Abstract
Neuroendocrine carcinoma (NEC) of the extrahepatic bile duct is rare, and only 22 cases have been reported. Only two of these were large-cell NEC (LCNEC); the vast majority were small-cell NEC. Here, we report a third case of LCNEC of the extrahepatic bile duct. A 76-year-old male presented to a local hospital with painless jaundice. Imaging studies revealed a tumor at the hepatic hilum. The patient underwent right hepatic lobectomy, bile duct resection, and cholecystectomy. The resection specimen showed a 5.0-cm invasive neoplasm involving the hilar bile ducts and surrounding soft tissue. Histologically, the tumor consisted of nests of medium to large cells with little intervening stroma. The tumor invaded a large portal vein branch. All four excised lymph nodes were positive for metastasis, and metastatic deposits were also present in the gallbladder wall. The tumor was diffusely positive for synaptophysin and focally positive for chromogranin A. Approximately 70%-80% of the tumor cells were positive for Ki-67, indicating strong proliferative activity. A diagnosis of LCNEC was made. A few bile ducts within and adjacent to the invasive tumor showed dysplasia of the intestinal phenotype and were focally positive for synaptophysin and chromogranin A, suggesting that the dysplastic intestinal-type epithelium played a precursor role in this case. A postoperative computer tomography scan revealed rapid enlargement of the abdominal and retroperitoneal lymph nodes. The patient died 21 d after the operation. NEC of the bile duct is an aggressive neoplasm, and its biological characteristics remain to be better defined.
Keywords: Neuroendocrine neoplasm, Large cell neuroendocrine carcinoma, Small cell neuroendocrine carcinoma, Extrahepatic bile duct, Dysplasia
Core tip: The authors report a case of large-cell neu-roendocrine carcinoma (LCNEC) of the hilar bile duct. Concurrent dysplasia with intestinal and neuroendocrine differentiation was suggested to be a precursor in this case. Neuroendocrine carcinoma (NEC) of the bile duct occurs more frequently in men (male:female ratio 1.9:1). The mid-portion of the common bile duct appears to be the commonest site of involvement. All three reported cases of LCNEC died within 12 mo and the prognosis of NEC of the bile duct appears to be equally poor in both small-cell NEC and LCNEC. Multimodal treatment may improve outcome in this highly aggressive cancer.
INTRODUCTION
Neuroendocrine neoplasms of the extrahepatic bile ducts are rare, with carcinoid tumors representing the most common type. Neuroendocrine carcinoma (NEC) is defined as a poorly differentiated, high-grade, malignant neuroendocrine neoplasm, which is classified as either small-cell NEC (SCNEC) or large-cell NEC (LCNEC)[1]. NEC of the extrahepatic bile ducts is exceedingly rare, and only 22 cases have been reported in the literature (Table 1)[2-23]. Of these, most cases are SCNEC, and only two cases of LCNEC have been reported to date. Since LCNEC was first described in the lung by Travis et al[24] in 1991, similar lesions have been described in extrapulmonary sites, including the gastrointestinal tract[25]. LCNEC shows immunohistochemical evidence of epithelial and neuroendocrine differentiation and is characterized by a diffuse growth pattern or neuroendocrine architecture (organoid, palisaded, rosettes, or trabeculae)[24,26]. Large cell size, low nuclear to cytoplasmic ratio, and frequent nucleoli are key cytologic features that help distinguish LCNEC from SCNEC. LCNEC of the biliary tract was first described in the gallbladder by Papotti et al[27] a little more than a decade ago; however, reported cases are still few, particularly in the extrahepatic bile ducts. Because of the paucity of cases, patient prognosis and responsiveness to anticancer treatments for LCNEC of the biliary tract largely remain to be elucidated. Most of the reported cases of LCNEC of the biliary tracts showed an aggressive course and short survival times; however, one case of LCNEC arising in the gallbladder achieved long survival (69 mo from the initial diagnosis) following multimodal treatment that included surgery, chemotherapy, and radiation therapy[28].
Table 1.
