Abstract
AIM: To investigate the potential roles of Delta-like ligand 4 (DLL4) on the biological behavior of gastric cancer cells and its molecular mechanisms.
METHODS: A recombinant eukaryotic expression vector containing human DLL4 gene was constructed and transfected into the human gastric cancer cell line SGC7901. Clones with up-regulated DLL4 were selected and amplified. The effect of DLL4 up-regulation on gastric cancer cell growth was assessed using cell growth assay. The migration and invasion were assessed using a transwell migration assay and matrigel invasion assay. Matrix metalloproteinases were detected using the zymogram technique. Cells were implanted subcutaneously into male BALB/c nu/nu mice. Tumor volumes were then calculated and compared. DLL4 staining in the implanted tumor was performed using immunohistochemistry technique.
RESULTS: Growth curves over a six-day time course showed significantly promoted cell proliferation of SGC7901 cells with up-regulated DLL4. DLL4 up-regulation in SGC7901 cells promoted the migration (205.4 ± 15.2 vs 22.3 ± 12.1, P < 0.05) and invasion (68.8 ± 5.3 vs 18.2 ± 6.0, P < 0.05) in vitro and tumorigenicity in vivo (2640.5 ± 923.6 mm3 vs 1115.1 ± 223.8 mm3, P < 0.05). Furthermore, significantly increased mRNA level and increased secretion of matrix metalloproteinase-2 (MMP-2) proenzyme were observed in SGC7901 cells with up-regulated DLL4. However, increased MMP-9 mRNA level but decreased extracellular MMP-9 proenzyme level was observed.
CONCLUSION: Our observations indicated a mechanism by which activation of DLL4-mediated Notch signaling promotes the expression and secretion of MMP-2 proenzyme and influences the progress of gastric cancer.
Keywords: Gastric cancer, Delta-like ligand 4/Notch, Matrix metalloproteinase, Migration, Invasion
Core tip: Delta-like ligand 4 (DLL4), one of the five notch signaling ligands in mammals, has been researched mainly with regard to vasculogenesis and tumor angiogenesis. To the best of our knowledge, there is rare study to investigate its role and mechanism in human gastric cancers. We found that DLL4 promotes cellular proliferation, migration, invasion and tumorigenicity in gastric cancer cells. Furthermore, increased mRNA level and increased secretion of matrix metalloproteinase-2 proenzyme, while increased matrix metalloproteinase (MMP)-9 mRNA levels but decreased extracellular MMP-9 proenzyme levels were observed. These results indicated a mechanism by which activation of DLL4-mediated Notch signaling promotes the expression and secretion of MMP-2 proenzyme and influences the progress of gastric cancer.
INTRODUCTION
Gastric cancer is one of the most common cancers and lethal malignancies worldwide[1,2]. Approximately 738000 patients with gastric cancer died in 2011[2]. Of these, 80% died within a short period after curative surgery due to locoregional recurrence (87%) and distant metastases (30%)[3,4]. The 5-year survival of gastric cancer is less than 40%, even with adjuvant chemo-radiotherapy[5]. Therefore, there is an urgent need for new therapeutic strategies to improve clinical outcomes of this disease.
Notch signaling, as an evolutionarily conserved signaling pathway, is involved in a variety of cellular processes including cell fate, differentiation, proliferation, survival rate, and apoptosis[1,6]. The Notch signaling in mammals consists of five ligands [Delta-like ligand 41/3/4 (DLL1/3/4) and Jagged 1/2] and four receptors (Notch 1-4). Notch proteins are synthesized as full-length unprocessed proteins and cleaved at the S1 site by furin-like convertase to generate the mature receptor. Notch-ligand binding induces the cleavages of a Notch receptor by metalloprotease and γ-secretase to release the Notch intracellular domain (NICD). The NICD subsequently translocates to the nucleus, where it forms a complex with the members of the CSL (C-promoter binding factor-1, suppressor of hairless in Drosophila and lag in Caenorhabditis elegans) transcription factor family and regulates the expression of downstream genes such as Hairy/Enhancer of Split (HES1/5/6/7) and the HES-related proteins (HEY1/2/L)[6-10].
Interestingly, Notch signaling may play distinct biological roles in different tumors. It acts as an oncogene in pancreatic cancer[11], colon cancer[12], breast cancer[13] and most other solid tumors[14,15], whereas it acts as an anti-oncogene in some types of skin cancer[16], lung cancer[17] and prostate cancer[18]. Compared with other solid tumors, less literature relates to the role of DLL4-mediated Notch signaling in gastric cancer.
Although most Notch-related genes are expressed in multiple tissue and cell types, DLL4 is largely restricted to the vascular endothelia and has been researched mainly with regard to vasculogenesis and tumor angiogenesis[7,19-21]. Blockade of DLL4 signaling has been shown to lead to inhibition of tumor angiogenesis by nonproductive angiogenesis in some types of murine tumor models[20,21]. However, the precise function and mechanism of DLL4 in gastric cancer remain unclear. In the present study, we up-regulated the expression of DLL4 in the gastric cancer cell line SGC7901, and assessed its biological function both in vitro and in vivo.
