-well plates at 4.5 × 105 cells per well and were transfected approximately 24 hours later with PolyJet transfection reagent (SignaGen Laboratories, Rockville, Maryland) according to the manufacturer's instructions.
For all experiments, cells were transfected with 300 ng of the indicated reporter plasmid, 100 ng of the Kiss1R expression vector to ensure consistent and adequate expression, the AFos expression vector where indicated, and 100 ng of a β-galactosidase reporter plasmid driven by the herpes virus thymidine kinase promoter to control for transfection efficiency (29). After 6 hours of transfection, cells were switched to serum-free DMEM (containing 0.1% BSA and 5 mg/L transferrin) for 24 hours. Cells were then treated for 12 hours with 100 nM kisspeptin-10 in dimethylsulfoxide (DMSO) and/or 10 nM GnRH in 0.1% BSA (again in serum-free media), as indicated. The concentration and treatment duration of kisspeptin were determined by dose-response and time-course studies (Supplemental Figure 3). Small interfering RNA (siRNA) for Egr-1 and the transfection reagent DharmaFECT Duo were purchased from Thermo Scientific (West Palm Beach, Florida), and transfections were performed according to the manufacturer's instructions.
For experiments using PKCi (Go 6983), after the 24 hours in serum-free media, cells were preincubated with or without 5 μM PKCi in DMSO for 30 minutes. Kisspeptin or control DMSO were then added directly to the wells for a further 12 hours. The appropriate concentration of PKCi was determined by a dose response study (Supplemental Figure 4). Vehicle control for kisspeptin and PKCi was 1:1000 DMSO and 0.1% BSA for GnRH.
Luciferase and β-galactosidase assays
After hormone treatment, luciferase and β-galactosidase activity were assayed as previously described (29). Briefly, cells were washed with PBS and lysed with 0.1 M potassium phosphate buffer (pH 7.8) containing 0.2% Triton X-100. Luciferase activity was measured using a buffer containing 100 mM Tris-HCl (pH 7.8), 15 mM MgSO4, 10 mM ATP, and 65 μM luciferin. β-Galactosidase activity was assayed using the Tropix Galacto-light assay (Applied Biosystems, Foster City, California), according to the manufacturer's instructions. Both assays were measured using a Veritas Microplate Luminometer (Promega Corp., Madison, Wisconsin).
Animals
All animal procedures were performed in accordance with the UCSD Institutional Animal Care and Use Committee regulations. All mice were on a C57BL/6J background and were group housed on a 12-hour light, 12-hour dark cycle with ad libitum chow (11% of calories fat, 17% of calories protein) and water.
Ovariectomy and estrogen replacement
Estradiol feedback paradigms for animals humanely destroyed in the morning (am) and the evening (pm) were tested in female mice, as previously described (30). Briefly, C57BL/6J female mice of 2–3 months of age were anesthetized by isoflurane inhalation and were OVX. At the time of surgery, animals were sc implanted with capsules containing either vehicle (name of vehicle, OVX) or 2.5 μg 17β-estradiol (OVX+17-β estradiol [E]). One week after surgery, OVX+E females were injected sc with 1 μg estradiol benzoate (E8515; Sigma Aldrich) in 100 μL of sesame oil. OVX females were injected with 100 μL of sesame oil. All injections occurred between 9:00 am and 10:00 am. The am. animals were humanely destroyed the following morning, before to 11:00 am, by CO2 inhalation followed by exsanguination. The pm animals were sacrificed that evening at 6:30 pm (30 min after lights out). Serum LH was measured from OVX and OVX+E animals by RIA (University of Virginia, Ligand and Assay Core).
Primary pituitary isolation
Two month-old C57BL/6J male mice were humanely destroyed and pituitaries were harvested (n = 10 animals/group) according to published methods (23). Isolated pituitaries were rinsed in cold PBS and were then minced with sharp scissors. Individual pituitaries were added to 10 mL 0.25% Trypsin-EDTA (Invitrogen/Life Technologies) containing 0.25% collagenase (Invitrogen/Life Technologies) in a 50-mL conical tube. Tubes were then agitated for 30 minutes at 80 rpm at 37°C at a 45° angle. Next, 10 mL of 10% fetal bovine serum in DMEM was added and the solution was mixed. DNase I (500 μL) (Sigma Aldrich) was added to the solution at a final concentration of 25 μg/mL, and the tube was agitated as described above for 15 minutes. After mixing, the solution containing the suspended cells was removed from the cellular debris and was centrifuged at room temperature for 8 minutes at 1000 rpm. The supernatant was discarded and the pelleted cells were resuspended in 2 mL of 10% fetal bovine serum in DMEM. Cells were plated at 5 × 105 cells per well in a 24-well plate (Nalge Nunc International, Rochester, New York), and were allowed to attach for 7 hours, after which the media were changed to serum-free DMEM. The next day, cells were treated with 30 nM GnRH, 100 nM kisspeptin, or vehicle (1:1000 DMSO for kisspeptin and 0.1% BSA for GnRH) for 6 hours. RNA was then harvested using TRIzol reagent (Invitrogen/Life Technologies). Genomic DNA was removed from all RNA samples using a DNA-free kit (Ambion, Austin, Texas; Invitrogen/Life Technologies). RT-PCR was performed using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, California). Quantitative PCR (qPCR) for LHβ, FSHβ, and TATA-binding protein (as an internal control) was performed using the primer sequences listed in Supplemental Table 1.
RT-PCR and qPCR
Pituitaries from C57BL/6J male and diestrous female mice (2–3 mo old) were harvested and frozen. Total RNA was extracted from pituitaries and skin biopsies using TRIzol reagent (Invitrogen/Life Technologies). RNA was also extracted from pituitaries from the OVX and OVX+E female mice. RNA was isolated from LβT2 cells using a QiaShredder and an RNeasy mini kit (QIAGEN, Valencia, California) according to the manufacturer's instructions. Genomic DNA was removed from all RNA samples using a DNA-free kit (Ambion; Invitrogen/Life Technologies). RT-PCR was performed using either an iScript cDNA synthesis kit (Bio-Rad Laboratories) or a SuperScript III First-Strand Synthesis kit (Invitrogen/Life Technologies), according to the manufacturer's instructions. qPCR for Kiss1R and glyceraldehyde 3-phosphate dehydrogenase (as an internal control) was performed using the primer sequences listed in Supplemental Table 1. PCR primer sequences for Kiss1R and Kiss1 are also listed in Supplemental Table 1.
Statistical analysis
All transient transfections were repeated independently at least 3 times. Data were normalized for luciferase activity relative to β-galactosidase to control for transfection efficiency. Data were also normalized to the pGL3 plasmid, as well as to vehicle control for all hormone treatments. Results are presented as mean ± SEM of the fold induction relative to vehicle control. Statistical tests were performed on luciferase activity normalized to β-galactosidase. Student's t tests, 1-way ANOVA, and 2-way ANOVA followed by Tukey post hoc tests were used as indicated, and P < .05 was considered statistically significant.
