Abstract
AIM: To investigate the contribution of the fibroblast growth factor receptor 4 (FGFR4) Gly388Arg polymorphism as a genetic risk factor for gastric cancer (GC) and to investigate any associations between this polymorphism and clinicopathological parameters and survival.
METHODS: Tumors and matched adjacent non-cancer tissues were collected from 304 GC patients, and 5 mL of venous blood was collected from 62 GC patients and 392 age- and sex-matched healthy controls without cancer history from the same ethnic population. DNA was extracted, and direct sequencing analyses were performed to genotype the FGFR4 Gly388Arg polymorphism in all the samples. Differences in the genotype frequencies of the FGFR4 Gly388Arg polymorphism between GC patients and healthy controls were estimated using the χ2 test. Binary logistic regression was used for all analysis variables to estimate risk as the ORs with 95%CIs. The relationships between the FGFR4 genotype and clinicopathological parameters were tested with the χ2 test. The Kaplan-Meier product-limit method, the log-rank test, and the Cox regression model were applied to evaluate the effect of the FGFR4 genotype on the overall survival of patients with GC.
RESULTS: In the present GC cohort, 118 patients (38.8%) were homozygous for the Gly388 allele, 124 patients (40.8%) were heterozygous, and 62 patients (20.4%) were homozygous for the Arg388 allele. The frequencies of the Gly/Gly, Gly/Arg, and Arg/Arg genotypes in the healthy controls were 33.6%, 48.0%, and 18.4%, respectively. The distributions of genotypes (χ2 = 3.589, P = 0.166) and alleles (χ2 = 0.342, P = 0.559) of the FGFR4 Gly388Arg polymorphism were not different between the GC patients and the healthy controls. Although we observed no correlation between the FGFR4 Gly388Arg polymorphism and clinicopathological parameters or survival in the total cohort of GC patients, the presence of the Arg388 allele was associated with shorter survival time in patients with GC if the tumor was small (log rank χ2 = 5.449, P = 0.020), well differentiated (log rank χ2 = 12.798, P = 0.000), T1 or T2 stage (log rank χ2 = 4.745, P = 0.029), without lymph node involvement (log rank χ2 = 6.647, P= 0.010), and at an early clinical stage (log rank χ2 = 4.615, P = 0.032).
CONCLUSION: Our results suggest that the FGFR4 Gly388Arg polymorphism is not a risk factor for GC cancer initiation but that it is a useful prognostic marker for GC patients when the tumor is relatively small, well differentiated, or at an early clinical stage.
Keywords: Fibroblast growth factor receptor 4, Gly388Arg, Genetic susceptibility, Single nucleotide polymorphism, Gastric cancer
Core tip: This study investigated the contribution of the fibroblast growth factor receptor 4 (FGFR4) Gly388Arg polymorphism as a genetic risk factor for gastric cancer (GC) and any associations between this polymorphism and clinicopathological parameters such as age, gender, clinical stage, tumor grade, human epidermal growth factor receptor 2 status and survival. The results suggested that the FGFR4 Gly388Arg polymorphism was not a risk factor for GC cancer initiation but that it was a useful prognostic marker for GC patients when the tumor was relatively small, well differentiated, or at an early clinical stage.
INTRODUCTION
Gastric cancer (GC) is one of the most common cancers in the world, and it has a complex etiology. Disease development is related to environmental factors, such as diet and Helicobacter pylori (H. pylori) infection, as well as genetic predisposition. In the last decade, GC incidence rates have steadily declined owing to changing diets and the application of antibiotics to treat H. pylori infection[1]. However, the treatment of patients with advanced GC remains a significant challenge. Surgical resection and chemotherapy are only effective for a fraction of patients, and their prognoses remain very poor. Recently, a number of molecularly targeted agents modulating different signal transduction pathways have been researched in clinical trials for many cancer types[2]. Trastuzumab, a monoclonal antibody against human epidermal growth factor receptor 2 (HER2; also known as ERBB2) was demonstrated to be effective in advanced HER2-positive GC. The addition of trastuzumab to chemotherapy improved survival in patients with advanced GC or gastroesophageal junction cancer compared with chemotherapy alone in a Trastuzumab for Gastric Cancer trial[3]. However, only 7% to 34% of GC cases are HER2 positive[4-6], meaning that only a fraction of GC patients can benefit from trastuzumab. Accordingly, there is an urgent need to better understand the genesis of GC to establish a sound basis for future treatments.
Fibroblast growth factor receptor 4 (FGFR4) is a member of the receptor tyrosine kinase family. These receptors have highly conserved structures containing an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. The FGFR4 protein interacts with specific growth factors, especially acidic fibroblast growth factor, and is critically involved in cell growth, differentiation, migration, angiogenesis, and tumorigenesis. Several prior studies have investigated the role of the FGFR4 signaling pathway in GC. Notably, a soluble variant of FGFR4 was first detected in human gastrointestinal epithelial cells and cancer cells in a study by Takaishi et al[7]. Shin et al[8] and Ye et al[9]. also demonstrated the upregulation of FGFR4 mRNA and protein in GC, suggesting the possibility that FGFR4 signaling could play a role in gastric carcinogenesis.
