Abstract
Bacteria-mediated gene transfer (‘bactofection’) has emerged as an alternative approach for genetic vaccination and gene therapy. Here, we assessed bactofection of airway epithelial cells in vitro and in vivo using an attenuated Escherichia coli genetically engineered to invade non-phagocytic cells. Invasive E. coli expressing green fluorescent protein (GFP) under the control of a prokaryotic promoter was efficiently taken up into the cytoplasm of cystic fibrosis tracheal epithelial (CFTE29o-) cells and led to dose-related reporter gene expression. In vivo experiments showed that following nasal instillation the vast majority of GFP-positive bacteria pooled in the alveoli. Further, bactofection was assessed in vivo. Mice receiving 5 × 108 E. coli carrying pCIKLux, in which luciferase (lux) expression is under control of the eukaryotic cytomegalovirus (CMV) promoter, showed a significant increase (P<0.01) in lux activity in lung homogenates compared to untransfected mice. Surprisingly, similar level of lux activity was observed for the non-invasive control strain indicating that the eukaryotic CMV promoter might be active in E. coli. Insertion of prokaryotic transcription termination sequences into pCIKLux significantly reduced prokaryotic expression from the CMV promoter allowing bactofection to be detected in vitro and in vivo. However, bacteria-mediated gene transfer leads to a significantly lower lux expression than cationic lipid GL67-mediated gene transfer. In conclusion, although proof-of-principle for lung bactofection has been demonstrated, levels were low and further modification to the bacterial vector, vector administration and the plassmids will be required.
Keywords: bactofection, lung, gene transfer, Escherichia coli, CMV promoter
Introduction
Gene therapy is currently being evaluated for a wide range of acute and chronic lung diseases, including acute respiratory distress syndrome, cancer, asthma, emphysema and cystic fibrosis (CF).1 Since the cloning of the CF transmembrane conductance regulator (CFTR) gene, we and others have carried out more than 20 gene therapy trials, using a variety of viral and non-viral gene transfer agents (GTAs).2 Most have demonstrated proof-of-principle for lung gene transfer and, in some cases have shown partial correction of the chloride transport defect.3,4 However, efficient gene transfer into airway epithelial cells, the target for CF gene therapy, has proven difficult and further optimization of existing GTAs and the development of novel GTAs are underway.
Over the last decade, delivery of plasmid DNA into mammalian cells using virulence-attenuated bacteria has emerged as an alternative approach for genetic vaccination and gene therapy. The term ‘bactofection’ has been applied to describe bacteria-mediated gene transfer into mammalian cells. Several studies have recently shown that bactofection is feasible both in vitro and in vivo using a broad range of intracellular bacteria, such as Shigella flexneri,5,6Salmonella typhimurium,7,8Listeria monocyto-genes,9,10Salmonella typhi11 and recombinant invasive Escherichia coli.12,13 Genetically engineered E. coli expressing the inv gene (encoding invasin from Yersinia pseudotuberculosis) and the hly gene (encoding listerioly-sin O (LLO) from L. monocytogenes) bind to β1-integrin on non-phagocytic cells leading to cell invasion.13 Following internalization into vacuoles, the bacteria undergo lysis due to diaminopimelic acid (dap) auxotrophy and the released LLO allows the escape of the bacterial content and plasmids into the cytosol of the mammalian cell.
Most in vitro studies assessing bactofection have been performed on poorly differentiated, immortalized cell lines such as HeLa, or macrophages, which have no direct relevance for airway gene therapy. However, Fajac et al.14 recently assessed uptake and intracellular trafficking of this E. coli vector into human CF tracheal/ bronchial cells, 16HBE (human bronchial epithelial) cells and explant outgrowths of non-CF bronchial tissue. This study showed efficient uptake of invasive E. coli into cells at the periphery of the outgrowth and in all airway cell lines tested and reported low efficiency gene transfer of GFP (green fluorescent protein) under control of the eukaryotic cytomegalovirus (CMV) immediate-early promoter/enhancer. Attempts at bactofection in vivo have mainly been directed at genetic vaccination, targeting macrophages and dendritic cells;5,7,8,11,15 fewer studies have assessed bacteria-mediated gene transfer into non-phagocytic cells. Castagliuolo et al.12 demonstrated that the invasive E. coli vector can efficiently deliver therapeutic genes to the intact intestinal mucosa in mice. However, bactofection of the airway epithelium in vivo, the target for CF gene therapy, has not yet been assessed.
In this study, the invasive E. coli was used to assess bactofection of murine lungs. In contrast to previously used first-generation bacterial vectors, which carry a plasmid encoding the inv and hly genes, this second-generation strain, E. coli BM4570, carries chromosomal copies of the genes (C Grillot-Courvalin, in preparation). We assessed the distribution and uptake of invasive E. coli BM4570 expressing GFP under the control of the prokaryotic Plac promoter in the lungs of mice. In addition, bactofection was assessed in pulmonary tissue using E. coli carrying a eukaryotic expression plasmid encoding a luciferase (lux) reporter gene.
Results
E coli mediates gene expression in vitro
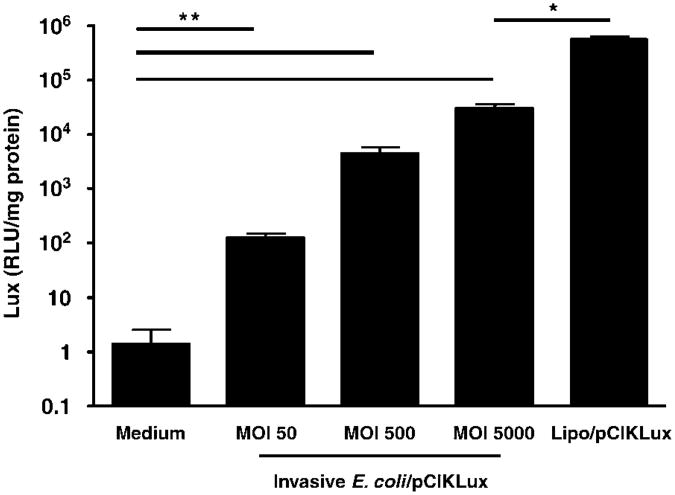
In order to assess E. coli-mediated lux gene transfer in vitro, CF tracheal epithelial (CFTE29o—) cells were incubated with invasive E. coli BM4570 carrying pCIK-Lux, a plasmid in which the lux gene is under control of the eukaryotic CMV promoter, for 2 h at multiplicity of infections (MOIs) ranging from 50 to 5000. After 48 h of infection, the cells were harvested and lux activity determined. As shown in Figure 1, all MOIs led to significant (P<0.01) dose-related lux activity compared to untreated cells. However, lux activity even at the highest MOI was significantly (P<0.05) lower than after Lipofectamine 2000-mediated (Invitrogen Ltd., Paisley, UK) gene transfer, despite the fact that a similar number of plasmid molecules was added to the cell preparation in each case.
