Summary
In the developing neocortex, progenitor cells expand through symmetric division before they generate cortical neurons through multiple rounds of asymmetric cell division. Here, we show that the orientation of the mitotic spindle plays a crucial role in regulating the transition between those two division modes. We demonstrate that the protein phosphatase PP4c regulates spindle orientation in early cortical progenitor cells. Upon removing PP4c, mitotic spindles fail to orient in parallel to the neuroepithelial surface and progenitors divide with random orientation. As a result, their divisions become asymmetric and neurogenesis starts prematurely. Biochemical and genetic experiments show that PP4c acts by dephosphorylating the microtubule binding protein Ndel1, thereby enabling complex formation with Lis1 to form a functional spindle orientation complex. Our results identify a key regulator of cortical development and demonstrate that changes in the orientation of progenitor division are responsible for the transition between symmetric and asymmetric cell division.
Highlights
-
•
PP4c is required for spindle orientation in cortical progenitors
-
•
Loss of PP4c leads to premature neuronal differentiation
-
•
Spindle misorientation causes layering defects during a critical time window
-
•
PP4c acts on Ndel1 and Lis1
Xie et al. address the importance of spindle orientation during cortical development. They show that horizontal spindle orientation is required during a distinct time window at the onset of neurogenesis to ensure symmetric division and maintain the progenitor pool.
Introduction
In developing mammalian brains, huge numbers of neurons are generated from a relatively small number of neural progenitor cells. For this, neural progenitors expand by symmetric division before switching to an asymmetric division mode to generate neurons (Götz and Huttner, 2005). In the developing mouse brain, neuroepithelial progenitors (NPs) span from the ventricular to the pial surface of the neural tube before the onset of neurogenesis. At around embryonic day 10.5 (E10.5), neurogenesis begins with the transformation of NPs into radial glial progenitors (RGPs), which express astroglial hallmarks such as brain lipid binding protein (BLBP), the astrocyte-specific glutamate transporter (GLAST), and Tenascin C (TN-C) (Haubensak et al., 2004; Hartfuss et al., 2001; Kriegstein and Alvarez-Buylla, 2009; Götz and Huttner, 2005). RGPs display apical-basal polarity and bear apical and basal processes that maintain their contacts with both the ventricular and pial surfaces. However, the cell bodies of RGPs are confined to the ventricular zone (VZ) that lines the lateral wall of the ventricles. In concert with their cell-cycle state, they undergo interkinetic nuclear migration (INM) (Taverna and Huttner, 2010): RGPs go through mitosis at the apical surface of the VZ. During the G1-S phase of the cell cycle, they migrate basally so that S phase reproducibly occurs on the basal edge of the VZ. RGPs display two modes of cell division. They divide symmetrically and generate two daughter cells that retain RGP properties to expand the number of neural progenitors. Alternatively, they divide asymmetrically giving rise to distinct daughter cell fates. Asymmetric RGP divisions produce either one RGP and one neuron or generate one RGP and one basal progenitor (BP, also called intermediate progenitor) (Noctor et al., 2004; Calegari et al., 2002; Miyata et al., 2004). BPs delaminate from the VZ and form the second germinal zone, the subventricular zone (SVZ), where they divide symmetrically to generate two neurons. In some cases, they can also generate two BPs to expand the basal progenitor pool (Noctor et al., 2004; Attardo et al., 2008). BPs emerge at E10.5 and become abundant from E13.5–E16.5, coinciding with the peak of neurogenesis (Englund et al., 2005). They are thought to be the source of most, if not all, neurons in the cortex (Sessa et al., 2008).
The transition of NPs into RGPs is a crucial event during mammalian brain development. Even subtle changes in progenitor cell numbers resulting from increased symmetric divisions at the onset of neurogenesis can have dramatic effects on the expansion of the cortical surface and ultimately on brain size (Rakic, 1995; Caviness et al., 1995). Mice expressing a stabilized form of β-Catenin in NPs, for example, display a significantly increased number of neural progenitors and show considerably increased cerebral cortical surface area and brain size (Chenn and Walsh, 2002). The timing of the NP to RGP transition is controlled by Notch signaling. Constitutively expressed activated Notch1, for example, promotes RGP cell fate in the developing mouse forebrain (Gaiano et al., 2000). In addition, Fgf10 has been shown to regulate the differentiation of NPs into RGPs (Sahara and O’Leary, 2009). Precisely how the transition between proliferative and neurogenic divisions is controlled to safeguard the proper number of neural progenitors is not clear.
Orientation of the mitotic spindle has been implicated in regulating symmetric and asymmetric cell division of neural progenitors, both in invertebrates and vertebrates (Morin and Bellaïche, 2011; Siller and Doe, 2009; Das and Storey, 2012; Lancaster and Knoblich, 2012). In Drosophila neuroblasts, spindle orientation is essential for correct asymmetric segregation of the cell fate determinants Numb, Brat, and Prospero into only one daughter cell and for correctly specifying neuronal and neuroblast fates (Knoblich, 2008). In the developing mouse brain, early symmetric NP divisions occur with a mitotic spindle that is oriented parallel to the ventricular surface during the neuroepithelial stages before neurogenesis begins. Spindle orientation is tightly controlled by Lis1 (also known as Pafah1b1), a gene that is mutated in lissencephaly (smooth brain) patients and Lis1 acts with its binding partners Ndel1 and dynein (Shu et al., 2004; Yingling et al., 2008). The Lis1/Ndel1/dynein complex interacts with the plus ends of astral microtubules and promotes microtubule capture at the cell cortex. Disruption of Lis1 leads to misorientation of the mitotic spindle in NPs and programmed cell death of NPs, suggesting a role of spindle orientation in the regulation of NP survival (Yingling et al., 2008). During the peak of neurogenesis, the fraction of obliquely/vertically oriented spindles rises with increasing neurogenesis rates (Huttner and Kosodo, 2005; Gauthier-Fisher et al., 2009). Recently, oblique spindle orientation mediated by overexpression of the mouse protein Inscuteable has been shown to regulate indirect neurogenesis rates (Postiglione et al., 2011). Collectively, orientation of the mitotic spindle plays various roles over the course of cortical development. How spindle orientation regulates neural progenitor proliferation and differentiation, especially at the onset of neurogenesis, however, is poorly understood.
Many of the key regulators of neurogenesis have been identified in the fruitfly Drosophila and were later shown to act similarly in the mouse neocortex (Brand and Livesey, 2011). Here, we make use of a genome-wide RNAi screen performed in Drosophila neuroblasts to identify the protein phosphatase PP4c (PP4c) as a regulator of mouse cortical neurogenesis. In Drosophila neuroblasts, PP4c is required for correct asymmetric cell division (Sousa-Nunes et al., 2009) and acts as a tumor suppressor that is required for proper control of neural stem cell number (Neumüller et al., 2011). We examined mice in which PP4c is deleted by Emx1Cre or NestinCre at different stages of cortical development. At the onset of neurogenesis, loss of PP4c resulted in a spindle misoriention phenotype in neural progenitors and caused them to switch from proliferative to neurogenic divisions. Eventually, this led to severe defects in cortical layering. When PP4c was removed during later stages, however, cortical layering is unaltered even though spindle orientation is randomized. Our data suggest that precise spindle orientation is required for cortical development during a critical time window to prevent asymmetric neurogenic divisions at the early stages of cortical development.