MATERIALS AND METHODS
Cell lines and animals
Human gastric cancer cell line SGC7901 was purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. Cells were propagated in RPMI-1640 medium (Gibco-Invitrogen, Carlsbad, CA, United States) supplemented with 10% fetal bovine serum (FBS) (Gibco-Invitrogen), penicillin (100 units/mL) and streptomycin (100 μg/mL) in a humidified atmosphere containing 5% CO2 at 37 °C.
Animal studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the protocol was approved by the Animal Research Committee of Zhejiang University, Hangzhou, China. Mouse protocols were conducted in accordance with stringent regulations laid out by Zhejiang University Laboratory Animal Center. Twenty-four 4-wk-old male BALB/c nu/nu mice (Shanghai SLAC Laboratory Animal Co, Shanghai, China) weighing 10-12 g used for subcutaneous tumor implantation were randomly divided into control, SGC7901-vector and SGC7901-DLL4 groups. Animals were housed in a sterile environment, and maintained on daily 12-h light/12-h dark cycle, which was controlled by qualified staff in the Zhejiang University Laboratory Animal Center.
Recombinant eukaryotic expression vector pEGFP-C1-DLL4 construction
A human full-length DLL4 cDNA fragment was amplified using a forward primer 5’-GGAATTCACCATGGCGGCAGCGTCC-3’ and a reverse primer 5’- CGGGATCCTTATACCTCCGTGGCAATGACAC-3’, verified by sequencing, and then EcoRI-BamHI-digested. The 2-kb fragment was purified using a AxyPrep DNA Gel Extraction Kit (Axygen, Union City, CA, United States) and was ligated to a EcoRI-BamHI-digested pEGFP-C1 vector DNA (Clontech, Mountain View, CA, United States). Positive clones were further confirmed by EcoRI-BamHI digestion and sequencing.
Transfection and clone selection
SGC7901 cells in logarithmic growth phase were collected and seeded into six-well plates at 4 × 105 cells/well to obtain approximately 80% confluence after overnight incubation. Cultured SGC7901 cells were divided into three groups: (1) transfected cells with recombinant pEGFP-C1-DLL4 vector (SGC7901-DLL4 group); (2) transfected cells with pEGFP-C1 vector (SGC7901-vector group); and (3) nontransfected cells (control group). Transfection was performed using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. The cells were then cultured in RPMI-1640 medium containing 10% FBS and G418 (600 μg/mL) for 21 d. G418-resistant clones were selected and amplified in complete medium containing G418 (300 μg/mL). Up-regulation of DLL4 was verified by real-time polymerase chain reaction (PCR) and Western blotting assay.
RNA extraction and real-time PCR
Extraction of total RNA from cultured cells was performed using the TRIzol method (Invitrogen). Total RNA (1 μg) was reverse-transcribed using RevertAid First Strand cDNA Synthesis Kit (Fermentas, Thermo-Fisher Scientific, Waltham, MA, United States). Real-time PCR was performed in triplicate using SYBR Green PCR Master Mix (TaKaRa, Tokyo, Japan) in an ABI PRISM Stepone Plus Sequence Detection System (Applied Biosystems, Foster City, CA, United States) according to the manufacturer’s instructions. Forty cycles were used to amplify DLL4/Notch-related genes (denaturation at 95 °C for 5 s, annealing and extension at 60 °C for 32 s). Relative quantitation of gene expression was detected using the method described by Pfaffl[22]. The primers for real-time PCR were described in Table 1.
Table 1.
Primer sequences for real-time polymerase chain reaction
| Genes | Forward sequences | Reverse sequences | Product size (bp) |
| DLL4 | 5’-CCCTGGCAATGTACTTGTGAT-3’ | 5’-TGGTGGGTGCAGTAGTTGAG-3’ | 73 |
| Notch1 | 5’-GCCTCAACATCCCCTACAAGA-3’ | 5’-CCACGAAGAACAGAAGCACAAA-3’ | 120 |
| HES1 | 5’-GTCAACACGACACCGGATAA-3’ | 5’-TTCAGCTGGCTCAGACTTTC-3’ | 113 |
| HES5 | 5’-TGGAGAAGGCCGACATCCT-3’ | 5’-GGCGACGAAGGCTTTGC-3’ | 65 |
| HEY1 | 5’-CGCGTTATCTGAGCATCATT-3’ | 5’-TGGGAAGCGTAGTTGTTGAG-3’ | 88 |
| MMP-2 | 5’-GCTGACGGTAAGGACGGACTC-3’ | 5’-CGTTGCCATTGAACAAGAAGG-3’ | 158 |
| MMP-9 | 5’-TTTGACAGCGACAAGAAGTGG-3’ | 5’-AGGGCGAGGACCATAGAGG-3’ | 189 |
| E-cadherin | CGTTAGAGGTGGGTGACTACAAA | GAACAGCAAGAGCAGCAGAAT | 220 |
| GAPDH | 5’-TGCCACTCCTCCACCTTTG-3’ | 5’-CGAACCACCCTGTTGCTGT-3’ | 104 |
DLL: Delta-like ligand 4; MMP: Matrix metalloproteinases; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
Western blotting analysis
Total protein extracts were prepared and run on 10% polyacrylamide gels. Fractionated proteins were electro-transferred to polyvinylidenefluoride membranes. Antibodies against human DLL4 (1:1000, Abcam, Cambridge, United Kingdom) and β-actin (1:1000, Abcam) were used for detection at 4 °C overnight. Horseradish peroxidase-conjugated anti-rabbit antibody was applied as a secondary antibody at 25 °C for 1 h. Antigens were identified by luminescent visualization using an ECL Western blotting Detection System (Millipore, Billerica, MA, United States). Signal intensity was measured using a Bio-Rad XRS chemiluminescence detection system (Bio-Rad).