Results
Kiss1r is expressed in pituitary gonadotropes and regulates gonadotropin gene expression
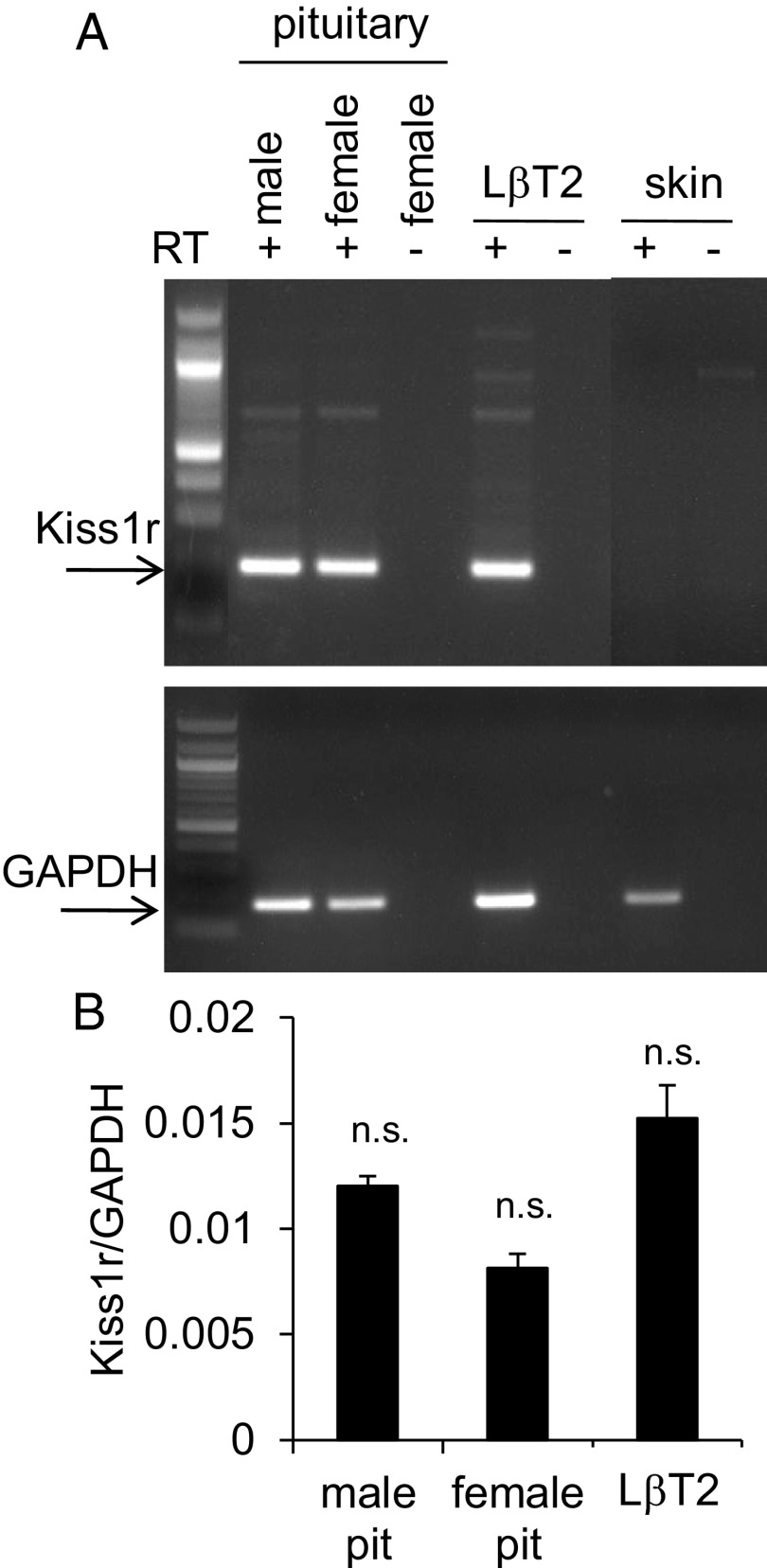
Kisspeptin has been shown to induce LH secretion by direct actions on pituitary cells in primary culture (14, 15), but the role of Kiss1R in mediating transcription of the gonadotropin genes, LHβ and FSHβ, has not been investigated. We performed RT-PCR for Kiss1r on pituitaries harvested from adult C57BL/6J male and female mice (2–3 mo of age) and found that it was expressed in both sexes (Figure 1A), supporting the in vivo relevance of kisspeptin at the level of the pituitary. Because the pituitary contains a heterogeneous cell population, we also determined that the LβT2 gonadotrope cell line expresses Kiss1r, indicating that it is an appropriate in vitro system with which to test the transcriptional role of Kiss1R (Figure 1A). These results were in agreement with a previous study in rats (14) and further confirm the presence of Kiss1r in pituitary gonadotropes. The relative levels of Kiss1r expression in the pituitaries and LβT2 cells were quantified by qPCR (Figure 1B). We also confirmed by in situ hybridization that Kiss1r mRNA is present in a population of LHβ-expressing gonadotropes in mouse pituitaries (data not shown). As expected, Kiss1 (encoding kisspeptin) was not detected in the LβT2 cells or mouse pituitary (Supplemental Figure 1).
Figure 1.
Kiss1r Is Expressed in LβT2 Gonadotropes. A, RT-PCR was performed on cDNA from female and male mouse pituitaries, mouse skin biopsies, and LβT2 cells, ± reverse transcriptase (RT). Mouse pituitaries served as positive controls, and skin served as a negative control. The primers yielded a 195-bp Kiss1r product indicated by the arrow. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to verify the presence of cDNA in all of the RT+ samples (lower panel). B, qPCR was performed for Kiss1r on male pituitary, female pituitary, and LβT2 cells (n = 3 animals per group and n = 3 different passages of cells). Data are normalized to GAPDH, and there was no significant difference between samples (1-way ANOVA). Pit, pituitary; n.s., not significant P > .05.