Results
Expression and Subcellular Localization of PP4c in the Developing Neocortex
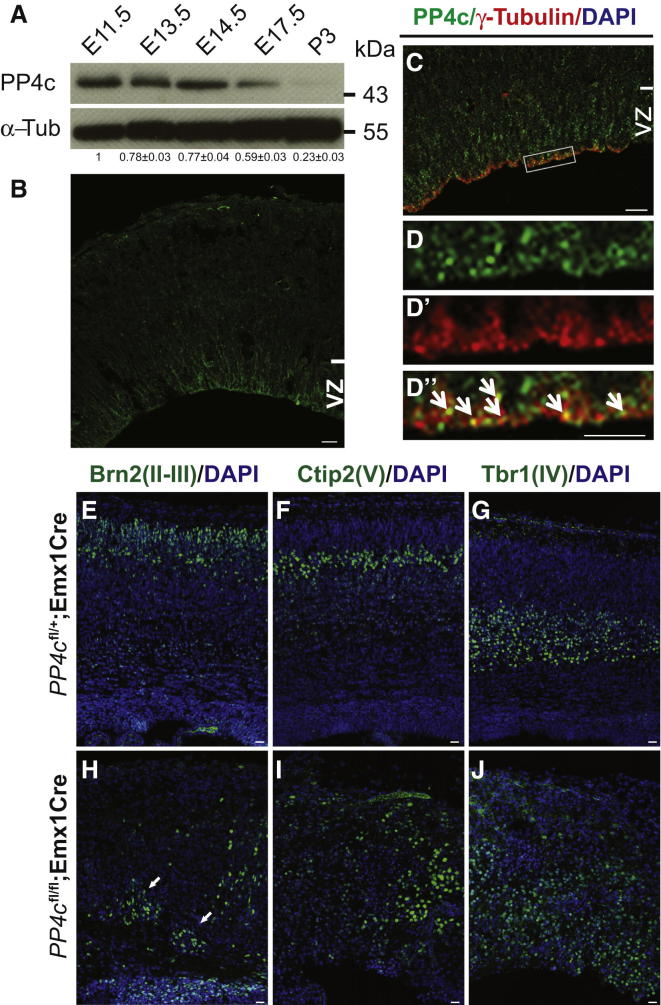
To evaluate the role of PP4c during cortical development, we first examined its expression in the developing mouse brain. In situ hybridization showed abundant PP4c mRNA expression in the VZ (Figures S1A–S1C available online). Western blot analysis revealed that PP4c expression was high at E11.5 when the cortical neuroepithelium is largely composed of neural progenitors, persisted until E17.5, and was downregulated in the postnatal stage (Figure 1A). Immunostaining of E14.5 cortical sections showed that PP4c expression was increased in the VZ compared to the more basal cortical areas (Figure 1B). Higher magnification and costaining with γ-Tubulin revealed that PP4c localized to the centrosomes of RGPs located at the VZ surface (Figures 1C and 1D). This is consistent with the localization of PP4c in Drosophila embryos (Helps et al., 1998) and mammalian cell lines (Figures S1D and S1E) (Toyo-oka et al., 2008; Brewis et al., 1993). The higher expression levels of PP4c during embryogenesis and its centrosomal localization in RGPs suggest that PP4c might be important for embryonic brain development.
Figure 1.
PP4c Localizes to Centrosomes and Is Essential for the Cortical Lamination in the Developing Mouse Brain
(A) Western blot of cortical lysates from various developmental stages probed for PP4c. The relative level of PP4c is quantified using Image J software and the level of PP4c at E11.5 is normalized to 1.
(B) Immunostaining of E14.5 brain sections shows higher PP4c expression in the ventricular zone (VZ).
(C) Coronal sections stained for PP4c (green) and the centrosomal marker γ-Tubulin (red).
(D–D″) Higher magnification of box marked in (C). Arrows in (D″) indicate colocalization of PP4c (green) with the centrosomal marker γ-Tubulin (red).
(E–J) Confocal images of E18.5 coronal sections from PP4cfl/+;Emx1Cre brains (Ctr) (E–G) and PP4cfl/fl;Emx1Cre brains (H–J) stained with layer-specific markers Brn2 (layer II-III), Ctip2 (layer V), and Tbr1 (layer VI) in green. Sections were counterstained with DAPI (blue). Arrows in (H) indicate mislocalized upper layer neurons in deep layers.
Scale bars represent 20 μm in (B) and (C), 10 μm in (D)–(D″), and 50 μm in (E)–(J).
See also Figures S1 and S2.
PP4c Is Essential for Cortical Layer Formation
To analyze the role of PP4c in cortical development, we conditionally inactivated PP4c in the developing neocortex by crossing mice with loxP-flanked alleles of PP4c (PP4cflox/flox) (Toyo-oka et al., 2008) with Emx1Cre. Emx1Cre drives Cre-mediated recombination in cortical progenitors and activates at E10.5 when neurogenesis begins (Gorski et al., 2002; Chou et al., 2009). PP4cflox/flox;Emx1Cre (PP4cfl/fl;Emx1Cre) mice died shortly after birth. Histological analysis of PP4cfl/fl;Emx1Cre brains showed global reduced cerebral cortex and brain size compared to heterozygous control brains PP4cfl/+;Emx1Cre (Ctr) (Figure S2). During development, cortical layers form in an “inside-out” fashion and deep layers are composed of early-born neurons, while superficial layers are composed of later-born neurons (Rakic, 2009). The cortical layers of E18.5 PP4cfl/fl;Emx1Cre brains were thinner and more disorganized than their control counterparts (Figure 1). Brn2-positive neurons, which are produced during late neurogenesis and form the upper cortical layers II-III, did not form a cohesive layer in the cortex of PP4cfl/fl;Emx1Cre brains. Some Brn2-positive neurons are found even in the deep cortical layers (Figures 1E and 1H). Layer V neurons, produced around the peak of neurogenesis (Molyneaux et al., 2007) and positive for Ctip2, were dispersed throughout the cortex in PP4c-deficient brains (Figures 1F and 1I). In contrast, the majority of neurons in the cerebral cortex of PP4cfl/fl;Emx1Cre brains are Tbr1 positive, although they are not organized into a cohesive layer (Figures 1G and 1J). Thus, PP4c is required in RGPs at the onset of neurogenesis for proper cortical layer formation.
PP4c Is Required to Maintain the Progenitor Pool at the Onset of Neurogenesis
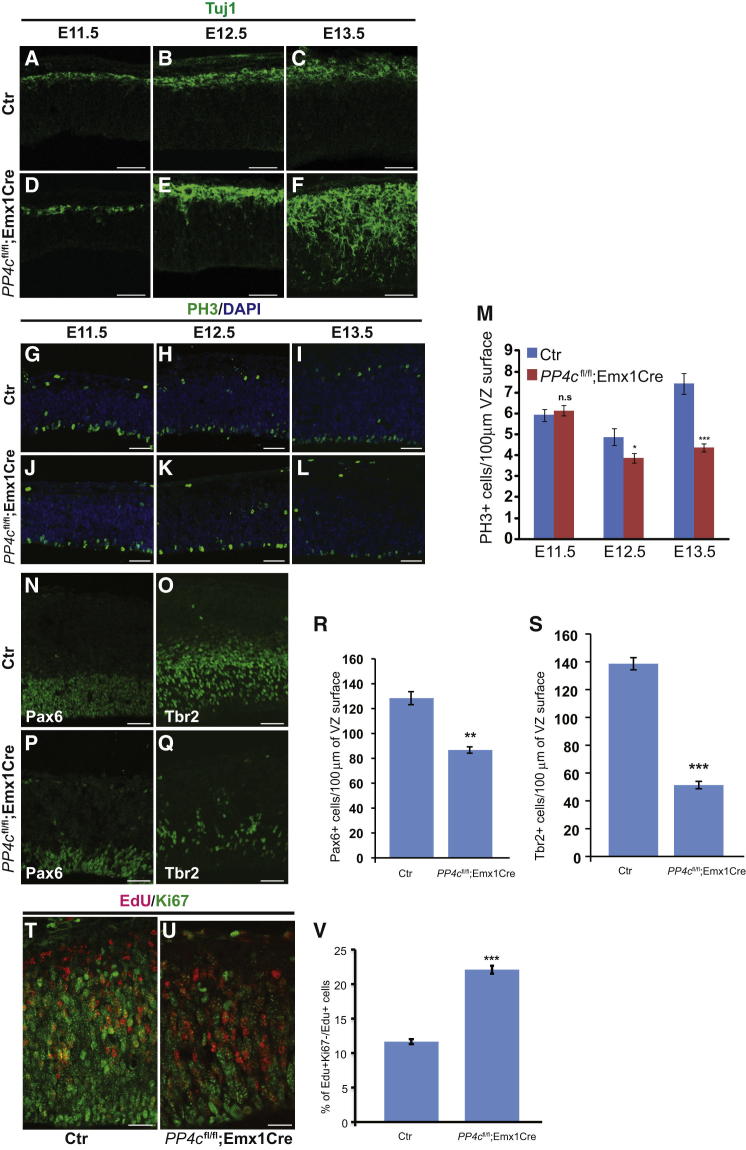
To identify the cellular basis for the layering defect, we examined neuronal differentiation by immunostaining for the early neuronal marker Tuj1. At E11.5, shortly after Emx1Cre activation, we did not observe any obvious differences in neuronal differentiation between heterozygous control and PP4c mutant mouse brains (Figures 2A and 2D). At E12.5, however, more neurons were generated in the mutants when compared to control brains (Figures 2B and 2E). This phenotype is even stronger at E13.5 when almost the entire cortex including the VZ and SVZ is occupied by differentiated neurons in mutant mice (Figure 2F), while only a few neurons in the cortical plate are seen in controls (Figure 2C).
Figure 2.
PP4c Is Required for Neural Progenitor Maintenance during Early Cortical Development
(A–L) Confocal images of coronal sections from PP4cfl/+;Emx1Cre brains (Ctr) (A–C and G–I) and mutants (D–F and J–L) at indicated developmental stages stained for Tuj1 (A–F, green) or PH3 (G–L, green) and DAPI (G–L, blue).
