Abstract
The release of insulin from pancreatic β-cells is regulated by a considerable number of G protein–coupled receptors. During the past several years, we have focused on the physiological importance of β-cell M3 muscarinic acetylcholine receptors (M3Rs). At the molecular level, the M3R selectively activates G proteins of the Gq family. Phenotypic analysis of several M3R mutant mouse models, including a mouse strain that lacks M3Rs only in pancreatic β-cells, indicated that β-cell M3Rs play a key role in maintaining blood glucose levels within a normal range. Additional studies with transgenic M3R mouse models strongly suggest that strategies aimed to enhance signaling through β-cell M3Rs may prove useful in the treatment of type 2 diabetes. More recently, we analyzed transgenic mice that expressed an M3R-based designer receptor in a β-cell–specific fashion, which enabled us to chronically activate a β-cell Gq-coupled receptor by a drug that is otherwise pharmacologically inert. Drug-dependent activation of this designer receptor stimulated the sequential activation of Gq, phospholipase C, ERK1/2, and insulin receptor substrate 2 signaling, thus triggering a series of events that greatly improved β-cell function. Most importantly, chronic stimulation of this pathway protected mice against experimentally induced diabetes and glucose intolerance, induced either by streptozotocin or by the consumption of an energy-rich, high-fat diet. Because β-cells are endowed with numerous receptors that mediate their cellular effects via activation of Gq-type G proteins, these findings provide a rational basis for the development of novel antidiabetic drugs targeting this class of receptors.
The proper regulation of insulin secretion from pancreatic β-cells is essential for the maintenance of normal blood glucose levels. In type 2 diabetes (T2D), the amount of insulin released from pancreatic β-cells is not sufficient to overcome peripheral insulin resistance, resulting in hyperglycemia and glucose intolerance (1, 2).
As is the case with virtually all other cell types, the activity of pancreatic β-cells is regulated by members of the superfamily of G protein–coupled receptors (GPCRs) (3, 4). After binding of extracellular ligands, most GPCRs selectively interact with and activate specific subgroups of heterotrimeric G proteins, such as Gs, Gq/11, or Gi/o. Whereas receptor-mediated activation of Gs and Gq/11 proteins is known to stimulate insulin release from pancreatic β-cells, GPCR-dependent activation of G proteins of the Gi/o family inhibits insulin secretion (3).
Because GPCRs are cell surface receptors, they represent excellent targets for drug therapy. During the past decade, antidiabetic drugs have been introduced in the clinic that mimic or enhance the actions of glucagon-like peptide-1 (GLP-1) at the GLP-1 receptor, a Gs-coupled GPCR (3, 5, 6). The GLP-1 receptor is expressed at relatively high levels by pancreatic β-cells but is also present in several other cell types and tissues (3, 5, 6). Drug-induced activation of the GLP-1 receptor promotes insulin release and can also stimulate β-cell proliferation and enhance β-cell mass, at least in certain animal models (5, 6).
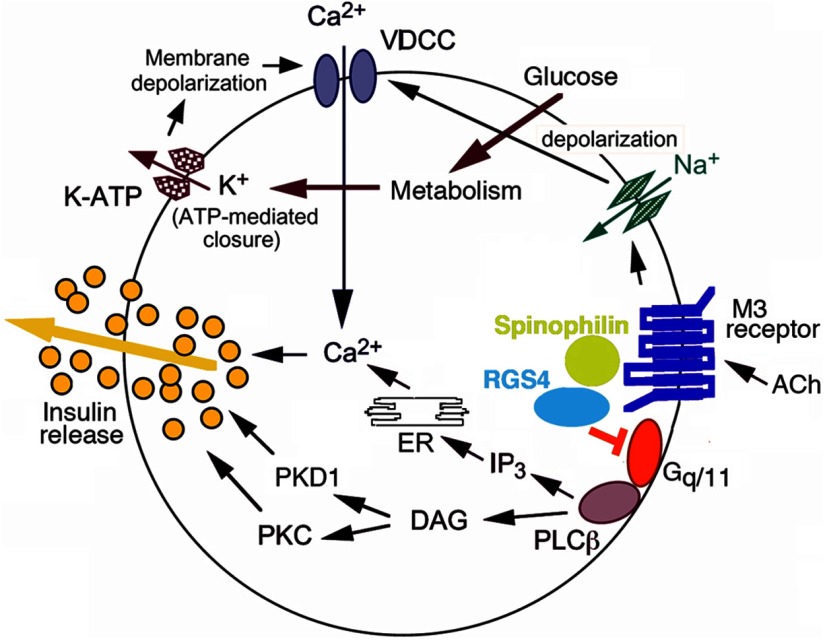
In an analogous fashion, many laboratories currently focus on the development of drugs predicted to improve β-cell function via activation of GPCRs that are linked to Gq-type G proteins. Such receptors include, for example, the M3 muscarinic receptor (M3R), GPR40 (FFA1), GPR120, and different P2Y receptor subtypes (3). At the cellular level, stimulation of this class of receptors triggers an inositol 1,4,5-trisphosphate (IP3)–dependent increase in intracellular calcium levels via Gq-meditated activation of phospholipase Cβ (PLCβ) (shown for the M3R in Figure 1). In addition to IP3, receptor-mediated activation of PLCβ leads to the generation of another major second messenger, diacylglycerol, which can activate different isoforms of protein kinase C (PKC) (shown for the M3R in Figure 1). As reviewed in detail previously (7), these biochemical events ultimately trigger enhanced glucose-dependent insulin release (shown for the M3R in Figure 1).
Figure 1.