Cell growth assay and growth curve
3-(4,5-dimethyl-2-yl)-5-(3-arboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assays using the CellTiter 96 AQueous nonradioactive cell proliferation MTS agent (Promega, Madison, WI, United States) were performed to evaluate cell growth. Approximately 500 cells in 100 μL of medium with 0.5% FBS were plated in 96-well plates and allowed to attach for 24 h. Then 20 μL of the Promega MTS reagent was added to each well, and further incubation was conducted in a humidified incubator for 2 h. Absorbance of each well at 490 nm was determined using a Microplate Reader (Bio-Rad). The cell-growth curve was plotted using the optical densities obtained over 6 consecutive days.
Transwell migration assay
Transwell units with 8.0-μm pore-size polycarbonate filters (Corning Costar, Tewksbury, MS, United States) were used to investigate chemotactic cell migration. Cells were harvested and suspended at approximately 4 × 105 cells/mL in RPMI 1640 medium containing 0.5% FBS. A total volume of 100 μL of suspension was added into the upper compartment of the transwell unit. After 30 min of attachment, the units were transferred to wells containing 600 μL RPMI 1640 medium with 20% FBS as a chemoattractant, and further incubation was conducted for 20 h. After removing the cells on the upper surface of the membrane with a cotton bud and 15-min staining with 0.1% crystal violet, cell numbers on the underside were determined using light microscopy. Five randomly selected fields were counted per insert.
Matrigel invasion assay
Transwell units with 8.0-μm pore-size polycarbonate filters (Corning Costar) were precoated with 50 μL of 1:5 diluted matrigel (Becton Dickinson Biosciences, Franklin Lakes, NJ, United States) and used to investigate cell invasion. A total volume of 100 μL of suspension containing approximately 40000 cells was added to each upper compartment of precoated units. After 30 min of attachment, the units were transferred to wells containing 600 μL RPMI 1640 medium with 20% FBS as a chemoattractant, and incubation was conducted for 20 h. After removing the cells and Matrigel on the upper surface of the membrane with a cotton bud and 15-min staining with 0.1% crystal violet, cell numbers on the underside were determined using light microscopy. Five randomly selected fields were counted per insert.
Gelatin zymography
Matrix metalloproteinases (MMPs) were detected using the zymogram technique according to the standard procedure[23]. Cells were allowed to grow in serum-free medium for 24 h. Then the medium was collected and diluted 1:1 with × 2 sample buffer and run on 10% polyacrylamide gels containing gelatin (1 mg/mL) until the bromophenol blue tracking dye reached the bottom of the gel. After electrophoresis, the gel was washed twice in Triton X-100 solution (2.5%) at room temperature for 15 min, transferred to 100 mL of development buffer containing 2 mL of Triton X-100 solution, and incubation was conducted at 37 °C for 72 h. Staining with Coomassie Blue R-250 solution proceeded overnight, then destaining was performed until the bands were clearly visible. Areas of protease activity appeared as clear bands against a dark blue background where the protease has digested the substrate.
Mouse tumor models
Twenty-four 4-wk-old male BALB/c nu/nu mice weighing 10-12 g were purchased and randomly divided into control, SGC7901-vector and SGC7901-DLL4 groups. Cells (1 × 106 cells/animal) in a total volume of 0.1 mL of PBS were implanted subcutaneously into the flank of each mice on day 0. Tumor size was measured on days 7, 14, 28, 35, 42. The tumor volume was calculated using the formula: volume = D × d2 × pi/6, where D and d represent the longer diameter and shorter diameter respectively.
Immunohistochemistry
For DLL4 staining, we used a rabbit monoclonal anti-human DLL4 antibody (1:200, Abcam). Paraffin-embedded tissue blocks were serially sectioned 4 μm in thickness, dewaxed, and rehydrated in serial alcohol washes. Endogenous peroxidase activity was blocked with 0.03% hydrogen peroxide in PBS for 20 min. Immunostaining for DLL4 was done by incubation for 1 h with primary antibody in blocking buffer and visualized using 3,3’-diaminobenzidine chromogen (Invitrogen) with hematoxylin (Invitrogen) counterstaining after treatment with HRP-conjugated Goat anti-rabbit immunoglobulin G (1:100 dilution).
Statistical analysis
Numerical results are shown as mean ± SD. Data were analyzed using SPSS ver. 13.0 statistical software (SPSS, Inc., Chicago, IL, United States). Differences among three groups were examined using one-way analysis of variance analysis. Means between two groups were compared using the Student’s t test. Statistical significance was considered a P value of < 0.05. All experiments were performed at least three times.