Figure 1.
Luciferase (lux) expression after bactofection of cystic fibrosis tracheal epithelial (CFTE29o—) cells with invasive E. coli. CFTE29o— cells were transfected with invasive E. coli BM4570 carrying the eukaryotic expression plasmid pCIKLux at MOI 50-5000. After 48 h of infection cells were harvested and lux activity assayed. Bacteria-mediated expression was compared to cells transfected with pCIKLux complexed to Lipofectamine 2000 (Lipo/pCIKLux) or untransfected cells. Data are expressed as mean±s.e.m. (n = 5 per group, **P<0.01 when compared to untransfected, *P<0.05 when compared to Lipofectamine).
These results indicate that invasive E. coli was able to mediate lux expression, defined as lux protein generated after bacteria infection, in a human CFTE29o—. Similar results were obtained with 293T cells (data not shown).
Bacteria are mainly localized in the alveoli
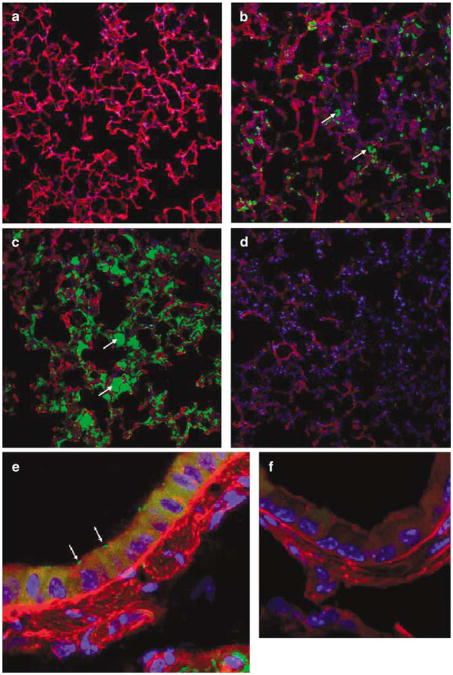
To visualize bacterial localization in vivo, the lungs of mice (n = 4) were inoculated via nasal ‘sniffing’ with 100 ml of invasive E. coli BM4570 carrying the pAT505 plasmid at 5 × 107 to 5 × 109 CFU (colony-forming unit) per mouse. The pAT505 plasmid contains the gfpmut1 gene encoding GFP under the control of the prokaryotic Plac promoter, resulting in GFP-expressing bacteria. The animals were killed 1 h post-infection and lung sections were examined for green fluorescent E. coli via confocal microscopy. A total of 16 mice were assessed (n = 4 per group), and at all doses studied the majority of bacteria were concentrated around the alveoli indicating bacterial pooling in this part of the lung (Figures 2a–d). For the conducting airway epithelium, the target for CF gene therapy, bacteria associated with the epithelium were quantified (n = 4 mice) and 16±3% of airway epithelial cells were associated with bacteria at the highest dose administered. However, for the vast majority of these cells only one bacterium (as judged by size and shape), was associated with each cell (Figures 2e and f).
Figure 2.
Green fluorescent protein (GFP)-expressing invasive E. coli in the mouse lung. Lungs of mice were infected with invasive E. coli BM4570 carrying the prokaryotic expression plasmid pAT505 (GFP expressed under the control of the prokaryotic Plac promoter) with doses ranging from 5 × 107 to 5 × 109 CFU (colony-forming unit) per mouse. The lungs were harvested 1 h post-infection. Invasive E. coli were associated with the alveoli in a dose-related manner ((a) 5 × 107 CFU per mouse, (b) 5 × 108 CFU per mouse, (c) 5 × 109 CFU per mouse, (d) PBS (phosphate buffered saline) control). Original magnification × 20. Bacteria associated with airway epithelial cells were also detected ((e) mouse transfected with 5 × 109 CFU, (f) PBS control). Original magnification × 63. GFP-expressing bacteria appear in green, alveoli in red and 4-6-diamidino-2-phenylindole (DAPI)-stained nuclei are shown in blue. Arrows indicate E. coli associated with alveoli and airway epithelial cells, respectively. Images are representative of 10 fields of views per section.
E. coli mediates lux expression in murine lung in vivo
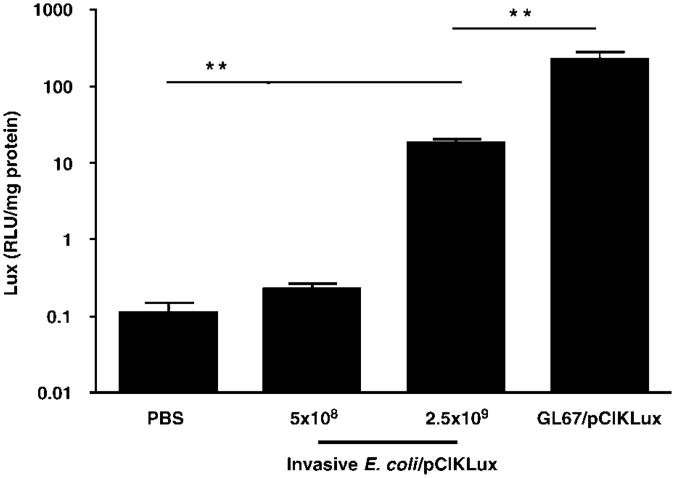
To assess whether plasmids could be transferred from the bacteria to the nucleus of lung cells in vivo, mice were inoculated intra-nasally with invasive E. coli BM4570 harbouring pCIKLux at doses ranging from 5 × 107 to 5 × 109 CFU per mouse. All mice (n = 8) receiving the highest dose died within 2 h and four out of eight mice treated with 2.5 × 109 E. coli died the following day. All remaining mice were culled 48 h post-infection and the lungs were harvested and assayed for lux activity. Figure 3 shows that lux expression was undetectable in mice that had received 5 × 108 CFU, but was significantly (P<0.01) increased in mice inoculated with 2.5 × 109, suggesting that E. coli-mediated gene transfer may have occurred in the murine lung. However, when compared to an already established non-viral GTA, cationic lipid GL67, bacteria-mediated gene transfer was 10-fold less efficient (P<0.01) in this model.