Recently, a single nucleotide polymorphism (SNP) at codon 388 (cDNA 1162) from G to A, which results in a change of amino acid from glycine to arginine, was identified in the transmembrane domain of the FGFR4 gene[10]. Significant scientific effort has been put into the investigation of this FGFR4 Gly388Arg polymorphism in cancer progression, and the results showed that patients with the Arg/Arg or Gly/Arg genotype (compared to those with a Gly/Gly genotype) had a shorter survival time or a higher proportion of nodal involvement in many types of cancer, including breast, lung, colon, prostate, soft tissue sarcoma, melanoma, and head and neck squamous cell carcinoma[10-19]. However, several researchers have opposed the association between this polymorphism and poor outcomes or lymph node involvement[20-23]. Furthermore, it has been reported that the FGFR4 Arg388 polymorphism may not be involved in tumor initiation in several different tumor types with the exception of prostate cancer[13,14,18,24]. Xu et al[25] conducted a meta-analysis of 2618 patients and 2305 controls and demonstrated that the FGFR4 Gly388Arg polymorphism was associated with both progression and risk in prostate cancer .
Recently, Ye et al[26] reported that the FGFR4 Arg allele was an independent prognostic factor in Chinese patients with GC. To our knowledge, this is the only report on the association between the FGFR4 Gly388Arg polymorphism and the progression of GC in Chinese patients, and it therefore needs further confirmation. Moreover, no study has been conducted on the correlation between this polymorphism and the risk of GC. In the present study, we expanded the sample sizes by enrolling 304 patients with GC and 392 healthy controls and genotyped every sample for the FGFR4 Gly388Arg polymorphism to confirm the findings of Ye et al[26] and investigate the association between the FGFR4 Gly388Arg polymorphism and the risk of GC.
MATERIALS AND METHODS
Patients and healthy controls
A total of 304 Chinese patients diagnosed with GC were recruited and underwent surgery between 2007 and 2009 at Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University. All GC cases were pathologically confirmed, and patient records were used to obtain clinical data. The study included a total of 223 males and 81 females. The age of the patients at the time of surgery ranged from 22 to 87 years, with a median age of 63.5 years. The clinical stage was determined according to the Union for International Cancer Control TNM staging system, and the tumor grade was based on the World Health Organization classification. The expression or amplification of HER2 in GC was detected using standard methods, including immunohistochemistry and fluorescence in situ hybridization. The HER2 status was determined according to the Hofmann HER2 scoring system[6]. Follow-up was performed regularly. The median follow-up time for patients still alive at analysis was 49 mo (range, 20-61 mo).
Tumors and matched adjacent non-cancer tissues were snap frozen in liquid nitrogen immediately after surgical removal and stored at -80 °C. Each tumor sample was evaluated microscopically, and macro-dissection was performed to ensure that more than 70% of the sample was tumor tissue before DNA extraction. Five milliliters of venous blood was collected from 62 GC patients and 392 age- and sex-matched healthy controls without cancer history from the same ethnic population. Informed consent was obtained from all the patients and controls for the use of their specimens and clinicopathologic data for this study. The study was approved by the Institutional Human Ethics Committee.
Genotyping of the FGFR4 Gly388Arg polymorphism
DNA samples from tumor and normal adjacent tissue were prepared with the Puregene DNA extraction kit (Qiagen, Valencia, CA, United States). Genomic DNA was extracted and purified from the blood of GC patients and the healthy control group using the QIAamp DNA Blood Midi kit (Qiagen, Germany). Sanger sequencing was applied to genotype the FGFR4 Gly388Arg polymorphism with the following PCR primers: 5’-GCGGCCAGTCTCACCACTGAC-3’ and 5’-TGGAGTCAGGCTCTTCCGGCA-3’. Each primer was tagged with the M13 primer as a uniform sequencing primer for individual PCR products to facilitate the sequencing process. All PCR assays were carried out in a 25 μL volume containing 10 ng genomic DNA, 0.1 μmol/L of each primer, and 1 × AmpliTaq Gold Pre Mix (AB). The PCR conditions were as follows: 95 °C for 10 min; 30 cycles of 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s; and 5 min at 72°C. The PCR product was 355 bp and was sequenced in both directions using the BigDye Terminator kit 3.1 (Applied Biosystems; Foster City, CA, United States) and ABI Genetic Analyzer3730 × l (Applied Biosystems) according to the manufacturer’s instructions. Sequence traces were analyzed for the polymorphism after assembly and quality calling with SeqScape2.5 sequence analysis software (Applied Biosystems).
Statistical analysis
Differences in the general characteristics and genotype frequencies of the FGFR4 Gly388Arg polymorphism between GC patients and healthy controls were estimated using the χ2 test. Hardy-Weinberg equilibrium analyses were performed to compare observed and expected genotype frequencies using the χ2 test. Binary logistic regression was used for all analysis variables to estimate risk as the ORs with 95%CIs. The relationships between the FGFR4 genotype and clinicopathological parameters were tested with the χ2 test. The Kaplan-Meier product-limit method, the log-rank test, and the Cox regression model were applied to evaluate the effect of the FGFR4 genotype on the overall survival of patients with GC. The covariates included in the models were age, gender, clinical stage, tumor grade, HER2 status and FGFR4 genotype. All analyses were carried out with SPSS for Windows software (SPSS, Chicago, IL, version 16.0). A P value < 0.05 was considered to be statistically significant.