RESULTS
Up-regulation of DLL4 changed downstream gene expression in SGC7901 cells
SGC7901 cells were transfected with vector encoding human DLL4 (SGC7901-DLL4 group) or empty vector (SGC7901-vector group) and were then selected by G418 for at least 3 wk. Non-transfected SGC7901 cells were used as a control group. The up-regulating effect of the vector on DLL4 protein levels in the SGC7901-DLL4 group was confirmed by western blot assay (Figure 1A). Real-time PCR was further used to assess the expression of Notch1, DLL4, and downstream genes including HES1, HES5, HEY1, as well as the mRNA levels of MMP-2, MMP-9 and the adhesion protein E-cadherin. The results show that the mRNA level of DLL4 in the SGC7901-DLL4 group was approximately 10-fold higher than in the SGC7901-vector group. Accordingly, up-regulation of DLL4 expression resulted in increased expression of HES5, MMP-2 and MMP-9 (Figure 1B).
Figure 1.
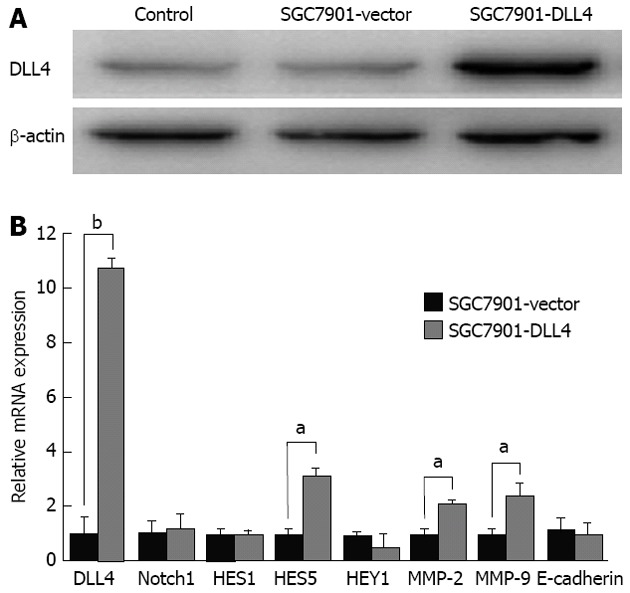
Up-regulation of Delta-like ligand 4 changed downstream gene expression in SGC7901 cells. A: Western blotting confirmed the up-regulation of Delta-like ligand 4 (DLL4) in the SGC7901-DLL4 group at the protein level; B: Real-time polymerase chain reaction was used to assess Notch1, DLL4, and downstream genes HES1, HES5, HEY1, as well as the matrix metalloproteinases-2 (MMP-2), MMP-9, and the adhesion protein E-cadherin at the mRNA level. The results showed increased expression of DLL4, HES5, MMP-2, and MMP-9, but no significant change of HES1 and HEY1 (aP < 0.05, bP < 0.01 vs the SGC7901-vector group). Data show mean ± SD of gene expression compared with the control group. Endogenous references was glyceraldehyde 3-phosphate dehydrogenase; SGC7901-vector: SGC7901 cells transfected with an empty vector; SGC7901-DLL4: SGC7901 cells transfected with a vector encoding human DLL4 gene; Control: Non-transfected SGC7901 cells.
Effects of DLL4 up-regulation on gastric cancer cell proliferation
MTS cell proliferation assays (Promega) were used to investigate the effect of DLL4 transfection on gastric cancer cells. A growth curve was plotted based on the optical densities obtained during the 6 d after attachment. The results showed that up-regulation of DLL4 resulted in significantly accelerated cell proliferation in the SGC7901-DLL4 group when compared to the SGC7901- vector group (P < 0.05, Figure 2).
Figure 2.
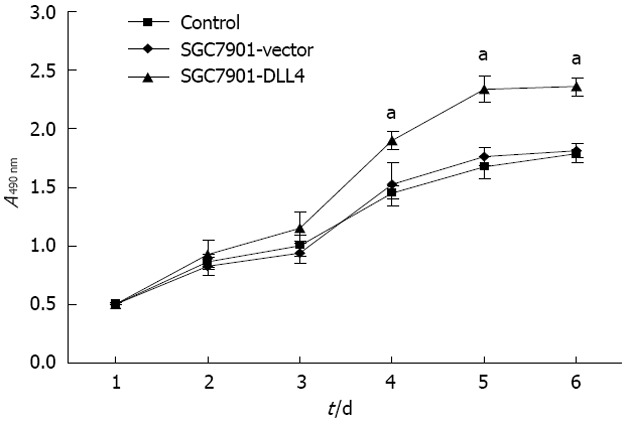
Up-regulation of Delta-like ligand 4 promoted cell proliferation in SGC7901 cells. Growth curve comparing SGC7901-Delta-like ligand 4 (DLL4), SGC7901-vector and SGC7901 cells over a 6-d time course. Up-regulation of DLL4 significantly promoted the proliferation of SGC7901 cells in vitro. Data are shown as the mean ± SD of three independent experiments (aP < 0.05 vs the SGC7901-vector group); SGC7901-vector: SGC7901 cells transfected with an empty vector; SGC7901-DLL4: SGC7901 cells transfected with a vector encoding human DLL4 gene; Control: Non-transfected SGC7901 cells.