M3R signaling in pancreatic β-cells. We recently demonstrated that RGS4 (16) and SPL (19) act as potent negative regulators of M3R signaling in pancreatic β-cells. BRET studies (19) suggest that SPL acts as an adaptor protein capable of recruiting RGS4 to the ligand-activated M3R/Gq protein complex, thus limiting the lifetime of M3R-activated G protein αq/11 subunits. Such a model is consistent with the observation that the lack of SPL or RGS4 affects M3R signaling to a similar degree (19). The figure was modified based on a scheme shown in Ref. 23. DAG, diacylglycerol; ER, endoplasmic reticulum; K-ATP, ATP-sensitive K+ channel; VDCC, voltage-dependent Ca2+ channel.
This review summarizes a series of studies aimed to better understand the physiological relevance of β-cell M3Rs and how signaling through these receptors is regulated at the molecular level. The focus of this review will be on work performed by our laboratory during the past few years.
Studies With M3R Mutant Mice
During the past few years, we have used different mutant mouse models to explore the role of the M3R in the regulation of insulin secretion and whole-body glucose homeostasis. Physiologically, β-cell M3Rs are activated by acetylcholine (ACh), which is released after stimulation of parasympathetic nerves innervating the endocrine pancreas (7, 8) (Figure 1). The M3R is not only expressed by pancreatic β-cells but also by several other peripheral cell types, including smooth muscle and glandular cells and in various regions of the brain (9–11). Like the M3R, the vast majority of GPCRs are expressed in multiple tissues and cell types (12).
To study the physiological relevance of β-cell M3Rs, we analyzed mutant mice that lacked M3Rs selectively in pancreatic β-cells only (β-M3R knockout [KO] mice) or transgenic (Tg) mice that overexpressed M3Rs in this particular cell type (β-M3R Tg mice) (13). We found that the β-M3R KO mice displayed impaired glucose tolerance and significantly reduced insulin release. In contrast, the β-M3R Tg mice showed the reverse phenotype (greatly improved glucose tolerance and increased insulin secretion). Moreover, overexpression of M3Rs in pancreatic β-cells rendered β-M3R Tg mice resistant to diet-induced glucose intolerance and hyperglycemia (13). These findings strongly support the concept that β-cell M3Rs play a central role in maintaining normal glucose homeostasis, raising the possibility that drugs that can enhance signaling through β-cell M3Rs may become clinically useful.
In β-M3R Tg mice, the activation of β-cell M3Rs is dependent on the endogenous release of ACh. At present, muscarinic agonists that can selectively activate the M3R subtype are not available. To mimic the effects of a drug that chronically activates β-cell M3Rs, we generated a mouse model that expresses a constitutively active version of the M3R selectively in pancreatic β-cells (14). This mutant receptor contains a point mutation at the bottom of transmembrane domain 6 (Q490L in the rat M3R sequence), which enables the receptor to activate Gq-type G proteins even in the absence of agonist ligands (15). Insulin release studies performed with isolated perifused islets prepared from these mutant mice (β-M3R[Q490L] Tg mice) confirmed that the Q490L mutant M3R exhibited pronounced ligand-independent signaling (constitutive activity) in mouse β-cells (14).
When we subjected β-M3R[Q490L] Tg mice to a panel of in vivo metabolic tests, they displayed a phenotype that closely resembled that of β-M3R Tg mice (14). Like the β-M3R Tg animals, the β-M3R[Q490L] Tg mice showed a pronounced reduction in blood glucose levels and a significant increase in plasma insulin levels compared with those of their wild-type (WT) littermates. Moreover, independent of the route of glucose administration (ip or oral), β-M3R[Q490L] Tg mice displayed striking improvements in glucose tolerance, associated with enhanced insulin release. Like β-M3R Tg mice, β-M3R[Q490L] Tg mice were also protected against the detrimental metabolic effects associated with the consumption of a high-fat diet (HFD). Taken together, these observations strongly support the concept that chronic activation of β-cell M3Rs does not result in a loss or significant reduction in M3R activity due to M3R desensitization or other counterregulatory cellular events.
Regulator of G Protein Signaling 4 (RGS4) and Spinophilin (SPL) as Negative Regulators of β-Cell M3R Function
RGS4
In an attempt to identify factors (proteins) that regulate M3R function in pancreatic β-cells, we recently demonstrated that RGS4 is enriched in MIN6 insulinoma cells and mouse pancreatic islets (16). RGS4 is a member of the B/R4 subfamily of RGS proteins, acting as a GTPase-activating protein for Gq- and Gi-type G protein α-subunits (17, 18). We showed that small interfering RNA (siRNA)–mediated knockdown of RGS4 in MIN6 cells enhanced M3R-mediated increases in intracellular calcium levels and glucose-stimulated insulin release. Moreover, studies with isolated islets prepared from RGS4 knockout mice showed that the absence of RGS4 was associated with a more pronounced M3R-mediated insulin response (16). In vivo studies demonstrated that mice selectively lacking RGS4 in pancreatic β-cells showed increased metabolic responses (enhanced insulin release and more pronounced decreases in blood glucose levels) after treatment with the peripherally acting muscarinic agonist, bethanechol (16). Control experiments with β-M3R KO mice indicated that these metabolic effects of bethanechol were due to the activation of β-cell M3Rs. Taken together, these results clearly indicate that RGS4 acts as a potent negative regulator of M3R function in pancreatic β-cells (Figure 1).
SPL
In a related study (19), we examined whether SPL, a scaffolding protein known to regulate various functions of the central nervous system (20, 21), was able to modulate signaling through β-cell M3Rs. This investigation was prompted by previous reports demonstrating that SPL can act as an adaptor molecule to recruit members of the RGS protein family to ligand-activated GPCR/G protein–signaling complexes (22).