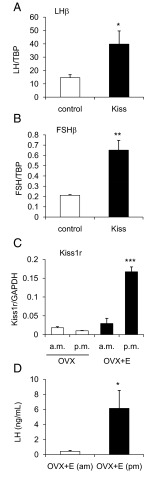
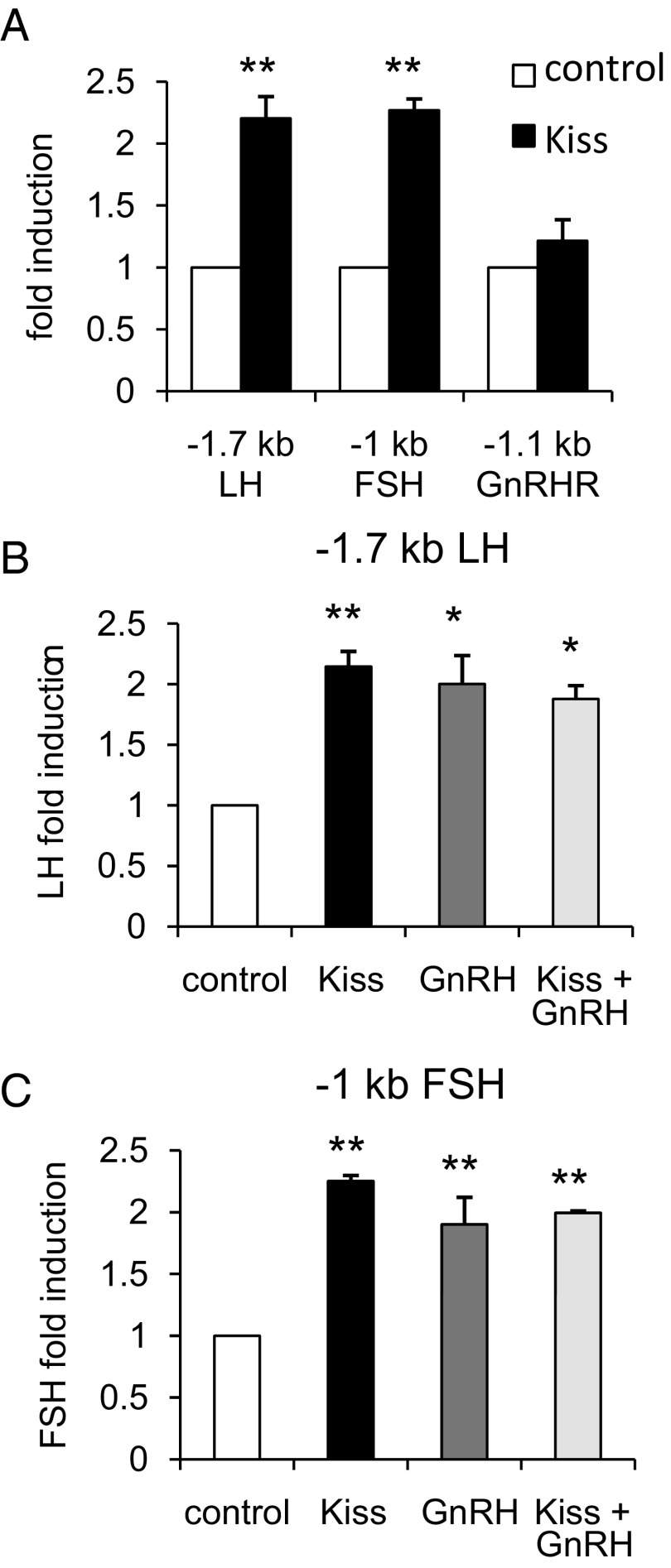
To initially determine whether Kiss1R can influence gene transcription in the gonadotrope, we tested whether kisspeptin could induce LHβ and FSHβ expression in mouse primary pituitary cultures. Kisspeptin treatment increased LHβ and FSHβ relative to vehicle (P < .05 and P < .01, respectively; Figure 7, A and B). In addition, expression of Kiss1r was detected in mouse primary pituitary cells, but was not influenced by GnRH treatment (data not shown). To determine the mechanism of kisspeptin action in gonadotropes, we first performed transient transfections in LβT2 cells with −1.7-kb LHβ, −1-kb FSHβ, and −1.1-kb GnRH receptor (GnRHR) promoter luciferase constructs with kisspeptin treatment. We found that a 12 hour treatment with 100 nM kisspeptin induced the LHβ and FSHβ promoters (P < .01 compared with control) but not GnRHR (Figure 2A). Because GnRH has been shown to act in synergy with other hormones to regulate FSHβ (20), we tested whether a combination of 10 nM GnRH and 100 nM kisspeptin treatment (for 12 h) could synergistically induce LHβ and FSHβ. Cotreatment with both GnRH and kisspeptin did not induce LHβ or FSHβ more than the individual treatments, suggesting a lack of synergy between these 2 hormones under our experimental conditions (Figure 2, B and C).
Figure 7.
Kisspeptin (Kiss) Induction of Gonadotrope Genes and Regulation of Pituitary Kiss1r Expression in Vivo. Primary pituitary cells from male mice (10 animals/group) were harvested and treated for 6 hours with 100 nM kisspeptin or vehicle. qPCR was performed for LHβ (A) and FSHβ (B). Data are normalized to TBP. *, P < .05; and **, P < .01 compared with control by Student's t test. C, Pituitaries were harvested from OVX and OVX+E females for both am and pm groups (4–9 animals/group). qPCR was performed for Kiss1r, and data are normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). ***, P < .001 by 2-way ANOVA. D, Serum LH was measured from am and pm OVX+E females. *, P < .05 by Student's t test. TBP, TATA-binding protein.
Figure 2.
Kisspeptin (Kiss) Induces LHβ and FSHβ but not GnRHR. LβT2 cells were transiently transfected with a Kiss1R expression vector and the indicated luciferase reporter. Cells were treated for 12 hours with vehicle (DMSO for kisspeptin and 0.1% BSA for GnRH), 100 nM kisspeptin, 10 nM GnRH, or cotreatment as indicated and subjected to luciferase assay. Data were normalized to vehicle-treated control. A, The −1.7-kb LHβ, −1-kb FSHβ, and −1.1-kb GnRHR promoters were transfected, cells were treated with vehicle or kisspeptin, and activity was compared between vehicle and kisspeptin. **, P < .01 by Student's t test. B, The −1.7-kb LHβ promoter was transfected into LβT2 cells, which were treated with vehicle, 100 nM kisspeptin, 10 nM GnRH, or cotreatment, and activity was compared across treatment conditions. **, P < .01; and *, P < .05 by 1-way ANOVA. C, The −1-kb FSHβ promoter was transfected into LβT2 cells, which were treated with vehicle, 100 nM kisspeptin, 10 nM GnRH, or cotreatment, and activity was compared across treatment conditions. *, P < .05 by 1-way ANOVA.
Kisspeptin induces LHβ through an Egr-1-dependent pathway
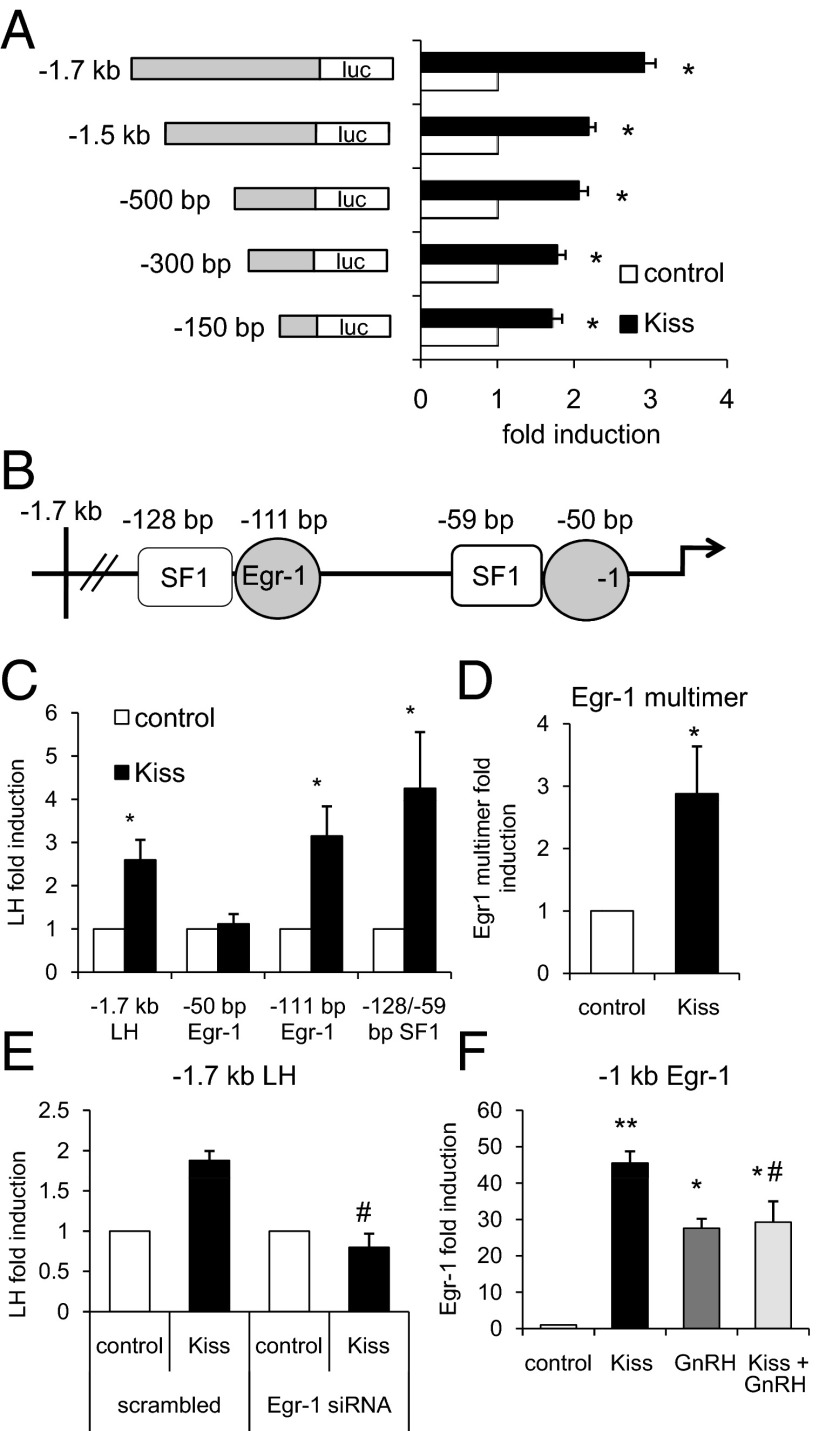
It has previously been shown that Egr-1, an immediate early gene, is necessary for LHβ induction by GnRH (31). Another transcription factor, steroidogenic factor 1 (SF1), is also involved in LHβ transcriptional regulation (32). Our finding that kisspeptin induced LHβ promoter activity led us to investigate the mechanism of this regulation and whether it occurred through Egr-1 or SF1. After previously determining the optimal time and dose, all subsequent experiments were treated for 12 hours, using the above concentrations for kisspeptin and GnRH. We first performed a truncation analysis using LHβ promoter constructs of decreasing lengths (−1.7 kb, −1.5 kb, −500 bp, −300 bp, and −150 