Abstract
AIM: To investigate activity, toxicity, and prognostic factors for survival of erlotinib and fixed dose-rate gemcitabine (FDR-Gem) in advanced pancreatic cancer.
METHODS: We designed a single-arm prospective, multicentre, open-label phase II study to evaluate the combination of erlotinib (100 mg/d, orally) and weekly FDR-Gem (1000 mg/m2, infused at 10 mg/m2 per minute) in a population of previously untreated patients with locally advanced, inoperable, or metastatic pancreatic cancer. Primary endpoint was the rate of progression-free survival at 6 mo (PFS-6); secondary endpoints were overall response rate (ORR), response duration, tolerability, overall survival (OS), and clinical benefit. Treatment was not considered to be of further interest if the PFS-6 was < 20% (p0 = 20%), while a PFS-6 > 40% would be of considerable interest (p1 = 40%); with a 5% rejection error (α = 5%) and a power of 80%, 35 fully evaluable patients with metastatic disease were required to be enrolled in order to complete the study. Analysis of prognostic factors for survival was also carried out.
RESULTS: From May 2007 to September 2009, 46 patients were enrolled (male/female: 25/21; median age: 64 years; median baseline carbohydrate antigen 19-9 (CA 19-9): 897 U/mL; locally advanced/metastatic disease: 5/41). PFS-6 and median PFS were 30.4% and 14 wk (95%CI: 10-19), respectively; 1-year and median OS were 20.2% and 26 wk (95%CI: 8-43). Five patients achieved an objective response (ORR: 10.9%, 95%CI: 1.9-19.9); disease control rate was 56.5% (95%CI: 42.2-70.8); clinical benefit rate was 43.5% (95%CI: 29.1-57.8). CA 19-9 serum levels were decreased by > 25% as compared to baseline in 14/23 evaluable patients (63.6%). Treatment was well-tolerated, with skin rash being the most powerful predictor of both longer PFS (P < 0.0001) and OS (P = 0.01) at multivariate analysis (median OS for patients with or without rash: 42 wk vs 15 wk, respectively, Log-rank P = 0.03). Additional predictors of better outcome were: CA 19-9 reduction, female sex (for PFS), and good performance status (for OS).
CONCLUSION: Primary study endpoint was not met. However, skin rash strongly predicted erlotinib efficacy, suggesting that a pharmacodynamic-based strategy for patient selection deserves further investigation.
Keywords: Pancreatic cancer, Gemcitabine, Fixed dose-rate, Erlotinib, Prognostic factors, Cutaneous rash, Phase II trial
Core tip: The most important finding reported in this study is the strong predictive value of the appearance of skin rash, related to epidermal growth factor receptor (EGFR)-pathway inhibition. Our data suggest that patients developing any grade of skin rash during the treatment, can achieve disease control and survival comparable to those obtained with more intensive and more toxic chemotherapy. These findings underline the relevance of further investigation of the biological mechanisms related to the occurrence of skin rash upon EGFR blockade in order to identify clinical/molecular biomarkers predicting toxicity and efficacy and to prospectively select a subset of patients who could potentially benefit from Gem/erlotinib.
INTRODUCTION
Pancreatic adenocarcinoma (PDAC) is arguably the most aggressive solid malignancy, with nearly as many deaths as the number of newly diagnosed cases each year. In 2012 an estimated 43920 new cases and an estimated 37390 deaths are expected to occur, making pancreatic carcinoma the fourth leading cause of cancer-related death in the United States. The prognosis of pancreatic cancer is extremely poor due to difficulties in early detection and early metastatic dissemination, with a 5-year survival rate of only 6%[1].
The majority of PDAC patients present with metastatic or inoperable disease. In this setting, systemic chemotherapy remains the treatment of choice, with a palliative objective and a disappointing, marginal, survival advantage. Single-agent gemcitabine (Gem), administered as weekly 30-min iv infusions, has become the standard care for advanced PDAC based on a small but statistically significant advantage over bolus 5-fluorouracil (5-FU), in terms of both clinical benefit (CB) and survival[2].
Until recently, efforts to improve on single-agent Gem efficacy[3], by combining Gem with either a second cytotoxic drug or a molecularly targeted agent, have failed[4,5]. The addition of erlotinib, an oral epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, to Gem has produced a minimal, albeit statistically significant, improvement in overall survival (OS), leading to FDA approval of the Gem/erlotinib combination in the setting of advanced, inoperable PDAC[6]. On the other hand, pharmacokinetic Gem modulation, achieved by prolonging its infusion time, is justified by the observation that deoxycytidine kinase, the enzyme converting Gem into its active triphosphate metabolite, is rapidly saturated at plasma concentrations achieved with the standard 30-min infusion. Consequently, the infusion of Gem over a prolonged period at the constant dose rate of 10 mg/m2 per min (FDR-Gem) avoids enzyme saturation and permits greater intracellular accumulation, possibly increasing Gem antitumor activity. This strategy has proven promising in a randomized phase II trial, in which FDR-Gem significantly improved time to treatment failure as compared with the standard 30-min infusion[7]. Although formally negative, a phase III trial comparing standard Gem with either FDR-Gem or the GEMOX combination, produced a clear signal in favor of FDR-Gem, which was as effective as the GEMOX combination[8].