In vitro studies with MIN6 insulinoma cells demonstrated that siRNA-mediated knockdown of SPL expression led to a pronounced augmentation of M3R-mediated increases in intracellular calcium levels and glucose-dependent insulin secretion (19). The magnitudes of these effects were very similar to those observed after siRNA-mediated RGS4 knockdown in the same cell type. Moreover, simultaneous knockdown of both SPL and RGS4 expression did not further enhance the M3R-mediated calcium and insulin responses. Knockdown of SPL expression in MIN6 cells had no significant effect on cell surface M3R expression levels, M3R internalization, or M3R/arrestin interactions (19). In agreement with the findings obtained with cultured MIN6 cells, M3R-mediated increases in insulin secretion were significantly augmented in pancreatic islets prepared from SPL−/− mice, compared with the corresponding responses observed with islets from WT littermates. Taken together, these observations suggest that SPL and RGS4 are components of the same cellular pathway that exerts an inhibitory function on M3R activity in β-cells.
Bioluminescence resonance energy transfer (BRET) studies performed with transfected COS-7 cells demonstrated that the M3R can interact with SPL even in the absence of ligands and that ligand-induced M3R activation further enhanced the affinity of the M3R for SPL (19). Additional BRET experiments strongly suggested that SPL acts as an adaptor protein that is able to recruit RGS4 to the ligand-activated M3R/G protein complex, thus limiting the lifetime of M3R-activated G protein αq/11 subunits (19) (Figure 1).
Systemic bethanechol treatment of SPL−/− mice led to enhanced insulin release and greater blood glucose–lowering effects, compared with the corresponding responses obtained with WT littermates (19). This finding strongly supports the notion that SPL also inhibits β-cell M3R signaling in vivo.
Because β-cell M3Rs play a key role in the maintenance of proper glucose homeostasis (13, 23), strategies aimed to target (inhibiting) M3R-associated proteins in pancreatic β-cells, such as RGS4 or SPL, may prove useful to enhance β-cell function for therapeutic purposes.
M3R-Based Designer Receptors as Novel Tools to Study GPCR Regulation of β-Cell Function
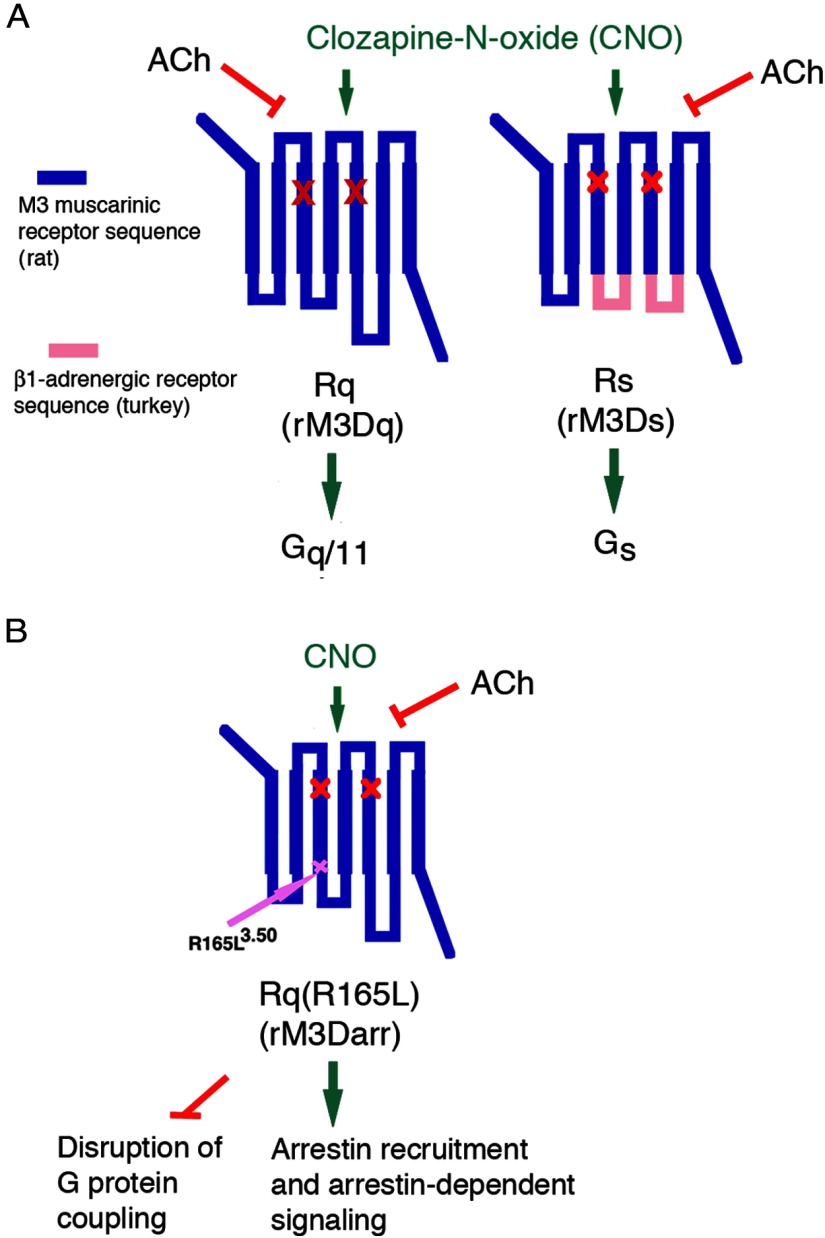
M3Rs, like other Gq-linked GPCRs expressed by pancreatic β-cells, are also present in many other tissues or cell types (9–11), and receptor subtype-selective agonists useful for in vivo studies are not available for the M3R and many GPCRs with similar G protein–coupling properties. For this reason, it has been difficult to monitor the metabolic consequences after the chronic administration of a drug that selectively stimulates a particular GPCR expressed by β-cells. To address this issue, we started to express a new class of muscarinic receptor–based designer GPCRs selectively in β-cells of transgenic mice (24, 25). Importantly, these engineered muscarinic receptors are unable to bind ACh, the endogenous muscarinic receptor ligand, but can be selectively activated by clozapine-N-oxide (CNO), a pharmacologically inactive metabolite of clozapine (26, 27) (Figure 2). Most commonly, these CNO-sensitive designer GPCRs are now referred to as designer receptors exclusively activated by designer drug (DREADDs) (26, 27). However, the term second-generation RASSLs (receptors activated solely by synthetic ligand) has also been used to describe this new class of designer receptors (28).