bp), and found that kisspeptin induction of LHβ is downstream of −150 bp, because all promoter truncations, including the −150 bp, were activated by kisspeptin treatment (P < .05, Figure 3A). Kisspeptin induced the −1.7-kb promoter more strongly than the other truncations (P < .001, Figure 3A), but no regulatory sites have been described in that region. Therefore, we focused our studies on the well-characterized proximal region. It has been shown that the proximal region of the LHβ promoter contains 2 sites each for SF1 (−128 bp and −59 bp) and Egr-1 (−111 bp and −50 bp), all downstream of −150 bp (Figure 3B) (25–27).
Figure 3.
Kisspeptin (Kiss) Induces LHβ via the Proximal −50-bp Egr-1 Site. LβT2 cells were transiently transfected with a Kiss1R expression vector and the indicated luciferase reporter. Cells were treated for 12 hours with vehicle (DMSO for kisspeptin and 0.1% BSA for GnRH), 100 nM kisspeptin, 10 nM GnRH, or cotreatment as indicated and subjected to luciferase assay. Data were normalized to vehicle-treated control. A, 5′-truncations of the LHβ promoter were transfected into LβT2 cells, which were treated with vehicle or 100 nM kisspeptin, and activity was compared between vehicle and kisspeptin treatment. *, P < .05 by Student's t test. B, Diagram of the proximal LHβ promoter containing 2 Egr-1 sites and 2 SF-1 sites. C, Mutations in either of the 2 Egr-1 sites (−111 bp or −50 bp) and a double mutation in the SF-1 sites (−128 bp and −59 bp) in the proximal region of the LHβ promoter (as indicated by the diagram) were transfected into LβT2 cells, which were treated with vehicle or 100 nM kisspeptin, and activity was compared between vehicle and kisspeptin treatment. *, P < .05 by Student's t test. D, An Egr-1 multimer was transfected into LβT2 cells, which were treated with vehicle or 100 nM kisspeptin, and activity was compared between vehicle and kisspeptin treatment. *, P < .05 by Student's t test. E, siRNA for Egr-1 (or scrambled control) was transfected into LβT2 cells, which were treated with vehicle or 100 nM kisspeptin. Activity was compared between vehicle and kisspeptin treatment for both scrambled control and siRNA for Egr-1. #, P < .05 by 2-way ANOVA. F, −1 kb of the Egr-1 promoter was transfected into LβT2 cells, which were treated with vehicle, 100 nM kisspeptin, 10 nM GnRH, or cotreatment, and activity was compared across treatment conditions. **, P < .01; and *, P < .05 by 1-way ANOVA. #, P < .01 by 2-way ANOVA.
To determine whether any of the SF1 or Egr-1 sites were required for kisspeptin induction of LHβ, we transfected LHβ promoter constructs containing mutations in either of the Egr-1 sites and both of the SF1 sites (25–27) into LβT2 cells. Reporter activation by kisspeptin was absent with the −50-bp Egr-1 site mutation, demonstrating the necessity of this site in LHβ induction by kisspeptin (Figure 3B). Conversely, we then tested whether the Egr-1 site alone was sufficient for kisspeptin activation. We transfected with an Egr-1 multimer construct containing tandem repeats of the Egr-1 binding sequence on a heterologous promoter and found a significant induction by kisspeptin (P < .05, Figure 3C). In addition, we showed that the −1-kb Egr-1 promoter was robustly induced by kisspeptin (P < .01, Figure 3D), further supporting that Kiss1R stimulates LHβ in an Egr-1-dependent manner. To directly test whether Egr-1 is required for kisspeptin induction of LHβ, we transfected with siRNA for Egr-1 along with the −1.7-kb LHβ promoter and found that the induction by kisspeptin was abolished in the presence of the siRNA (P < .05, Figure 3E). Egr-1 siRNA decreased endogenous Egr-1 expression in LβT2 cells by approximately 40% compared with scrambled and untreated controls (Supplemental Figure 2). GnRH-dependent activation of Egr-1 is critical for LHβ transcription (33), so we cotreated with GnRH and kisspeptin and found a significant interaction (P < .01 Figure 3F) but no synergistic activation.