Recently, a four-drug combination including 5-FU, folinic acid, oxaliplatin and irinotecan (FOLFIRINOX regimen) has demonstrated to improve objective response rate (ORR), progression-free survival (PFS) and OS over single-agent Gem administered by standard 30-min infusion in metastatic PDAC patients[9]. However, such improved efficacy comes at the price of significantly higher toxicity (both hematological and non-hematological), which restricts the use of such regimen to accurately selected, young and fit patients.
Based on our previous experience with a modified FDR-Gem regimen, which utilizes a lower Gem dose (1000 mg/m2) as compared with the original FDR-Gem described by Tempero et al[7] (1500 mg/m2) resulting in reduced hematological toxicity[10,11], we prospectively investigated the activity and tolerability of FDR-Gem in combination with erlotinib in advanced, inoperable PDAC patients.
MATERIALS AND METHODS
Patient population
Patients with cytologically or histologically proven, treatment-naïve, unresectable or metastatic PDAC and measurable disease were eligible for the study. Prior radiation for the management of local disease was allowed, provided that disease progression had been documented, all toxicities had resolved and treatment was completed at least 4 wk before study enrollment. Prior chemotherapy was not permitted, except for fluorouracil or Gem given concurrently with RT for radiosensitization purposes. Other inclusion criteria included: age > 18 years; Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤ 3; life expectancy > 12 wk; adequate hematological, renal, hepatic function; absence of other relevant medical conditions, potentially precluding the delivery of the planned treatment. The study was reviewed and approved by the institutional review board of the Regina Elena National Cancer Institute (Rome, Italy), and written informed consent, according to Institutional requirements, was obtained from all patients before entering the study.
Treatment and study design
This was a single-arm, open-label, multicenter phase II study, evaluating the activity and tolerability of the combination of FDR-Gem and erlotinib in patients with advanced PDAC. Study patients received Gem at the dose of 1000 mg/m2, administered as a 10 mg/m2 per min FDR iv infusion (100 min total infusion time)[10,11], weekly for 7 consecutive weeks and on days 1, 8, and 15 of a 4-wk cycle thereafter for a maximum of 6 cycles in the absence of progressive disease (PD) or unacceptable toxicity; erlotinib was administered as a daily oral dose of 100 mg from day 1 until PD or unacceptable toxicity. Toxicities were recorded according to the National Cancer Institute-Common Toxicity Criteria Version 3.0. Appropriate dose reductions of each study agent were planned in case of severe toxicities. Tumor assessments were performed at the end of cycle 1 and every 2 cycles thereafter.
Response and progression were evaluated using the Response Evaluation Criteria in Solid Tumours (RECIST 1.0)[12]. All patients who had measurable lesions and who had at least one objective tumour assessment after baseline were considered evaluable for response. The composite end point of CB was evaluated according to the criteria established by Burris et al[2] and included the assessment of pain (pain intensity and analgesic consumption) and functional impairment (assessed by Karnofsky PS) as primary measures and weight change (assessed by body weight) as a secondary measure. Each patient was classified as positive, stable, or negative for each of the primary CB measures (pain intensity or PS)[2]. For all patients, positive indicated a sustained (≥ 4 wk) improvement over baseline. If the patient was stable on both primary measures of clinical benefit, the patient was then classified as either positive or non-positive on the basis of the secondary clinical benefit measure of weight. For patients to achieve an overall rating of a positive CB, they had to be positive for at least one parameter without being negative for any of the others.