Figure 2.
Structure of M3R-based DREADDs used to explore GPCR regulation of β-cell function. All 3 designer GPCRs shown in this figure contain the Y3.33C and A5.46G point mutations in transmembrane domains TM3 and TM5, respectively (red X marks). They lack the ability to bind ACh but can be activated by CNO with high potency and efficacy. A, Structure of the Rq and Rs DREADDs (alternative names: rM3Dq and rM3Ds, respectively) used in the study by Guettier et al (24). B, Structure of the Rq(R165L) DREADD (alternative name: rM3Darr). This designer receptor is unable to activate heterotrimeric G proteins but promotes arrestin-dependent signaling (47). It contains the R165L3.50 point mutation at the bottom of TM3 within the highly conserved DRY motif. In the presence of CNO, the Rq(R165L) DREADD is able to recruit arrestin-2 and -3 and to mediate arrestin-dependent downstream signaling (47).
In an initial study (24), we generated a transgenic mouse line (β-Rq) that expressed a Gq-coupled, M3R-based DREADD (nomenclature used in Ref. 24; Figure 2A) selectively in pancreatic β-cells (24, 26). For control purposes, we designed an M3R/β1-adrenergic receptor hybrid DREADD that no longer couples to Gq but preferentially activates Gs. We then generated a transgenic mouse line (β-Rs) that expressed this Gs-coupled DREADD (nomenclature used in Ref. 24: Rs; Figure 2A) selectively in pancreatic β-cells at expression levels that were comparable to those observed with β-Rq mice. Initially, we focused on the metabolic effects observed after acute administration of CNO to β-Rq and β-Rs mice (24). We found that acute stimulation of β-cell Gq and Gs signaling led to striking increases in insulin release, reduced blood glucose levels, and greatly improved glucose tolerance.
In general, the β-Rq–mediated in vivo metabolic effects were more pronounced than the corresponding responses after activation of β-Rs. However, we noted that β-Rs showed a certain degree of agonist-independent signaling (constitutive activity), which made it difficult to directly compare the metabolic effects observed with the 2 different transgenic mouse lines (24). For example, it is possible that ligand-independent Rs signaling may have triggered counterregulatory responses in the β-Rs mice (see, eg, Ref. 29), thus complicating the proper interpretation of the experimental data. In any case, the robust, CNO-dependent in vivo metabolic changes observed with the β-Rq transgenic mice strongly suggest that drugs that can chronically activate β-cell Gq/11-coupled receptors may prove useful in the treatment of T2D and glucose intolerance, similar to GLP-1 receptor agonists.
More recently, we performed more detailed metabolic studies with transgenic β-Rq mice chronically treated with CNO (for up to 10 weeks) (25). When β-Rq mice were maintained on regular mouse chow, chronic CNO treatment resulted in a significant increase in β-cell mass (24, 25), compared with that of WT littermates. Additional studies strongly suggested that this effect was primarily due to enhanced β-cell proliferation (25).
Chronic CNO treatment of β-Rq mice was also associated with a significant increase in pancreatic insulin content and enhanced expression of the genes coding for preproinsulin (Ins2) and proprotein convertases 1 and 2 (Pcks1 and Pcks2, respectively) (25). These findings are consistent with the concept that the increase in pancreatic insulin content displayed by CNO-treated β-Rq mice is caused by enhanced β-cell proliferation as well as transcriptional processes that promote insulin synthesis.
In addition, we found (25) that chronic CNO treatment of β-Rq mice led to pronounced increases in the expression of many β-cell transcription factors (Pdx1, MafA, Ngn3, NeuroD1, and Nkx6.1) that are critical for β-cell differentiation and/or maintaining normal β-cell function and β-cell mass (30–34) (Figure 3). Pdx1, for example, plays a critical role in β-cell differentiation and replication (30, 32, 33) and stimulates the expression of many genes that are of fundamental importance for β-cell function, including Ins2, Pcks1, Pcks2, and Glut2 (35, 36). It is therefore likely that the Rq-mediated increases in the expression of Pdx1 and other β-cell transcription factors are responsible for (at least partially) the enhanced expression of the Ins2, Pcks1, Pcks2, and Glut2 genes observed with β-Rq mice chronically treated with CNO.