Up-regulation of DLL4 accelerated migration of SGC7901 cells
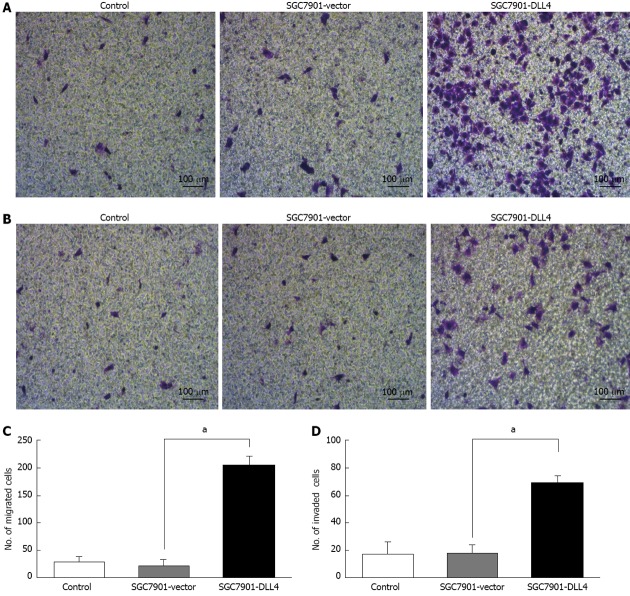
The effects of DLL4 up-regulation on SGC7901 cell migration were investigated using 8.0-μm pore-size Corning Costar Transwell units. Approximately 4 × 104 cells from each of the groups were plated on the insert. The results show that the number of SGC7901 cells transfected with DLL4, which migrated across the insert, was 8.3 times higher than those transfected with empty vector (205.4 ± 15.2 vs 22.3 ± 12.1, P < 0.05) (Figure 3).
Figure 3.
Up-regulation of Delta-like ligand 4 promoted cell migration and invasion in SGC7901 cells. A: Crystal violet staining revealed the migrated cells of each group; B: Crystal violet staining reveals invaded cells from each of the groups; C: The number of cells in the SGC7901-Delta-like ligand 4 (DLL4) group that had migrated was 8.31 times higher than those transfected with an empty vector (205.4 ± 15.2 vs 22.3 ± 12.1, aP < 0.05); D: The number of cells in the SGC7901-DLL4 group that had migrated across both the Matrigel and the insert was 2.83 times higher than that in the SGC7901-vector group (68.8 ± 5.3 vs 18.2 ± 6.0, aP < 0.05). SGC7901-vector: SGC7901 cells transfected with an empty vector; SGC7901-DLL4: SGC7901 cells transfected with vector encoding human DLL4 gene; Control: Non-transfected SGC7901 cells.
Up-regulation of DLL4 accelerated invasion of SGC7901 cells
Matrigel invasion assays were performed using 8.0-μm pore-size Corning Costar transwell units precoated with 50 μL Matrigel (1:5 diluted), which permitted cell migration across the filter. Approximately 4 × 104 cells from each group were plated in the insert. The results show that the number of SGC7901 cells transfected with DLL4, which migrated across both the Matrigel and the insert, was 2.83 times higher than those transfected with an empty vector (68.8 ± 5.3 vs 18.2 ± 6.0, P < 0.05) (Figure 3).
Effect of DLL4 up-regulation on the secretion and activation of extracellular MMPs
Gelatin zymography was used to analyze the effect of DLL4 up-regulation on the secretion and activation of MMPs, such as MMP-2 and MMP-9. From each group, 20 μL of culture supernatant were diluted 1:1 with × 2 sample buffer, incubated for 30 min at 25°C, and added to a zymogram comprising 10% polyacrylamide, 1 mg/mL gelatin, and electrophoresed at a constant voltage of 110 V until the bromophenol blue tracking dye reached the bottom of the gel. Coomassie Blue R-250 staining of the zymogram revealed significantly increased MMP-2 proenzyme level but decreased MMP-9 proenzyme level in the medium of the SGC7901-DLL4 group compared to the SGC7901-vector group (Figure 4).
Figure 4.
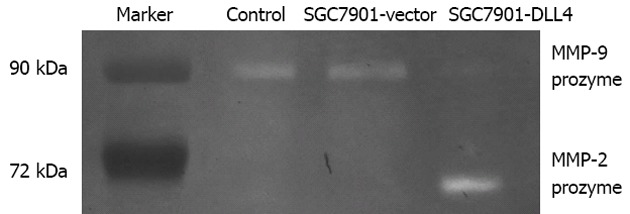
Effect of Delta-like ligand 4 up-regulation on the secretion and activation of extracellular matrix metalloproteinases. Twenty microlitres of culture supernatant from each group was used. Coomassie Blue R-250 staining of the zymogram revealed significantly increased matrix metalloproteinases (MMP)-2 proenzyme but decreased MMP-9 proenzyme in the SGC7901-Delta-like ligand 4 (DLL4) group vs the SGC7901-vector group. SGC7901-vector: SGC7901 cells transfected with an empty vector; SGC7901-DLL4: SGC7901 cells transfected with vector encoding human DLL4 gene; Control: Non-transfected SGC7901 cells.