Statistical analysis
PFS rate at 6 mo (PFS-6) was selected as the primary study endpoint. Secondary endpoints were ORR, response duration, tolerability, OS, and CB. Sample size was computed according to the exact single-stage Phase II design described by A’Hern[13]. The treatment was not considered to be of further interest if the PFS rate at 6 mo was < 20% (p0 = 20%). The alternate hypothesis assumed that a PFS rate at 6 mo of > 40% would be of considerable interest (p1 = 40%). With a 5% rejection error (α = 5%) and a power of 80%, a total of 35 fully evaluable patients were needed to complete the study. In order to have enough power to also analyze the ‘pure’ metastatic sub-population separately, 46 patients were planned to enter the study, taking into account a dropout rate of approximately 15%. The Kaplan-Meier method was used to estimate PFS and OS[14]. PFS was defined as the time from the first day of treatment to the first observation of disease progression or death due to any cause and OS was defined as the time from the first day of treatment to death from any cause. ORR was estimated as the proportion of patients evaluable for response who met RECIST criteria for complete or partial response (CR or PR). Response duration was calculated for all patients achieving a PR or CR as the time from first objective status assessment of CR/PR to the first time documented PD or death. Cox proportional hazards models were used to compare survival among different patient/disease characteristics and treatment response groups[15]; hazard ratios were appropriately derived from these models. The SPSS statistical software package version 20.0 (SPSS, Inc, Chicago, IL, United States) was used for all statistical analyses.
RESULTS
Between May 2007 and September 2009, 46 patients with advanced-stage PDAC were enrolled in the study from 3 institutions. Patient characteristics are shown in Table 1.
Table 1.
Patients’ characteristics n (%)
| Characteristics | Categories | Data |
| Age (yr) | Median (range) | 64 (35-81) |
| Gender | Male | 25 (54) |
| Female | 21 (46) | |
| Stage | Locally advanced | 5 (11) |
| Metastatic | 41 (89) | |
| ECOG PS | 0 | 10 (22) |
| 1 | 26 (56) | |
| 2 | 9 (20) | |
| 3 | 1 (2) | |
| Basal CA 19-9 (U/mL) | Median (range) | 897 (1-49, 483) |
| Interval between symptoms and treatment (wk) | Median (range) | 12 (2-179) |
| Clinical benefit | Evaluable | 33 (75) |
| Not evaluable | 13 (25) | |
| Follow-up (wk) | Median (range) | 21.5 (2-91) |
| Number of administrations | Median (range) | 9 (1-29) |
PS: Performance status; ECOG: Eastern Cooperative Oncology Group; CA 19-9: Carbohydrate antigen 19-9.
Treatment outcome
All 46 patients were evaluable for response according to RECIST criteria. PR and stable disease (SD) were observed in 5/46 (10.9%) and 21/46 (45.7%) patients, respectively, for an overall disease control rate (DCR), defined as the percentage of patients who had CR, PR or SD as their best response, of 56.5% (95%CI: 42.2-70.8); PD was documented at the first response assessment in 20 patients (43.5%). Median response duration was 27.4 wk (range 11-45 wk); median duration of stable disease was 27.6 wk (range 10-85 wk). Fifteen out of 35 evaluable patients (42.9%) experienced a positive CB. CA 19-9 serum levels were decreased by > 25% as compared to baseline in 14/23 evaluable patients (63.6%). Similar results were obtained in the pure metastatic population (data not shown). At a median follow-up of 23.6 wk (range 2-139 wk), the median PFS and 1-year PFS rate were 14 wk (95%CI: 10-19) and 10.9%, respectively; PFS-6 (primary study endpoint) was 30.4% (Figure 1A). The median OS and 1-year OS rate were 26 wk (95%CI: 8-43 wk) and 20.2%, respectively (Figure 1B). In the pure metastatic population the corresponding figures were: median PFS: 14 wk, PFS-6: 24.4 %, median OS: 20 wk, 1-year OS: 12.7% (Table 2, data not shown).
Figure 1.
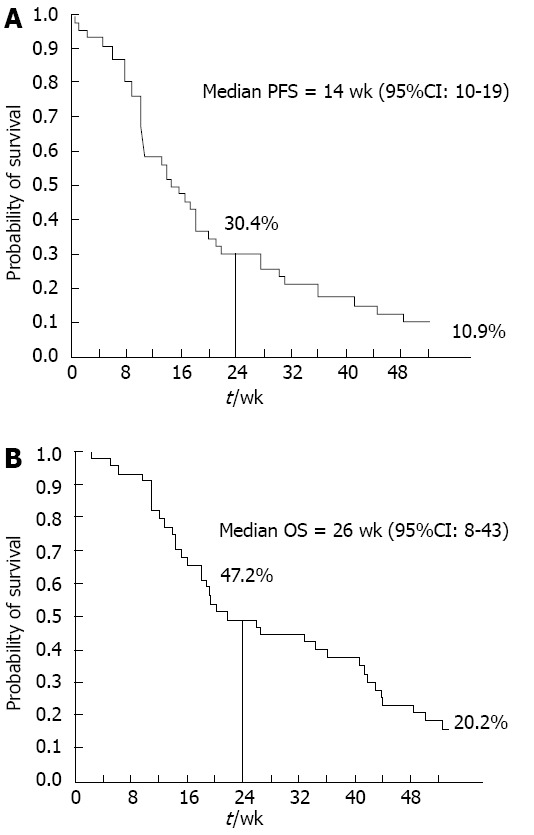
Kaplan-Meier analysis of progression-free survival and overall survival in the intent to treat population. A: Progression-free survival (PFS); B: Overall survival (OS).