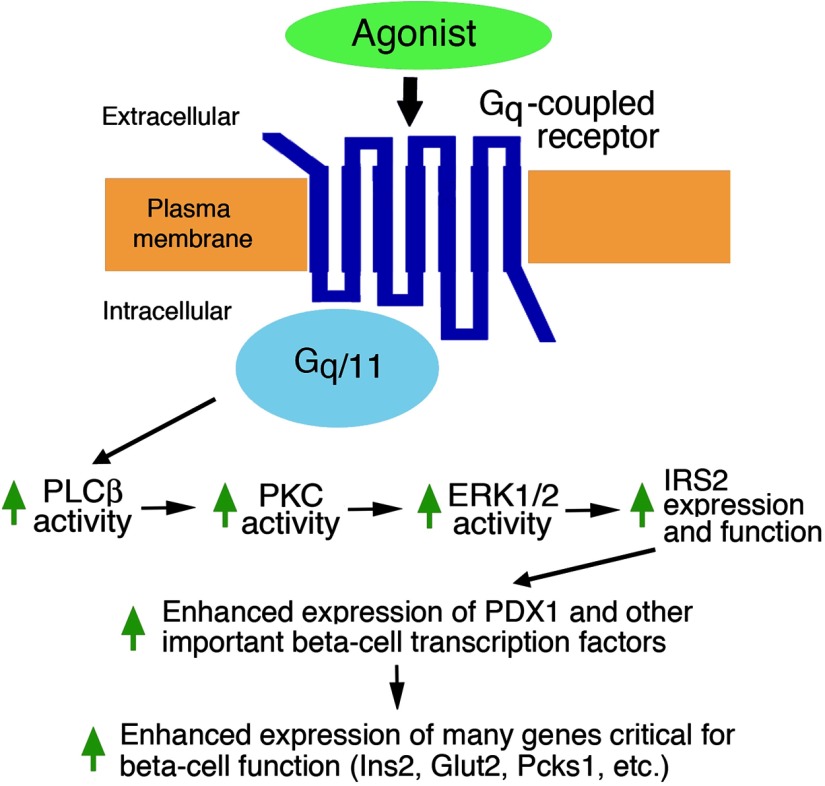
Figure 3.
A GPCR/Gq-dependent signaling cascade predicted to be operative in pancreatic β-cells. The scheme shown here is based primarily on data reported by Jain et al (25). It depicts some of the key signaling molecules involved in Gq-mediated activation of IRS2 expression and function in β-cells. Enhanced IRS2 activity is predicted to stimulate the expression of Pdx1 and other key β-cell transcription factors, activating the promoters of many genes important for β-cell function (25).
To map the molecular pathways that link Rq activation with the pronounced changes in β-cell gene expression levels, we performed a series of additional in vitro and in vivo experiments (25). We noted that chronic CNO treatment of β-Rq mice led to greatly increased insulin receptor substrate 2 (IRS2) protein and transcript levels in pancreatic islets derived from these transgenic mice. It is well known that IRS2, an adaptor protein that mediates many of the cellular actions of insulin and IGF-I, plays a central role in maintaining normal β-cell function (37–41). We therefore speculated that persistent stimulation of β-Rq signaling may improve β-cell function by activation of IRS2-dependent signaling pathways. Consistent with this concept, we found that the expression levels of the phosphorylated, signaling-competent forms of Akt and ERK1/2 were also enhanced in islets derived from β-Rq mice chronically treated with CNO (25). Notably, many of the important cellular functions of IRS2, including the stimulatory effects of IRS2 on β-cell replication (37), are mediated by these 2 kinases. These data strongly suggest that chronic activation of β-cell Gq-coupled receptors can promote signaling through the IRS2-Akt-ERK1/2 cascade, thus promoting β-cell replication and function. It should also be noted that IRS2 signaling is known to promote Pdx1 expression (38, 40). These findings provide additional support for the concept that the increase in Pdx1 transcript levels observed after chronic CNO treatment of β-Rq mice is due, at least in part, to enhanced IRS2 signaling (Figure 3).
To explore how the activation of a Gq-coupled receptor leads to increased IRS2 expression in β-cells, we performed a series of additional studies with rat INS1-M3 insulinoma cells and pancreatic islets prepared from CNO-treated β-Rq mice (as well as various control groups) (25). The outcome of studies using kinase inhibitors and RNA interference–based gene knockdown technology strongly supported a model in which receptor-induced stimulation of the PLCβ/PKC pathway enhances the activity of the Irs2 promoter in an ERK1/2-dependent fashion (Figure 3). This concept is further supported by the recent observation that ERK activation greatly enhances the activity the Irs2 gene by stimulating the binding of the SP1 and NFI transcription factors to a short region of the Irs2 promoter (42).
Studies with IRS2-deficient mice (IRS2−/− mice) further confirmed that the beneficial metabolic effects observed after stimulation of β-cell Rq receptors require the presence of IRS2 (25). In agreement with published data (38), 1- to 2-month-old IRS2−/− mice developed a very severe diabetes phenotype, characterized by pronounced hyperglycemia and glucose intolerance and striking reductions in pancreatic insulin content and β-cell mass (25). Studies performed by Morris White and his colleagues (39–41) strongly suggest that these metabolic deficits are caused primarily by the lack of IRS2 in pancreatic β-cells. Importantly, we demonstrated that CNO treatment of IRS2−/− mice that expressed the Rq receptor in a β-cell–selective fashion did not lead to any improvements in glucose homeostasis or β-cell function or to any significant increases in pancreatic insulin content and β-cell mass (25).
To investigate whether chronic activation of β-cell Rq receptors was able to improve glucose homeostasis in a mouse model of diabetes, we induced diabetes in β-Rq mice via a series of streptozotocin (STZ) injections that reduced β-cell mass by ∼70% to 80% (25). Notably, a pronounced reduction in β-cell mass has also been demonstrated in autopsy studies of obese patients with T2D (43). We made the striking observation that STZ-injected β-Rq mice that had been chronically treated with CNO were largely protected against the detrimental metabolic effects observed with STZ-treated control mice, including hyperglycemia and glucose intolerance (25). These beneficial metabolic CNO effects observed with STZ-injected β-Rq mice were accompanied by pronounced increases in pancreatic insulin content and β-cell mass and elevated expression levels of many genes critical for β-cell function and replication. It is likely that these changes in gene expression levels induced by chronic activation of β-Rq play a key role in CNO-dependent prevention of STZ-induced diabetes.