RESULTS
FGFR4 genotype in healthy controls and GC patients
Data from the 62 patients who provided both tumor tissue and blood samples showed that the Gly388Arg genotypes were identical between tumor tissue and blood from the same individual. This result confirms that there were no somatic mutations at this locus in any of these patients.
The clinical characteristics of all the subjects are shown in Table 1. There were no significant differences in age, gender, smoking, hypertension, or diabetes status between the GC patients and healthy controls. The frequencies of the FGFR4 Gly388Arg polymorphism genotypes are summarized in Table 2. Among the 304 GC patients, 118 patients (38.8%) were homozygous for the Gly388 allele, 124 were heterozygous (40.8%), and 62 were homozygous (20.4%) for the Arg388 allele. In the healthy controls, the frequencies of the Gly/Gly, Gly/Arg, and Arg/Arg genotypes and the Arg allele were 33.6%, 48.0%, 18.4% and 42.3%, respectively. The frequencies were consistent with the Hardy-Weinberg equilibrium in the healthy controls. The genotype (χ2= 3.589, P = 0.166) and allele frequencies (χ2 = 0.342, P = 0.559) of the FGFR4 Gly388Arg polymorphism were not different between the GC patients and the healthy controls. Binary logistic regression analysis indicated that the ORs for the carriers of Gly/Arg, carriers of Arg/Arg, and carriers of the Gly/Arg or Arg/Arg genotypes were 0.738 (95%CI: 0.527-1.033), 0.963 (95%CI: 0.633-1.466) and 0.800 (95%CI: 0.586-1.093), respectively. No differences were observed between patients and healthy controls. The distribution of the Gly388 and Arg388 alleles was not different between patients and healthy controls (OR = 0.938, 95%CI: 0.756-1.163). These results suggest that the FGFR4 Gly388Arg polymorphism is not an independent risk factor for GC in Chinese patients.
Table 1.
General characteristics of gastric cancer patients and healthy controls n (%)
| Characteristics | GC n = 304 | Healthy controls n = 392 | Pearson’s χ2 value | P value |
| Age (yr) | 2.548 | 0.110 | ||
| < 60 | 125 (41.12) | 138 (35.20) | ||
| ≥ 60 | 179 (58.88) | 254 (64.80) | ||
| Sex | 0.115 | 0.735 | ||
| Male | 223 (73.36) | 292 (74.49) | ||
| Female | 81 (26.64) | 100 (25.51) | ||
| Smoker | 0.314 | 0.575 | ||
| Yes | 92 (30.26) | 111 (28.32) | ||
| No | 212 (69.74) | 281 (71.68) | ||
| Hypertension | 0.012 | 0.931 | ||
| Yes | 78 (25.66) | 102 (26.02) | ||
| No | 226 (74.34) | 290 (73.98) | ||
| Diabetes | 2.591 | 0.108 | ||
| Yes | 52 (17.11) | 50 (12.76) | ||
| No | 252 (82.89) | 342 (87.24) |
GC: Gastric cancer.
Table 2.
Distribution and regression analysis of the fibroblast growth factor receptor 4 Gly388Arg genotype in gastric cancer patients and healthy controls n (%)
| Gly388Arg | GC n = 304 | Healthy controls n = 392 | OR (95%CI) | P value |
| Genotype | ||||
| GG | 118 (38.8) | 132 (33.6) | 1 | |
| AG | 124 (40.8) | 188 (48.0) | 0.738 (0.527-1.033) | 0.076 |
| AA | 62 (20.4) | 72 (18.4) | 0.963 (0.633-1.466) | 0.862 |
| AA + AG | 186 (61.2) | 260 (66.3) | 0.800 (0.586-1.093) | 0.161 |
| Allele | ||||
| G | 360 (59.2) | 452 (57.7) | 1 | |
| A | 248 (40.8) | 332 (42.3) | 0.938 (0.756-1.163) | 0.559 |
FGFR4: Fibroblast growth factor receptor 4; GC: Gastric cancer.
FGFR4 Gly388Arg polymorphism is not associated with any clinicopathological parameters in GC patients
Based on previous reports and due to the requirements for accurate statistical analysis, the 304 GC patients were divided into two groups: patients with the Gly/Gly genotype and patients with the Arg/Arg or Arg/Gly genotypes. As shown in Table 3, no correlation was observed between the FGFR4 Gly388Arg polymorphism and any of the following clinicopathological parameters: age at diagnosis, gender, tumor size, clinical stage, differentiation and HER2 status.
Table 3.