Table 2.
Objective response, clinical benefit response and carbohydrate antigen 19-9 reduction in the overall population n (%)
| Parameter | Patients (n = 46) |
| CR/PR | 5 (10.9) |
| SD | 21 (45.7) |
| PD | 20 (43.4) |
| CB | |
| Pos | 15 (42.9) |
| Neg | 20 (57.1) |
| CA 19-9 reduction > 25% | 14 (63.6)1 |
In 23 evaluable patients. CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease; CB: Clinical benefit; Pos: Positive; Neg: Negative; CA 19-9: Carbohydrate antigen 19-9.
Toxicity
All patients were evaluable for toxicity. Main hematological and non-hematological toxicities are summarized in Table 3. Treatment protocol was well tolerated, with only 3 serious adverse events that required hospitalization: 2 episodes of GI bleeding and 1 duodenal perforation. Three patients (7%) reported grade 4 toxicities (neutropenia in 2 patients and asymptomatic transaminase elevation in 1 patient). Grade 3 hematological toxicity was also rare: anemia in 4 patients (9%), neutropenia in 8 patients (18%), thrombocytopenia in 5 patients (11%); only 1 patient (2%) experienced febrile neutropenia. The main non-hematological toxicity were: asymptomatic serum transaminase elevation and hyperbilirubinemia (grade 3 in 9% of patients); grade 3 diarrhea in 1 patient (2%); grade 2 and 3, erlotinib-related skin rash in 11% and 4% of patients, respectively. Median time to rash was 7 d. Gem and erlotinib doses were reduced in 14 and 3 patients, respectively. No toxic deaths were recorded.
Table 3.
Toxicity (maximum toxicity per patient) n (%)
| Variables | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Haemoglobin | 15 (32.6) | 17 (37.0) | 10 (21.7) | 4 (8.7) | - |
| Leucopenia | 29 (63.0) | 3 (6.5) | 9 (19.6) | 5 (10.9) | - |
| Neutropenia | 25 (54.3) | 2 (4.3) | 9 (19.6) | 8 (17.4) | 2 (4.3) |
| Febrile neutropenia | 44 (95.7) | - | 2 (4.3) | - | - |
| Platelet | 32 (69.6) | 6 (13.0) | 2 (4.3) | 6 (13.0) | - |
| Fever | 35 (76.1) | 9 (19.6) | 2 (4.3) | - | - |
| Bleeding | 45 (97.8) | - | - | 1 (2.2) | - |
| Alopecia | 41 (89.1) | 4 (8.7) | 1 (2.2) | - | - |
| Anorexia | 38 (82.6) | 7 (15.2) | 1 (2.2) | - | - |
| Asthenia | 22 (47.8) | 12 (26.1) | 11 (23.9) | 1 (2.2) | - |
| Cardiac | 45 (97.8) | - | 1 (2.2) | - | - |
| Skin | 24 (52.2) | 15 (32.6) | 5 (10.9) | 2 (4.3) | - |
| Diarrhoea | 18 (39.1) | 16 (34.8) | 11 (23.9) | 1 (2.2) | - |
| Constipation | 46 (100.0) | - | - | - | - |
| Stomatitis | 42 (91.3) | 1 (2.2) | 3 (6.5) | - | - |
| ALT | 24 (52.2) | 13 (28.3) | 6 (13.0) | 3 (6.5) | - |
| AST | 19 (41.3) | 12 (26.1) | 10 (21.7) | 4 (8.7) | 1 (2.2) |
| Bilirubine | 42 (91.3) | 2 (4.3) | 2 (4.3) | - | - |
| Renal | 43 (93.5) | 3 (6.5) | - | - | - |
| Neurological | 45 (97.8) | 1 (2.2) | - | - | - |
| Nausea | 39 (84.8) | 3 (6.5) | 4 (8.7) | - | - |