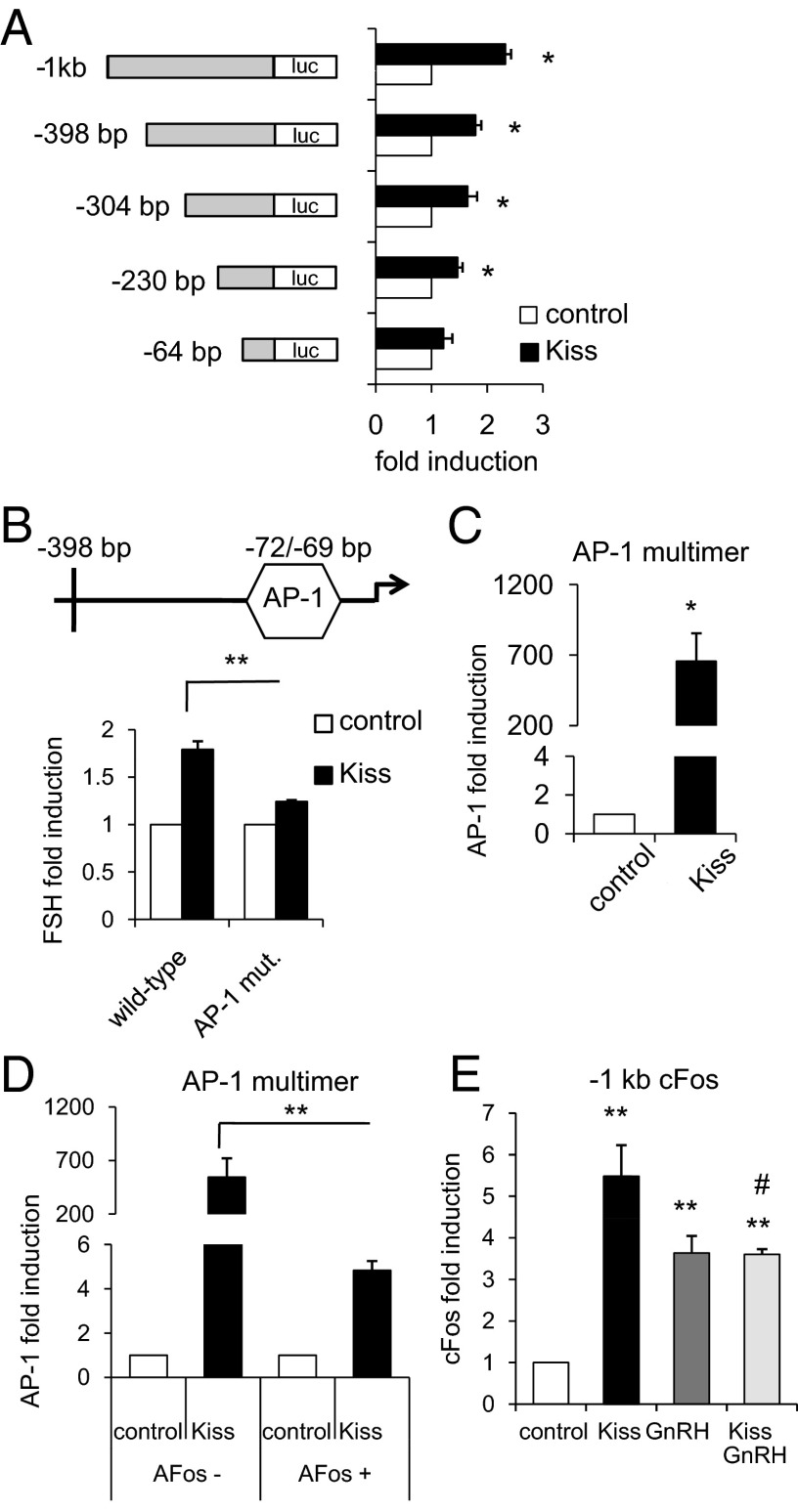
Kisspeptin induces FSHβ via AP-1
Having determined that Kiss1R-mediated activation of LHβ occurs through Egr-1, we next explored the mechanism of FSHβ induction, because both β-gonadotropin subunits are differentially regulated at the transcriptional level (20). We approached this question in the same manner as for LHβ, with a truncation analysis using FSHβ promoter truncations of −1 kb, −398 bp, −304 bp, −230 bp, and −64 bp in length. All reporters were significantly induced by kisspeptin except −64 bp (Figure 4A), suggesting that Kiss1R signaling is acting downstream of −230 bp. FSHβ is in part regulated by AP-1, which is comprised of dimers of Fos and Jun isoforms (20), acting through an AP-1 site located at −72/−69 bp (34). We transfected a reporter with a mutation in this AP-1 site within the −398-bp FSHβ promoter and found that the induction by kisspeptin was significantly lower than in the wild-type −398-bp FSHβ promoter (P < .01, Figure 4B).
Figure 4.
Kisspeptin (Kiss) Induced FSHβ via cFos-Dependent AP-1 Activation. LβT2 cells were transiently transfected with a Kiss1R expression vector and the indicated luciferase reporter. Cells were treated for 12 hours with vehicle (DMSO for kisspeptin and 0.1% BSA for GnRH), 100 nM kisspeptin, 10 nM GnRH, or cotreatment as indicated and subjected to luciferase assay. Data were normalized to vehicle-treated control. A, 5′-truncations of the FSHβ promoter were transfected into LβT2 cells, which were treated with vehicle or kisspeptin, and activity was compared between vehicle and 100 nM kisspeptin treatment. *, P < .05 by Student's t test. B, The wild-type −398-bp FSHβ promoter and the −398-bp promoter containing a mutation in the −72/−69 bp AP-1 half-site were transfected into LβT2 cells, which were treated with vehicle or 100 nM kisspeptin, and activity was compared between kisspeptin treatment for the 2 promoters and between kisspeptin and vehicle. **, P < .01 by 2-way ANOVA. C, An AP-1 multimer on a luciferase reporter was transfected into LβT2 cells, which were treated with vehicle or 100 nM kisspeptin, and activity was compared between vehicle and kisspeptin treatment. *, P < .05 by Student's t test. D, The AP-1 multimer was transfected into LβT2 cells with or without a dominant-negative cFos, AFos. Cells were treated with vehicle or 100 nM kisspeptin, and activity was compared for the kisspeptin treatment in the presence or absence of AFos. **, P < .01 by 1-way ANOVA. E, −1 kb of the cFos promoter was transfected into LβT2 cells, which were treated with vehicle, 100 nM kisspeptin, 10 nM GnRH, or cotreatment, and activity was compared across treatment conditions. **, P < .01 by 1-way ANOVA. #, P < .001 by 2-way ANOVA. mut., mutation.
The requirement of the AP-1 site for FSHβ promoter induction by kisspeptin led us to test its sufficiency by transfecting an AP-1 multimer containing tandem repeats of the AP-1 sequence on a luciferase reporter. The AP-1 multimer was highly activated by kisspeptin, reaching approximately 700-fold that of the control treatment (P < .05, Figure 4C). We further confirmed the necessity of the functional c-Fos/c-Jun heterodimer in mediating kisspeptin induction by cotransfecting AFos (a dominant-negative cFos) along with the AP-1 multimer and found that AFos reduced the induction by kisspeptin (P < .01, Figure 4D). Lastly, because cFos is an important component of AP-1, we transfected with −1 kb of the cFos promoter driving luciferase and found an increase with kisspeptin treatment (P < .01, Figure 4E). GnRH is also known to induce FSHβ through AP-1, and cotreatment with kisspeptin and GnRH showed a significant interaction (P < .001, Figure 4E) but no synergistic activation. Additionally, kisspeptin alone caused a greater induction than cotreatment with GnRH (P < .05, Figure 4E). Taken together, these results demonstrate that Kiss1R mediates FSHβ induction through cFos-dependent AP-1 activation.