Association analysis of the fibroblast growth factor receptor 4 Gly388Arg polymorphism and clinicopathological parameters in gastric cancer patients
| Variables | Total n = 304 | Gly/Gly n = 118 | Gly/Arg + Arg/Arg n = 186 | Pearson’s χ2 value | P value |
| Age (yr) | 0.013 | 0.909 | |||
| < 60 | 125 | 49 | 76 | ||
| ≤ 60 | 179 | 69 | 110 | ||
| Sex | 1.398 | 0.237 | |||
| Male | 223 | 91 | 132 | ||
| Female | 81 | 27 | 54 | ||
| Tumor size | 0.198 | 0.656 | |||
| ≤ 3 cm | 58 | 24 | 34 | ||
| > 3 cm | 246 | 94 | 152 | ||
| Differentiation | 2.122 | 0.145 | |||
| G1 + G2 | 100 | 33 | 67 | ||
| G3 + G4 | 204 | 85 | 119 | ||
| Invasion depth | 0.000 | 0.995 | |||
| T1 + T2 | 49 | 19 | 30 | ||
| T3 + T4 | 255 | 99 | 156 | ||
| N stage | 0.640 | 0.200 | |||
| N0 | 82 | 27 | 55 | ||
| N1 + N2 + N3 | 222 | 91 | 131 | ||
| M stage | 1.089 | 0.297 | |||
| M0 | 274 | 109 | 165 | ||
| M1 | 30 | 9 | 21 | ||
| Clinical stage | 0.980 | 0.322 | |||
| I + II | 103 | 36 | 67 | ||
| III + IV | 201 | 82 | 119 | ||
| HER2 status | 0.391 | 0.532 | |||
| Negative | 180 | 63 | 117 | ||
| Positive | 45 | 18 | 27 |
FGFR4: Fibroblast growth factor receptor 4; GC: Gastric cancer; HER2: Human epidermal growth factor receptor 2.
Impact of the FGFR4 Gly388Arg polymorphism on GC survival
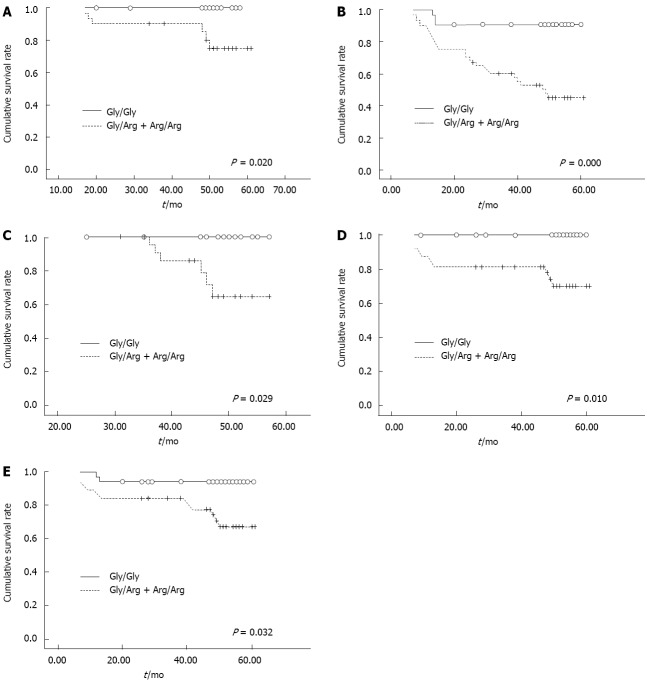
When analyzing the entire patient cohort using Kaplan-Meier survival analysis, no difference was observed in survival between patients with the Gly/Gly genotype and patients with the Gly/Arg or Arg/Arg genotypes (log rank χ2 = 0.047, P = 0.829). When the patient population was stratified by clinicopathological parameters, such as age at diagnosis, gender, tumor size, differentiation, clinical stage, HER2 status and chemotherapy history (5-fluorouracil and cisplatinum), we found that the presence of the Arg388 allele was associated with a shorter survival time in GC patients if the tumor was small (less than or equal to 3 cm in size) (log rank χ2 = 5.449, P = 0.020, Figure 1A), well differentiated (log rank χ2 = 12.798, P = 0.000, Figure 1B), of T1 or T2 stage invasion depth (log rank χ2 = 4.745, P = 0.029, Figure 1C), without lymph node involvement (log rank χ2 = 6.647, P = 0.010, Figure 1D), and at an early clinical stage (log rank χ2 = 4.615, P = 0.032, Figure 1E). No survival differences were observed in any of the other subgroups (Table 4). In addition, the Cox proportional hazard analysis of survival demonstrated that the FGFR4 Gly388Arg polymorphism was not an independent prognostic factor for GC patients (data not shown).
Figure 1.
A considerable difference was found between patients with the Gly/Gly genotype and patients with Gly/Arg or Arg/Arg genotypes after stratified Kaplan-Meier survival analysis. A: Patients with tumor size ≤ 3 cm; B: Patients with well-differentiated gastric cancer (grades I and II); C: Patients classified as stage T1 or T2; D: Patients with no lymph node involvement; E: Patients at an early clinical stage (I/II).
Table 4.