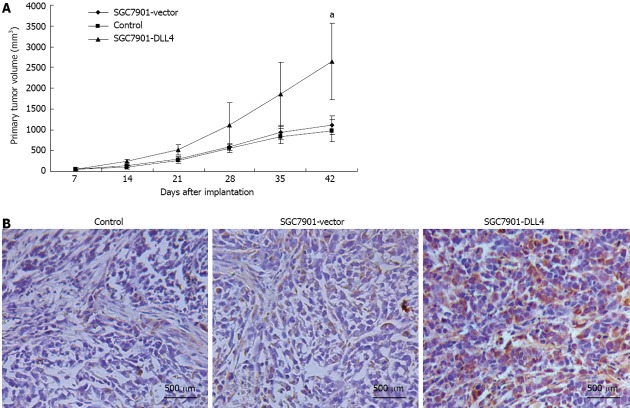
Up-regulation of DLL4 accelerated tumor growth in vivo
To examine the effect of DLL4 up-regulation on gastric cancer growth in vivo, 24 four-wk-old male BALB/c nu/nu mice were divided randomly and averagely into three groups and implanted subcutaneously with control/SGC7901-vector/SGC7901-DLL4 cells. Six weeks after inoculation, the gastric cancer cells grew as subcutaneous implants in each nude mouse (100%). The size of subcutaneously formed tumor masses of the SGC7901-DLL4 group (2640.5 ± 923.6 mm3) (P < 0.05 relative to the SGC7901-vector group) was significantly larger than the control (1011.1 ± 273.6 mm3) and SGC7901-vector groups (1115.1 ± 223.8 mm3) (Figure 5A). Immunohistochemistry staining of DLL4 confirmed the up-regulation of DLL4 expression in the SGC7901-DLL4 group (Figure 5B).
Figure 5.
Up-regulation of Delta-like ligand 4 promoted tumorigenesis of SGC7901 cells in vivo. Twenty-four 4-wk-old male BALB/c nu/nu mice were divided randomly into three groups and implanted subcutaneously with control/SGC7901-vector/SGC7901-Delta-like ligand 4 (DLL4) cells. A: Six weeks after inoculation, the size of subcutaneous formed tumor masses of the SGC7901-DLL4 group (2640.5 ± 923.6 mm3) was significantly larger than the control (1011.1 ± 273.6 mm3) and SGC7901-vector groups (1115.1 ± 223.8 mm3); B: Immunohistochemistry staining of DLL4 confirmed the up-regulation of DLL4 in the SGC7901-DLL4 group. aP < 0.05 vs the SGC7901-vector group.
DISCUSSION
The finding that DLL4/Notch signaling participates in vascular development and homeostasis, as well as the evidence that DLL4 is predominantly expressed in the developing endothelium and in some tumor endothelia, suggest that DLL4-mediated Notch signaling activation is involved in tumor angiogenesis[24-26]. However, literature regarding the biological properties of DLL4-mediated Notch signaling in gastric cancer is very limited. The aim of our study is to investigate the potential roles of DLL4 on the biological behavior of gastric cancer cells and its molecular mechanisms.
In this study, we investigated the effect of DLL4 up-regulation on cell growth, migration and invasion in vitro and tumor growth in vivo. The results showed that up-regulation of DLL4 significantly promotes proliferation, migration, and invasion of SGC7901 gastric cancer cells in vitro and tumor growth in vivo. Our findings indicate the oncogenic function of DLL4/Notch signaling in gastric cancers, which is in accord with previous studies on other cancers. For example, small interfering RNA (siRNA)-induced knockdown of DLL4 resulted in decreased proliferation, increased apoptosis and retarded growth of tumor in vitro and in vivo[27-31]. Other Notch-targeting approaches such as chemical inhibitors of gamma-secretase significantly suppressed cell growth in colon cancer cell lines[32,33] and sensitized oxaliplatin- and 5-Fu-induced apoptosis and growth inhibition[34]. These findings indicate that DLL4/Notch signaling is an important molecular pathway involved in oncogenesis and chemoresistance. Targeting DLL4/Notch signaling might constitute a novel molecular therapy for cancers.
Wang et al[31] reported that inactivation of Notch signaling in prostate cancer cells leads to decreased expression and activity of MMP-9, which contributes to the inhibition of cell migration and invasion. Our study shows that up-regulation of DLL4 leads to decreased activity of MMP-9 with increased MMP-9 expression at mRNA level. One explanation for this observation could be that Notch signaling targeting genes, as well as MMP-9, have complex post-transcriptional regulations. This explanation might also contribute to diverse roles of Notch signaling in different types of cancers. Another possible explanation is that the activation of MMP-9 is inhibited by other signaling pathways or molecules induced by DLL4 up-regulation, such as tissue inhibitor of metalloproteinase-1 (TIMP-1), TIMP-2, or other TIMPs. Furthermore, significantly increased mRNA levels and secretion of MMP-2 proenzymes were observed in gastric cancer cells with up-regulated DLL4. These results suggest that MMP-9 signaling might not be sufficient to exert an effect alone in gastric cancer progression[35], while other molecules such as MMP-2 might play a major role.
In summary, to our knowledge, activation of DLL4-mediated Notch signaling effectively promotes proliferation, migration, and invasion of gastric cancer cells, and desensitizes the cells to chemotherapeutically induced cell senescence. Our data suggest that DLL4-mediated Notch signaling may play an important role in the progression of gastric cancer, and that DLL4-mediated Notch signaling could be a potential target for gastric cancer biotherapy. Meanwhile, we identified MMP-2 as a novel target of DLL4-mediated Notch signaling in gastric cancer cells. However, the precise molecular mechanism of DLL4-mediated Notch signaling in gastric cancer remains unclear. Further studies are required to elucidate possible downstream target genes or potential interacting molecules of DLL4-mediated Notch signaling.