| Vomiting | 40 (87.0) | 3 (6.5) | 3 (6.5) | - | - |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
Clinical predictors of response and survival
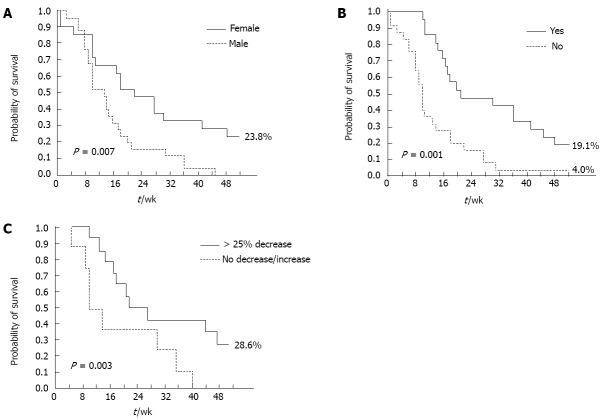
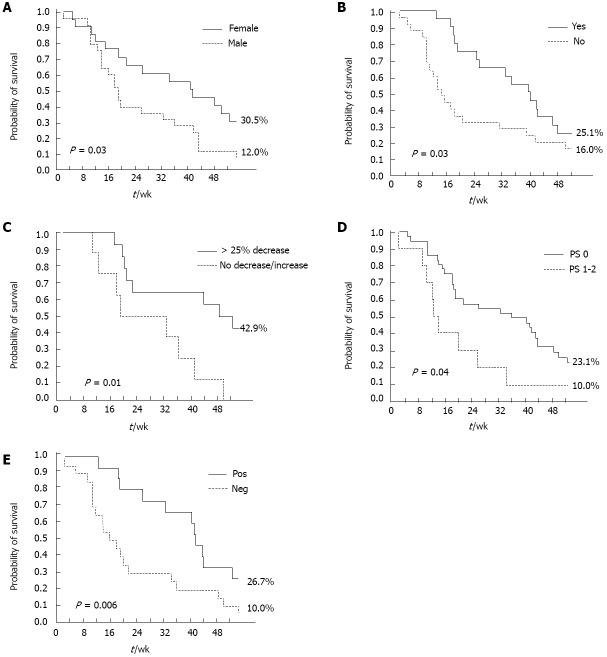
A positive CB and skin rash (any grade) were significant, independent predictors of DCR at multivariate analysis, in both the overall and pure metastatic populations. Female gender (P = 0.03) and skin rash (any grade, P < 0.0001) were significant, independent predictors of longer PFS (Table 4). ECOG PS (0-1, P = 0.01), skin rash (any grade, P = 0.03), and carbohydrate antigen (CA 19-9) decrease (> 25% relative to baseline, P = 0.04) were significantly associated with longer OS at multivariate analysis (Table 4). Conversely, the occurrence of other Gem- or erlotinib-related toxicities, such as hematological toxicity and diarrhea, did not significantly impact on survival outcomes. The impact of these factors on PFS and OS was further confirmed by Kaplan-Meier analysis (Figures 2 and 3): in particular, median PFS and median OS were both significantly longer in patients experiencing any grade of skin rash (21 wk vs 10 wk, Log-rank P = 0.001, and 42 wk vs 15 wk, Log-rank P = 0.03, respectively) (Figures 2A and 3A). In the “pure metastatic” population, gender and skin rash (P = 0.007 and P = 0.002), and gender, PS, CB and skin rash (P = 0.01, P = 0.06, P = 0.02 and P = 0.02) were significant, independent predictors of PFS and OS, respectively, at multivariate analysis (data not shown).
Table 4.
Multivariate analysis for progression-free survival and overall survival
| Variables |
PFS |
OS |
||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| PS | NA | 8.78 (1.60-48.2) | 0.01 | |
| Gender | 2.99 (1.13-7.90) | 0.03 | 2.66 (0.85-8.35) | 0.09 |
| CB | NA | 3.07 (0.87-10.8) | 0.08 | |
| Skin rash1 | 8.66 (2.65-28.32) | < 0.0001 | 5.10 (1.41-18.4) | 0.01 |
| CA 19-9 decrease2 | 2.64 (0.93-7.45) | 0.07 | 3.36 (1.05-10.6) | 0.04 |
None vs any grade;
Carbohydrate antigen 19-9 (CA 19-9) decrease of ≥25%. PFS: Progression-free survival; OS: Overall survival; PS: Performance status according to Eastern Cooperative Oncology Group (PS 0-1 vs PS 2-3); CB: Clinical benefit; NA: Not applicable.
Figure 2.
Kaplan-Meier analysis of independent progression-free survival predictors. A: Progression-free survival (PFS) by sex; B: PFS by skin rash; C: PFS by carbohydrate antigen 19-9 decrease.
Figure 3.
Kaplan-Meier analysis of independent overall survival predictors. A: Overall survival (OS) by sex; B: OS by skin rash; C: OS by carbohydrate antigen 19-9 decrease; D: OS by Eastern Cooperative Oncology Group performance status (PS); E: OS by clinical benefit. Pos: Positive; Neg: Negative.