Influence of fibroblast growth factor receptor 4 Gly388Arg polymorphism on gastric cancer survival
| Total n = 257 | Gly/Gly n = 100 | Gly/Arg + Arg/Arg n = 157 | Log rank χ2 value | P value | |
| Age (yr) | |||||
| < 60 | 98 | 37 | 61 | 1.459 | 0.227 |
| ≥ 60 | 159 | 63 | 96 | 1.734 | 0.188 |
| Sex | |||||
| Male | 188 | 79 | 109 | 0.041 | 0.839 |
| Female | 69 | 21 | 48 | 0.018 | 0.894 |
| Tumor size | |||||
| ≤ 3 cm | 55 | 24 | 31 | 5.449 | 0.020 |
| > 3 cm | 202 | 76 | 126 | 0.308 | 0.579 |
| Differentiation | |||||
| G1 + G2 | 94 | 33 | 61 | 12.798 | 0.000 |
| G3 + G4 | 163 | 67 | 96 | 2.637 | 0.104 |
| Invasion depth | |||||
| T1 + T2 | 46 | 19 | 27 | 4.745 | 0.029 |
| T3 + T4 | 211 | 81 | 130 | 0.037 | 0.848 |
| N stage | |||||
| N0 | 73 | 24 | 49 | 6.647 | 0.010 |
| N1 + N2 + N3 | 184 | 76 | 108 | 0.024 | 0.876 |
| M stage | |||||
| M0 | 235 | 94 | 141 | 0.027 | 0.869 |
| M1 | 22 | 6 | 16 | 0.139 | 0.710 |
| Clinical stage | |||||
| I + II | 91 | 33 | 58 | 4.615 | 0.032 |
| III + IV | 166 | 67 | 99 | 0.048 | 0.827 |
| Chemotherapy (5-fluorouracil and cisplatinum) | |||||
| Yes | 156 | 55 | 101 | 0.019 | 0.891 |
| No | 51 | 21 | 30 | 0.442 | 0.506 |
| HER2 status | |||||
| Negative | 157 | 54 | 103 | 0.458 | 0.499 |
| Positive | 36 | 15 | 21 | 1.014 | 0.314 |
FGFR4: Fibroblast growth factor receptor 4; GC: Gastric cancer; HER2: Human epidermal growth factor receptor 2.
DISCUSSION
In the present study, the overall frequencies of the Gly/Gly, Gly/Arg, Arg/Arg genotypes and Arg388 allele in healthy controls were 33.6%, 48.0%, 18.4% and 42.3%, respectively. As shown in Table 5, the frequencies of the Arg allele or Arg/Arg genotypes in our study were similar to those of the Chinese patient populations reported by Chen et al[27], Zhu et al[28], Ma et al[29]and Yang et al[30]. Interestingly, the Arg388 allele frequencies in the Chinese population are much higher than those in Caucasian cohorts. In a meta-analysis by Xu et al[31], the Arg allele was more highly represented among controls of Asian descent than controls of European and African-American descent. Our findings also support this result, which is contrary to a previous study that reported approximately 50% homo- or hetero-zygous carriers of the Arg allele in healthy controls independent of ethnic background[26].
Table 5.
Frequency of the codon 72 genotype
| Gly/Gly | Gly/Arg | Arg/Arg | |
| This study (China) | 132 (33.6) | 188 (48.0) | 72 (18.4) |
| Chen et al[27] (China) | 133 (29.1) | 229 (50.1) | 95 (20.8) |
| Zhu et al[28] (China) | 231 (33.4) | 346 (50.0) | 115 (16.6) |
| Ma et al[29] (China) | 243 (33.2) | 368 (50.3) | 121 (16.5) |
| Yang et al[30] (China) | 123 (32.0) | 195 (50.6) | 67 (17.4) |
| Ma et al[14] (Japan) | 67 (37.4) | 87 (48.6) | 25 (14.0) |
| Morimoto et al[15] (Japan) | 39 (38.2) | 50 (49.0) | 13 (12.7) |
| Ho et al[34] (Singapore) | 30 (34.1) | 38 (43.2) | 20 (22.7) |
| Bange et al[10] (Italy) | 55 (44.7) | 60 (48.9) | 8 (6.5) |
| Spinola et al[11] (Italy) | 112 (50.9) | 83 (37.7) | 25 (11.4) |
| Ho et al[35] (United Kingdom) | 150 (51.5) | 117 (40.2) | 24 (8.2) |
| Wang et al[36] (United States-European) | 53 (54.6) | 40 (41.2) | 4 (4.1) |
| Wang et al[36] (United States-African) | 76 (80.9) | 18 (19.1) | 0 (0.0) |
| FitzGerald et al[13] (United States-European) | 631 (50.4) | 496 (39.6) | 124 (9.9) |
| FitzGerald et al[13] (United States-African) | 60 (75.0) | 18 (22.5) | 2 (2.5) |
For the first time, we report the distribution of the FGFR4 Gly388Arg genotypes and alleles in both GC patients and matched healthy controls. No differences were found between GC patients and healthy controls. Our findings suggest that this polymorphism is not a risk factor for GC initiation in the Chinese population. This result is consistent with previous reports on several cancer types, including breast cancer and lung cancer, in different races[10,18,24]. However, it has been reported that the Arg388 allele is associated with an increased risk of prostate cancer[13,14]. Moreover, this polymorphism also plays a role in some types of non-cancer disease initiation. Zhu et al[28] and Ma et al[29] found that the FGFR4 Gly388Arg polymorphism can act as a protective factor against coronary artery disease in the Chinese population. Based on a recent case-control study, the FGFR4 Gly388Arg polymorphism is also considered to be a genetic risk factor that contributes to the aggravation of gallstone disease[27]. Therefore, these findings may reflect a tissue-specific effect of this polymorphism.