(M) Quantification of PH3-positive cells per 100 μm VZ surface at different developmental stages in controls (blue bar) and mutants (red bar) (at least three brains were analyzed for each genotype).
(N–Q) RGPs and BPs are identified by staining for Pax6 (N and P) and Tbr2 (O and Q), respectively, in controls (N and O) and PP4c mutants (P and Q).
(R and S) Quantification of Pax6-positive RGPs (R) and Tbr2-positive BPs (S).
(T and U) Mitotic exit rates in control brains (T) and PP4c-deficient brains (U): neural progenitors double labeled for EdU (for 24 hr) and Ki67. Cells that exited the cell cycle during the past 24 hr are EdU positive but Ki67 negative.
(V) Number of cells exiting the cell cycle was increased in PP4cfl/fl;Emx1Cre brains compared to controls (n = 3 for each genotype).
Data are presented as mean ± SEM; ns, not significant; ∗p < 0.05, ∗∗p < 005, ∗∗∗p < 0.001; Student’s t test for two-tailed distribution with unequal variance.
Scale bars represent 50 μm in (A)–(Q) and 20 μm in (T) and (U).
To examine whether supernumerary neurons are generated at the expense of progenitors, we stained control and mutant brain sections for phospho-Histone H3 (PH3) to mark mitotic progenitors. At E11.5, the numbers of PH3-positive progenitors in mutant and control brains were comparable (Figures 2G, 2J, and 2M). At E12.5, however, the number of mitotic progenitors was significantly reduced in the mutant brains and this effect became even stronger at E13.5 (Figures 2H, 2I, 2K, 2L, and 2M). When we examined the number of Pax6-positive RGPs and Tbr2-positive BPs, we found that both types of progenitors were depleted in the PP4c mutant brains (Figures 2N–2S). Interestingly, loss of PP4c seems to have stronger effects on Tbr2-positive BPs (37.5% of control) compared to Pax6-positive RGPs (67.5% of control). The reduction in BP cell number could be an indirect consequence of the reduction in RGPs, although we cannot rule out that PP4c directly regulates BPs. To test whether the observed generation of supernumerary neurons correlates with increased cell-cycle exit, we labeled cycling progenitors by EdU incorporation at E12.5 and stained for the proliferation marker Ki67 24 hr later, at E13.5. Cells that have exited the cell cycle during those 24 hr are expected to be EdU positive but Ki67 negative. Indeed, we found that more cells exit the cell cycle in PP4c-deficient brains (22.08%) compared to control brains (11.65%) (Figures 2T–2V). Thus, our data demonstrate that PP4c is necessary to prevent neuronal differentiation and maintain the progenitor pool during the early stages of mouse cortical development.
PP4c Is Required for Proper Spindle Orientation in RGPs
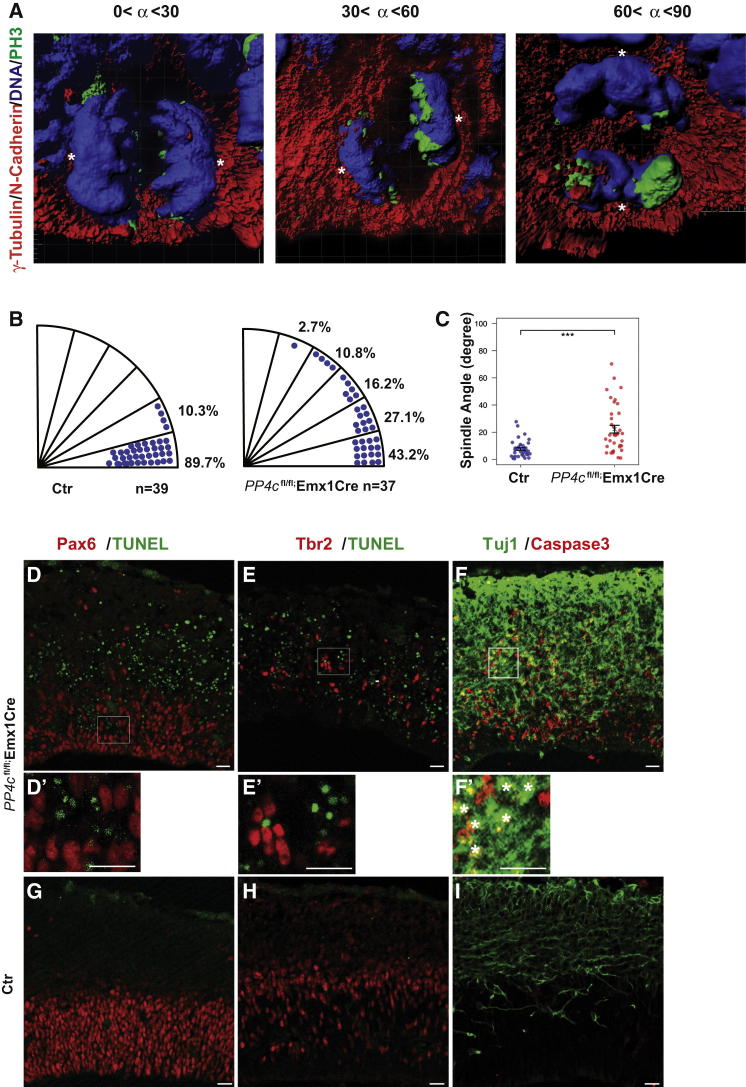
As PP4c is concentrated at centrosomes (Figure 1), we tested whether the defects observed in the knockout mice are due to spindle morphology or orientation defects in cortical progenitor cells. Tubulin staining demonstrated that the overall morphology of mitotic spindles in cortical progenitors is unaffected (Figures S3A–SH). In addition, the number of centrosomes was not altered obviously in PP4c mutant brains (Figures S3I–SL). Using established methodology (Figures S3M–SO) (Postiglione et al., 2011), we determined the orientation of mitotic spindles in wild-type and PP4c mutant cortical progenitors in three dimensions (3D) (Figure 3A). Spindle orientation was analyzed at E11.5 to avoid indirect effects from the cell-fate transformations observed at E12.5. Brain sections were stained for PH3 (to mark mitotic progenitors), γ-Tubulin (for centrosomes), and N-Cadherin (to outline cells). Only cells in ana- or early telophase were analyzed. In control brains, nearly 90% of the mitotic spindles are between 0° and 15° relative to the ventricular surface and only 10% are between 15° and 30° (Figure 3B). This is consistent with previous studies, although the method of spindle orientation measurement is different (Haydar et al., 2003; Konno et al., 2008; Kosodo et al., 2004). In PP4c knockout brains, however, only 43.2% of the spindles are between 0° and 15°, 27.1% are between 15° and 30°, and 27% are between 30° and 60°; 2.7% of the mitotic spindles are between 60° and 90°, a close to vertical orientation that we never observed in controls (Figure 3B). Statistical analysis indicates that these defects are highly significant (Figure 3C). Thus, PP4c is essential for proper horizontal spindle orientation during the early phases of cortical development.
Figure 3.
Depletion of PP4c Leads to Misorientation of the Mitotic Spindle of Neural Progenitors
(A) Samples of 3D-reconstructed mitotic progenitors from three different classes of divisions. Cell outline and centrosomes (asterisks) are marked by N-cadherin (red) and γ-Tubulin (red), respectively. PH3 (green) marks mitotic DNA (DAPI, blue).
(B and C) Distribution of spindle orientation in RGCs from PP4cfl/+;Emx1Cre (Ctr) and mutant brains (B) and statistic analysis of the distribution of spindle orientation (C). Data are presented as Circular Standard Deviation. ∗∗∗p < 0.001, Wilcoxon rank-sum test.
(D–I) TUNEL (green, D and E) and caspase-3 (red, F) staining in PP4cfl/fl;Emx1Cre brains. (D′–F′) Enlargements of the areas indicated in (D)–(F) show that Pax6-positive RGPs (red) and Tbr2-positive BPs (red) are negative for TUNEL staining. Newborn neurons labeled with Tuj1 (green), however, are positive for caspase-3, whereas either TUNEL or caspase-3 is barely detectable in control brains (G–I). Scale bars represent 20 μm.
See also Figure S3.
Previous experiments have demonstrated that altering spindle orientation in neuroepithelial cells results in widespread apoptosis and led to the conclusion that spindle orientation is essential for neuroepithelial cell survival (Yingling et al., 2008). Consistent with those data, both TUNEL labeling and staining for activated caspase-3 reveal extensive cell death in PP4cfl/fl; Emx1Cre mice. Costaining those mice for lineage markers, however, shows that the vast majority of dying cells have neuronal identity, while progenitor cells are not affected (0.79% of Pax6-positive cells are positive for TUNEL, 2% of Tbr2-positive cells are positive for TUNEL, whereas nearly 90% of caspase-3-positive cells are neurons) (Figures 3D–3I). Thus, we propose that apoptosis is a secondary consequence of premature neurogenesis that might occur because neuronal survival factors might be missing at those early stages of cortical development.