DISCUSSION
In this study, performed in an unselected patient population, the administration of FDR-Gem in combination with erlotinib proved to be feasible, well tolerated, and moderately active. However, the planned goal to obtain a PFS-6 > 40% was not reached (PFS-6: 31.8%). Thus, the addition of erlotinib to an FDR-Gem backbone in unselected patients is unlikely to improve on historical result; indeed 1-year OS (21.6%), median OS (26 wk, 95%CI: 9-43), and activity in terms of responses, with a DCR of 59% are within the ranges reported with single-agent Gem, administered either as a 30-min iv infusion or as FDR, or with the combination of Gem and erlotinib[2,6-8,10].
The safety profile of the tested FDR-Gem/erlotinib combination is extremely manageable, an important issue in advanced PDAC patients, who are often frail and at a high risk of an adverse impact of treatment on quality of life. In particular, we confirm here that administering FDR-Gem at 1000 mg/m2, as in previous experiences from our group[10,11], decreases hematological toxicity in comparison with the original FDR-Gem schedule developed by Tempero et al[7], where FDR-Gem was administered at the 1500 mg/m2 dose level (grade 3-4 neutropenia 23% in the present trial vs 48.8% in Tempero’s trial). Other experiences with a different EGFR-TKI (gefitinib) have also confirmed an extremely safe and manageable toxicity profile of Gem-FDR at a lower dose (1200 mg/m2), thus suggesting these combinations as feasible platforms for associations with additional chemotherapeutics or different targeted agents[16].
Though the combination under study proved feasible and well tolerated, the question remains as to whether such a strategy (i.e., adding an EGFR kinase inhibitor to a FDR-Gem backbone in unselected patients) is worthy pursuing if it does not improve efficacy. As the results of the trial are technically negative (primary endpoint was not met), the easiest answer would be that this combination does not merit further investigation, particularly in a scenario, such as that of advanced PDAC treatment, where novel polychemotherapy strategies (FOLFIRINOX and Gem/nab-paclitaxel combinations) are moving the field forward and, for the first time in almost 20 years, show increased efficacy and improved survival as compared with single-agent Gem. However, survival analysis of the present trial and of two other recently reported experiences[17,18] clearly show that, at least in some patients, the addition of erlotinib to Gem has both biological and clinical activity: indeed, the most relevant finding reported herein is the strong predictive value of the appearance of skin rash. Patients developing erlotinib-related skin toxicity experience a more than doubled median OS (42 wk vs 15 wk, P = 0.03), and PFS (21 wk vs 10 wk, P = 0.001); conversely, the occurrence of other toxicities, such as hematologic toxicity or diarrhea, has no impact on treatment activity and/or survival outcomes. A similar predictive effect had already been described in the registration trial of erlotinib in PDAC, where patients experiencing grade 2 skin rash had a 1-year survival of 43%[6] and is shared by other agents targeting the EGFR pathway, either small molecules or monoclonal antibodies, regardless of the disease setting[19-25]. The trial exploring the addition of bevacizumab to Gem and erlotinib, also showed a significantly better outcome for patients developing skin rash, regardless of the treatment arm[26]. A more recent randomized trial showed that skin rash is able to dichotomize patients receiving erlotinib between good and poor prognosis[27].
In addition to skin rash, survival analysis of the current study also underlines the importance of two other treatment-modified factors to guide the management of advanced PDAC patients: clinical benefit and decline in CA 19-9 levels. Though chosen as the primary end-point in the Gemcitabine registration trial by Burris et al[2], the relationship between CB and OS has never been validated. Interestingly, in the present trial the occurrence of CB was a significant independent predictor of longer OS, while objective response, as assessed by RECIST criteria, was not, a finding of great clinical relevance in the context of a disease with dismal prognosis, where symptom control represents a real issue for clinical practice. A reduction in CA 19-9 levels > 25% from baseline was also an independent prognostic factor for survival, thus adding to the numerous evidence supporting the prognostic role (and clinical utility) of a CA 19-9 reduction, regardless of the chosen cut-off point[28-31].
In conclusion, although the study reported herein failed to meet its primary endpoint of prolonging PFS with the addition of erlotinib to FDR-Gem, intriguing data on skin rash do suggest that a subset of advanced PDAC patients could actually achieve disease control and survival comparable to those obtained with more intensive (and more toxic) polychemotherapy approaches, such as FOLFIRINOX and Gem/nab-paclitaxel combinations, with a well tolerated and easily manageable regimen, potentially also suitable for elderly and unfit patients. However, in order for this strategy to become a concrete treatment option, an in-depth investigation of the biological mechanisms underlying the occurrence of skin rash upon EGFR blockade is required to identify clinical/molecular biomarkers predicting toxicity and efficacy and to prospectively select patients who could potentially benefit from Gem/erlotinib combinations.