In summary, up-regulation of DLL4 significantly promoted cellular proliferation, migration, and invasion in vitro and tumor growth in vivo. Significantly increased MMP-2 expression at both the mRNA and the protein level was observed in gastric cancer with up-regulated DLL4. Our observations indicated a mechanism by which activation of DLL4-mediated Notch signaling promotes the expression and secretion of MMP-2 proenzyme and influences the progress of gastric cancer.
COMMENTS
Background
Gastric cancer is one of the most common cancers and lethal malignancies worldwide. Discovering novel biomarkers that correlate with gastric cancer may present opportunities to reduce the severity of this disease. As one of the five Notch signaling ligands in mammals, Delta-like ligand 4 (DLL4) has been researched mainly with regard to vasculogenesis and tumor angiogenesis. However, the precise function and mechanism of DLL4 in gastric cancer remain unclear.
Research frontiers
Notch signaling, as an evolutionarily conserved signaling pathway, is involved in a variety of cellular processes, including cell fate, differentiation, proliferation and apoptosis. Previous data indicates its distinct biological roles in different tumors. It may work as an oncogene or anti-oncogene. Their observations indicated a mechanism by which activation of DLL4-mediated Notch signaling promotes the expression and secretion of matrix metalloproteinase (MMP)-2 proenzyme and influences the progress of gastric cancer.
Innovations and breakthroughs
Previous studies indicated that DLL4 is largely restricted to the vascular endothelia. Recent reports were focused on its roles on vasculogenesis and tumor angiogenesis. The authors discovered that DLL4 up-regulation promotes cellular proliferation, migration, invasion and tumorigenicity in gastric cancer cells. Increased mRNA level and increased secretion of matrix metalloproteinase-2 proenzyme, while increased MMP-9 mRNA level but decreased extracellular MMP-9 proenzyme level were observed.
Applications
In understanding the role and mechanism of DLL4-mediated Notch signaling in gastric cancer, this study may represent a future strategy as a therapeutic target and/or a way to improve clinical treatment for gastric cancer.
Peer review
The authors found that ectopic expression of DLL4 significantly promoted cellular proliferation, migration, invasion in vitro and tumor growth in vivo. Significantly increased mRNA levels and secretion of MMP-2 proenzymes were observed, increased MMP-9 mRNA but decreased extracellular MMP-9 proenzyme were observed. The paper is well presented and the results are interesting.
Footnotes
Supported by The Key Project of Science and Technology of Zhejiang Province, No. 2009C14017; the National Natural Science Foundation of China, No. 81071959 and No. 81101837; the Zhejiang Provincial Medical and Healthy Science Foundation of China, No. 2013KYA100 and No. 2009B043; and the Public Welfare Technology Research Project of Zhejiang Province, No. 2010C34001
P- Reviewers Ricci V, Zhu YL S- Editor Gou SX L- Editor O’Neill M E- Editor Li JY
References
- 1.Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC, Li AF, Wang AM, Kuo ML, Chi CW. The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase-2. Cancer Res. 2009;69:5039–5048. doi: 10.1158/0008-5472.CAN-08-4021. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Kim DW, Park SA, Kim CG. Detecting the recurrence of gastric cancer after curative resection: comparison of FDG PET/CT and contrast-enhanced abdominal CT. J Korean Med Sci. 2011;26:875–880. doi: 10.3346/jkms.2011.26.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767–2773. doi: 10.1200/JCO.2004.10.184. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Katoh M, Katoh M. Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int J Mol Med. 2006;17:681–685. [PubMed] [Google Scholar]
- 7.Yan M. Therapeutic promise and challenges of targeting DLL4/NOTCH1. Vasc Cell. 2011;3:17. doi: 10.1186/2045-824X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsujinaka T, Fujitani K, Hirao M, Kurokawa Y. Current status of chemoradiotherapy for gastric cancer in Japan. Int J Clin Oncol. 2008;13:117–120. doi: 10.1007/s10147-007-0743-1. [DOI] [PubMed] [Google Scholar]
- 9.Katoh M, Katoh M. Notch signaling in gastrointestinal tract (review) Int J Oncol. 2007;30:247–251. [PubMed] [Google Scholar]
- 10.Lahmar M, Catelain C, Poirault S, Dorsch M, Villeval JL, Vainchenker W, Albagli O, Lauret E. Distinct effects of the soluble versus membrane-bound forms of the notch ligand delta-4 on human CD34+CD38low cell expansion and differentiation. Stem Cells. 2008;26:621–629. doi: 10.1634/stemcells.2007-0428. [DOI] [PubMed] [Google Scholar]