PP4c Regulates Neurogenesis through Spindle Orientation during a Critical Time Window
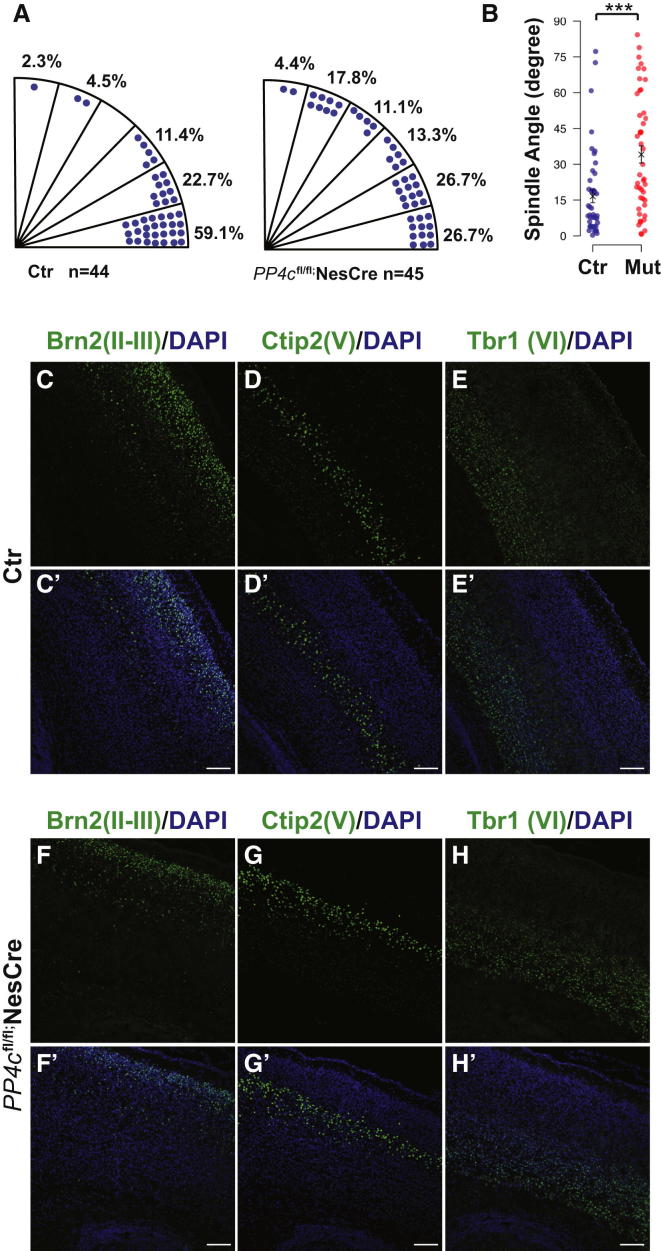
To ask whether PP4c is required for lineage specification throughout cortical development or only during a specific time window, we crossed PP4cflox/flox mice with a NestinCre line (Nes11Cre) to conditionally inactive PP4c in the forebrain at E11.5 (PP4cfl/fl;NesCre), 1 day later than Emx1Cre (Chou et al., 2009; Backman et al., 2005). Immunostaining confirmed the loss of PP4c at E12.5 in PP4cfl/fl;NesCre brains, 1 day later compared to Emx1Cre-mediated PP4c depletion (Figure S4). Just like in earlier embryos, we detected profound and highly significant defects in the orientation of mitotic spindles in progenitor cells from those mice (Figures 4A and 4B). While more than 50% of mitotic spindles are horizontal in controls, this number is strongly decreased and the number of oblique and vertical spindles increased in PP4cfl/fl;NesCre mice. Surprisingly, however, randomization of spindle orientation at this later stage of development does not result in the abnormal cortical organization observed at earlier stages. Both superficial layer and deep layer neurons are generated and positioned correctly (Figures 4C–4H) and the drastic reduction in progenitor numbers observed in PP4cfl/fl;Emx1Cre is no longer observed (data not shown). Thus, our results define a critical time period within cortical development (E10.5–E12.5), during which planar orientation of mitotic spindles mediated by PP4c maintains symmetric divisions of cortical progenitors. Proliferative symmetric division of progenitors during this time is crucial for cortical organization and correct cortical layering.
Figure 4.
Cortical Integrity Is Maintained upon the Depletion of PP4c at a Later Developmental Stage
(A and B) Orientation of the mitotic spindle is randomized in RGPs of PP4cfl/fl;NesCre (mut) brains, while the majority of spindles of RGPs are orientated parallel to the ventricular surface in PP4cfl/+;NesCre (Ctr) brains at E12.5. Distribution (A) and statistic analysis (B) of spindle orientation are presented. Data are presented as Circular Standard Deviation. ∗∗∗p < 0.001, Wilcoxon rank-sum test.
(C–H) Cortical layers are formed correctly at postnatal day 3 (P3) in PP4cfl/+;NesCre (Ctr) and PP4cfl/fl;NesCre brains as revealed by the staining of layer-specific markers Brn2 (C, C′, F, and F′), Ctip2 (D, D′, G, and G′), and Tbr1 (E, E′, H, and H′). DNA (DAPI) is blue. Scale bars represent 50 μm.
See also Figure S4.
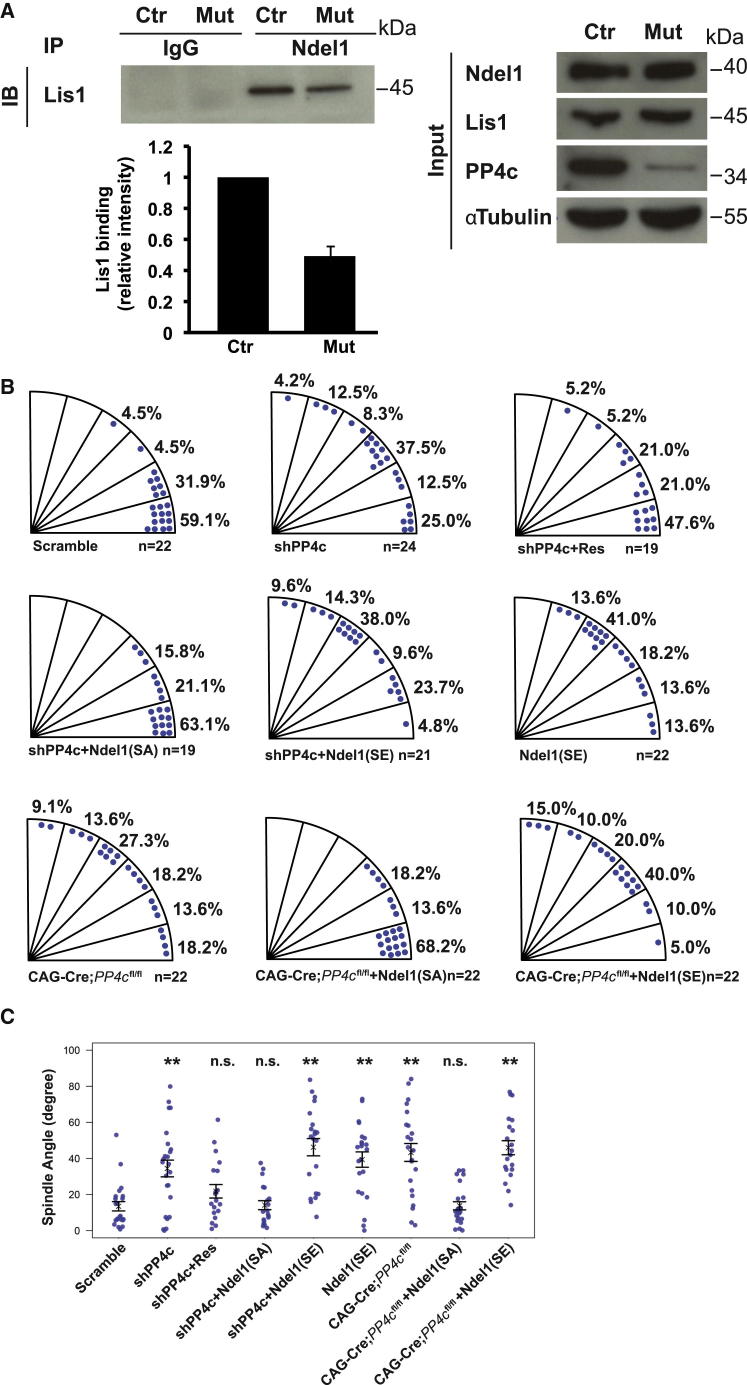
PP4c Acts through Ndel1 and Lis1 to Regulate Spindle Orientation
Previous experiments have shown that PP4c can dephosphorylate Ndel1, a Lis1 binding partner that is localized at centrosomes (Toyo-oka et al., 2008; Shu et al., 2004). As Lis1 has recently been found to regulate spindle microtubule capture at the cell cortex and to regulate spindle orientation (Yingling et al., 2008), we analyzed whether PP4c might regulate the interaction between Ndel1 and Lis1. Lis1 and Ndel1 can be coimmunoprecipitated from both wild-type and PP4c knockout brains. Quantification shows, however, that the interaction is significantly weaker in knockout brains than in controls (Figure 5A). To test whether increased Ndel1 phosphorylation is indeed responsible for the PP4c mutant spindle orientation phenotype, we performed rescue experiments using a version of Ndel1 in which the Cdk5/Cdk1 phosphorylation sites are mutated to Alanine (Niethammer et al., 2000). For this, we electroporated an shRNA construct efficiently targeting PP4c (Figure S5) into cortical progenitor cells in utero at E14.5 and analyzed the electroporated brains at E17.5. Like the knockout, PP4c RNAi (but not a scrambled control construct) causes a significant defect in spindle orientation (Figure 5B). The spindle defect can be rescued to wild-type by coelectroporating an RNAi-resistant PP4c construct, indicating that it is specific. Importantly, the phenotype can also be rescued by a nonphosphorylatable form of Ndel1 (Ndel1SA) in which three of the key PP4c target residues have been mutated to Alanine. However, the phosphomimetic form of Ndel1 (Ndel1SE) cannot rescue the spindle orientation defect (Figures 5B and 5C). In addition, we observed similar spindle orientation defects when plasmids expressing Cre driven by a CAG promoter were electroporated into PP4cfl/fl embryonic brains, indicating that the phenotype is specific to the loss of PP4c. Ndel1SA, but not Ndel1SE, could rescue the spindle orientation defects caused by the loss of PP4c (Figures 5B and 5C). To examine whether the nonphosphorylatable form of Ndel1 can also rescue the lineage defects at the onset of neurogenesis, we performed in utero electroporation at E11.5. Downregulation of PP4c leads to an increase of neuronal differentiation with the depletion of the progenitor pool, which is consistent with what we observed in PP4cfl/fl;Emx1Cre brains (Figures S6A, S6B, S6D, and S6E). This phenotype was again rescued by coelectroporation of Ndel1SA (Figures S6C, S6D, and S6E). Thus, our data suggest that excessive phosphorylation of Ndel1 results in disruption of the Ndel1/Lis1 complex in PP4c mutant mice and is responsible for the spindle orientation defect. In the future, it will be interesting to examine which regulatory subunit forms the complex with PP4c to regulate spindle orientation.