Figure 3.
Luciferase (lux) expression in the murine lung after transfection with invasive E. coli. The lungs of mice were infected with invasive E. coli BM4570 carrying the eukaryotic expression plasmid pCIKLux (5 × 108 and 2.5 × 109 CFU (colony-forming unit) per mouse). Lungs were harvested 48 h post-infection and lux activity assayed. Bacteria-mediated lux expression was compared to mice transfected with pCIKLux complexed to the cationic lipid GL67 (GL67/pCIKLux) or PBS (phosphate buffered saline) controls. Data are expressed as mean±s.e.m. (n = 4–8, **P<0.01, when compared to PBS and GL67/pCIKLux).
Invasive, but not non-invasive, E. coli enters CFTE29o– cells efficiently
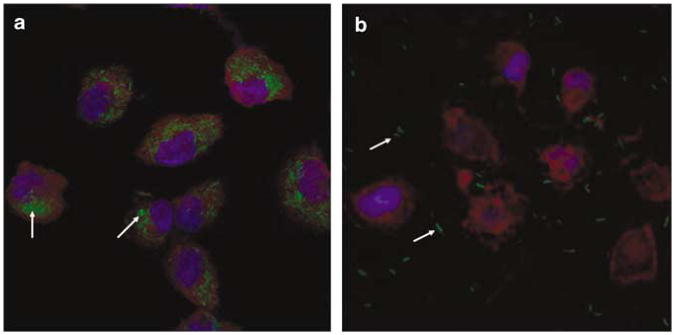
To determine more accurately, if lux expression in the mouse lung is due to bactofection, we compared the efficiency of invasive and non-invasive bacteria in vivo. In preparation for these experiments, we repeated in vitro experiments previously published by Fajac et al.14 to ensure that the invasive and non-invasive bacteria behaved appropriately in our hands. We first compared the uptake of invasive E. coli BM4570 and its non-invasive counterpart BM2710, each carrying the prokaryotic expression plasmid pAT505 (resulting in GFP-expressing green fluorescent bacteria) in CFTE29o— cells (MOI 50-5000) in vitro. Cells were harvested 2 h post-infection, fixed and slides examined for GFP-expressing E. coli via confocal microscopy. In contrast to the invasive strain, the non-invasive E. coli strain lacks the inv gene from Y. pseudotuberculosis and hence does not express invasin, responsible for bacterial uptake into non-phagocytic cells. This strain would, therefore, not be expected to enter CFTE29o— cells. Figure 4a shows that the vast majority of invasive E. coli BM4570 was taken up into the cytoplasm of CFTE29o— cells at MOI 500, whereas non-invasive E. coli BM2710 did not enter the cells (a few residual bacteria remained attached to slides after fixing) (Figure 4b). These results support previous published data showing that invasin is required for the uptake of E. coli into non-phagocytic epithelial cells.13,14
Figure 4.
Uptake of green fluorescent protein (GFP)-expressing invasive and non-invasive E. coli into cystic fibrosis tracheal epithelial (CFTE29o—) cells. CFTE29o— cells were infected with (a) invasive E. coli BM4570 (MOI 500) and (b) its non-invasive counterpart E. coli BM2710 (MOI 500) carrying the prokaryotic expression plasmid pAT505 (GFP expressed under the control of the prokaryotic Plac promoter). The cells were harvested 2 h post-infection. GFP-expressing bacteria appear in green, the cytosol and 4-6-diamidino-2-phenylindole (DAPI)-stained nuclei are shown red and blue, respectively. Original magnification × 63. Arrows indicate GFP-expressing E. coli.
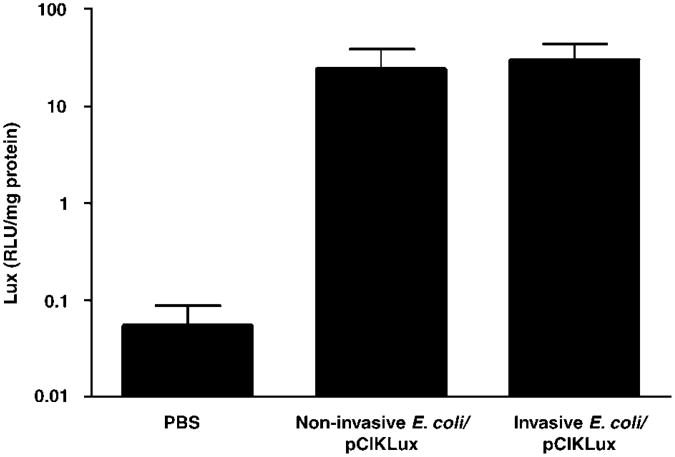
Invasive and non-invasive E. coli lead to similar levels of lux activity in vivo
Mice were infected with invasive E. coli BM4570 and non-invasive E. coli BM2710 carrying pCIKLux (2.5 × 109 CFU per mouse). Mice were culled 48 h post-infection and lux activity was determined in lung homogenates. Similar levels of lux expression were observed when comparing the invasive E. coli to its non-invasive counterpart (Figure 5), suggesting that the eukaryotic CMV promoter may be active in E. coli and lux expression observed in vivo may therefore, not be due to plasmid transfer from the bacteria to the nuclei of the lung, but rather via prokaryotic read through.
Figure 5.
Luciferase (lux) expression in murine lung after transfection with invasive and non-invasive E. coli. Mice were infected with invasive E. coli BM4570 and its non-invasive counterpart E. coli BM2710 carrying pCIKLux (2.5 × 109 CFU (colony-forming unit) per mouse). Lungs were harvested 48 h post-infection and lux activity assayed. Data are expressed as mean ± s.e.m. (n = 8–9).