Mechanisms of kisspeptin induction of the immediate early genes
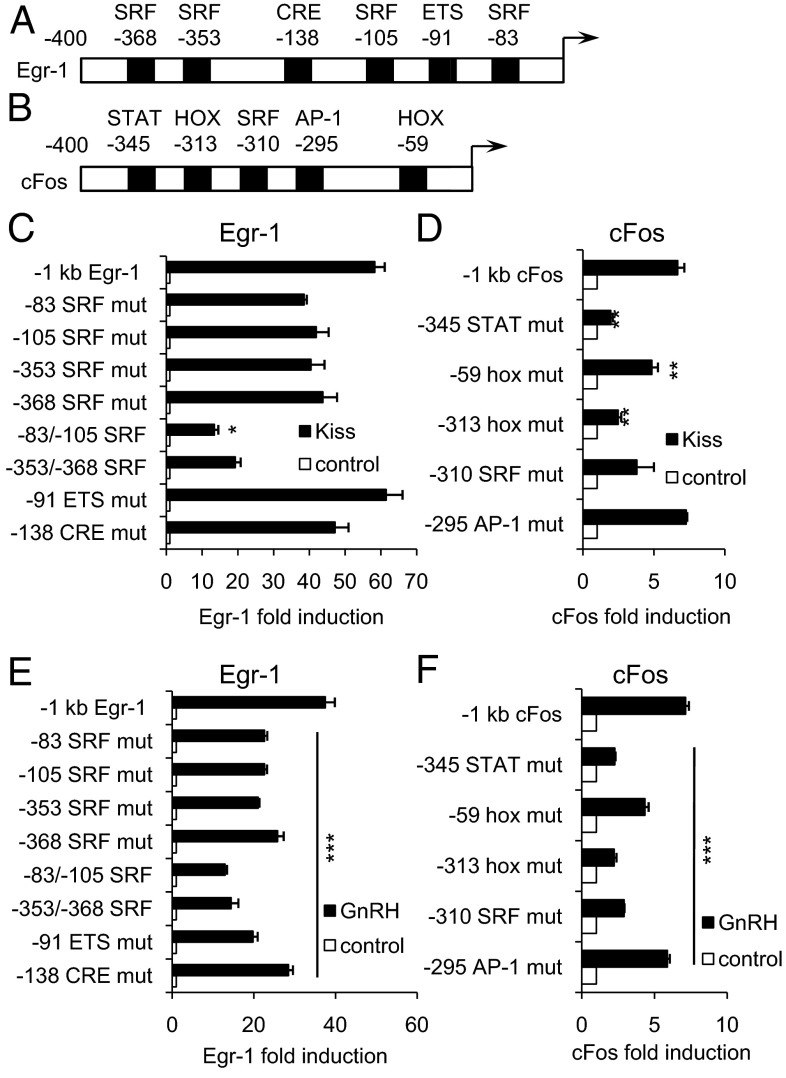
We next sought to explore the transcriptional mechanism by which the Egr-1 promoter is activated by kisspeptin. Transfections with mutations in 4 SRF sites as well as an ETS and a CRE site within the Egr-1 promoter (Figure 5A) showed that a double mutation in the SRF sites at −83/−105 resulted in a decreased effect of kisspeptin (P < .05, Figure 5C). We found a similar effect of this double mutation for GnRH (P < .001, Figure 5E). ETS and CRE, as well as each of the single SRF sites, were also required for the full effect of GnRH on the Egr-1 promoter (P < .001, Figure 5E).
Figure 5.
Kisspeptin Induced Egr-1 through SRF Sites and cFos through Homeobox and STAT Sites. LβT2 cells were transiently transfected with a Kiss1R expression vector and the indicated luciferase reporter. Cells were treated for 12 hours with vehicle (DMSO for kisspeptin and 0.1% BSA for GnRH), 100 nM kisspeptin, or 10 nM GnRH as indicated and subjected to luciferase assay. Data were normalized to vehicle-treated control. A, Diagram of the proximal Egr-1 promoter containing SRF, CRE, and ETS sites. B, Diagram of the proximal cFos promoter containing SRF, AP-1, STAT, and hox sites. C and E, Mutations in the Egr-1 promoter were transfected into LβT2 cells, which were treated with vehicle, 100 nM kisspeptin, or 10 nM GnRH. Kisspeptin and GnRH induction for all mutants were compared across to wild-type. *, P < .05 by Student's t test; ***, P < .001 by 1-way ANOVA. D and F, Mutations in the cFos promoter were transfected into LβT2 cells, which were treated with vehicle, 100 nM kisspeptin, or 10 nM GnRH. Kisspeptin and GnRH induction for all mutants were compared across to wild-type. **, P < .01; ***, P < .001 by 1-way ANOVA. mut., mutation.
To investigate the mechanism of kisspeptin-mediated cFos induction, we transfected with mutations in AP-1, SRF, STAT, and 2 Hox sites within the cFos promoter (Figure 5B) and found that the −313 Hox site, the −59 Hox site, and the −345 STAT site were all required for kisspeptin induction of the cFos promoter (P < .01, Figure 5D). These sites, as well as the AP-1 site, were also required for GnRH induction of the cFos promoter (P < .001, Figure 5F). The −345 STAT has previously been shown not to be required for cFos induction after 6 hours of GnRH treatment (23) whereas we found the requirement of this site after 12 hours of treatment.
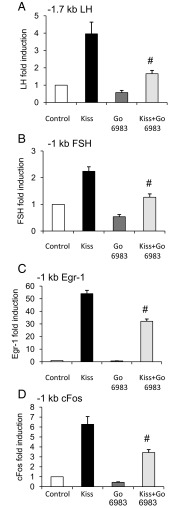
Kisspeptin induction of the LHβ, FSHβ, cFos, and Egr-1 promoters is PKC dependent
As a Gq/11-coupled receptor, Kiss1R exerts its transcriptional effects by the activation of intracellular signaling pathways including phospholipase C/calcium mobilization, protein kinase C (PKC), and MAPK (35, 36). In GnRH neurons, kisspeptin is thought to stimulate GnRH release via PKC (37, 38) but this has not been investigated in pituitary gonadotropes. We therefore tested whether PKC signaling was required for kiss-mediated induction of the LHβ, FSHβ, Egr-1, and cFos promoters by treating with a PKC inhibitor (PKCi). We found that 12 hours treatment with the PKCi Go 6983 at 5 μM significantly decreased the effect of kisspeptin on each of these promoters (P < .05, Figure 6).
Figure 6.
Kisspeptin (Kiss) Induction of LHβ, FSHβ, Egr-1, and cFos Was PKC Dependent. LβT2 cells were transiently transfected with a Kiss1R expression vector and the indicated luciferase reporter. The −1.7-kb LHβ (A), −1-kb FSHβ (B), −1-kb Egr-1 (C), and −1-kb cFos (D) promoters were transfected into LβT2 cells, and the cells were treated for 30 minutes with 5 μM Go 6983 or vehicle followed by a 12-hour treatment with DMSO vehicle or 100 nM kisspeptin. Cells were then subjected to luciferase assay and data were normalized to vehicle-treated control. Promoter activity was compared across the treatment conditions. #, P < .05 compared with kisspeptin treatment by 2-way ANOVA.
Kisspeptin regulates LHβ and FSHβ gene expression in mouse pituitary cells
To demonstrate the relevance of our finding that kisspeptin can induce gonadotropin promoter activity in transfected LβT2 gonadotropes, we next tested whether kisspeptin could induce LHβ and FSHβ expression in mouse primary pituitary cultures. Kisspeptin treatment increased LHβ and FSHβ relative to vehicle (P < .05 and P < .01, respectively; Figure 7, A and B).
Pituitary Kiss1r expression increases during the E-induced LH surge
Our results thus far demonstrate that kisspeptin/Kiss1R signaling acts through specific transcriptional mechanisms in LβT2 gonadotropes to induce LHβ and FSHβ via the activation of immediate early genes Egr-1 and cFos, respectively. This increase in kisspeptin-mediated transcription also occurs in primary mouse pituitary cells. To extend our study of the transcriptional role of Kiss1R, we tested whether Kiss1r itself was regulated in the mouse pituitary. A previous study suggested that pituitary Kiss1r is responsive to regulation by estradiol in female OVX rats (19), but it is unknown whether this occurs in mice, and whether Kiss1r expression is influenced by a combination of either circadian factors or high estradiol levels that are present during the LH surge leading to ovulation.