Neuroendocrine carcinoma of the extrahepatic bile ducts-review of the literature
| No. | Ref. | Age (yr) | Sex | Histology | Location | Maximal dimension (cm) | Treatment | Follow-up information | Other findings |
| 1 | Sabanathan et al[2] | 67 | M | SCNEC | Bm | 5 | Palliative bypass and chemotherapy | Alive 6 mo | |
| 2 | Miyashita et al[3] | 85 | F | SCNEC | Bi | 3 | Palliative bypass | DOD 5 mo after surgery | |
| 3 | Kuraoka et al[4] | 75 | M | SCNEC | Bi | 4.5 | Resection | Alive 5 mo after surgery | Dysplasia |
| 4 | Hazama et al[5] | 60 | M | SCNEC | CBD | 0.3 | Neoadjuvant chemotherapy and resection | DOD 12 mo after surgery | |
| 5 | Arakura et al[6] | 70 | F | SCNEC | Bm | 3 | Resection and chemotherapy | DOD 14 mo after surgery | |
| 6 | Park et al[7] | 60 | F | SCNEC | Bs-Bm | 3 | Resection | DOD 5 mo after surgery | |
| 7 | Thomas et al[8] | 54 | M | SCNEC | Bh-CBD | NA | Resection | Alive With Metastasis, 6 mo | Clonorchis sinensis infestation |
| 8 | Viana Miguel et al[9] | 76 | M | SCNEC | Bm | NA | Resection, chemotherapy and irradiation | Alive 5 mo after surgery | Gallstone |
| 9 | Jeon et al[10] | 65 | M | SCNEC | Bs-Bm | 2 | Presection and chemotherapy | DOD 12 mo after surgery | |
| 10 | Nakai et al[11] | 32 | M | SCNEC | CBD | NA | NA, diagnosed by autopsy | NA | |
| 11 | Arakura et al[12] | 75 | M | SCNEC | Bh, Bs, Bm, Bi | 6.5 | Chemotherapy and irradiation | DOD 10 mo after therapy | |
| 12 | Hosonuma et al[13] | 69 | F | SCNEC | Bs-Bm | 3 | Biliary drainage | Alive 2 mo after biliary drainage | |
| 13 | Okamura et al[14] | 62 | M | SCNEC | Bm | 3 | Preoperative chemotherapy, resection and irradiation | DOD 20 mo after surgery | |
| 14 | Yamaguchi et al[15] | 77 | F | NEC | Bi | NA | Resection and chemotherapy | Alive 27 mo | |
| 15 | van der Wal et al[16] | 55 | M | SCNEC + atypical carcinoid + AD | Bm | 4 | Resection | NA | Dysplasia/carcinoma in situ |
| 16 | Nishihara et al[17] | 64 | M | SCNEC + AD | Bh-Bs | 1.9 | Resection | Alive 8 mo after surgery | |
| 17 | Yamamoto et al[18] | 71 | F | SCNEC + AD | Bh | 6 | Resection | DOD 7 mo after surgery | Common bile duct stones |
| 18 | Kim et al[19] | 64 | M | SCNEC + AD | Bm | 3 | Resection | Alive 30 d after surgery | Clonorchis sinensis infestation |
| 19 | Edakuni et al[20] | 82 | F | SCNEC + AD | Bm | 6 | Resection | Alive 45 mo after surgery | |
| 20 | Kaiho et al[21] | 66 | F | SCNEC + AD | Bm | 3.5 | Resection and chemotherapy | DOD 8 mo after surgery | |
| 21 | Sato et al[22] | 68 | M | LCNEC + AD | Bi | 2 | Resection and chemotherapy | DOD 3 mo after surgery | |
| 22 | Demoreuil et al[23] | 73 | M | LCNEC + AD | Bh-Bs | 3 | Resection and chemotherapy | DOD 12 mo after surgery | |
| 23 | Current report, 2013 | 76 | M | LCNEC | Bh-Bs | 5 | Resection | DOD 21 d after surgery | Dysplasia, intestinal type |
SCNEC: Small cell neuroendocrine carcinoma; NEC: Nneuroendocrine carcinoma, not otherwise specified; AD: Adenocarcinoma; LCNEC: Large cell neuroendocrine carcinoma; Bm: Mid portion of common bile duct; Bi: Inferior or distal common bile duct; CBD: Common bile duct, not otherwise specified; Bs; Superior or proximal common bile/hepatic duct; Bh: Hilar bile duct; DOD: Died of disease. N/A: Information not available; F: Female; M: Male.
The origin of neuroendocrine neoplasms of the biliary tracts is unclear. They may arise from metaplastic epithelia, where there are a variety of epithelial cells (including neuroendocrine cells, goblet cells, and gastric-type epithelial cells) that are not found in the normal biliary epithelium. In fact, intestinal and/or gastric-type metaplasias are not uncommon in non-neoplastic mucosa adjacent to a variety of neuroendocrine neoplasms of the gallbladder, including carcinoid tumor, SCNEC, and LCNEC[27,29,30].
Here, we report a case of LCNEC of the hilar bile duct. The bile duct mucosa adjacent to the LCNEC showed high-grade dysplasia of the intestinal phenotype, which may have been the precursor in this case. While the presence of concurrent nearby dysplasia was described in 2 previously reported cases of SCNEC[4,16], neuroendocrine differentiation or metaplastic change has not been demonstrated in dysplastic epithelium. Therefore, we aimed to perform detailed immunophenotypic characterization of the dysplastic epithelium and demonstrated intestinal and neuroendocrine differentiation in the dysplastic epithelium. Another objective of this article is to provide a comprehensive literature review on NEC of the bile duct. Despite the paucity of cases, an attempt was made to compare the clinicopathologic characteristics and outcomes between SCNECs and LCNECs.