In our study, we found a significant difference following stratification by tumor size, differentiation, invasion depth, lymph node involvement or clinical stage (P < 0.05). Clinical stage depends on invasion depth and lymph node involvement. Therefore, our results demonstrate that the FGFR4 Gly388Arg polymorphism is a prognostic factor in relatively small (less than 3 cm), well-differentiated (grades I and II) and early-stage GC (stages I and II) tumors but not in the total cohort of GC patients, which is in contrast to previous reports by Ye et al[26]. Because this study (and that of Ye et al[26]) focused on patients of Chinese origin, we suggest two possibilities to explain the conflicting results. The first possibility is the sample size. One major strength of our study is its large size. Our study included a higher proportion of patients with large tumors and late-stage disease than the study by Ye et al[26]. Thus, our results are more reliable given the isolation of the target patients from a pool of varied cases. Second, another possible explanation for the discord is that different DNA analysis methods were used in these two studies. Our study used a direct sequencing approach for genotype analysis, which was a more reliable method than the PCR-RFLP approach used by Ye et al[26]. Moreover, we detected the association between HER2 status and the FGFR4 Gly388Arg polymorphism in GC for the first time. No correlation was observed between HER2 status and the FGFR4 genotype (P = 0.532), which was consistent with previous reports in breast cancer[32].
The biochemical function of the FGFR4 Gly388Arg polymorphism in GC is still unclear. No correlation was found between the FGFR4 genotype and mRNA expression by Ye et al[26], and therefore, we do not attribute the polymorphism’s effect to FGFR4 up-regulation. The FGFR4 Arg388 allele may be in linkage disequilibrium with other genetic changes that contribute to poor prognosis in GC. A previous study indicated that 39 head and neck cancer cell lines harboring the FGFR4 Arg388 allele exhibited an increased sensitivity to cisplatinum[33]. In breast cancer, no significant survival differences between FGFR4 genotypes were found in patients without adjuvant systemic therapy. However, with adjuvant systemic therapy, breast cancer patients with the Gly/Gly genotype exhibited better disease-free survival and overall survival duration. Notably, this association seemed to be attributable to relatively poor therapy response in Arg388 carriers[32]. In our study, 156 patients underwent chemotherapy (5-fluorouracil and cisplatinum) following surgery. However, the median survival time of patients with the Gly/Arg and Arg/Arg genotypes did not differ from that of patients with the Gly/Gly genotype who underwent chemotherapy. Therefore, our data do not provide any evidence to support the theory that the FGFR4 Gly388Arg polymorphism is associated with sensitivity to chemotherapy.
In conclusion, our results demonstrate that the FGFR4 Gly388Arg polymorphism plays no role in the initiation of GC in Chinese patients, but it may be a factor in the survival of patients harboring relatively small, well-differentiated tumors in early GC stages.
COMMENTS
Background
Gastric cancer (GC) is one of the most common cancers in the world. Surgical resection and chemotherapy are only effective for a limited number of patients, and their prognoses remain very poor. Accordingly, there is an urgent need for a better understanding of the genesis of GC to establish a sound basis for future treatments.
Research frontiers
A germline polymorphism in the fibroblast growth factor receptor 4 (FGFR4) gene resulting in an amino acid change from glycine to arginine was identified several years ago. The presence of the FGFR4 Arg388 allele is associated with decreased disease-free survival in many cancers, including breast, lung, colon, prostate, soft tissue sarcoma, melanoma, and head and neck squamous cell carcinoma. Recently, Ye et al reported that the FGFR4 Arg388 allele was an independent prognostic factor in Chinese patients with GC. To our knowledge, this is the only report on the association between the FGFR4 Gly388Arg polymorphism and the progression of GC in Chinese patients, and this association needs further confirmation. Moreover, no study has been conducted on the correlation between this polymorphism and the risk of GC.
Innovations and breakthroughs
The correlation between the FGFR4 Gly388Arg polymorphism and the risk of GC was investigated for the first time, and the authors suggested that this polymorphism was not a risk factor for GC cancer initiation. Another interesting finding was that the Arg388 allele frequency was much higher in this Chinese population than in Caucasian cohorts. The authors also investigated the associations between the FGFR4 Gly388Arg polymorphism and clinicopathological parameters and survival in a larger sample series using more reliable methods. This result demonstrates that this polymorphism may contribute to the survival of patients with relatively small, well-differentiated tumors in the early stages of GC.
Applications
The FGFR4 Gly388Arg polymorphism is helpful for predicting the prognosis of early-stage GC patients with relatively small, well-differentiated tumors.
Terminology
Single nucleotide polymorphism (SNP): Genetic polymorphisms are natural variants in the genomic DNA sequence that are present in more than 1% of the population. One SNP represents the DNA variations in a single nucleotide. SNPs are widely used to better understand disease processes, thereby paving the way for genetic-based diagnostics and therapeutics.
Peer review
The results are interesting and may convey a useful prognostic marker for some GC patients.