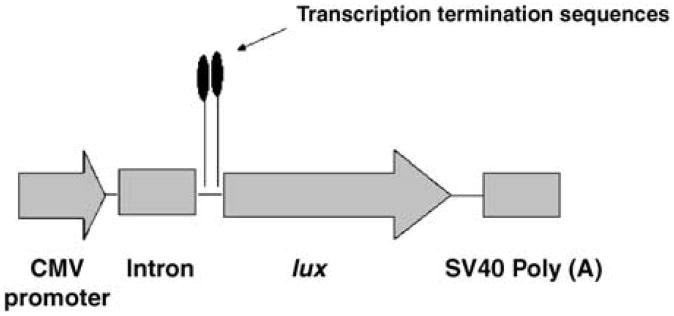
Incorporation of prokaryotic termination sequences into pCIKLux reduces prokaryotic reporter gene expression
In an attempt to abolish lux expression potentially generated by E. coli via prokaryotic read through, a new plasmid (pCIKLux-mod) was created by inserting two copies of the transcription terminator sequence of gene 32 from bacteriophage T4 downstream of the hybrid intron (Figure 6), a procedure that was previously successful for E. coli and Salmonella.16 To assess if prokaryotic lux expression was reduced after generation of the modified plasmid (pCIKLux-mod), invasive E. coli BM4570 carrying the eukaryotic lux expression plasmids pCIKLux-mod or pCIKLux (3 × 106 and 3 × 108 CFU) were lysed and the lysates assayed for lux activity. Insertion of the transcription terminator sequences lead to an ∼20-fold reduction in lux activity (pCIKLux: 201.39 RLU (relative light unit) per 3 × 106CFU and pCIKLux-mod: 9.55 RLU per 3 × 106 CFU, pCIKLux: 25323.85 RLU per 3 × 108 CFU and pCIKLux-mod: 1011.98 RLU per 3 × 108 CFU). However, lux activity from invasive E. coli/ pCIKLux-mod was still considerably higher than background levels (control plasmid; 0.0005 RLU per 3 × 109 CFU) indicating that the CMV promoter still has some residual activity in E. coli.
Figure 6.
Modification of pCIKLux to abolish CMV-driven lucifer-ase (lux) expression in E. coli. A new plasmid (pCIKLux-mod) was created by inserting two copies of the prokaryotic transcription terminator of gene 32 from bacteriophage T4 downstream from the hybrid intron of pCIKLux.
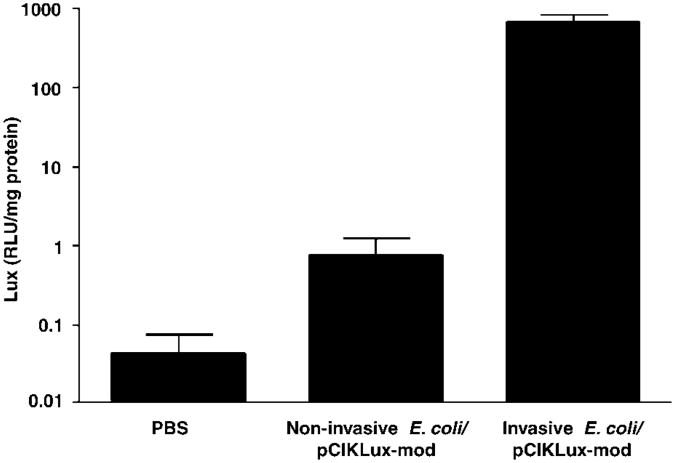
Low-level bactofection occurs in vitro and in vivo following transfection with E. coli carrying modified pCIKLux
We next assessed bactofection in vitro and in vivo using invasive E. coli carrying the plasmid pCIKLux-mod. CFTE29o— cells were transfected with invasive E. coli BM4570 or its non-invasive counterpart E. coli BM2710 carrying the pCIKLux-mod at MOI 50. The cells were harvested 48 h post-infection, lysed and assayed for lux activity. Cells treated with the invasive E. coli carrying pCIKLux-mod showed high levels of lux activity (Figure 7). As part of these experiments, we also assessed survival of intracellular bacteria 48 h after infection in vitro and showed that only a very small number of bacteria survived intracellularly (∼0.0001% of the initial dose, n=4; data not shown). At an MOI of 50, ∼15 bacteria per well would, therefore, have survived. The previous experiment showed that 3 × 106 bacteria generated ∼10 RLU lux activity when bacteria were lysed. It is, therefore, unlikely that this low level of prokaryotic lux expression derived from the small number of bacteria, surviving 48 h after bactofection, generated the high levels of lux activity observed in this experiment (∼8.4 RLU per 15 CFU). The data, therefore, suggest that bacteria-mediated gene transfer into the nuclei of CFTE29o— cells has occurred. Experiments using the non-invasive E. coli carrying the unmodified plasmid were also carried out. There was no significant difference in lux expression when comparing the non-invasive E. coli carrying the unmodified plasmid to those with the modified plasmid.
Figure 7.
Luciferase (lux) expression after bactofection of cystic fibrosis tracheal epithelial (CFTE29o—) cells with invasive and noninvasive E. coli carrying the modified pCIKLux (pCIKLux-mod). CFTE29o— cells were infected at MOI 50 with invasive E. coli BM4570 and non-invasive E. coli BM2710 carrying the pCIKLux-mod (pCIKLux-mod). After 48 h of infection cells were harvested and lux activity assayed. Data are expressed as mean ± s.e.m. (n = 6).
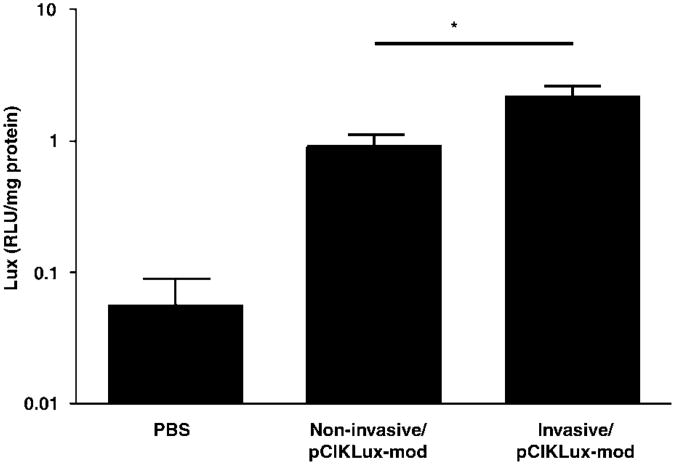
For the in vivo corollary of these studies, mice were infected with invasive E. coli BM4570 and non-invasive E. coli BM2710 carrying the pCIKLux-mod (2.5 × 109 CFU per mouse). Mice were culled 48 h post-infection and lux activity was determined in lung homogenates. A small but significant (P<0.05) increase in lux expression was observed in mice treated with invasive E. coli when compared to mice treated with non-invasive E. coli (Figure 8), suggesting that low-level bactofection had occurred in vivo.