CASE REPORT
A 76-year-old male presented to a local hospital with a 2 wk history of increasing yellowish discoloration of the skin and dark-colored urine. The patient had no abdominal pain, nausea, or vomiting. He had a history of hypertension, rheumatoid arthritis, peptic ulcer disease, status post-stent placement for ischemic heart disease, and status post-right inguinal hernia repair. The patient denied any family history of cancer. His medication included metoprolol and benazepril. The patient was admitted to the local hospital for 7 d, during which time an abdominal computed tomography (CT) scan revealed a tumor involving the hepatic hilum. Endoscopic retrograde cholangiopancreatography demonstrated a stricture at the common hepatic duct, and two biliary stents were placed. The imaging studies suggested a malignant biliary stricture, but this could not be confirmed with biopsy. After the stent placement, the patient’s serum bilirubin, which was initially reported to be 10 mg/dL, decreased to approximately 5 mg/dL and the yellowish discoloration of his skin normalized. The patient was transferred to the department of Surgery at the University of Pittsburgh Medical Center for further work-up and treatment. On admission, the patient’s vital signs were within normal limits. The patient was obese with a body mass index of 34 kg/m2. The sclerae were slightly icteric. The patient had normal cardiac and respiratory examinations and did not have cervical, axillary, or supraclavicular lymphadenopathy. Examination of the abdomen showed mild epigastric tenderness to deep palpation with no rebound tenderness or guarding. The right inguinal area had a well-healed scar from an inguinal hernia repair in the 1950s. The patient’s complete blood count and serum biochemistry data on admission were as follows: white blood cells, 11300/mm3; hemoglobin, 13.4 g/dL; hematocrit, 39.5%; platelets, 377000/mm3; blood glucose, 122 mg/dL; total bilirubin, 3.5 mg/dL; aspartate aminotransferase, 106 IU/L; alanine aminotransferase, 88 IU/L; and gamma-glutamyl transpeptidase, 80 IU/L. Carbohydrate antigen 19-9 was 32.9 U/mL. Chest X-ray was normal. The patient underwent an operation in October 2011 with the presumed diagnosis of hilar cholangiocarcinoma (Klatskin tumor). The tumor of the hepatic hilum was resected with a right hepatic lobectomy, bile duct resection, and cholecystectomy. The patient had markedly enlarged lymph nodes in the hepatic hilum and retropancreatic area, which were also resected. There was no evidence of extrahepatic disease other than lymphadenopathy. Reconstruction was performed with a Roux-en-Y hepaticojejunostomy. In the resection specimen, the neoplasm diffusely involved the perihilar bile ducts and the surrounding portal connective tissue and measured 5.0 cm at its greatest dimension (Figure 1). The tumor had also invaded a large, right portal vein branch and formed an intraluminal mass. Histologically, the tumor cells were poorly differentiated with no evidence of glandular or squamous differentiation. The tumor cells were arranged in cellular nests and sheets with a small amount of intervening fibrovascular stroma (Figure 2A). The tumor cells had medium to large hyperchromatic nuclei with fine to coarse granular chromatin and occasional small nucleoli. The tumor cells had a small to moderate amount of amphophilic cytoplasm (Figure 2B). They showed brisk apoptotic activity and frequent mitotic figures (15-18 mitoses per 10 high-power fields). The tumor showed perineural and angiolymphatic invasion. All four lymph nodes were positive for metastatic tumor. The resected gallbladder had microscopic metastatic deposits in the perimuscular layer. No gallstones were present. The tumor cells were negative for mucicarmine stain. Immunohistochemical stains revealed that the tumor cells were strongly positive for synaptophysin (Figure 2C). They were also focally positive for chromogranin A, pancytokeratin, cytokeratin (CK) 7, CK 19, and MOC-31. Fewer than 1% of the tumor cells were weakly positive for thyroid transcription factor-1. They were negative for napsin-A, surfactant apoprotein A, alpha-fetoprotein, vimentin, CK5/6, p63, leukocyte common antigen, and S100. Approximately 70%-80% of the tumor cells were positive for Ki-67 (Figure 2D). The histologic findings and the immunoprofile of the neoplasm were consistent with LCNEC. A few foci of intermediate- to high-grade dysplasia were also identified in the perihilar bile ducts located within and adjacent to the invasive neoplasm (Figure 3A). The dysplastic epithelium contained some goblet cells. The dysplastic epithelial cells were immunoreactive for CK19, CK20, and CDX2 (Figure 3B and C). The goblet cells were positive for mucin (MUC)2 and negative for CK7, MUC1, MUC5AC, and MUC6. The immunoprofile of the dysplastic epithelium, together with the presence of goblet cells expressing MUC2 was consistent with an intestinal phenotype. The epithelial cells of this lesion were also positive for synaptophysin (Figure 3D) and chromogranin A but with less extensive and intense immunoreactivity compared to the invasive neoplasm. The findings of the in situ component were suggestive of a premalignant or preinvasive lesion rather than intraepithelial spread from the invasive neoplasm. The patient’s immediate postoperative course was uncomplicated; however, a CT scan of the abdomen eight days after the operation revealed extensive enlargement of his abdominal and retroperitoneal lymph nodes, which was suggestive of rapid metastasis. The patient had poor oral intake, hypovolemia, and significant back pain. Postoperative chemotherapy or radiation therapy was not performed because of the patient’s poor general condition. The patient died 21 d after operation. An autopsy was not performed.