COMMENTS
Background
Pancreatic adenocarcinoma has a dismal prognosis. Although the disappointing survival advantage obtained in many studies, chemotherapy is the only treatment option for the majority of patients, and single agent gemcitabine (Gem) remains standard care for many of them. Recently, the polychemotherapy regimen named FOLFIRINOX has produced an improvement in survival over single agent Gem but require an accurate selection of young and fit patients to limit treatment-related toxicities. In order to improve Gem efficacy, pharmacokinetic Gem modulation and combination with other chemotherapeutic agent has been proposed. To this regard, the prolonged infusion at constant dose rate has shown promising results in phase II and III trials and the addition of erlotinib to Gem has provided a minimal, albeit statistically significant, improvement in survival.
Research frontiers
In the field of advanced pancreatic adenocarcinoma (PDAC), the research hotspot is to find active regimen, for patients not suitable for aggressive combination chemotherapy, able to improve survival over single agent Gem, without worsening tolerability. In the context of targeted therapies, applied to PDAC, but also any other malignancy, the opportunity of prospectively select patients who could benefit from targeted therapy plays a fundamental role.
Innovations and breakthroughs
The combination of fixed dose-rate (FDR)-Gem at 1000 mg/m2 and erlotinib appears feasible, well tolerated and extremely manageable. In comparison to other FDR-Gem schedules with different doses (1500 or 1200 mg/m2), the regimen shows a reduced hematological toxicity profile. This suggests that the schedule is a feasible platform for combining targeted therapies. In the study, a strong predictive value of the appearance of skin rash is demonstrated. Patients developing erlotinib-related skin toxicity experienced a more than doubled median overall survival, comparable to that obtained with more intensive polychemotherapy approaches. This relation has not been reported for other toxicities (hematologic toxicities or diarrhea). Moreover, occurrence of clinical benefit and reduction in carbohydrate antigen levels > 25% from baseline also proved to be an independent prognostic factor for survival. All these data confirm these factors as an important guide for the management of advanced PDAC patients.
Applications
The study results suggest the importance of investigating the biological mechanisms underlying the occurrence of skin rash upon epidermal growth factor receptor blockade. The identification of clinical/molecular biomarkers is strongly required to predict toxicity and efficacy and to prospectively select patients who could potentially benefit from Gem/erlotinib combinations.
Peer review
This study investigated activity, toxicity, and prognostic factors for survival of erlotinib and FDR-Gem in advanced pancreatic cancer. They highlighted the correlation between the rash and efficacy. The similar studies were published in the past and they had the similar results, furthermore there were randomized controlled trials among them. This study is the confirmation of result of those studies, but it has reference to clinical practice.
Footnotes
Supported by A Grant of the National Ministry of Health and the Italian Association for Cancer Research (AIRC); An AIRC fellowship to Vaccaro V
P- Reviewer Kukongviriyapan V S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Kris MG, Benowitz SI, Adams S, Diller L, Ganz P, Kahlenberg MS, Le QT, Markman M, Masters GA, Newman L, et al. Clinical cancer advances 2010: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2010;28:5327–5347. doi: 10.1200/JCO.2010.33.2742. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bria E, Milella M, Gelibter A, Cuppone F, Pino MS, Ruggeri EM, Carlini P, Nisticò C, Terzoli E, Cognetti F, et al. Gemcitabine-based combinations for inoperable pancreatic cancer: have we made real progress? A meta-analysis of 20 phase 3 trials. Cancer. 2007;110:525–533. doi: 10.1002/cncr.22809. [DOI] [PubMed] [Google Scholar]
- 5.Vaccaro V, Gelibter A, Bria E, Iapicca P, Cappello P, Di Modugno F, Pino MS, Nuzzo C, Cognetti F, Novelli F, et al. Molecular and genetic bases of pancreatic cancer. Curr Drug Targets. 2012;13:731–743. doi: 10.2174/138945012800564077. [DOI] [PubMed] [Google Scholar]