Figure 8.
Luciferase (lux) expression in the murine lung after transfection with invasive and non-invasive E. coli carrying the modified pCIKLux (pCIKLux-mod). The lungs of mice were infected with invasive E. coli BM4570 and non-invasive E. coli BM2710 carrying the pCIKLux-mod (2.5 × 109 CFU (colony-forming unit) per mouse). After 48 h of infection mice were killed and lux activity was determined in lung homogenates. Bacteria-mediated lux expression from invasive E. coli was compared to non-invasive E. coli. Data are expressed as mean ± s.e.m. (n = 8–9, *P<0.05).
Discussion
Here, we have assessed the distribution and uptake of invasive E. coli BM4570 expressing GFP under the control of the prokaryotic Plac promoter in the murine lung. In addition, bactofection was assessed in pulmonary tissues using E. coli carrying a eukaryotic expression plasmid encoding a lux reporter gene. Bacteria were mainly located in the alveolar epithelium and only rarely associated with respiratory epithelial cells. Bacteria-mediated lux expression was detected in the murine lung, but was less efficient than GL67-mediated gene transfer.
To date, attempts at using bacteria as vectors have mainly concentrated on delivering DNA for the purpose of genetic vaccination. More recently, the use of bacteria as gene transfer vectors for gene therapy applications has been suggested. The ability to spread from cell to cell, in addition to the absence of insert size restrictions make bacteria an interesting vector for gene therapy. Finally, repeated administration, a major limitation for viral vectors, may be feasible as some bacteria can colonize or recurrently infect humans. Proof-of-principle for bactofection in a range of non-phagocytic cell lines, including airway epithelial cells, has been established in vitro.13,14,17–19 These studies have mainly focused on transfer of the GFP reporter gene. However, more relevant to our work, recombinant E. coli and L. monocytogenes were recently used to transfer artificial chromosomes carrying the CFTR gene locus20 or a pCMV-CFTR plasmid,10,21 respectively, to cell lines in vitro.
We first assessed E. coli-mediated bactofection of our lux reporter gene in CFTE29o— cells in vitro to ensure that all bacterial proteins required for bactofection are efficiently produced and that the eukaryotic expression plasmid pCIKLux is fully functional. CFTE29o— cells were used because they resemble the target cell for CF gene therapy and are known to express β1-integrin, which is required for efficient bacterial uptake into non-phagocytic cells.14 Although cell survival decreased with increasing MOI, even at an MOI of 5000, a small proportion of the cells survived to allow lux detection 48 h after bactofection. In general cell lines do not tolerate MOIs above 500 very well (C Grillot-Courvalin, personal communication). In contrast to previously published in vitro studies we included FBS (fetal bovine serum) during bactofection, which may to a degree have protected the cells from bacteria induced toxicity.
As expected, we detected a dose-related increase in lux expression. This is likely to be a combination of prokaryotic and eukaryotic gene expression as the subsequent in vivo experiment revealed that the CMV promoter in combination with the lux gene was leaky in E. coli. However, only a very small number of bacteria survive inside 293T cells (∼0.0001% of the initial dose) after 48 h (data not shown), suggesting that a significant proportion of lux measured at this time point must have been generated by plasmid DNA reaching the nuclei of the CFTE29o— cells. As proof-of-principle that bactofection can occur in CFTE29o— cells, Fajac et al.14 recently demonstrated bacteria mediated gene transfer in this cell type using E. coli carrying a plasmid in which GFP expression was under the control of the eukaryotic CMV promoter as pCMV-EGFP is not leaky in bacteria. As this is the first study using a lux plasmid for bactofection, comparisons of absolute lux expression with other studies were not possible. However, compared to standard Lipofectamine 2000-mediated transfection, bactofection was less efficient in this cell line.
As noted above invasive E. coli requires β1-integrin expression on the cell surface and Blundell et al.22 have shown that β1-integrin is expressed in murine airways. Although we saw a dose-related deposition of bacteria within the alveolar region, there was little evidence for bacterial contact with conducting airways, and the limited number of bacteria associated with airway epithelial cells did not appear to be inside the cell. The ‘nasal sniffing’ method has been widely used for transfecting murine lungs,23,24 being rapid, non-invasive and well tolerated. However, using this technique contact between the bacteria and the airway epithelium is likely short, and may have been insufficient for binding of the bacteria to β1-integrin. Nasal sniffing may lead to pooling of liquid in the alveolar region, possibly ensuring longer contact time between the bacteria and the cells and, therefore, leading to increased deposition of bacteria in the alveolar region. Alternative methods such as nebulization may be more appropriate in targeting the airways, but are currently comparatively inefficient in mouse models. In vivo infection with invasive E. coli generated dose-related lux expression in the lung. The toxicity of E. coli BM4570 at high doses (>2.5×109 CFU per mouse), likely related to septic shock, restricted the use of higher titres and highlighted a narrow toxicity/efficacy window.
Total protein levels were generally higher in bacteria and lipid-treated mice when compared to phosphate buffered saline (PBS)-treated animals, which in part is likely to be a reflection of inflammatory cells entering the lung. In addition bacterial proteins may contribute to total lung protein. However, a fivefold increase in bacterial load did in our experiment not affect total protein content. Importantly, normalization of lux expression for total lung protein may have slightly underestimated gene expression in bacteria-treated mice.
To determine more accurately, if lux expression observed in the mouse lung was due to bactofection, we compared expression levels following infection with invasive and non-invasive E. coli in vivo. In contrast to the invasive strain, the non-invasive bacteria lack both the inv and hly genes and do, therefore, not enter non-phagocytic cells. Surprisingly, similar levels of lux expression were detected indicating that lux expression might not be due to bactofection, but more likely was due to CMV promoter-mediated expression of lux in the bacteria. Proof-of-principle for low-level CMV promoter-driven reporter gene expression in Gram-negative bacteria such as E. coli and S. typhimurium has previously been reported for β-galactosidase and GFP reporter genes, and techniques to reduce the inappropriate expression have been described. Goussard et al.16 inserted a prokaryotic termination sequence from bacteriophage T4 downstream of the CMV promoter, which successfully abolished both β-galactosidase and GFP expression in those bacterial species. Using this technique, the transcription terminator sequences from T4 inserted into pCIKLux significantly reduced lux expression from the bacteria, but did not completely abolish expression. Prokaryotic recognition of the CMV promoter and the phage termination sequences may be context dependent and may in part depend on the sequence of the reporter gene. These results may have implications for other studies, and the degree of bacteria-derived gene expression in the context of bactofection studies has to be carefully monitored and controlled. The study therefore highlights that bacteria can function as useful vectors for the application of ‘alternative’ gene therapy that is the in situ production of therapeutic proteins by the genetically modified bacteria.