Figure 1.
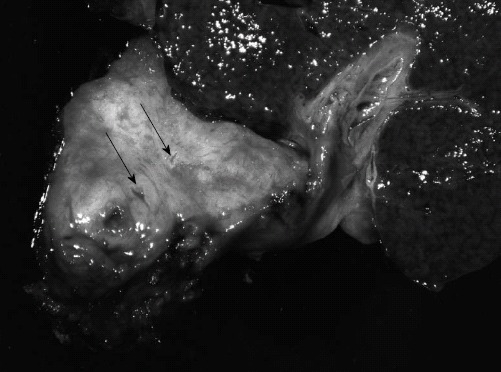
Gross appearance of the hilar neoplasm. The resected specimen showed a firm, tan-grey tumor measuring 5.0-cm in greatest dimension with extensive involvement of the perihilar bile ducts and surrounding soft tissue. Some of the small ducts and vessels within the lesion showed severe luminal narrowing (arrows).
Figure 2.
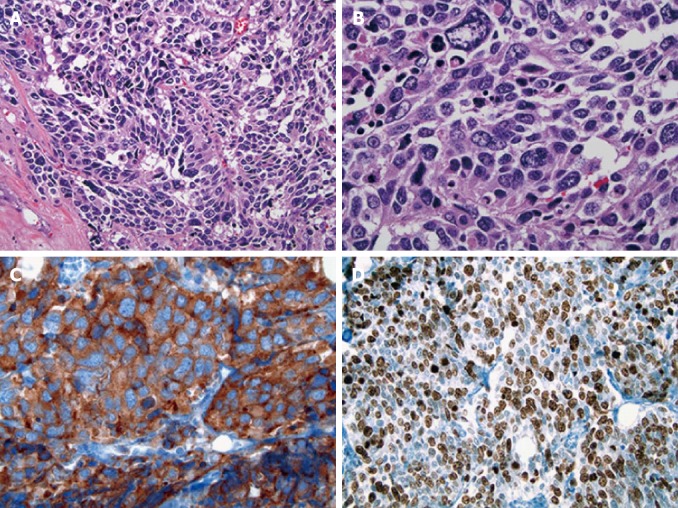
Histologic features of the hilar neoplasm. A: The tumor cells were arranged in cellular nests and sheets with little intervening fibrovascular stroma [Hematoxylin and eosin (HE), × 200]; B: The tumor cells had a small to moderate amount of amphophilic cytoplasm and medium to large hyperchromatic nuclei with fine to coarse granular chromatin and occasional small nucleoli (HE, × 400); C: The tumor cells were strongly positive for synaptophysin (× 400); D: 70%-80% of the tumor cells were positive for Ki-67 (× 200).
Figure 3.
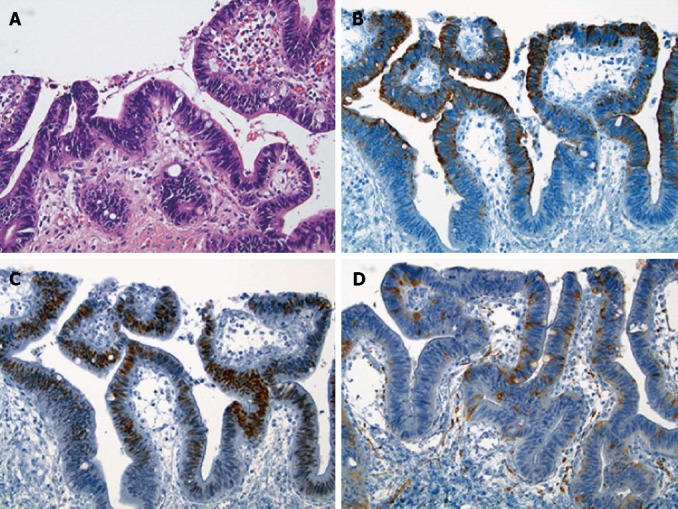
Histologic features of the dysplastic epithelium found in a medium-sized perihilar bile duct located within the invasive neoplasm. A: The dysplastic epithelium contained some goblet cells [Hematoxylin and eosin (HE), × 200]; B, C: The dysplastic epithelial cells were immunoreactive for CK20 (B, × 200) and CDX2 (C, × 200); D: Some of the dysplastic epithelial cells were positive for synaptophysin with less extensive and weak immunoreactivity compared to the invasive neoplasm (× 200).