Figure 5.
PP4c Acts through Ndel1-Lis1 to Regulate Spindle Orientation
(A) Binding of Ndel1-Lis1 was tested in E15.5 brains by coimmunoprecipitation. Ndel1-Lis1 binding was reduced in PP4cfl/fl;NesCre brains (Mut) compared to PP4cfl/+;NesCre brains (Ctr). Data are presented as mean ± SEM.
(B and C) In utero electroporation was performed at E14.5 using various constructs indicated. Distribution (B) and statistic analysis (C) of spindle orientation are presented. Spindle angles were analyzed at E17.5. Data are presented as Circular Standard Deviation. ∗∗p < 0.005, ns, not significant, Wilcoxon rank-sum test.
See also Figures S5 and S6.
Notch Signaling Is Disrupted in PP4c-Depleted Progenitors
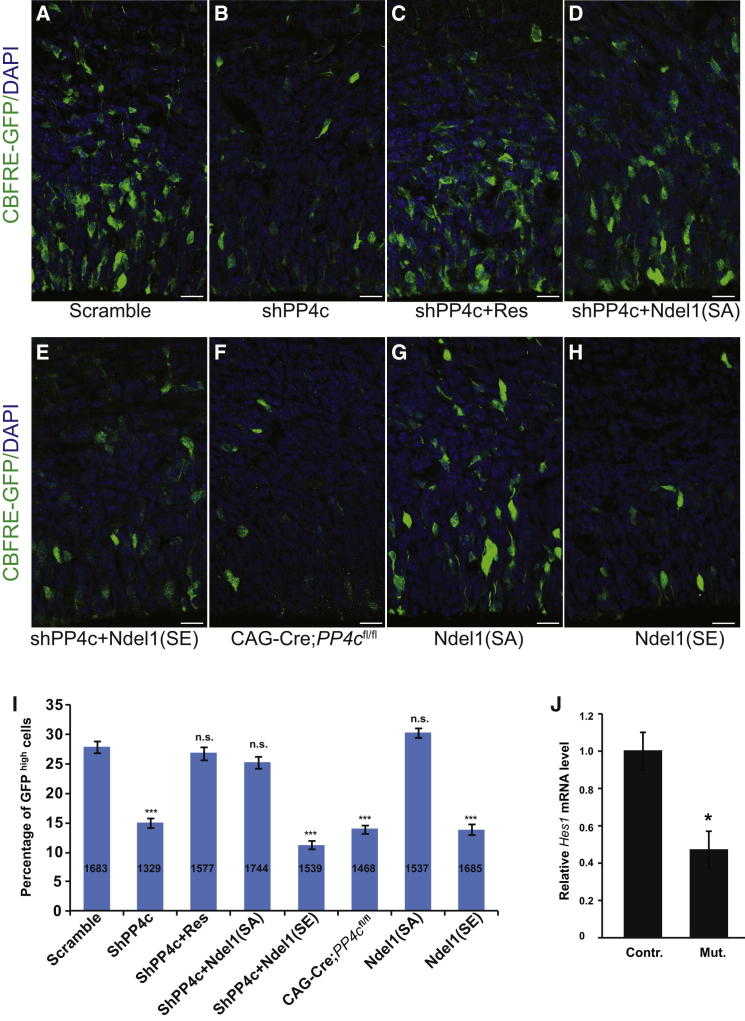
To address how cell fates might be affected by the spindle orientation defects, we analyzed the Notch signaling pathway. Notch signaling plays an essential role in regulating neural progenitor proliferation and differentiation (Pierfelice et al., 2011) and has been proposed to be an important downstream mediator of the asymmetric cell division machinery in the mammalian epidermis (Williams et al., 2011). To determine Notch activity, we used a Notch reporter (CBFRE-EGFP) that carries a CBF1-reponse element upstream of EGFP. The intensity of EGFP in the cell reflects endogenous Notch activity (Mizutani et al., 2007; Bultje et al., 2009). The CBFRE-EGFP construct was coelectroporated with either a PP4c or a scrambled shRNA into E13.5 mouse brains. The resulting downregulation of PP4c by shRNA or genetic removal of PP4c via Cre expression in PP4cfl/fl background caused a significant reduction in EGFP fluorescence when compared to controls (Figures 6A and 6F). Counting the numbers of highly GFP-positive cells in which the Notch pathway is active showed that this effect is highly significant (Figure 6I). The defect is specific as the decreased Notch activity can be restored by coelectroporation of an RNAi-resistant PP4c construct (Figures 6C and 6I). We then asked whether overexpression of Ndel1SA could rescue the Notch signaling defect caused by the downregulation of PP4c. Indeed, the Notch activity was rescued upon expression of Ndel1SA, but not Ndel1SE (Figures 6D, 6E, and 6I). We noticed that expression of Ndel1SE, but not Ndel1SA, led to a defect on the Notch activity (Figures 6G, 6H, and 6I), which most likely is due to the spindle orientation defect (Figures 5B and 5C). We also examined whether the Notch targets, like Hes1, are reduced in PP4c mutant brains. Quantitative real-time PCR revealed that the level of Hes1 mRNA is significantly reduced in PP4c mutant cortex compared to the control cortex (Figure 6J). These results suggest that the misoriented spindle in the neural progenitors of PP4c knockouts could affect Notch signaling activity. Given that Notch signaling activity regulates neural progenitor proliferation and differentiation in the developing neocortex (Gaiano et al., 2000; Pierfelice et al., 2011), these data indicate that PP4c regulates neural progenitor proliferation through spindle orientation-dependent Notch signaling activity.
Figure 6.
Notch Activity Is Reduced in Progenitors upon PP4c RNAi
(A–H) Notch activity was analyzed 2 days after in utero electroporation performed at E13.5 using a Notch reporter construct (CBFRE-GFP, green) together with constructs expressing Scramble (A), shPP4c (B), shPP4c and an RNAi resistant form of PP4c (Res) (C), shPP4c and Ndel1(SA) (D), shPP4c and Ndel1(SE) (E), Cre under a CAG promoter in PP4cfl/fl background (F), Ndel1(SA) (G), or Ndel1(SE) (H).
(I) Quantification of the percentage of GFPhigh cells in the neocortex (the number of cells quantified for each condition is indicated in the bars; at least three animals were analyzed for each condition). ∗∗∗p < 0.001, data are shown as mean ± SEM, Student’s t test for two-tailed distribution with unequal variance.