- 11.Mullendore ME, Koorstra JB, Li YM, Offerhaus GJ, Fan X, Henderson CM, Matsui W, Eberhart CG, Maitra A, Feldmann G. Ligand-dependent Notch signaling is involved in tumor initiation and tumor maintenance in pancreatic cancer. Clin Cancer Res. 2009;15:2291–2301. doi: 10.1158/1078-0432.CCR-08-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jubb AM, Turley H, Moeller HC, Steers G, Han C, Li JL, Leek R, Tan EY, Singh B, Mortensen NJ, et al. Expression of delta-like ligand 4 (Dll4) and markers of hypoxia in colon cancer. Br J Cancer. 2009;101:1749–1757. doi: 10.1038/sj.bjc.6605368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jubb AM, Soilleux EJ, Turley H, Steers G, Parker A, Low I, Blades J, Li JL, Allen P, Leek R, et al. Expression of vascular notch ligand delta-like 4 and inflammatory markers in breast cancer. Am J Pathol. 2010;176:2019–2028. doi: 10.2353/ajpath.2010.090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel NS, Li JL, Generali D, Poulsom R, Cranston DW, Harris AL. Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res. 2005;65:8690–8697. doi: 10.1158/0008-5472.CAN-05-1208. [DOI] [PubMed] [Google Scholar]
- 15.Donnem T, Andersen S, Al-Shibli K, Al-Saad S, Busund LT, Bremnes RM. Prognostic impact of Notch ligands and receptors in nonsmall cell lung cancer: coexpression of Notch-1 and vascular endothelial growth factor-A predicts poor survival. Cancer. 2010;116:5676–5685. doi: 10.1002/cncr.25551. [DOI] [PubMed] [Google Scholar]
- 16.Dotto GP. Notch tumor suppressor function. Oncogene. 2008;27:5115–5123. doi: 10.1038/onc.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB, Ball DW. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 2001;61:3200–3205. [PubMed] [Google Scholar]
- 18.Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao WQ. Dynamics of notch expression during murine prostate development and tumorigenesis. Cancer Res. 2001;61:7291–7297. [PubMed] [Google Scholar]
- 19.Suchting S, Freitas C, le Noble F, Benedito R, Bréant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oishi H, Sunamura M, Egawa S, Motoi F, Unno M, Furukawa T, Habib NA, Yagita H. Blockade of delta-like ligand 4 signaling inhibits both growth and angiogenesis of pancreatic cancer. Pancreas. 2010;39:897–903. doi: 10.1097/MPA.0b013e3181ce7185. [DOI] [PubMed] [Google Scholar]
- 21.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkes SP, Li H, Taniguchi GT. Zymography and reverse zymography for detecting MMPs, and TIMPs. Methods Mol Biol. 2001;151:399–410. doi: 10.1385/1-59259-046-2:399. [DOI] [PubMed] [Google Scholar]
- 24.Kuhnert F, Kirshner JR, Thurston G. Dll4-Notch signaling as a therapeutic target in tumor angiogenesis. Vasc Cell. 2011;3:20. doi: 10.1186/2045-824X-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwasaki J, Nihira S. Anti-angiogenic therapy against gastrointestinal tract cancers. Jpn J Clin Oncol. 2009;39:543–551. doi: 10.1093/jjco/hyp062. [DOI] [PubMed] [Google Scholar]
- 26.Gurney A, Hoey T. Anti-DLL4, a cancer therapeutic with multiple mechanisms of action. Vasc Cell. 2011;3:18. doi: 10.1186/2045-824X-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding XY, Ding J, Wu K, Wen W, Liu C, Yan HX, Chen C, Wang S, Tang H, Gao CK, et al. Cross-talk between endothelial cells and tumor via delta-like ligand 4/Notch/PTEN signaling inhibits lung cancer growth. Oncogene. 2012;31:2899–2906. doi: 10.1038/onc.2011.467. [DOI] [PubMed] [Google Scholar]
- 28.Hu W, Lu C, Dong HH, Huang J, Shen DY, Stone RL, Nick AM, Shahzad MM, Mora E, Jennings NB, et al. Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer. Cancer Res. 2011;71:6030–6039. doi: 10.1158/0008-5472.CAN-10-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer M, Yen WC, Kapoun AM, Wang M, O’Young G, Lewicki J, Gurney A, Hoey T. Anti-DLL4 inhibits growth and reduces tumor-initiating cell frequency in colorectal tumors with oncogenic KRAS mutations. Cancer Res. 2011;71:1520–1525. doi: 10.1158/0008-5472.CAN-10-2817. [DOI] [PubMed] [Google Scholar]
- 30.Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, Fitch-Bruhns M, Lazetic S, Park IK, Sato A, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V, Hay N, Sarkar FH. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem. 2010;109:726–736. doi: 10.1002/jcb.22451. [DOI] [PubMed] [Google Scholar]
- 32.Zheng H, Pritchard DM, Yang X, Bennett E, Liu G, Liu C, Ai W. KLF4 gene expression is inhibited by the notch signaling pathway that controls goblet cell differentiation in mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G490–G498. doi: 10.1152/ajpgi.90393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghaleb AM, Aggarwal G, Bialkowska AB, Nandan MO, Yang VW. Notch inhibits expression of the Krüppel-like factor 4 tumor suppressor in the intestinal epithelium. Mol Cancer Res. 2008;6:1920–1927. doi: 10.1158/1541-7786.MCR-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng RD, Shelton CC, Li YM, Qin LX, Notterman D, Paty PB, Schwartz GK. gamma-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 2009;69:573–582. doi: 10.1158/0008-5472.CAN-08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mrena J, Wiksten JP, Nordling S, Kokkola A, Ristimäki A, Haglund C. MMP-2 but not MMP-9 associated with COX-2 and survival in gastric cancer. J Clin Pathol. 2006;59:618–623. doi: 10.1136/jcp.2005.033761. [DOI] [PMC free article] [PubMed] [Google Scholar]