DISCUSSION
According to the most recent World Health Organization (WHO) classification, neuroendocrine neoplasms of the digestive system are classified into three general categories based on histologic features and proliferation fraction[1,31]. These include NET, NEC, and mixed adenoneuroendocrine carcinoma (MANEC). A NET is defined as well-differentiated neuroendocrine neoplasm with mild to moderate nuclear atypia and a low proliferation fraction (≤ 20 mitoses per 10 high-power fields or ≤ 20% Ki67 index). This category encompasses neoplasms termed carcinoid tumors. A NEC is a poorly differentiated, high-grade malignant neuroendocrine neoplasm composed of either small or intermediate to large cells with marked nuclear atypia and a high proliferation fraction (> 20 mitoses per 10 high-power fields or > 20% Ki67 index). This category includes SCNEC and LCNEC, but a dichotomous subclassification of small cell vs non-small cell may also apply[25]. MANEC has a phenotype that is morphologically recognizable as both gland-forming epithelial and neuroendocrine carcinomas. Arbitrarily, at least 30% of either component should be identified to qualify for this definition.
Neuroendocrine neoplasms of the extrahepatic bile ducts are rare, and the majority of the reported cases are NET/carcinoid, which represent 0.1%-0.2% of all gastrointestinal carcinoids/NET[32]. According to the data obtained from the Surveillance, Epidemiology, and End Results program of the National Cancer Institute, there were 31 cases of carcinoid, 17 of SCNECs, and 10 of NECs of not-otherwise-specified type of the gallbladder and extrahepatic bile ducts between 1973 and 2005[33]. Thus, NEC of the extrahepatic bile duct is exceedingly rare and is probably less frequent than NET/carcinoid. According to the literature, 23 cases of NEC of the extrahepatic bile ducts, including the case described here, have been reported. The most common histologic subtype of NEC of the extrahepatic bile ducts is SCNEC (19 of 23 cases; Table 1). Only two cases of LCNEC of the common bile duct were previously reported (Table 1, cases 21 and 22); therefore, this is the third reported case of LCNEC arising in the extrahepatic bile duct. The reported cases of NEC include 15 males and 8 females, with a male to female ratio of 1.9: 1. Patient age ranged from 32-85 years with a mean age of 67.2 years. The mean age of LCNEC was higher than that of SCNEC (72.3 years vs 65.9 years), but this difference was not statistically significant. NEC can occur anywhere in the extrahepatic bile duct, but the mid portion of the common bile duct appears to be the most common site of involvement. Two cases had biliary stones (Table 1, cases 8 and 17). Concurrent Clonorchis sinensis infestation was seen in two cases (Table 1, cases 7 and 18). The presence of concurrent nearby dysplasia was described in three of 23 cases, including ours (Table 1, cases 3, 15 and 23). Eight cases were composite neuroendocrine and adenocarcinoma (Table 1, cases 15-22). Some of these composite cases may be classified as MANEC rather than NEC according to the current WHO classification system, depending on the proportion of the adenocarcinoma component. When NEC coexisted with adenocarcinoma, a gradual transition between areas of NEC and adenocarcinoma was observed in six of eight cases (Table 1, cases 15-17 and 19-21). In three of eight cases (Table 1, cases 18, 19 and 21), the adenocarcinoma component was located in the superficial portion of the tumor, and the NEC component was located mainly in the deeper portion of the tumor. No other particular spatial relationships between NEC and adenocarcinoma components have been described.
NEC of the gastrointestinal tract can show a spectrum of morphologic features ranging from classic SCNEC to LCNEC, and some cases have features between these two types. As Shia et al[25] previously pointed out, there were no criteria for classifying NEC of the GI tract with non-small cell morphology before the most recent WHO classification. Therefore, NEC of the extrahepatic bile ducts and gallbladder with non-small cell morphology may have been diagnosed inconsistently. In fact, histologic features of some of the previously reported cases of SCNEC of the extrahepatic bile ducts are not morphologically typical of SCNEC. For example, the NEC tumor component reported as a part of composite adenocarcinoma and SCNEC of the hilar bile duct by Yamamoto et al[18] (Figure 2B in their manuscript) showed prominent nucleoli and moderately abundant cytoplasm, which is not typical of SCNEC (Table 1, case 17). Thus, some of the non-small cell type NECs or LCNEC may have been diagnosed or reported as SCNEC because of the previous lack of a distinct diagnostic category.