We performed qPCR on pituitaries from OVX mice with or without estradiol replacement that were humanely destroyed in the morning (am, representing low LH levels due to negative feedback) or the evening (pm, representing the LH surge due to positive feedback). Pituitary Kiss1r was unchanged in the OVX animals between the am and pm groups, but was significantly higher in the OVX+E pm animals compared with OVX+E am mice and both groups of OVX females (P < .001, Figure 7C). Serum LH was measured to confirm the effectiveness of the hormonal paradigm in promoting the LH surge in OVX+E animals (P < .05, Figure 7D).
Discussion
Many lines of evidence have demonstrated the importance of kisspeptin signaling in the hypothalamus, including animal and clinical studies showing the necessity of the kisspeptin system in promoting reproductive function (9). Only recently, however, has a role for kisspeptin signaling begun to be described in the pituitary. Although a limited number of studies have shown that kisspeptin can stimulate LH secretion at the level of the pituitary, it is unknown whether Kiss1R can also regulate transcription of gonadotrope-specific genes, and if so, by what mechanism.
Kiss1R signaling induces gonadotropin transcription via immediate early gene activation
We initially tested whether kisspeptin can activate the biologically specific subunits of the gonadotropin genes, LHβ and FSHβ, as well as GnRHR. We found that the effect of kisspeptin treatment was specific to the gonadotropin β-subunits. Truncation analysis of the LHβ promoter showed that the induction by kisspeptin mapped to a proximal area of the promoter containing 2 Egr-1 sites. The more proximal of these 2 sites was found to be required for kisspeptin induction of LHβ and is also important for GnRH-mediated activation of LHβ (26). Egr-1 itself was induced by kisspeptin in part by SRF and is both necessary and sufficient for kisspeptin-mediated LHβ induction, as indicated by Egr-1 mutation and multimer studies, as well as siRNA for Egr-1, which abolished the induction by kisspeptin.
Our studies of Kiss1R-mediated LHβ induction have implications for our understanding of the LH surge, a critical event in the female reproductive cycle. A sharp rise in GnRH triggers the increase in LH transcription, but our findings open the possibility that Kiss1R may also be influencing the fluctuations in LH levels. In addition to LH, FSH also increases at the time of the surge. We therefore next investigated the mechanism for FSHβ induction, which is regulated differently by GnRH than LHβ. Truncation analysis of the FSHβ promoter mapped the effect of kisspeptin to a region between −64 bp and −230 bp, which contains an AP-1 half-site required for GnRH induction of FSHβ (34). An AP-1 multimer responded robustly to kisspeptin in a cFos-dependent manner, demonstrating that AP-1 is both necessary and sufficient to activate FSHβ via Kiss1R-mediated signaling. Kisspeptin and GnRH may act via different mechanisms to induce Egr-1 and cFos, as demonstrated by differences in the involvement of STAT, AP-1, homeobox factors, ETS, and CRE in our studies of mutations in these regulatory regions.
However, cotreatment with kisspeptin and GnRH did not result in a synergistic induction of the LHβ, FSHβ, Egr-1, or cFos promoters, which leads to the possibility that both of these hormones may be activating the same signaling pathway. This is supported by our finding that a PKC inhibitor prevented the induction of these promoters by kisspeptin, which is consistent with previous reports that GnRH also signals through PKC and the fact that the GnRH receptor is also coupled to Gq/11 (39). There are many forms of PKC (most of which are expressed in LβΤ2 cells); thus it is possible that kisspeptin and GnRH are activating different isoforms. PKC signaling has been shown to activate the MAPK-dependent ERK1/2 phosphorylation cascade, and ERK1/2 induces both cFos and Egr-1 (40–42). Although the LH surge results from the rapid secretion of LH, it is plausible that kisspeptin may augment GnRH to stimulate gonadotropin transcription as a means to replace the LH and FSH that are released. As immediate early genes, cFos and Egr-1 are rapidly activated; this is consistent with their induction by kisspeptin to replenish the gonadotropins that are secreted during the surge, which also occurs over a very short period of time. We therefore propose that in pituitary gonadotropes, similar to GnRH, Kiss1R activates PKC signaling, which leads to both cFos and Egr-1 induction and, ultimately, expression of FSHβ and LHβ, respectively.
In addition to transfected LβΤ2 gonadotropes, we have also shown that kisspeptin induces gonadotropin gene expression in mouse primary pituitary cells. The endogenous Kiss1R in mouse pituitaries was sufficient for this effect, providing physiologically relevant evidence that this occurs in vivo as well as in vitro.
Pituitary Kiss1r is induced at the time of the LH surge
Pituitary Kiss1r has previously been shown to respond to estradiol status in OVX female rats (19), but it is unknown whether pituitary Kiss1r responds to circadian regulation and/or positive feedback by estradiol, as occurs at the time of the LH surge (30), a crucial component for the induction of ovulation in both rodents and humans. Our finding that pituitary Kiss1r was increased during the LH surge in OVX+E females provides a physiologic context for furthering the understanding of kisspeptin's role in the pituitary. Because the levels of estradiol were consistent between the am and pm OVX+E groups (the only difference being the time of day when the animals were humanely destroyed), it is likely that estradiol was not the sole cause for the increased pituitary Kiss1r expression in the pm group. Moreover, Kiss1r expression in the OVX+E am group was similar to that of the OVX am group, further suggesting a lack of a direct effect of estradiol. Likewise, the increase in pituitary Kiss1r required both the presence of estradiol and the pm time point, suggesting that any influence of circadian regulation in the absence of estradiol was not sufficient (as is the case for the LH surge itself). Furthermore, primary pituitary cells treated with GnRH did not show an increase in Kiss1r expression, suggesting that GnRH alone is also not sufficient. Given these considerations, we therefore propose that both circadian factors and an estradiol-mediated GnRH surge are required in combination for the in vivo increase in pituitary Kiss1r observed in pm OVX+E mice. This increase in pituitary Kiss1r, coupled with the kisspeptin-mediated gonadotropin induction from our in vitro studies, is strong evidence that Kiss1R in the pituitary is involved in the reproductively important LH surge. Overall, an evening increase in Kiss1r in the pituitary may augment the actions of GnRH to stimulate transcription to replenish the massive levels of gonadotropins released during the LH surge. The generation of gonadotrope-specific Kiss1R-null mice would allow for more extensive studies of the in vivo role of pituitary Kiss1R.
In conclusion, we have shown, for the first time, that Kiss1R signaling can regulate the gonadotropins at the transcriptional level both in vivo and in vitro. We have shown that in LβT2 gonadotropes, kisspeptin acts similarly to GnRH, by inducing PKC-dependent expression of LHβ and FSHβ via Egr-1 and AP-1, respectively. Although the integration of various physiologic factors that regulate the LH surge cannot be fully recapitulated in vitro, our transcriptional studies do suggest that kisspeptin/Kiss1R may be involved in regulating the increase in gonadotropin gene expression during this important reproductive stage. To that end, we also report a novel finding that pituitary Kiss1r expression is regulated in accordance with the circadian- and GnRH-mediated LH surge in female mice. The transcriptional mechanisms activated by kisspeptin to influence gonadotropin expression, as well as its own up-regulation during the LH surge in females, shows that Kiss1R has functional roles in the pituitary, which will further our understanding of how the reproductive axis is regulated.
Supplementary Material
Acknowledgments
We thank Sheila J. Semaan, Matthew C. Poling, Sunamita Leming, and Ping Gong (University of California, San Diego) for providing assistance with this project. University of Virginia Center for Research in Reproduction provided hormone measurement services.