Footnotes
P- Reviewers Kim JJ, Shimoyama S, Zagouri F S- Editor Huang XZ L- Editor A E- Editor Zhang DN
References
- 1.Fenoglio-Preiser C, Carneiro F, Correa P, Guilford P, Lambert R, Megraud F, Munoz N, Powell SM, Rugge M, Sasako M, et al. Gastric carcinoma. In: Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumors, Pathology and genetics of tumors of the digestive system. Lyon: LARC Press; 2000. pp. 39–40. [Google Scholar]
- 2.Zagouri F, Papadimitriou CA, Dimopoulos MA, Pectasides D. Molecularly targeted therapies in unresectable-metastatic gastric cancer: a systematic review. Cancer Treat Rev. 2011;37:599–610. doi: 10.1016/j.ctrv.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 4.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 5.Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 7.Takaishi S, Sawada M, Morita Y, Seno H, Fukuzawa H, Chiba T. Identification of a novel alternative splicing of human FGF receptor 4: soluble-form splice variant expressed in human gastrointestinal epithelial cells. Biochem Biophys Res Commun. 2000;267:658–662. doi: 10.1006/bbrc.1999.2010. [DOI] [PubMed] [Google Scholar]
- 8.Shin EY, Lee BH, Yang JH, Shin KS, Lee GK, Yun HY, Song YJ, Park SC, Kim EG. Up-regulation and co-expression of fibroblast growth factor receptors in human gastric cancer. J Cancer Res Clin Oncol. 2000;126:519–528. doi: 10.1007/s004320000128. [DOI] [PubMed] [Google Scholar]
- 9.Ye YW, Zhang X, Zhou Y, Wu J, Zhao C, Yuan L, Wang G, Du C, Wang C, Shi Y. The correlations between the expression of FGFR4 protein and clinicopathological parameters as well as prognosis of gastric cancer patients. J Surg Oncol. 2012;106:872–879. doi: 10.1002/jso.23153. [DOI] [PubMed] [Google Scholar]
- 10.Bange J, Prechtl D, Cheburkin Y, Specht K, Harbeck N, Schmitt M, Knyazeva T, Müller S, Gärtner S, Sures I, et al. Cancer progression and tumor cell motility are associated with the FGFR4 Arg(388) allele. Cancer Res. 2002;62:840–847. [PubMed] [Google Scholar]
- 11.Spinola M, Leoni VP, Tanuma J, Pettinicchio A, Frattini M, Signoroni S, Agresti R, Giovanazzi R, Pilotti S, Bertario L, et al. FGFR4 Gly388Arg polymorphism and prognosis of breast and colorectal cancer. Oncol Rep. 2005;14:415–419. [PubMed] [Google Scholar]
- 12.Spinola M, Leoni V, Pignatiello C, Conti B, Ravagnani F, Pastorino U, Dragani TA. Functional FGFR4 Gly388Arg polymorphism predicts prognosis in lung adenocarcinoma patients. J Clin Oncol. 2005;23:7307–7311. doi: 10.1200/JCO.2005.17.350. [DOI] [PubMed] [Google Scholar]
- 13.FitzGerald LM, Karlins E, Karyadi DM, Kwon EM, Koopmeiners JS, Stanford JL, Ostrander EA. Association of FGFR4 genetic polymorphisms with prostate cancer risk and prognosis. Prostate Cancer Prostatic Dis. 2009;12:192–197. doi: 10.1038/pcan.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Z, Tsuchiya N, Yuasa T, Inoue T, Kumazawa T, Narita S, Horikawa Y, Tsuruta H, Obara T, Saito M, et al. Polymorphisms of fibroblast growth factor receptor 4 have association with the development of prostate cancer and benign prostatic hyperplasia and the progression of prostate cancer in a Japanese population. Int J Cancer. 2008;123:2574–2579. doi: 10.1002/ijc.23578. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto Y, Ozaki T, Ouchida M, Umehara N, Ohata N, Yoshida A, Shimizu K, Inoue H. Single nucleotide polymorphism in fibroblast growth factor receptor 4 at codon 388 is associated with prognosis in high-grade soft tissue sarcoma. Cancer. 2003;98:2245–2250. doi: 10.1002/cncr.11778. [DOI] [PubMed] [Google Scholar]
- 16.Streit S, Mestel DS, Schmidt M, Ullrich A, Berking C. FGFR4 Arg388 allele correlates with tumour thickness and FGFR4 protein expression with survival of melanoma patients. Br J Cancer. 2006;94:1879–1886. doi: 10.1038/sj.bjc.6603181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Costa Andrade VC, Parise O, Hors CP, de Melo Martins PC, Silva AP, Garicochea B. The fibroblast growth factor receptor 4 (FGFR4) Arg388 allele correlates with survival in head and neck squamous cell carcinoma. Exp Mol Pathol. 2007;82:53–57. doi: 10.1016/j.yexmp.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki H, Okuda K, Kawano O, Yukiue H, Yano M, Fujii Y. Fibroblast growth factor receptor 4 mutation and polymorphism in Japanese lung cancer. Oncol Rep. 2008;20:1125–1130. [PubMed] [Google Scholar]
- 19.Tanuma J, Izumo T, Hirano M, Oyazato Y, Hori F, Umemura E, Shisa H, Hiai H, Kitano M. FGFR4 polymorphism, TP53 mutation, and their combinations are prognostic factors for oral squamous cell carcinoma. Oncol Rep. 2010;23:739–744. [PubMed] [Google Scholar]