LCNEC of the extrahepatic bile ducts is extremely rare. Both of the previously reported cases contained a minor component of adenocarcinoma (10%-20% of the entire tumor). One tumor was located in an intra-pancreatic portion of the common bile duct (Table 1, case 21), and another was located in the perihilar bile duct (Table 1, case 22). The adenocarcinoma component of one case (Table 1, case 21) showed focal expression of a neuroendocrine marker (chromogranin A), and the other case did not express neuroendocrine markers (Table 1, case 22). The coexistence of adenocarcinoma in these cases, as well as in the aforementioned six cases of composite adenocarcinoma and SCNEC suggests that NEC of the common bile duct may arise from pluripotent progenitor cells. This idea is further supported by the observation of transitional zones between NEC and adenocarcinoma components in the majority of cases. The invasive tumor described here was composed entirely of LCNEC. Although no adenocarcinoma component was identified, a few perihilar bile ducts located within and adjacent to the LCNEC showed dysplasia of the intestinal phenotype with focal endocrine differentiation. Although data on the histogenesis of neuroendocrine neoplasm of the biliary tracts in the literature are limited, neuroendocrine neoplasm of the bile duct and gallbladder may arise from neuroendocrine cells in intestinal or gastric metaplasia, where there may be progenitor cells with a greater ability to differentiate into neuroendocrine cells[29,34]. The presence of intestinal type dysplasia in our case suggests that LCNEC may have arisen from metaplastic epithelium, although we cannot exclude the possibility that the metaplastic/neuroendocrine phenotype may have been acquired at the time of or subsequent to the dysplasia. Regardless, the progression from dysplasia with an intestinal/neuroendocrine phenotype to an aggressive NEC may have occurred in the case described.
The prognosis of NEC of the bile duct appears to be poor. Among the 21 cases with follow-up data, 57% (12/21) of the patients died of disease 3 to 20 mo after surgery, and only two patients have been reported to survive more than 2 years (Table 1, cases 14 and 19). Among the seven patients who survived at least 12 mo, five were treated with multidisciplinary treatment, including surgical resection, adjuvant or neoadjuvant chemotherapy, and radiation. The longest survival was 45 mo in a patient who was treated with surgical treatment alone (Table 1, Case 19). According to Edakuni et al[20], that tumor was a composite adenocarcinoma (-40%) and SCNEC (-60%). They speculated that the reason for the long survival may have been the low proliferative fraction (9.6% Ki-67-positive tumor cells) of the SCNEC. Recently, most neuroendocrine neoplasm grading systems rely extensively on the proliferation rate, which has been shown to provide significant prognostic information[31]. Based on the current WHO grading system, the NEC component of the case reported by Edakuni et al[20] may be best classified as intermediate-grade NET rather than SCNEC, and this appears to at least in part explain the long survival time. According to Iype et al[35], LCNECs appear to have a worse prognosis than SCNEC in the gallbladder. Although only two cases of LCNEC of the bile duct have been previously reported, both patients died within 12 mo of surgery despite postoperative chemotherapy (Table 1, cases 21 and 22). In our case, the patient died 21 d after the operation with radiographic evidence of rapid progression of metastatic disease. Thus, the survival for patients with LCNEC of the extrahepatic bile duct appears to be equally poor as for those with SCNEC. Multidisciplinary management appears to be effective and provide longer survival time for SCNEC of the bile duct (Table 1, cases 13 and 14). Recently, a long surviving case of LCNEC of the gallbladder was reported by Shimono et al[28]. That patient received multimodal treatment consisting of chemotherapy, radiation therapy, surgical resection, and γ-knife irradiation for brain metastases, which resulted in 69 mo of survival. This case suggests that multimodal treatment is potentially effective in treating LCNEC patients. The effectiveness of chemotherapy and radiotherapy in both SCNEC and LCNEC of the biliary tract needs to be further investigated in a larger number of cases to confirm this observation.
In summary, we reported a case of high-grade neuroendocrine neoplasm arising in the perihilar bile ducts that was best classified as LCNEC. The coexistent dysplasia with intestinal and neuroendocrine differentiation may represent a LCNEC precursor. We feel that histologic subtyping of NECs into SCNEC and LCNEC (or even non-small cell NEC), rather than grouping all types of high-grade neuroendocrine neoplasms together as NECs, is necessary because biologic characteristics of each subtype need to be more clearly defined for better prognostication and selection of therapy.