(J) Decreased Notch target Hes1 mRNA in PP4cfl/fl;Emx1Cre (Mut.) brains compared to PP4cfl/fl (Contr.) brains (n = 3 for each condition). ∗p < 0.05, data are shown as mean ± SEM, Student’s t test for two-tailed distribution with unequal variance.
Discussion
In this study, we have examined the role of PP4c in regulating neural progenitor proliferation and differentiation in the mouse neocortex. Our data suggest that PP4c regulates Ndel1 phosphorylation and its interaction with Lis1 to control the orientation of the mitotic spindle in cortical progenitors. When PP4c is deleted at the onset of neurogenesis, the resulting spindle orientation defects lead to premature differentiation of cortical progenitors into neurons and severe defects in cortical layering and brain cytoarchitecture. When deleted at E12.5, however, these defects are no longer observed, although mitotic spindles in progenitor cells assume a random orientation. Thus, our data indicate that correct spindle orientation is essential in RGPs during a critical time window at the onset of neurogenesis to prevent differentiation of neural progenitors and the maintenance of cortical integrity.
A Critical Role of Spindle Orientation for the Maintenance of the Progenitor Pool at the Onset of Neurogenesis
During the initial neuroepithelial stages of cortical development (E8.5–E9.5), neural progenitors expand by dividing symmetrically. Neurogenesis ensues at E10.5 and gradually increases until E14.5, when most neuroepithelial divisions are asymmetric, generating one progenitor and one neuron or basal progenitor. This transition is accompanied by a gradual change of spindle orientation: while most mitotic spindles are parallel to the epithelial surface at E10.5, almost half of them show an oblique orientation at E14.5 (Postiglione et al., 2011). We propose that this gradual transition in spindle orientation is important to allow a gradual increase in neurogenesis rates and proper cortical morphogenesis. Upon the removal of PP4c at the onset of neurogenesis through Emx1Cre-mediated recombination, the number of progenitors with oblique orientation of the mitotic spindle was significantly increased. As a consequence, huge numbers of neurons were generated at the expense of the progenitor pool, suggesting that planar spindle orientation is important for the regulation of neural progenitor self-renewing divisions during early cortical development. This regulation seems to be temporally controlled as a similarly dramatic change in neuronal numbers was not observed when PP4c is removed by NestinCre recombination at E12.5. Interestingly, these phenotypic differences are not due to different effects on spindle orientation. In fact, a statistical analysis of 3D spindle orientation data reveals essentially complete randomization of spindles in PP4cfl/fl;NesCre mice, suggesting that PP4c activity itself is not regulated in time but the sensitivity toward spindle manipulation decreases over time.
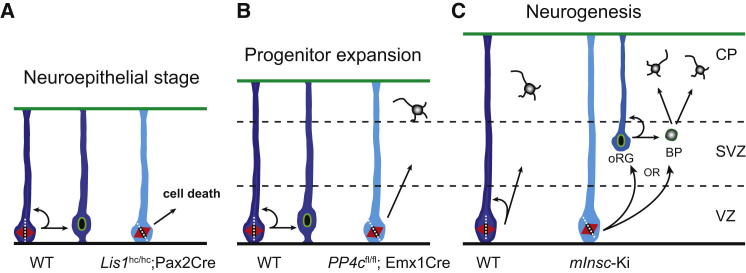
Together with previous data, our findings suggest a model in which three different stages can be distinguished for the role of spindle orientation for lineage specification in the developing cortex (Figure 7). During the early neuroepithelial stages (Figure 7A), before neurogenesis, correct spindle orientation is required for the survival of neuroepithelial progenitors (Yingling et al., 2008). At the onset of neurogenesis (Figure 7B), spindle orientation is no longer required for progenitors to survive but is essential to maintain a symmetric division mode in a fraction of those progenitors and to maintain the progenitor pool, which essentially contributes to the cortical lamination. As the rate of neurogenesis increases, the importance of spindle orientation for progenitor maintenance decreases and, during the peak of neurogenesis (Figure 7C), oblique orientation of the mitotic spindle (as observed upon overexpression of mInsc or mutation of LGN) is correlated with the production of intermediate progenitors or outer radial glial progenitors (oRGs) (Postiglione et al., 2011). How these distinct modes of cell-fate regulation in the developing cortex and their connections to spindle orientation are brought about is currently unclear. Most likely, daughter cells arising from the division of neural progenitors respond differently to the various signaling pathways acting at different developmental stages, such as Notch and FGF (Pierfelice et al., 2011; Guillemot and Zimmer, 2011).
Figure 7.
A Mode that Illustrates Developmental Roles of Spindle Orientation in the Mouse Brain
During early neuroepithelial stages before neurogenesis (A), planar spindle orientation is essential for the survival of neuroepithelial progenitors. Misoriented spindles resulting from disruption of Lis1 function lead to dramatic apoptosis in progenitor cells (Yingling et al., 2008). During a critical time window at the onset of neurogenesis (B), planar spindle orientation is required for expansion of the RGP pool and to prevent premature neuronal differentiation. Loss of PP4c mediated by Emx1Cre leads to dramatically increased neuronal differentiation accompanied by depletion of the progenitor pool. At the peak of neurogenesis (C), oblique spindle orientation promotes indirect neurogensis, as observed upon overexpression of mInsc (Postiglione et al., 2011).
Although we observed spindle randomization in PP4cfl/fl;NesCre, we did not observe an increase in intermediate progenitors as seen in mInsc knockin mice or LGN mutants (Postiglione et al., 2011; Konno et al., 2008). This could potentially be explained, because mInsc overexpression results in an increase of oblique/vertical divisions when analyzed by an improved methodology to model the random distribution of spindle orientation (C.J., Y.X., and J.A.K., unpublished data), whereas PP4cfl/fl;NesCre mice show random spindle orientation. Alternatively, activities of the mutant genes other than spindle orientation could be responsible. In Drosophila, redundant pathways regulating spindle orientation have been observed (Siller and Doe, 2009). Mud (known as NuMA in mammals), for example, competes with Insc (known as mInsc in mice) to bind to Pins (known as LGN/AGS3 in mammals) (Mapelli and Gonzalez, 2012). In addition, an astral microtubule-dependent pathway, the Dlg-Pins-Gai pathway, can compensate for the Mud-Pins-Gai pathway (Siegrist and Doe, 2005). As Lis1, Ndel1, and Dynein are downstream of both of those pathways, similar redundancies in vertebrates could explain the seemingly different phenotypic outcome.
Correct Spindle Orientation at the Onset of Neurogenesis Is Required for Cortical Development
During brain development, the birth date of neurons is correlated with distinct laminar fates in the cerebral cortex (Molyneaux et al., 2007). Therefore, it is crucial for the establishment of cortical lamination that the correct number of neurons is generated at a given developmental stage. Our data suggest that PP4c-mediated spindle orientation is important for cortical lamination during early neurogenesis but dispensable during later stages. Depletion of PP4c in PP4cfl/fl;Emx1Cre brains led to a reduction of the brain size. Importantly, cortical layers were completely disrupted in PP4cfl/fl;Emx1Cre brains with no coherent layers formed. We found upper layer neurons positive for Brn2 within deep layers, while Tbr1-positive deep layer neurons were distributed throughout the cortex. We attribute those defects to the spindle orientation defect and the premature neuronal differentiation at the expense of proliferating progenitors at the early stage of cortical development. When PP4c was removed later by NestinCre-mediated recombination, cortical layers were formed correctly. Therefore, our data suggest that the expansion of the progenitor pool during a critical period at the onset of neurogenesis is essential for cortical layer development.
A potential explanation for the cortical layering defects is provided by the recent discovery of a new population of neural progenitors that express Cux2 and exclusively generate upper layer neurons (Franco et al., 2012). These progenitors arise at the onset of neurogenesis from bipotent progenitors that first expand during an initial prolioferation phase and then generate both Cux2-positive and -negative cells. It is conceivable that spindle misorientation in PP4cfl/fl;Emx1Cre mice could deplete the bipotent progenitors during the initial proliferation phase. This could explain the apparent depletion of upper layer neurons that we observe in the PP4cfl/fl;Emx1Cre mice. It could also explain the layering defects as layer formation at those later stages could be compromised due to the depletion of radial glial progenitors that provide the scaffold for radial neuronal migration. In support of this, Cux2 has been shown as a downstream target of Notch signaling (Iulianella et al., 2009) and Notch signaling is disrupted in those mice.