- 6.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 7.Tempero M, Plunkett W, Ruiz Van Haperen V, Hainsworth J, Hochster H, Lenzi R, Abbruzzese J. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 8.Poplin E, Feng Y, Berlin J, Rothenberg ML, Hochster H, Mitchell E, Alberts S, O’Dwyer P, Haller D, Catalano P, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778–3785. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 10.Gelibter A, Malaguti P, Di Cosimo S, Bria E, Ruggeri EM, Carlini P, Carboni F, Ettorre GM, Pellicciotta M, Giannarelli D, et al. Fixed dose-rate gemcitabine infusion as first-line treatment for advanced-stage carcinoma of the pancreas and biliary tree. Cancer. 2005;104:1237–1245. doi: 10.1002/cncr.21286. [DOI] [PubMed] [Google Scholar]
- 11.Milella M, Gelibter AJ, Pino MS, Bossone G, Marolla P, Sperduti I, Cognetti F. Fixed-dose-rate gemcitabine: a viable first-line treatment option for advanced pancreatic and biliary tract cancer. Oncologist. 2010;15:e1–e4. doi: 10.1634/theoncologist.2008-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.A’Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859–866. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Cox DR. Regression models and life-Tables. J Royal Stat Soc. 1972;34:187–200. [Google Scholar]
- 16.Carneiro BA, Brand RE, Fine E, Knop RH, Khandekar JD, Uhlig W, Locker GY. Phase I trial of fixed dose rate infusion gemcitabine with gefitinib in patients with pancreatic carcinoma. Cancer Invest. 2007;25:366–371. doi: 10.1080/15368370701357957. [DOI] [PubMed] [Google Scholar]
- 17.Aranda E, Manzano JL, Rivera F, Galán M, Valladares-Ayerbes M, Pericay C, Safont MJ, Mendez MJ, Irigoyen A, Arrivi A, et al. Phase II open-label study of erlotinib in combination with gemcitabine in unresectable and/or metastatic adenocarcinoma of the pancreas: relationship between skin rash and survival (Pantar study) Ann Oncol. 2012;23:1919–1925. doi: 10.1093/annonc/mdr560. [DOI] [PubMed] [Google Scholar]
- 18.Klapdor R, Klapdor S, Bahlo M. Combination therapy with gemcitabine (GEM) and erlotinib (E) in exocrine pancreatic cancer under special reference to RASH and the tumour marker CA19-9. Anticancer Res. 2012;32:2191–2197. [PubMed] [Google Scholar]
- 19.Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913–3921. doi: 10.1158/1078-0432.CCR-06-2610. [DOI] [PubMed] [Google Scholar]
- 20.Lynch TJ, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610–621. doi: 10.1634/theoncologist.12-5-610. [DOI] [PubMed] [Google Scholar]
- 21.O’Byrne KJ, Bondarenko I, Barrios C, Eschbach C, Martens U, Hotko Y, Kortsik C, Celik I, Stroh C, Pirker R. Molecular and clinical predictors of outcome for cetuximab in non-small cell lung cancer (NSCLC): Data from the FLEX study. ASCO Meeting Abstracts. 2009;27:8007. [Google Scholar]
- 22.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, Vynnychenko I, Park K, Yu CT, Ganul V, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 24.Tejpar S, Peeters M, Humblet Y, Gelderblom H, Vermorken J, Viret F, Glimelius B, Ciardiello F, Kisker O, Van Cutsem E. Phase I/II study of cetuximab dose-escalation in patients with metastatic colorectal cancer (mCRC) with no or slight skin reactions on cetuximab standard dose treatment (EVEREST): Pharmacokinetic (PK), Pharmacodynamic (PD) and efficacy data. ASCO Meeting Abstracts. 2007;25:4037. [Google Scholar]
- 25.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 26.Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 27.Heinemann V, Vehling-Kaiser U, Waldschmidt D, Kettner E, Märten A, Winkelmann C, Klein S, Kojouharoff G, Gauler TC, von Weikersthal LF, et al. Gemcitabine plus erlotinib followed by capecitabine versus capecitabine plus erlotinib followed by gemcitabine in advanced pancreatic cancer: final results of a randomised phase 3 trial of the 'Arbeitsgemeinschaft Internistische Onkologie' (AIO-PK0104) Gut. 2013;62:751–759. doi: 10.1136/gutjnl-2012-302759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko AH, Hwang J, Venook AP, Abbruzzese JL, Bergsland EK, Tempero MA. Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer. 2005;93:195–199. doi: 10.1038/sj.bjc.6602687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halm U, Schumann T, Schiefke I, Witzigmann H, Mössner J, Keim V. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer. 2000;82:1013–1016. doi: 10.1054/bjoc.1999.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziske C, Schlie C, Gorschlüter M, Glasmacher A, Mey U, Strehl J, Sauerbruch T, Schmidt-Wolf IG. Prognostic value of CA 19-9 levels in patients with inoperable adenocarcinoma of the pancreas treated with gemcitabine. Br J Cancer. 2003;89:1413–1417. doi: 10.1038/sj.bjc.6601263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinemann V, Schermuly MM, Stieber P, Schulz L, Jüngst D, Wilkowski R, Schalhorn A. CA19-9: a pedictor of response in pancreatic cancer treated with gemcitabine and cisplatin. Anticancer Res. 1999;19:2433–2435. [PubMed] [Google Scholar]