Footnotes
P- Reviewer Zhang WH S- Editor Huang XZ L- Editor A E- Editor Zhang DN
References
- 1.Komminoth P, Arnold R, Capella C, Klimstra DS, Kloppel G, Rindi G, Albores-Saavedra J, Solcia E. Neuroendocrine neoplasms of the gallbladder and extrahepatic bile ducts. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC Press; 2010. pp. 274–276. [Google Scholar]
- 2.Sabanathan S, Hashimi H, Nicholson G, Edwards AS. Primary oat cell carcinoma of the common bile duct. J R Coll Surg Edinb. 1988;33:285–286. [PubMed] [Google Scholar]
- 3.Miyashita T, Konishi K, Noto M, Taniguchi K, Kaji M, Kimura H, Maeda K, Yabushita K, Miwa A. A case of small cell carcinoma of the common bile duct. Nihon Shokakibyo Gakkai Zasshi. 2001;98:1195–1198. [PubMed] [Google Scholar]
- 4.Kuraoka K, Taniyama K, Fujitaka T, Nakatsuka H, Nakayama H, Yasui W. Small cell carcinoma of the extrahepatic bile duct: case report and immunohistochemical analysis. Pathol Int. 2003;53:887–891. doi: 10.1046/j.1440-1827.2003.01575.x. [DOI] [PubMed] [Google Scholar]
- 5.Hazama K, Suzuki Y, Takahashi M, Takahashi Y, Yoshioka T, Takano S, Kondoh S, Katoh H. Primary small cell carcinoma of the common bile duct, in which surgical treatment was performed after neoadjuvant chemotherapy: report of a case. Surg Today. 2003;33:870–872. doi: 10.1007/s00595-003-2594-3. [DOI] [PubMed] [Google Scholar]
- 6.Arakura N, Hasebe O, Yokosawa S, Imai Y, Furuta S, Hosaka N. A case of small cell carcinoma of the extrahepatic bile duct which could be diagnosed before operation. Nihon Shokakibyo Gakkai Zasshi. 2003;100:190–194. [PubMed] [Google Scholar]
- 7.Park HW, Seo SH, Jang BK, Hwang JY, Park KS, Cho KB, Hwang JS, Ahn SH, Kang KJ, Kang YN, et al. A case of primary small cell carcinoma in the common bile duct. Korean J Gastroenterol. 2004;43:260–263. [PubMed] [Google Scholar]
- 8.Thomas NE, Burroughs FH, Ali SZ. Small-cell carcinoma of the extrahepatic bile duct and concurrent clonorchiasis. Diagn Cytopathol. 2005;32:92–93. doi: 10.1002/dc.20162. [DOI] [PubMed] [Google Scholar]
- 9.Viana Miguel MM, García-Plata Polo E, Vidal Doce O, Aldea Martínez J, de la Plaza Galindo M, Santamaría García JL. Oat cell carcinoma of the common bile duct. Cir Esp. 2006;80:43–45. doi: 10.1016/s0009-739x(06)70915-0. [DOI] [PubMed] [Google Scholar]
- 10.Jeon WJ, Chae HB, Park SM, Youn SJ, Choi JW, Kim SH. A case of primary small cell carcinoma arising from the common bile duct. Korean J Gastroenterol. 2006;48:438–442. [PubMed] [Google Scholar]
- 11.Nakai N, Takenaka H, Hamada S, Kishimoto S. Identical p53 gene mutation in malignant proliferating trichilemmal tumour of the scalp and small cell carcinoma of the common bile duct: the necessity for therapeutic caution? Br J Dermatol. 2008;159:482–485. doi: 10.1111/j.1365-2133.2008.08631.x. [DOI] [PubMed] [Google Scholar]
- 12.Arakura N, Muraki T, Komatsu K, Ozaki Y, Hamano H, Tanaka E, Kawa S. Small cell carcinoma of the extrahepatic bile duct diagnosed with EUS-FNA and effectively treated with chemoradiation. Intern Med. 2008;47:621–625. doi: 10.2169/internalmedicine.47.0663. [DOI] [PubMed] [Google Scholar]
- 13.Hosonuma K, Sato K, Honma M, Kashiwabara K, Takahashi H, Takagi H, Mori M. Small-cell carcinoma of the extrahepatic bile duct: a case report and review of the literature. Hepatol Int. 2008;2:129–132. doi: 10.1007/s12072-007-9027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura Y, Maeda A, Matsunaga K, Kanemoto H, Boku N, Furukawa H, Sasaki K, Uesaka K. Small-cell carcinoma in the common bile duct treated with multidisciplinary management. J Hepatobiliary Pancreat Surg. 2009;16:575–578. doi: 10.1007/s00534-009-0051-4. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Shimizu H, Yoneda E. A case of recurrent endocrine cell carcinoma of the common bile duct successfully treated by hepatic artery infusion with CPT-11 and CDDP. Gan To Kagaku Ryoho. 2009;36:823–825. [PubMed] [Google Scholar]
- 16.van der Wal AC, Van Leeuwen DJ, Walford N. Small cell neuroendocrine (oat cell) tumour of the common bile duct. Histopathology. 1990;16:398–400. doi: 10.1111/j.1365-2559.1990.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 17.Nishihara K, Tsuneyoshi M, Niiyama H, Ichimiya H. Composite glandular-endocrine cell carcinoma of the extrahepatic bile duct: immunohistochemical study. Pathology. 1993;25:90–94. doi: 10.3109/00313029309068910. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto J, Abe Y, Nishihara K, Katsumoto F, Takeda S, Abe R, Toyoshima S. Composite glandular-neuroendocrine carcinoma of the hilar bile duct: report of a case. Surg Today. 1998;28:758–762. doi: 10.1007/BF02484625. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Park YN, Yoon DS, Lee SJ, Yu JS, Noh TW. Composite neuroendocrine and adenocarcinoma of the common bile duct associated with Clonorchis sinensis: a case report. Hepatogastroenterology. 2000;47:942–944. [PubMed] [Google Scholar]