We also found that β-Rq mice chronically consuming CNO with the drinking water were protected against the negative metabolic consequences caused by maintaining mice on a HFD (25). Specifically, we observed that CNO-treated β-Rq mice, in contrast to non–CNO-treated control mice, did not develop hyperglycemia, glucose intolerance, and impaired glucose-induced insulin release. Somewhat surprisingly, when mice were maintained on a HFD, the CNO-treated β-Rq mice showed a smaller increase in β-cell mass than the 3 control groups (nontreated β-Rq and WT mice and CNO-treated WT mice). One possible explanation for this observation is that the CNO-dependent improvement in β-cell function, which is most likely initiated by changes at the transcriptional level (25), is responsible for the beneficial metabolic effects observed with CNO-treated HFD β-Rq mice.
The findings summarized above further support the concept that agents that can enhance signaling through Gq-coupled receptors expressed by pancreatic β-cells, including the M3R, GPR40, GPR120, and different P2Y receptor subtypes (3), represent interesting new targets for the development of novel antidiabetic drugs.
Stimulation of Insulin Release by an Arrestin-Biased Designer Receptor
In most cases, ligand-activated GPCRs are rapidly phosphorylated by GPCR kinases (44), which increases the receptors' affinity for members of the arrestin protein family (arrestin-2 and -3; also known as β-arrestin-1 and -2), thus promoting receptor desensitization (44). However, accumulating evidence indicates that arrestin-2 and -3 can also act as scaffolding proteins that enable GPCRs to transduce intracellular signals in a G protein–independent manner (45, 46). At present, little is known about the physiological relevance of these arrestin-dependent signaling pathways in the regulation of β-cell function and other important physiological processes. However, such knowledge may prove useful for the development of novel therapeutic agents, including arrestin-biased agonists (45, 46).
To facilitate studies aimed to explore the physiological consequences of arrestin-/2/3–dependent signaling, we recently developed an M3R-based DREADD that can recruit arrestins in a CNO-dependent fashion but is unable to couple to G proteins (Figure 2B) (47). Several years ago, we reported that a rat mutant M3R that contained the R165L3.50 point mutation virtually lost its ability to activate G proteins (48). Prompted by this observation, we introduced the R165L3.50 point mutation into the Rq DREADD (alternative name: rM3Dq). As expected, CNO treatment of cultured cells expressing the resulting designer receptor [Rq(R165L); alternative name: rM3Darr] did not result in any changes in intracellular calcium, inositol phosphate, or cAMP levels, suggesting that Rq(R165L) lost the ability to activate heterotrimeric G proteins such as Gq/11, Gs, or Gi/o. However, BRET assays performed with transfected COS-7 cells demonstrated that Rq(R165L) was able to interact with arrestin-2 and -3 after CNO activation of the designer receptor (47). In agreement with these findings, CNO treatment of Rq(R165L)-expressing human embryonic kidney 293T cells stimulated ERK1/2 phosphorylation in a CNO- and arrestin-dependent fashion. Taken together, these findings strongly support the concept that the Rq(R165L) receptor represents an arrestin-biased DREADD.
To study the potential role of arrestin signaling in regulating insulin secretion, we expressed the Rq(R165L) designer receptor in mouse MIN6 insulinoma cells (47). As expected, CNO treatment of Rq(R165L)-expressing MIN6 cells had no significant effect on intracellular calcium and cAMP levels, indicating that Rq(R165L) is unable to activate heterotrimeric G proteins in this insulinoma cell line. However, incubation of Rq(R165L)-expressing MIN6 cells with CNO resulted in a concentration-dependent release of insulin. The magnitude of this response was significantly reduced after siRNA-mediated knockdown of either arrestin-2 or -3 (47). These data strongly support the concept that activation of arrestin-dependent signaling pathways promotes insulin release from pancreatic β-cells. In agreement with our findings, Sonoda et al (49) recently reported that arrestin-2 plays a role in GLP-1–induced insulin secretion in cultured INS-1 rat insulinoma cells. Moreover, phenotypic analysis of a novel M3R mutant mouse model strongly suggested that arrestin signaling contributes to M3R-mediated augmentation of insulin release (50). We are currently in the process of generating transgenic mice that express the Rq(R165L) designer receptor in a β-cell selective fashion. Studies with this novel mouse model should provide important new information about the potential physiological relevance of arrestin-dependent signal pathways that are operational in pancreatic β-cells.
Potential Clinical Use of Antidiabetic Drugs Targeting the M3R
The administration of an M3R agonist would represent the most direct approach to enhance signaling through β-cell M3Rs. However, as mentioned earlier in this review, selective M3R agonists are not available at present. The high-resolution structure of the M3R (51), together with that of the M2 muscarinic receptor (52), indicates that the amino acid side chains forming the orthosteric binding pocket are fully conserved among all muscarinic receptor subtypes, explaining why it has been so difficult to develop subtype-selective M3R ligands in the past. Interestingly, the M2 muscarinic receptor and M3R structures also reveal the presence of a relatively large extracellular vestibule, which is located just above the orthosteric binding domain (51, 52). The amino acids lining this outer cavity are less well conserved than those that form the orthosteric muscarinic binding pocket. By exploiting these structural differences, it should be possible to develop allosteric agonists which selectively activate the M3R (ectopic M3R agonists) or compounds that act as positive allosteric modulators (PAMs) at the M3R (M3R PAMs). In fact, such compounds have recently been developed for other muscarinic receptor subtypes, including the M1, M4, and M5 receptors (53, 54).