- 20.Jézéquel P, Campion L, Joalland MP, Millour M, Dravet F, Classe JM, Delecroix V, Deporte R, Fumoleau P, Ricolleau G. G388R mutation of the FGFR4 gene is not relevant to breast cancer prognosis. Br J Cancer. 2004;90:189–193. doi: 10.1038/sj.bjc.6601450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang YC, Lu ML, Rao JY, Wallerand H, Cai L, Cao W, Pantuck A, Dalbagni G, Reuter V, Figlin RA, et al. Joint association of polymorphism of the FGFR4 gene and mutation TP53 gene with bladder cancer prognosis. Br J Cancer. 2006;95:1455–1458. doi: 10.1038/sj.bjc.6603456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mawrin C, Kirches E, Diete S, Wiedemann FR, Schneider T, Firsching R, Kropf S, Bogerts B, Vorwerk CK, Krüger S, et al. Analysis of a single nucleotide polymorphism in codon 388 of the FGFR4 gene in malignant gliomas. Cancer Lett. 2006;239:239–245. doi: 10.1016/j.canlet.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Matakidou A, El Galta R, Rudd MF, Webb EL, Bridle H, Eisen T, Houlston RS. Further observations on the relationship between the FGFR4 Gly388Arg polymorphism and lung cancer prognosis. Br J Cancer. 2007;96:1904–1907. doi: 10.1038/sj.bjc.6603816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naidu R, Har YC, Taib NA. Polymorphism of FGFR4 Gly388Arg does not confer an increased risk to breast cancer development. Oncol Res. 2009;18:65–71. doi: 10.3727/096504009789954609. [DOI] [PubMed] [Google Scholar]
- 25.Xu B, Tong N, Chen SQ, Hua LX, Wang ZJ, Zhang ZD, Chen M. FGFR4 Gly388Arg polymorphism contributes to prostate cancer development and progression: a meta-analysis of 2618 cases and 2305 controls. BMC Cancer. 2011;11:84. doi: 10.1186/1471-2407-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye Y, Shi Y, Zhou Y, Du C, Wang C, Zhan H, Zheng B, Cao X, Sun MH, Fu H. The fibroblast growth factor receptor-4 Arg388 allele is associated with gastric cancer progression. Ann Surg Oncol. 2010;17:3354–3361. doi: 10.1245/s10434-010-1323-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen Q, Li WJ, Wan YY, Yu CD, Li WG. Fibroblast growth factor receptor 4 Gly388Arg polymorphism associated with severity of gallstone disease in a Chinese population. Genet Mol Res. 2012;11:548–555. doi: 10.4238/2012.March.8.3. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Q, Liu T. Fibroblast growth factor receptor 4 polymorphisms and coronary artery disease: a case control study. Mol Biol Rep. 2012;39:8679–8685. doi: 10.1007/s11033-012-1723-8. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Zhang H, Han C, Tong D, Zhang M, Yao Y, Luo Y, Liu X. Fibroblast growth factor receptor 4 polymorphisms and susceptibility to coronary artery disease. DNA Cell Biol. 2012;31:1064–1069. doi: 10.1089/dna.2011.1552. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Yang Y, Zhou Y, Lu M, An Y, Li R, Chen Y, Lu DR, Jin L, Zhou WP, Qian J, et al. Association between fibroblast growth factor receptor 4 polymorphisms and risk of hepatocellular carcinoma. Mol Carcinog. 2012;51:515–521. doi: 10.1002/mc.20805. [DOI] [PubMed] [Google Scholar]
- 31.Xu W, Li Y, Wang X, Chen B, Wang Y, Liu S, Xu J, Zhao W, Wu J. FGFR4 transmembrane domain polymorphism and cancer risk: a meta-analysis including 8555 subjects. Eur J Cancer. 2010;46:3332–3338. doi: 10.1016/j.ejca.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Thussbas C, Nahrig J, Streit S, Bange J, Kriner M, Kates R, Ulm K, Kiechle M, Hoefler H, Ullrich A, et al. FGFR4 Arg388 allele is associated with resistance to adjuvant therapy in primary breast cancer. J Clin Oncol. 2006;24:3747–3755. doi: 10.1200/JCO.2005.04.8587. [DOI] [PubMed] [Google Scholar]
- 33.Farnebo L, Jedlinski A, Ansell A, Vainikka L, Thunell LK, Grénman R, Johansson AC, Roberg K. Proteins and single nucleotide polymorphisms involved in apoptosis, growth control, and DNA repair predict cisplatin sensitivity in head and neck cancer cell lines. Int J Mol Med. 2009;24:549–556. doi: 10.3892/ijmm_00000264. [DOI] [PubMed] [Google Scholar]
- 34.Ho HK, Pok S, Streit S, Ruhe JE, Hart S, Lim KS, Loo HL, Aung MO, Lim SG, Ullrich A. Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J Hepatol. 2009;50:118–127. doi: 10.1016/j.jhep.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Ho CK, Anwar S, Nanda J, Habib FK. FGFR4 Gly388Arg polymorphism and prostate cancer risk in Scottish men. Prostate Cancer Prostatic Dis. 2010;13:94–96. doi: 10.1038/pcan.2009.49. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Yu W, Cai Y, Ren C, Ittmann MM. Altered fibroblast growth factor receptor 4 stability promotes prostate cancer progression. Neoplasia. 2008;10:847–856. doi: 10.1593/neo.08450. [DOI] [PMC free article] [PubMed] [Google Scholar]