- 20.Edakuni G, Sasatomi E, Satoh T, Tokunaga O, Miyazaki K. Composite glandular-endocrine cell carcinoma of the common bile duct. Pathol Int. 2001;51:487–490. doi: 10.1046/j.1440-1827.2001.01227.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaiho T, Tanaka T, Tsuchiya S, Yanagisawa S, Takeuchi O, Miura M, Saigusa N, Hayasaka A, Matsuzaki O, Miyazaki M. A case of small cell carcinoma of the common bile duct. Hepatogastroenterology. 2005;52:363–367. [PubMed] [Google Scholar]
- 22.Sato K, Waseda R, Tatsuzawa Y, Fujinaga H, Wakabayashi T, Ueda Y, Katsuda S. Composite large cell neuroendocrine carcinoma and adenocarcinoma of the common bile duct. J Clin Pathol. 2006;59:105–107. doi: 10.1136/jcp.2005.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demoreuil C, Thirot-Bidault A, Dagher C, Bou-Farah R, Benbrahem C, Lazure T, Gayral F, Buffet C. Poorly differentiated large cell endocrine carcinoma of the extrahepatic bile ducts. Gastroenterol Clin Biol. 2009;33:194–198. doi: 10.1016/j.gcb.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Travis WD, Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB, Nieman L, Chrousos G, Pass H, Doppman J. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15:529–553. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Shia J, Tang LH, Weiser MR, Brenner B, Adsay NV, Stelow EB, Saltz LB, Qin J, Landmann R, Leonard GD, et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am J Surg Pathol. 2008;32:719–731. doi: 10.1097/PAS.0b013e318159371c. [DOI] [PubMed] [Google Scholar]
- 26.Gollard R, Jhatakia S, Elliott M, Kosty M. Large cell/neuroendocrine carcinoma. Lung Cancer. 2010;69:13–18. doi: 10.1016/j.lungcan.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Papotti M, Cassoni P, Sapino A, Passarino G, Krueger JE, Albores-Saavedra J. Large cell neuroendocrine carcinoma of the gallbladder: report of two cases. Am J Surg Pathol. 2000;24:1424–1428. doi: 10.1097/00000478-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Shimono C, Suwa K, Sato M, Shirai S, Yamada K, Nakamura Y, Makuuchi M. Large cell neuroendocrine carcinoma of the gallbladder: long survival achieved by multimodal treatment. Int J Clin Oncol. 2009;14:351–355. doi: 10.1007/s10147-008-0843-6. [DOI] [PubMed] [Google Scholar]
- 29.Barrón-Rodríguez LP, Manivel JC, Méndez-Sánchez N, Jessurun J. Carcinoid tumor of the common bile duct: evidence for its origin in metaplastic endocrine cells. Am J Gastroenterol. 1991;86:1073–1076. [PubMed] [Google Scholar]
- 30.Kuwabara H, Uda H. Small cell carcinoma of the gall-bladder with intestinal metaplastic epithelium. Pathol Int. 1998;48:303–306. doi: 10.1111/j.1440-1827.1998.tb03910.x. [DOI] [PubMed] [Google Scholar]
- 31.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 32.Albores-Saavedra J, Henson DE, Klimstra D. Endocrine and benign mesenchymal tumors of the extrahepatic bile ducts. In: Rosai J, Sobin LH, editors. Tumor of the Gallbladder, Extrahepatic Bile Ducts, and Ampulla of Vater. 3rd ed. Washington DC: Armed Forces Institute of Pathology; 2000. pp. 217–221. [Google Scholar]
- 33.Albores-Saavedra J, Batich K, Hossain S, Henson DE, Schwartz AM. Carcinoid tumors and small-cell carcinomas of the gallbladder and extrahepatic bile ducts: a comparative study based on 221 cases from the Surveillance, Epidemiology, and End Results Program. Ann Diagn Pathol. 2009;13:378–383. doi: 10.1016/j.anndiagpath.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Eltawil KM, Gustafsson BI, Kidd M, Modlin IM. Neuroendocrine tumors of the gallbladder: an evaluation and reassessment of management strategy. J Clin Gastroenterol. 2010;44:687–695. doi: 10.1097/MCG.0b013e3181d7a6d4. [DOI] [PubMed] [Google Scholar]
- 35.Iype S, Mirza TA, Propper DJ, Bhattacharya S, Feakins RM, Kocher HM. Neuroendocrine tumours of the gallbladder: three cases and a review of the literature. Postgrad Med J. 2009;85:213–218. doi: 10.1136/pgmj.2008.070649. [DOI] [PubMed] [Google Scholar]