This mechanism that regulates the expansion of the early progenitor pool may be evolutionarily conserved. During human brain development, the cortical surface is expanded dramatically compared to the mouse brain (Lui et al., 2011). Expansion of the progenitor pool during early stages of brain development may be essential for the generation of a sufficient number of progenitors, which in turn differentiate into the large number of neurons during brain development (Howard et al., 2006). This is accompanied by a change in progenitor cell lineage. In the mouse cortex, two neurons typically arise from each progenitor division, because intermediate progenitors typically divide only once. Human neurogenesis, in contrast, involves a transit-amplifying population called outer radial glia (oRG) cells (also called outer subventricular zone progenitors or basal radial glia), so that many more neurons can arise from each progenitor division. Modeling a similar lineage divergence in the Drosophila brain (Bowman et al., 2006) has shown that the existence of a transit-amplifying population not only changes neuron number but also kinetics of neurogenesis: neurogenesis rates increase exponentially rather than linearly over time and fewer neurons are generated during early stages, while neurogenesis is dramatically increased in later stages. Besides simply increasing neuron numbers, therefore, the lineage changes that occurred during mammalian evolution may also affect the cortex by modifying the numbers of neurons generated at specific times of neurogenesis.
Several microcephaly-associated proteins, such as MCPH1, CDK5RAP2, and Nde1, have been shown to regulate spindle orientation and progenitor proliferation in rodent brains (Gruber et al., 2011; Feng and Walsh, 2004). Mutation of these genes leads to severe microcephaly disease in humans (Manzini and Walsh, 2011). It is likely that imbalanced progenitor proliferation and differentiation mediated by misoriented mitotic spindles are causal for those various microcephalies. Given that PP4c is a key regulator of proliferative divisions of neural progenitors during early cortical development, it is of great interest to examine whether such a role of PP4c is conserved during human brain development.
Experimental Procedures
Mice
Homozygous PP4cfl/fl mice (Toyo-oka et al., 2008) were crossed to Emx1-Cre mice (Gorski et al., 2002) to generate PP4cfl/+;Emx1Cre mice, which were further crossed with PP4cfl/fl mice to generate PP4cfl/fl;Emx1Cre mice and the littermate controls phenotypic WT embryos, PP4cflox/+; Emx1Cre (Ctr). To obtain PP4cfl/fl;NesCre mice and the littermate controls PP4cfl/+;NesCre (Ctr), we used Nes11Cre mice (generously provided by Dr. Ondrej Machon, Olso University Hospital, Norway).
In Utero Electroporation
In utero electroporations were carried out essentially as described previously in Postiglione et al. (2011). Briefly, for experiments at E14.5, timed pregnant C57BL/6J mice were anesthetized, uterine horns were exposed, and 1.5 μg/μl DNA solution was injected in the lateral ventricle. Platinum electrodes (5 mm, BTX) were positioned on either side of the embryonic head and five 50 ms pulses of 33 mV with 950 ms intervals were applied with an electroporator (BTX, ECM830). After electroporation, uterine horns were placed back into the abdominal cavity and wounds were sutured. Brains were analyzed 3 days later. For experiments at E11.5, the procedure was altered in that injections were guided by ultrasound visualization (Vevo 770, scanhead RMV711, Visualsonics) and electrode pulses were adjusted to 25 mV. Embryo positioning was identified by ultrasound signal and visually using a highpower lightsource. All procedures were performed in accordance with protocols approved by the institutional animal care.
Immunohistochemistry
Mouse brains were fixed in 4% paraformaldehyde overnight at 4°C followed by cryoprotection in 30% sucrose until they sunk to the bottom. Coronal sections were prepared using a cryostat (Leica Micro-systems). Brain sections were permeabilized with 0.1% Triton X-100 for 15 min and then incubated with blocking solution (5% normal goat serum, 0.1% Triton X-100, and 5% BSA in PBS) for 1 hr at room temperature, followed by the incubation of primary antibody at 4°C overnight. The following primary antibodies have been used: goat anti-PP4c (1:50, Santa Cruz), mouse anti-γ-Tubulin (1:1,000, Sigma), mouse anti-N-Cadherin (1:500, BD Biosciences), rabbit anti-PH3 (1:300, Millipore), rabbit anti-Pax6 (1:300, Covance), mouse anti-Tuj1 (1:1,000, Covance), rabbit anti-Caspase-3 (1:300, Cell Signaling), chicken anti-GFP (1:1,000, Abcam), rabbit anti-Stab2 (1:300, Abcam), rabbit anti-Brn2 (1:200, Santa Cruz), rabbit anti-Tbr1 (1:250, Abcam), rabbit anti-Tbr2 (1:300, Abcam), and mouse anti-Ki67 (1:100, Cell Signaling).
After incubation with the primary antibody, sections were washed in PBS, followed by the incubation with appropriate fluorescence-conjugated secondary antibodies for 1 hr at room temperature before mounting.
Real-Time qPCR
mRNA was isolated from both control and PP4cfl/fl;Emx1Cre cerebral cortex using TRIzol reagent (Invitrogen) and cDNA was synthesized from 3 μg of total RNA using Superscript II with random primers (Invitrogen). Real-time PCR was performed on a C1000 Thermal Cycler (Bio-Rad). Quantification was performed using CFX Manager software (Bio-Rad) with data normalized to the level of Actin mRNA. The following primer sequences were used: Actin Forward: 5′-TTTGCAGCTCCTTCGTTGC-3′, reverse: 5′-CCATTCCCACCATCACACC-3′ and Hes1 Forward: 5′-TCCAAGCTAGAGAAGGCAGACA-3′, reverse: 5′-CGCGGTATTTCCCCAACA-3′.
Spindle Angle Analysis
Brain sections were stained with N-Cadherin to outline the cell shape and PH3 to identify the anaphase and early telophase dividing cells. γ-Tubulin was used to mark centrosomes. Images of z stack sections were taken by Zeiss LSM780 confocal microscopy and 3D reconstruction of the confocal stacks was done with IMARIS software (BITPLANE scientific software) as described previously in Postiglione et al. (2011). Briefly, we define x, y, and z coordinates of the two centrosomes and five points within the ventricular surface of the 3D-rendered mitotic progenitors. These five points are used to determine the best-fitting plane by orthogonal distance regression. Then, we calculate the angle φ between the vector connecting two centrosomes and the normal vector of the best-fitting plane. The angle α of spindle orientation is calculated as 90° minus the angle φ. To estimate the uncertainty of each spindle orientation angle, we repeat this calculation for all possible combinations using only four out of the five points within the plane and determine the angular SD of the resulting normal vectors.
Immunoprecipitation and Western Blot
PP4cfl/fl;NesCre and control forebrains were homogenized with lysis buffer (10 mM Tris [pH 7.5], 135 mM NaCl, 5 mM EDTA, 0.5% Triton X-100) containing protease inhibitor mixture and protein phosphatase inhibitor (Roche). The lysates were subjected to immunoprecipitation with anti-Ndel1 antibody or IgG control with Dynabeads protein G (Invitrogen). The elution and input were loaded onto a 3%–8% Bis-Tris gel (Invitrogen), blotted with primary and secondary antibodies, and visualized with ECL Plus (Amersham Biosciences).
Primary antibodies used were rabbit anti-Lis1 (1:500, Santa Cruz), rabbit anti-Ndel1 (1:2,000; Toyo-oka et al., 2008), goat anti-PP4c (1:500, Santa Cruz), and mouse anti-α-Tubulin (1:5,000, Sigma).
Cell-Cycle Exit Assay
EdU labeling was carried out by intraperitoneal injection of 100 μm of 1 mg/ml EdU in PBS into pregnant mice carrying E12.5 embryos. Twenty-four hours later, mice were killed and embryonic brains were dissected and sectioned. Sections were stained with anti-Ki67 antibody (1:100, BD pharmingen) and postfixed with 4% PFA before the EdU detection (Invitrogen).
Acknowledgments
We thank Angela Peer for excellent technical assistance, Karin Paiha and Pawel Pasierbek for excellent bio-optics support and image analysis, and N. Gaiano for providing the CBFRE-GFP construct. We are grateful to all members of the J.A.K. laboratory for discussions and particularly to Madeline Lancaster for comments on the manuscript. Y.X. was supported by a Lise Meitner postdoctoral fellowship (FWF, M1147-B09). Work in J.A.K.’s laboratory is supported by the Austrian Academy of Sciences, the Austrian Science Fund (FWF, Z153-B09, I552-B19), and an advanced grant from the European Research Council (ERC, NeuroSystem PN 250342). Experiments were conceived and designed by Y.X. and J.A.K. and carried out by Y.X. S.H. provided the PP4c knockout mice and reagents. C.J. performed 3D spindle analysis. C.E. and Y.X. performed ultrasound-guided in utero electroporation. The manuscript was written by Y.X. and J.A.K.
Published: July 3, 2013
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes six figures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2013.05.027.