This work was supported by National Institutes of Health (NIH) Grants R01 DK044838, R01 HD020377, and R01 HD072754 (to P.L.M.), R01 HD057549 (to D.C.), R01 HD065856 (to A.S.K), and by National Institute of Child Health and Human Development/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (P.L.M.). P.L.M. was partially supported by P30 DK063491, P42 ES101337, and P30 CA023100. E.A.W. was partially supported by T32 GM008666, P42 ES101377, and T32 DA007315 and is in the Biomedical Sciences Graduate Program.
Disclosure Summary: The authors have no conflict of interest.
Footnotes
- AP-1
- activator protein-1
- CRE
- cAMP response element
- DMSO
- dimethylsulfoxide
- E
- 17-β estradiol
- Egr-1
- early growth response factor 1
- ETS
- E-twenty six
- GnRHR
- GnRH receptor
- Kiss1R
- kisspeptin receptor
- OVX
- ovariectomized
- PKC
- protein kinase C
- PKCi
- PKC inhibitor
- qPCR
- quantitative PCR
- SF1
- steroidogenic factor 1
- siRNA
- small interfering RNA
- SRF
- serum response factor
- STAT
- signal transducer and activator of transcription.
References
- 1. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Funes S, Hedrick JA, Vassileva G, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363 [DOI] [PubMed] [Google Scholar]
- 3. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 4. Kauffman AS, Park JH, McPhie-Lalmansingh AA, et al. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27:8826–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 6. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. d'Anglemont de Tassigny X, Fagg LA, Dixon JP, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kauffman AS. Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol. 2010;324:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272 [DOI] [PubMed] [Google Scholar]
- 12. Richard N, Corvaisier S, Camacho E, Kottler ML. KiSS-1 and GPR54 at the pituitary level: overview and recent insights. Peptides. 2009;30:123–129 [DOI] [PubMed] [Google Scholar]
- 13. Caraty A, Smith JT, Lomet D, et al. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148:5258–5267 [DOI] [PubMed] [Google Scholar]
- 14. Gutiérrez-Pascual E, Martínez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagón MM, Castaño JP. Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol. 2007;19:521–530 [DOI] [PubMed] [Google Scholar]
- 15. Luque RM, Córdoba-Chacón J, Gahete MD, et al. Kisspeptin regulates gonadotroph and somatotroph function in nonhuman primate pituitary via common and distinct signaling mechanisms. Endocrinology. 2011;152:957–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology. 2008;149:1951–1959 [DOI] [PubMed] [Google Scholar]
- 17. Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157 [DOI] [PubMed] [Google Scholar]
- 18. Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150:5530–5538 [DOI] [PubMed] [Google Scholar]
- 19. Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;20:381–393 [DOI] [PubMed] [Google Scholar]
- 20. Thackray VG, Mellon PL, Coss D. Hormones in synergy: Regulation of the pituitary gonadotropin genes. Mol Cell Endocrinol. 2010;314:192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spady TJ, Shayya R, Thackray VG, Ehrensberger L, Bailey JS, Mellon PL. Androgen regulates follicle-stimulating hormone β gene expression in an activin-dependent manner in immortalized gonadotropes. Mol Endocrinol. 2004;18:925–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawson MA, Tsutsumi R, Zhang H, et al. Pulse sensitivity of the luteinizing hormone β promoter is determined by a negative feedback loop involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21:1175–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ely HA, Mellon PL, Coss D. GnRH Induces the c-Fos gene via phosphorylation of SRF by the calcium/calmodulin kinase II pathway. Mol Endocrinol. 2011;25:669–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thackray VG, Hunnicutt JL, Memon AK, Ghochani Y, Mellon PL. Progesterone inhibits basal and gonadotropin-releasing induction of luteinizing hormone β-subunit gene expression. Endocrinology. 2009;150:2395–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halvorson LM, Kaiser UB, Chin WW. Stimulation of luteinizing hormone β gene promoter activity by the orphan nuclear receptor, steroidogenic factor-1. J Biol Chem. 1996;271:6645–6650 [DOI] [PubMed] [Google Scholar]
- 26. Kaiser UB, Halvorson LM, Chen MT. Sp1, steroidogenic factor 1 (SF-1), and early growth response protein 1 (egr-1) binding sites form a tripartite gonadotropin-releasing hormone response element in the rat luteinizing hormone-β gene promoter: an integral role for SF-1. Mol Endocrinol. 2000;14:1235–1245 [DOI] [PubMed] [Google Scholar]
- 27. Quirk CC, Lozada KL, Keri RA, Nilson JH. A single Pitx1 binding site is essential for activity of the LHβ promoter in transgenic mice. Mol Endocrinol. 2001;15:734–746 [DOI] [PubMed] [Google Scholar]
- 28. Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–3329 [DOI] [PubMed] [Google Scholar]
- 29. Ghochani Y, Saini JK, Mellon PL, Thackray VG. FOXL2 is involved in the synergy between activin and progestins on the follicle-stimulating hormone β-subunit promoter. Endocrinology. 2012;153:2023–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci U S A. 2007;104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thackray VG, McGillivray SM, Mellon PL. Androgens, progestins, and glucocorticoids induce follicle-stimulating hormone β-subunit gene expression at the level of the gonadotrope. Mol Endocrinol. 2006;20:2062–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jorgensen JS, Nilson JH. AR suppresses transcription of the LHbeta subunit by interacting with steroidogenic factor-1. Mol Endocrinol. 2001;15:1505–1516 [DOI] [PubMed] [Google Scholar]
- 33. Weck J, Anderson AC, Jenkins S, Fallest PC, Shupnik MA. Divergent and composite gonadotropin-releasing hormone-responsive elements in the rat luteinizing hormone subunit genes. Mol Endocrinol. 2000;14:472–485 [DOI] [PubMed] [Google Scholar]
- 34. Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castaño JP, Martínez-Fuentes AJ, Gutiérrez-Pascual E, Vaudry H, Tena-Sempere M, Malagón MM. Intracellular signaling pathways activated by kisspeptins through GPR54: do multiple signals underlie function diversity? Peptides. 2009;30:10–15 [DOI] [PubMed] [Google Scholar]
- 36. Stathatos N, Bourdeau I, Espinosa AV, et al. KiSS-1/G protein-coupled receptor 54 metastasis suppressor pathway increases myocyte-enriched calcineurin interacting protein 1 expression and chronically inhibits calcineurin activity. J Clin Endocrinol Metab. 2005;90:5432–5440 [DOI] [PubMed] [Google Scholar]
- 37. Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology. 2009;150:341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choe HK, Kim HD, Park SH, et al. Synchronous activation of gonadotropin-releasing hormone gene transcription and secretion by pulsatile kisspeptin stimulation. Proc Natl Acad Sci U S A. 2013;110:5677–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Naor Z, Harris D, Shacham S. Mechanism of GnRH receptor signaling: combinatorial cross-talk of Ca2+ and protein kinase C. Front Neuroendocrinol. 1998;19:1–19 [DOI] [PubMed] [Google Scholar]
- 40. Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. GnRH activates ERK1/2 leading to the induction of c-fos and LHβ protein expression in LβT2 cells. Mol Endocrinol. 2002;16:419–434 [DOI] [PubMed] [Google Scholar]
- 41. Zhang T, Wolfe MW, Roberson MS. An early growth response protein (Egr) 1 cis-element is required for gonadotropin-releasing hormone-induced mitogen-activated protein kinase phosphatase 2 gene expression. J Biol Chem. 2001;276:45604–45613 [DOI] [PubMed] [Google Scholar]
- 42. Herbison AE, de Tassigny Xd, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:312–321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.