It should be noted that M3Rs also play a key role in mediating ACh-induced smooth muscle contraction and activation of most endocrine and exocrine glands (9, 10, 55). Thus, the potential clinical use of agents that selectively promote signaling through the M3R (eg, M3R ectopic agonists or PAMs) may be limited by peripheral side effects. However, because β-cell M3Rs appear to be coupled to downstream effectors with particularly high efficacy, it might be possible to apply low doses of such agents that enhance the activity of β-cell M3Rs but have little effect on other M3R-expressing tissues. The future development of allosteric M3R agonists or PAMs would allow us to test this hypothesis experimentally.
Recent studies suggest that so-called “biased GPCR agonists” may offer therapeutic benefits for the treatment of various clinical disorders (56). In the GPCR field, this term primarily refers to agonists that are able to preferentially activate G protein– or arrestin-dependent signaling pathways. It is likely that the development of biased M3R agonists, together with the generation of new mouse models in which distinct M3R signaling pathways can be activated in a drug-dependent fashion, will guide the development of novel classes of M3R ligands that may prove useful for improving β-cell function while having reduced adverse effects on other M3R-expressing tissues.
Besides the M3R, pancreatic β-cells express several other Gq/11-coupled receptors including GPR40 (FFA1), GPR120, and different P2Y receptor subtypes (P2Y1 and P2Y6) (3, 57, 58). The development of novel compounds targeting these receptors for therapeutic purposes is currently the focus of many industrial and academic laboratories. It should be of great interest to compare the beneficial metabolic effects of these various compounds in animal models of diabetes and, eventually, in patients with T2D.
Concluding Remarks
Studies with new mutant mouse models have led to novel insights into the physiological relevance of β-cell M3Rs. Various lines of evidence suggest that the beneficial metabolic effects observed after chronic stimulation of β-cell M3Rs involve G protein-dependent and, perhaps, G protein-independent pathways requiring arrestin signaling. Moreover, recent data indicate that 2 M3R-associated proteins, RGS4 and SPL, exert inhibitory effects on agonist-induced M3R signaling in pancreatic β-cells. These observations may pave the way for the development of novel classes of drugs that are able to modulate β-cell M3R function for therapeutic purposes.
Acknowledgments
We thank all of our collaborators for their invaluable contributions to the studies summarized in this minireview.
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACh
- acetylcholine
- BRET
- bioluminescence resonance energy transfer
- CNO
- clozapine-N-oxide
- DREADD
- designer receptors exclusively activated by designer drug
- GLP-1
- glucagon-like peptide-1
- GPCR
- G protein–coupled receptor
- HFD
- high-fat diet
- IP3
- inositol 1,4,5-trisphosphate
- IRS2
- insulin receptor substrate 2
- KO
- knockout
- M3R
- M3 muscarinic receptor
- PAM
- positive allosteric modulator
- PKC
- protein kinase C
- PLCβ
- phospholipase Cβ
- RGS4
- regulator of G protein signaling 4
- siRNA
- small interfering RNA
- SPL
- spinophilin
- STZ
- streptozotocin
- T2D
- type 2 diabetes
- Tg
- transgenic
- WT
- wild-type.
References
- 1. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 2. Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346 [DOI] [PubMed] [Google Scholar]
- 3. Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385 [DOI] [PubMed] [Google Scholar]
- 4. Regard JB, Kataoka H, Cano DA, et al. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest. 2007;117:4034–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 6. Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113:546–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocr Rev. 2001;22:565–604 [DOI] [PubMed] [Google Scholar]
- 8. Duttaroy A, Zimliki CL, Gautam D, Cui Y, Mears D, Wess J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in M3 muscarinic acetylcholine receptor-deficient mice. Diabetes. 2004;53:1714–1720 [DOI] [PubMed] [Google Scholar]
- 9. Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–565 [PubMed] [Google Scholar]
- 10. Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290 [PubMed] [Google Scholar]
- 11. Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA. Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience. 1994;63:207–221 [DOI] [PubMed] [Google Scholar]
- 12. Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135:561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gautam D, Han SJ, Hamdan FF, et al. A critical role for β cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3:449–461 [DOI] [PubMed] [Google Scholar]
- 14. Gautam G, Ruiz de Azua I, Li JH, et al. Beneficial metabolic effects caused by persistent activation of β-cell M3 muscarinic acetylcholine receptors in transgenic mice. Endocrinology. 2010;151:5185–5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmidt C, Li B, Bloodworth L, Erlenbach I, Zeng FY, Wess J. Random mutagenesis of the M3 muscarinic acetylcholine receptor expressed in yeast. Identification of point mutations that “silence” a constitutively active mutant M3 receptor and greatly impair receptor/G protein coupling. J Biol Chem. 2003;278:30248–30260 [DOI] [PubMed] [Google Scholar]
- 16. Ruiz de Azua I, Scarselli M, Rosemond E, et al. RGS4 is a negative regulator of insulin release from pancreatic β-cells in vitro and in vivo. Proc Natl Acad Sci USA. 2010;107:7999–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827 [DOI] [PubMed] [Google Scholar]
- 18. Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–559 [DOI] [PubMed] [Google Scholar]
- 19. Ruiz de Azua I, Nakajima K, Rossi M, et al. Spinophilin as a novel regulator of M3 muscarinic receptor-mediated insulin release in vitro and in vivo. FASEB J. 2012;26:4275–4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Allen P B, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci USA. 1997;94:9956–9961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sarrouilhe D, di Tommaso A, Métayé T, Ladeveze V. Spinophilin: from partners to functions. Biochimie. 2006;88:1099–1113 [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Zeng W, Soyombo AA, et al. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol. 2005;7:405–411 [DOI] [PubMed] [Google Scholar]