Supplemental Information
References
- Attardo A., Calegari F., Haubensak W., Wilsch-Bräuninger M., Huttner W.B. Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. PLoS ONE. 2008;3:e2388. doi: 10.1371/journal.pone.0002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman M., Machon O., Mygland L., van den Bout C.J., Zhong W., Taketo M.M., Krauss S. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev. Biol. 2005;279:155–168. doi: 10.1016/j.ydbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Bowman S.K., Neumüller R.A., Novatchkova M., Du Q., Knoblich J.A. The Drosophila NuMA Homolog Mud regulates spindle orientation in asymmetric cell division. Dev. Cell. 2006;10:731–742. doi: 10.1016/j.devcel.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Brand A.H., Livesey F.J. Neural stem cell biology in vertebrates and invertebrates: more alike than different? Neuron. 2011;70:719–729. doi: 10.1016/j.neuron.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Brewis N.D., Street A.J., Prescott A.R., Cohen P.T. PPX, a novel protein serine/threonine phosphatase localized to centrosomes. EMBO J. 1993;12:987–996. doi: 10.1002/j.1460-2075.1993.tb05739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultje R.S., Castaneda-Castellanos D.R., Jan L.Y., Jan Y.N., Kriegstein A.R., Shi S.H. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari F., Haubensak W., Yang D., Huttner W.B., Buchholz F. Tissue-specific RNA interference in postimplantation mouse embryos with endoribonuclease-prepared short interfering RNA. Proc. Natl. Acad. Sci. USA. 2002;99:14236–14240. doi: 10.1073/pnas.192559699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness V.S.J., Jr., Takahashi T., Nowakowski R.S. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. doi: 10.1016/0166-2236(95)93933-o. [DOI] [PubMed] [Google Scholar]
- Chenn A., Walsh C.A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chou S.J., Perez-Garcia C.G., Kroll T.T., O’Leary D.D. Lhx2 specifies regional fate in Emx1 lineage of telencephalic progenitors generating cerebral cortex. Nat. Neurosci. 2009;12:1381–1389. doi: 10.1038/nn.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R.M., Storey K.G. Mitotic spindle orientation can direct cell fate and bias Notch activity in chick neural tube. EMBO Rep. 2012;13:448–454. doi: 10.1038/embor.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C., Fink A., Lau C., Pham D., Daza R.A., Bulfone A., Kowalczyk T., Hevner R.F. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Walsh C.A. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron. 2004;44:279–293. doi: 10.1016/j.neuron.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Franco S.J., Gil-Sanz C., Martinez-Garay I., Espinosa A., Harkins-Perry S.R., Ramos C., Müller U. Fate-restricted neural progenitors in the mammalian cerebral cortex. Science. 2012;337:746–749. doi: 10.1126/science.1223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N., Nye J.S., Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Gauthier-Fisher A., Lin D.C., Greeve M., Kaplan D.R., Rottapel R., Miller F.D. Lfc and Tctex-1 regulate the genesis of neurons from cortical precursor cells. Nat. Neurosci. 2009;12:735–744. doi: 10.1038/nn.2339. [DOI] [PubMed] [Google Scholar]
- Gorski J.A., Talley T., Qiu M., Puelles L., Rubenstein J.L., Jones K.R. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M., Huttner W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Gruber R., Zhou Z., Sukchev M., Joerss T., Frappart P.O., Wang Z.Q. MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nat. Cell Biol. 2011;13:1325–1334. doi: 10.1038/ncb2342. [DOI] [PubMed] [Google Scholar]
- Guillemot F., Zimmer C. From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron. 2011;71:574–588. doi: 10.1016/j.neuron.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Hartfuss E., Galli R., Heins N., Götz M. Characterization of CNS precursor subtypes and radial glia. Dev. Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Haubensak W., Attardo A., Denk W., Huttner W.B. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydar T.F., Ang E.J., Jr., Rakic P. Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc. Natl. Acad. Sci. USA. 2003;100:2890–2895. doi: 10.1073/pnas.0437969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helps N.R., Brewis N.D., Lineruth K., Davis T., Kaiser K., Cohen P.T. Protein phosphatase 4 is an essential enzyme required for organisation of microtubules at centrosomes in Drosophila embryos. J. Cell Sci. 1998;111:1331–1340. doi: 10.1242/jcs.111.10.1331. [DOI] [PubMed] [Google Scholar]
- Howard B., Chen Y., Zecevic N. Cortical progenitor cells in the developing human telencephalon. Glia. 2006;53:57–66. doi: 10.1002/glia.20259. [DOI] [PubMed] [Google Scholar]
- Huttner W.B., Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr. Opin. Cell Biol. 2005;17:648–657. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Iulianella A., Sharma M., Vanden Heuvel G.B., Trainor P.A. Cux2 functions downstream of Notch signaling to regulate dorsal interneuron formation in the spinal cord. Development. 2009;136:2329–2334. doi: 10.1242/dev.032128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich J.A. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Konno D., Shioi G., Shitamukai A., Mori A., Kiyonari H., Miyata T., Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Kosodo Y., Röper K., Haubensak W., Marzesco A.M., Corbeil D., Huttner W.B. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A., Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Knoblich J.A. Spindle orientation in mammalian cerebral cortical development. Curr. Opin. Neurobiol. 2012;22:737–746. doi: 10.1016/j.conb.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui J.H., Hansen D.V., Kriegstein A.R. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini M.C., Walsh C.A. What disorders of cortical development tell us about the cortex: one plus one does not always make two. Curr. Opin. Genet. Dev. 2011;21:333–339. doi: 10.1016/j.gde.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli M., Gonzalez C. On the inscrutable role of Inscuteable: structural basis and functional implications for the competitive binding of NuMA and Inscuteable to LGN. Open Biol. 2012;2:120102. doi: 10.1098/rsob.120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T., Kawaguchi A., Saito K., Kawano M., Muto T., Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- Mizutani K., Yoon K., Dang L., Tokunaga A., Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Molyneaux B.J., Arlotta P., Menezes J.R., Macklis J.D. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Morin X., Bellaïche Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell. 2011;21:102–119. doi: 10.1016/j.devcel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Neumüller R.A., Richter C., Fischer A., Novatchkova M., Neumüller K.G., Knoblich J.A. Genome-wide analysis of self-renewal in Drosophila neural stem cells by transgenic RNAi. Cell Stem Cell. 2011;8:580–593. doi: 10.1016/j.stem.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M., Smith D.S., Ayala R., Peng J., Ko J., Lee M.S., Morabito M., Tsai L.H. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Noctor S.C., Martínez-Cerdeño V., Ivic L., Kriegstein A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Pierfelice T., Alberi L., Gaiano N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron. 2011;69:840–855. doi: 10.1016/j.neuron.2011.02.031. [DOI] [PubMed] [Google Scholar]
- Postiglione M.P., Jüschke C., Xie Y., Haas G.A., Charalambous C., Knoblich J.A. Mouse inscuteable induces apical-basal spindle orientation to facilitate intermediate progenitor generation in the developing neocortex. Neuron. 2011;72:269–284. doi: 10.1016/j.neuron.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara S., O’Leary D.D. Fgf10 regulates transition period of cortical stem cell differentiation to radial glia controlling generation of neurons and basal progenitors. Neuron. 2009;63:48–62. doi: 10.1016/j.neuron.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa A., Mao C.A., Hadjantonakis A.K., Klein W.H., Broccoli V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron. 2008;60:56–69. doi: 10.1016/j.neuron.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T., Ayala R., Nguyen M.D., Xie Z., Gleeson J.G., Tsai L.H. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron. 2004;44:263–277. doi: 10.1016/j.neuron.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Siegrist S.E., Doe C.Q. Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell. 2005;123:1323–1335. doi: 10.1016/j.cell.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Siller K.H., Doe C.Q. Spindle orientation during asymmetric cell division. Nat. Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- Sousa-Nunes R., Chia W., Somers W.G. Protein phosphatase 4 mediates localization of the Miranda complex during Drosophila neuroblast asymmetric divisions. Genes Dev. 2009;23:359–372. doi: 10.1101/gad.1723609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna E., Huttner W.B. Neural progenitor nuclei IN motion. Neuron. 2010;67:906–914. doi: 10.1016/j.neuron.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Toyo-oka K., Mori D., Yano Y., Shiota M., Iwao H., Goto H., Inagaki M., Hiraiwa N., Muramatsu M., Wynshaw-Boris A. Protein phosphatase 4 catalytic subunit regulates Cdk1 activity and microtubule organization via NDEL1 dephosphorylation. J. Cell Biol. 2008;180:1133–1147. doi: 10.1083/jcb.200705148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.E., Beronja S., Pasolli H.A., Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingling J., Youn Y.H., Darling D., Toyo-Oka K., Pramparo T., Hirotsune S., Wynshaw-Boris A. Neuroepithelial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell. 2008;132:474–486. doi: 10.1016/j.cell.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.