Despite the incomplete inhibition of prokaryotic lux expression, we assessed lux expression in vitro after infection with the invasive and non-invasive bacteria carrying the modified plasmid. High levels of lux activity was observed with the invasive strain suggesting that bacteria-mediated gene transfer into the nuclei of CFTE29o— cells had occurred, as we and others have shown13 that only a very small number of bacteria survive intracellular after 48 h, which are unlikely to be responsible for the high transfection level seen.
We then compared the non-invasive and invasive E. coli carrying the pCIKLux-mod in vivo and detected a modest, but significant increase in lux expression in mice treated with the invasive strain. This may indicate that low-level bactofection had occurred in vivo, which was masked by high levels of prokaryotic lux expression in experiments using the unmodified pCIKLux plasmid. However, overall the efficiency of E. coli-mediated bactofection in the mouse lung was low and gene expression was significantly lower than after transfection with our gold-standard non-viral GTA lipid GL67.
In summary, we have demonstrated successful bactofection in vitro using lux as a reporter gene. In vivo data indicated that E. coli-mediated lung bactofection, at least in our model, does not currently lead to easily detectable eukaryotic gene expression, and is less efficient than the well-established liposome-mediated gene transfer. In addition, this study has highlighted a narrow toxicity/efficacy window, which restricted the dose range, which could be used. In future studies, insertion of genes encoding for additional cell adhesion molecules suitable for epithelial cell uptake into E. coli might be useful. Alternatively obligate intracellular pulmonary bacteria such as Legionella, Mycoplasma pneumoniae, Chlamydia pneumoniae or Coxiella burnetti could be considered as vectors for lung bactofection, as these bacteria may have developed more efficient mechanisms for uptake into lung epithelial cells.
Materials and methods
Bacterial strains and plasmids
Non-invasive E. coli BM2710 and invasive E. coli BM4570 were generated as previously described25 (and C Grillot-Courvalin, in preparation,). Invasive E. coli BM4570 and non-invasive E. coli BM2710 were transformed with a pCIKLux plasmid carrying a lux reporter gene or a pCIKLux-mod, in which two copies of the transcription terminator sequence of gene 32 from bacteriophage T4 were inserted downstream of the hybrid intron. To obtain pCIKLux-mod, a NheI linker containing the T4 terminator was created by hybridization of two complementary linkers, T4-F (5′-CTAGCGGTACCATTATAT TACTAATTAATTGGGGACCCTAGAGGTCCCCTTTTTT ATTTTAAAAAG-3′) and T4-R (5′-CTAGCTTTTTAAAA TAAAAAAGGGGACCTCTAGGGTCCCCAATTAATTAG TAATATAATGGTACCG-3′) and the fragment obtained was cloned into the NheI site of the pCIKLux. Lux expression in pCIKLux and pCIKLux-mod is controlled by the eukaryotic CMV immediate-early promoter/ enhancer. Bacteria expressing GFP were obtained by transforming E. coli BM4570 and E. coli BM2710 with plasmid pAT505 in which the gfpmut1 gene is under the control of the prokaryotic Plac promoter.13
Bacterial growth
About 5 ml of brain heart infusion (BHI) medium containing 0.5 mM diaminopimelic acid (dap) and 50 mg ml−1 ampicillin were inoculated with a single bacteria colony and grown for 8 h at 37 °C, while shaking. The starter culture was used to inoculate 250 ml of BHI containing dap and ampicillin. Overnight cultures were harvested by centrifugation (4000 r.p.m.) in the middle logarithmic phase of growth (OD600 = 1-2) and re-suspended at a final concentration of 5 × 109 to 5 × 1010 CFU per ml in minimum essential medium (MEM) containing 0.5 mM dap and 10% FBS or PBS containing 0.5 mM dap for in vitro and in vivo experiments, respectively. All chemicals were from Sigma-Aldrich Com. Ltd., Poole, UK unless otherwise stated.
In vitro bactofection
A human CF tracheal epithelial cell line (CFTE29o—) was cultured in MEM supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin. Cells were maintained at 37 °C in a 5% CO2 atmosphere. CFTE29o— cells were seeded 24 h prior to bacterial infection in 6-well plates or 8-well chamber slides at a density of 3 × 105 and 8 × 103 cells per well, respectively.
Bacterial uptake into CFTE29o cells
CFTE29o-cells seeded on chamber slides were first washed with serum-free MEM and then incubated with invasive E. coli BM4570 and non-invasive E. coli BM2710 carrying plasmid pAT505 (GFP expression under control of the prokaryotic Plac promoter) for 2 h at 37 °C at a MOI of bacteria ranging from 50 to 5000 in 100 ml MEM containing 0.5 mM dap and 10% FBS (n = 8 wells per dose). After infection, the cells were washed and fixed in 4% paraformaldehylde (PFA) for 30 min at room temperature and then stained with 0.1% Evans blue prior to mounting in Vectashield containing 4-6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Peterborough, UK). Uptake of GFP-expressing fluorescent bacteria into the cell cytosol was examined using a confocal microscope (LEICATCS SP) equipped with a × 20-63/1.32-0.6 numerical aperture (NA) oil immersion objective.
Reporter gene expression in CFTE29o— cells
To assess bactofection efficiency, CFTE29o— cells were first washed with serum-free MEM. E. coli BM4570 and E. coli BM2710 carrying pCIKLux or pCIKLux-mod (as described above) were then added at MOIs ranging from 50 to 5000 in 2 ml MEM containing 0.5 mM dap and 10% FBS (n = 5 wells per dose). As a positive control, CFTE29o— cells were transfected with pCIKLux com-plexed to Lipofectamine 2000 according to the manufacturer's recommendations. Briefly, 4 μg pCIKLux and 10 μg Lipofectamine 2000 were each diluted in 250 μl Opti-MEM (Invitrogen), gently mixed and incubated for 20 min at room temperature. About 0.5 ml Lipofectamine 2000-pCIKLux complex in a total volume of 2 ml Opti-MEM was then added and cells were incubated with liposome complexes or bacterial suspension for 5 and 2 h at 37 °C, respectively. The medium was then removed and the cells were washed three times in MEM prior to further incubation in complete medium containing gentamicin (20 μg ml−1) to kill remaining extra-cellular bacteria. After 48 h of transfection, cells were trypsi-nized, centrifuged twice for 10 min at 11 000 r.p.m. and the supernatants were discarded. The pellets were assayed for lux activity as described below.
