Abstract
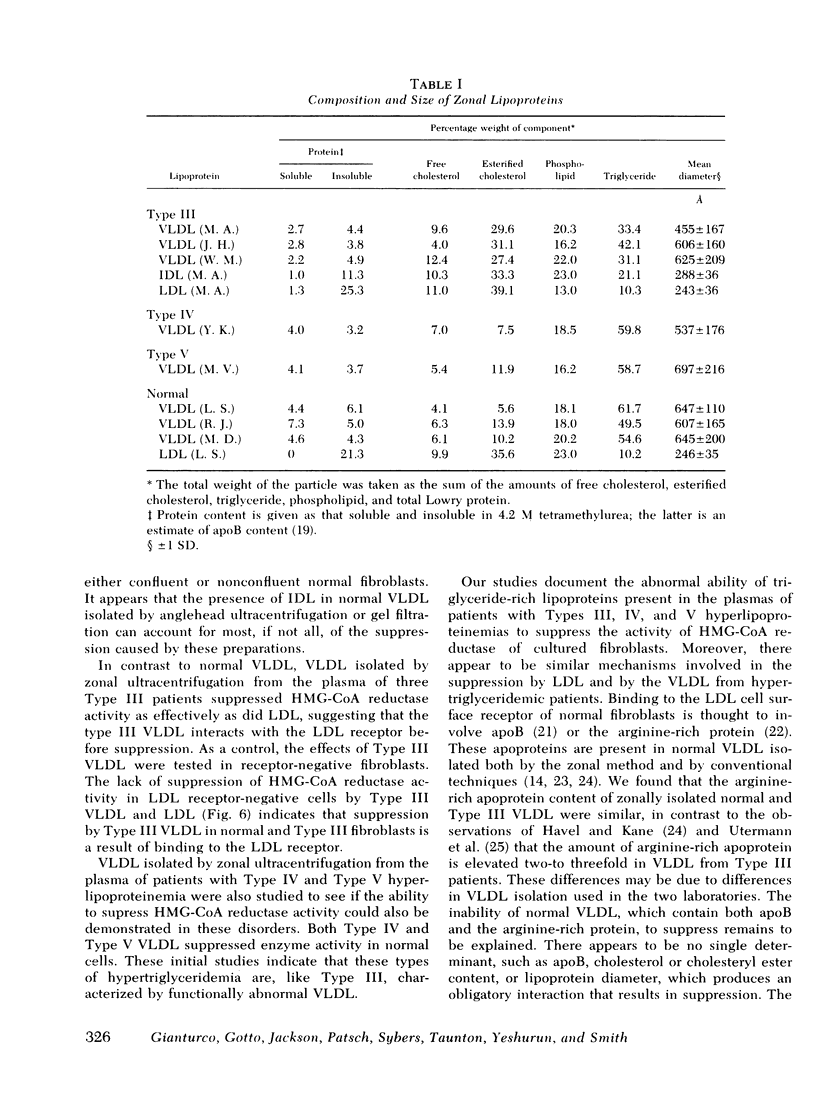
Very low density lipoproteins (VLDL) and low density lipoproteins (LDL) from human normolipemic plasma, and the VLDL, the intermediate density lipoprotein (IDL), and LDL from patients with Type III hyperlipoproteinemic plasma were tested for their abilities to suppress the activity of 3-hydroxy-3-methylglutaryl-Coenzyme A (HMG-CoA) reductase in cultured human fibroblasts from normal subjects and a Type III patient. Regulation of cholesterol synthesis in the fibroblasts of a patient with Type III hyperlipoproteinemia appears to be normal. VLDL from normal subjects, isolated by angle head ultracentrifugation (d < 1.006) or by gel filtration on BioGel A-5m, were about 5 times less effective than LDL in suppressing HMG-CoA reductase activity, based on protein content, in agreement with previous reports with normal fibroblasts. Zonal centrifugation of normal VLDL isolated by both methods showed that the VLDL contained IDL. Normal VLDL from the angle head rotor, refractionated by the zonal method, had little, if any, ability to suppress the HMG-CoA reductase activity in either normal or Type III fibroblasts. VLDL, IDL, and LDL fractionated by zonal ultracentrifugation from Type III plasma gave half-maximum inhibition at 0.2-0.5 μg of protein/ml, indistinguishable from the suppression caused by normal LDL. Type III VLDL did not suppress HMG-CoA reductase in mutant LDL receptor-negative fibroblasts. Zonally isolated VLDL obtained from one Type IV and one Type V patient gave half-maximal suppression at 5 and 0.5 μg of protein/ml, respectively. Molecular diameters and apoprotein compositions of the zonally isolated normal and Type III VLDL were similar; the major difference in composition was that Type III VLDL contained more cholesteryl esters and less triglyceride than did normal VLDL. The compositions and diameters of the Type IV and Type V VLDL were similar to normal VLDL. These findings show that the basic defect in Type III hyperlipoproteinemia is qualitatively different from the cellular defect found in familial hypercholesterolemia, since the regulation of HMG-CoA reductase activity is normal in Type III fibroblasts. The metabolic defect in hypertriglyceridemia is related to the triglyceriderich lipoproteins which, free of other lipoproteins, have an enhanced ability to interact with cultured fibroblasts to regulate HMG-CoA reductase activity. These studies suggest that, in hypertriglyceridemia, there is a mechanism for direct cellular catabolism of VLDL which is not functional for normal VLDL.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bersot T. P., Mahley R. W., Brown M. S., Goldstein J. L. Interaction of swine lipoproteins with the low density lipoprotein receptor in human fibroblasts. J Biol Chem. 1976 Apr 25;251(8):2395–2398. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science. 1976 Jan 16;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Lipoprotein receptors, cholesterol metabolism, and atherosclerosis. Arch Pathol. 1975 Apr;99(4):181–184. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P. Primary dysbetalipoproteinemia: predominance of a specific apoprotein species in triglyceride-rich lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2015–2019. doi: 10.1073/pnas.70.7.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazzard W. R., Bierman E. L. Delayed clearance of chylomicron remnants following vitamin-A-containing oral fat loads in broad-beta disease (type III hyperlipoproteinemia). Metabolism. 1976 Jul;25(7):777–801. doi: 10.1016/0026-0495(76)90149-9. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Removal of lipids from human plasma low-density lipoprotein by detergents. Biochemistry. 1971 Jun 22;10(13):2542–2547. doi: 10.1021/bi00789a019. [DOI] [PubMed] [Google Scholar]
- Kane J. P. A rapid electrophoretic technique for identification of subunit species of apoproteins in serum lipoproteins. Anal Biochem. 1973 Jun;53(2):350–364. doi: 10.1016/0003-2697(73)90081-x. [DOI] [PubMed] [Google Scholar]
- Kostner G. M., Patsch J. R., Sailer S., Braunsteiner H., Holasek A. Polypeptide distribution of the main lipoprotein density classes separated from human plasma by rate zonal ultracentrifugation. Eur J Biochem. 1974 Jun 15;45(2):611–621. doi: 10.1111/j.1432-1033.1974.tb03587.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moses R. E. Replicative deoixyribonucleic acid synthesis in a system diffusible for macromolecules. J Biol Chem. 1972 Oct 10;247(19):6031–6038. [PubMed] [Google Scholar]
- Patsch J. R. Lipoprotein of the density 1.006-1.020 in the plasma of patients with type III hyperlipoproteinaemia in the postabsorptive state. Eur J Clin Invest. 1975 Feb;5(1):45–55. doi: 10.1111/j.1365-2362.1975.tb00427.x. [DOI] [PubMed] [Google Scholar]
- Patsch J. R., Sailer S., Braunsteiner H., Forte T. Electron microscopic characterization of lipoproteins from patients with familial type III hyperlipoproteinaemia. Eur J Clin Invest. 1976 Jul 30;6(4):307–310. doi: 10.1111/j.1365-2362.1976.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Patsch J. R., Sailer S., Kostner G., Sandhofer F., Holasek A., Braunsteiner H. Separation of the main lipoprotein density classes from human plasma by rate-zonal ultracentrifugation. J Lipid Res. 1974 Jul;15(4):356–366. [PubMed] [Google Scholar]
- Rudel L. L., Lee J. A., Morris M. D., Felts J. M. Characterization of plasma lipoproteins separated and purified by agarose-column chromatography. Biochem J. 1974 Apr;139(1):89–95. doi: 10.1042/bj1390089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röschlau P., Bernt E., Gruber W. Enzymatische Bestimmung des Gesamt-Cholesterins im Serum. Z Klin Chem Klin Biochem. 1974 Sep;12(9):403–407. [PubMed] [Google Scholar]
- Shore V. G., Shore B. Heterogeneity of human plasma very low density lipoproteins. Separation of species differing in protein components. Biochemistry. 1973 Jan 30;12(3):502–507. doi: 10.1021/bi00727a022. [DOI] [PubMed] [Google Scholar]
- Utermann G., Jaeschke M., Menzel J. Familial hyperlipoproteinemia type III: deficiency of a specific apolipoprotein (apo E-III) in the very-low-density lipoproteins. FEBS Lett. 1975 Aug 15;56(2):352–355. doi: 10.1016/0014-5793(75)81125-2. [DOI] [PubMed] [Google Scholar]