- 23. Ruiz de Azua I, Gautam D, Guettier JM, Wess J. Novel insights into the function of β-cell M3 muscarinic acetylcholine receptors: therapeutic implications. Trends Endocrinol Metab. 2011;22:74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guettier JM, Gautam D, Scarselli M, et al. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci USA. 2009;106:19197–19202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jain S, Ruiz de Azua I, Lu H, White MF, Guettier JM, Wess J. Chronic activation of a designer Gq-coupled receptor improves β cell function. J Clin Invest. 2013;123:1750–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–5168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conklin BR, Hsiao EC, Claeysen S, et al. Engineering GPCR signaling pathways with RASSLs. Nat. Methods. 2008;5:673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma YH, Landis C, Tchao N, et al. Constitutively active stimulatory G-protein αs in β-cells of transgenic mice causes counterregulation of the increased adenosine 3′,5′-monophosphate and insulin secretion. Endocrinology. 1994;134:42–47 [DOI] [PubMed] [Google Scholar]
- 30. Cerf ME. Transcription factors regulating β-cell function. Eur J Endocrinol. 2006;155:671–679 [DOI] [PubMed] [Google Scholar]
- 31. Schisler JC, Jensen PB, Taylor DG, et al. The Nkx6.1 homeodomain transcription factor suppresses glucagon expression and regulates glucose-stimulated insulin secretion in islet beta cells. Proc Natl Acad Sci USA. 2005;102:7297–7302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernardo AS, Hay CW, Docherty K. Pancreatic transcription factors and their role in the birth, life and survival of the pancreatic β cell. Mol Cell Endocrinol. 2008;294:1–9 [DOI] [PubMed] [Google Scholar]
- 33. Kaneto H, Miyatsuka T, Kawamori D, et al. PDX-1 and MafA play a crucial role in pancreatic β-cell differentiation and maintenance of mature β-cell function. Endocr J. 2008;55:235–252 [DOI] [PubMed] [Google Scholar]
- 34. Rukstalis JM, Habener JF. Neurogenin3: a master regulator of pancreatic islet differentiation and regeneration. Islets. 2009;1:177–184 [DOI] [PubMed] [Google Scholar]
- 35. Zangen DH, Bonner-Weir S, Lee CH, et al. Reduced insulin, GLUT2, and IDX-1 in beta-cells after partial pancreatectomy. Diabetes. 1997;46:258–264 [DOI] [PubMed] [Google Scholar]
- 36. Hart AW, Baeza N, Apelqvist A, Edlund H. Attenuation of FGF signalling in mouse β-cells leads to diabetes. Nature. 2000;408:864–868 [DOI] [PubMed] [Google Scholar]
- 37. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96 [DOI] [PubMed] [Google Scholar]
- 38. Withers DJ, Gutierrez JS, Towery H, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904 [DOI] [PubMed] [Google Scholar]
- 39. Kushner JA, Ye J, Schubert M, et al. Pdx1 restores β cell function in Irs2 knockout mice. J Clin Invest. 2002;109:1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hennige AM, Burks DJ, Ozcan U, et al. Upregulation of insulin receptor substrate-2 in pancreatic β cells prevents diabetes. J Clin Invest. 2003;112:1521–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin X, Taguchi A, Park S, et al. Dysregulation of insulin receptor substrate 2 in β cells and brain causes obesity and diabetes. J Clin Invest. 2004;114:908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Udelhoven M, Leeser U, Freude S, et al. Identification of a region in the human IRS2 promoter essential for stress induced transcription depending on SP1, NFI binding and ERK activation in HepG2 cells. J Mol Endocrinol. 2010;44:99–113 [DOI] [PubMed] [Google Scholar]
- 43. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 44. Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650 [DOI] [PubMed] [Google Scholar]
- 45. Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakajima K, Wess J. Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol Pharmacol. 2012;82:575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li B, Nowak NM, Kim SK, et al. Random mutagenesis of the M3 muscarinic acetylcholine receptor expressed in yeast: identification of second-site mutations that restore function to a coupling-deficient mutant M3 receptor. J Biol Chem. 2005;280:5664–5675 [DOI] [PubMed] [Google Scholar]
- 49. Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, Olefsky JM. β-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic β cells. Proc Natl Acad Sci USA. 2008;105:6614–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kong KC, Butcher AJ, McWilliams P, et al. M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase D1. Proc Natl Acad Sci USA. 2010;107:21181–21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kruse AC, Hu J, Pan AC, et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature. 2012;482:552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haga K, Kruse AC, Asada H, et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature. 2012;482:547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Digby GJ, Shirey JK, Conn PJ. Allosteric activators of muscarinic receptors as novel approaches for treatment of CNS disorders. Mol Biosyst. 2010;6:1345–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Keov P, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology. 2011;60:24–35 [DOI] [PubMed] [Google Scholar]
- 55. Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733 [DOI] [PubMed] [Google Scholar]
- 56. Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Balasubramanian R, Ruiz de Azua I, Wess J, Jacobson KA. Activation of distinct P2Y receptor subtypes stimulates insulin secretion in MIN6 mouse pancreatic beta cells. Biochem Pharmacol. 2010;79:1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yelovitch S, Barr HM, Camden J, et al. Identification of a promising drug candidate for the treatment of type 2 diabetes based on a P2Y1 receptor agonist. J Med Chem. 2012;55:7623–7635 [DOI] [PMC free article] [PubMed] [Google Scholar]