In vivo bactofection
Bacterial localization in murine airways
Invasive E. coli BM4570 (5 × 107 to 5 × 109 CFU per mouse) harbouring pAT505, in which GFP expression is under control of the prokaryotic Plac promoter, was delivered to the lungs of gut-corrected CF-knockout mice26 (∼12 weeks old females, n = 4 per group, bred in house) in a total volume of 100 μl. All animal procedures adhered to the UK Home Office Animals (Scientific Procedures) Act 1986. Briefly, mice were anaesthetised with metophane (Medical Development International Ltd., Springvale, Australia) and a single bolus (100 ml) was applied to the nose and rapidly ‘sniffed’ into the lung. After 1 hr of infection mice were killed. The trachea was exposed and lungs were inflated with 4% PFA. The inflated lungs were excised, submerged overnight in 4% PFA and then cryopreserved in 15% sucrose at 4 °C for a period of 12 h before embedding the left lobe in OCT compound (Bayer PLC, Newbury, UK). The embedded lobe was trimmed to the point where the airways were fully branched and 7 μm sections were cut, mounted on to glass slides and stained with 0.1% Evans blue prior to mounting in Vectashield containing DAPI. Localization of GFP-expressing fluorescent bacteria was examined using a confocal microscope (LEICA TCS SP) equipped with a × 20–63/1.32–0.6 NA oil immersion objective. A total of ten fields of views per section were analysed for each mouse.
Reporter gene expression in murine airways
CF knockout mice were transfected with 5 × 107 to 5 × 109E. coli BM4570 carrying pCIKLux (∼12 weeks old female/male mice, n = 6–8 per group) or with invasive E. coli BM4570 or non-invasive E. coli BM2710 (5 × 108 CFU per mouse, n = 8–12 per group) carrying the original pCIKLux or the modified plasmid pCIKLux-mod. Control groups received PBS or GL67/pCIKLux complexes (see below). After 48 h of infection, all mice were culled. Lungs were harvested, snap-frozen in liquid nitrogen and stored at —80 °C for detection of lux activity.
Cationic lipid GL67 (Genzyme Corporation, Framing-ham, MA, USA) was re-suspended in water for injection (Arnolds Veterinary Products, Shrewsbury, UK) to a final concentration of 1.2 mM and incubated on ice for 10 min. Equal volumes of lipid GL67 and pCIKLux (4.8 mM, 1.6mgml−1) were mixed at a molar ratio of 1:4 (both equilibrated to 30 °C for 5 min) and complexes were incubated at 30 °C for a further 15 min before use.
Reporter gene expression in bacteria
To assess prokaryotic lux expression from the eukaryotic CMV promoter, E. coli BM4570 carrying the eukaryotic lux expression plasmids pCIKLux-mod and pCIKLux (1.5 × 107 and 1.5 × 109 CFU corresponding to MOI 50 and 5000, respectively) were lysed according to the manufacturer's recommendation (Luciferase Assay Kit; Promega, Southampton, UK) and the lysates assayed for lux activity. Briefly, 300 μl bacteria were centrifuged for 10 min at 6200 r.p.m., re-suspended in 2.5 μl 1M K2HPO4, 20 mM EDTA and 22.5 μl PBS and incubated for 10 min at room temperature in 75 μl freshly prepared lysis mix (1.25 mg ml−1 lysozyme, 2.5 mg ml−1 bovine serum albumin, 1 × cell culture lysis reagent). The lysate was centrifuged for 10 min at 13000 r.p.m. and 20 ml of the supernatant was assayed for lux activity according to the manufacturer's recommendations. E. coli BM4570/ pAT505 expressing GFP under the control of a prokaryotic promoter was included as a negative control.
Luciferase assay
Cells and lung tissue were homogenized in appropriate volumes of lysis buffer (1 × RL buffer; Roche, UK). Following three freeze/thaw cycles, the lysates were spun for 10 min at 13000 c.p.m. and the supernatant assayed for lux activity according to the manufacturer's recommendations (Promega). In addition, total protein concentration (Bio-Rad DC-Protein Assay Kit; Bio-Rad, Hemel Hempstead, UK) was photometrically measured in all supernatants. Reporter gene expression was expressed as lux activity (RLU) per mg total protein.
Statistical analysis
Results are expressed as mean ± s.e.m. Data were compared using analysis of variance plus Dunnett post hoc analysis or independent sample t-test where appropriate. Where necessary, raw data were log10 transformed to ensure normal distribution and equal variances between groups. A non-parametric Mann–Whitney test or a Kruskal–Wallis with Dunn's post hoc correction was carried out when the assumptions were not met after transformations. The null hypothesis was rejected at P<0.05.
Acknowledgments
We thank Lucinda Somerton (Department of Gene Therapy, Faculty of Medicine, Imperial College, London, UK) and Ed Inett (Royal Brompton Hospital, London, UK) for their help with this project. The study was funded by the Cystic Fibrosis Trust and the CF Trust Dr Benjamin Angel Fellowship (UG).
References
- 1.Davies JC, Alton EW. Airway gene therapy. Adv Genet. 2005;54:291–314. doi: 10.1016/S0065-2660(05)54012-4. [DOI] [PubMed] [Google Scholar]
- 2.Griesenbach U, Geddes DM, Alton EW. Gene therapy progress and prospects: cystic fibrosis. Gene Therapy. 2006;13:1061–1067. doi: 10.1038/sj.gt.3302809. [DOI] [PubMed] [Google Scholar]
- 3.Alton EW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J, et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet. 1999;353:947–954. doi: 10.1016/s0140-6736(98)06532-5. [DOI] [PubMed] [Google Scholar]
- 4.Zabner J, Couture LA, Gregory RJ, Graham SM, Smith AE, Welsh MJ. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]
- 5.Sizemore DR, Branstrom AA, Sadoff JC. Attenuated bacteria as a DNA delivery vehicle for DNA-mediated immunization. Vaccine. 1997;15:804–807. doi: 10.1016/s0264-410x(96)00252-6. [DOI] [PubMed] [Google Scholar]
- 6.Vecino WH, Quanquin NM, Martinez-Sobrido L, Fernandez-Sesma A, Garcia-Sastre A, Jacobs WR, Jr, et al. Mucosal immunization with attenuated Shigella flexneri harboring an influenza hemagglutinin DNA vaccine protects mice against a lethal influenza challenge. Virology. 2004;325:192–199. doi: 10.1016/j.virol.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 7.Bauer H, Darji A, Chakraborty T, Weiss S. Salmonella-mediated oral DNA vaccination using stabilized eukaryotic expression plasmids. Gene Therapy. 2005;12:364–372. doi: 10.1038/sj.gt.3302423. [DOI] [PubMed] [Google Scholar]
- 8.Darji A, Zur LS, Garbe AI, Chakraborty T, Weiss S. Oral delivery of DNA vaccines using attenuated Salmonella typhimurium as carrier. FEMS Immunol Med Microbiol. 2000;27:341–349. doi: 10.1111/j.1574-695X.2000.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich G, Bubert A, Gentschev I, Sokolovic Z, Simm A, Catic A, et al. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat Biotechnol. 1998;16:181–185. doi: 10.1038/nbt0298-181. [DOI] [PubMed] [Google Scholar]
- 10.Krusch S, Domann E, Frings M, Zelmer A, Diener M, Chakraborty T, et al. Listeria monocytogenes mediated CFTR transgene transfer to mammalian cells. J Gene Med. 2002;4:655–667. doi: 10.1002/jgm.313. [DOI] [PubMed] [Google Scholar]
- 11.Pasetti MF, Anderson RJ, Noriega FR, Levine MM, Sztein MB. Attenuated deltaguaBA Salmonella typhi vaccine strain CVD 915 as a live vector utilizing prokaryotic or eukaryotic expression systems to deliver foreign antigens and elicit immune responses. Clin Immunol. 1999;92:76–89. doi: 10.1006/clim.1999.4733. [DOI] [PubMed] [Google Scholar]
- 12.Castagliuolo I, Beggiao E, Brun P, Barzon L, Goussard S, Manganelli R, et al. Engineered E. coli delivers therapeutic genes to the colonic mucosa. Gene Therapy. 2005;12:1070–1078. doi: 10.1038/sj.gt.3302493. [DOI] [PubMed] [Google Scholar]
- 13.Grillot-Courvalin C, Goussard S, Huetz F, Ojcius DM, Courvalin P. Functional gene transfer from intracellular bacteria to mammalian cells. Nat Biotechnol. 1998;16:862–866. doi: 10.1038/nbt0998-862. [DOI] [PubMed] [Google Scholar]
- 14.Fajac I, Grosse S, Collombet JM, Thevenot G, Goussard S, Danel C, et al. Recombinant Escherichia coli as a gene delivery vector into airway epithelial cells. J Control Release. 2004;97:371–381. doi: 10.1016/j.jconrel.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Shiau AL, Chu CY, Su WC, Wu CL. Vaccination with the glycoprotein D gene of pseudorabies virus delivered by nonpathogenic Escherichia coli elicits protective immune responses. Vaccine. 2001;19:3277–3284. [Google Scholar]
- 16.Goussard S, Grillot-Courvalin C, Courvalin P. Eukaryotic promoters can direct protein synthesis in Gram-negative bacteria. J Mol Microbiol Biotechnol. 2003;6:211–218. doi: 10.1159/000077252. [DOI] [PubMed] [Google Scholar]
- 17.Critchley RJ, Jezzard S, Radford KJ, Goussard S, Lemoine NR, Grillot-Courvalin C, et al. Potential therapeutic applications of recombinant, invasive E.coli. Gene Therapy. 2004;11:1224–1233. doi: 10.1038/sj.gt.3302281. [DOI] [PubMed] [Google Scholar]
- 18.Hense M, Domann E, Krusch S, Wachholz P, Dittmar KE, Rohde M, et al. Eukaryotic expression plasmid transfer from the intracellular bacterium Listeria monocytogenes to host cells. Cell Microbiol. 2001;3:599–609. doi: 10.1046/j.1462-5822.2001.00138.x. [DOI] [PubMed] [Google Scholar]
- 19.Pilgrim S, Stritzker J, Schoen C, Kolb-Maurer A, Geginat G, Loessner MJ, et al. Bactofection of mammalian cells by Listeria monocytogenes:improvement and mechanism of DNA delivery. Gene Therapy. 20010:2036–2045. doi: 10.1038/sj.gt.3302105. [DOI] [PubMed] [Google Scholar]
- 20.Laner A, Goussard S, Ramalho AS, Schwarz T, Amaral MD, Courvalin P, et al. Bacterial transfer of large functional genomic DNA into human cells. Gene Therapy. 2005;12:1559–1572. doi: 10.1038/sj.gt.3302576. [DOI] [PubMed] [Google Scholar]
- 21.Zelmer A, Krusch S, Koschinski A, Rohde M, Repp H, Chakraborty T, et al. Functional transfer of eukaryotic expression plasmids to mammalian cells by Listeria monocytogenes: a mechanistic approach. J Gene Med. 2005;7:1097–1112. doi: 10.1002/jgm.764. [DOI] [PubMed] [Google Scholar]
- 22.Blundell RA, Harrison DJ. Integrin characterization in pulmonary bronchioles. Exp Mol Pathol. 2005;79:74–78. doi: 10.1016/j.yexmp.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Griesenbach U, Boyton RJ, Somerton L, Garcia SE, Ferrari S, Owaki T, et al. Effect of tolerance induction to immunodominant T-cell epitopes of Sendai virus on gene expression following repeat administration to lung. Gene Therapy. 2006;13:449–456. doi: 10.1038/sj.gt.3302677. [DOI] [PubMed] [Google Scholar]
- 24.Griesenbach U, Kitson C, Escudero GS, Farley R, Singh C, Somerton L, et al. Inefficient cationic lipid-mediated siRNA and antisense oligonucleotide transfer to airway epithelial cells in vivo. Respir Res. 2006;7:26. doi: 10.1186/1465-9921-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courvalin P, Goussard S, Grillot-Courvalin C. Gene transfer from bacteria to mammalian cells. C R Acad Sci III. 1995;318:1207–1212. [PubMed] [Google Scholar]
- 26.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]