Abstract
Victims of infection are expected to suffer increasingly as parasite population growth increases. Yet, under some conditions, faster growing parasites do not appear to cause more damage and infections can be quite tolerable. We studied these conditions by assessing how the relationship between parasite population growth and host health is sensitive to environmental variation. In experimental infections of the crustacean Daphnia magna and its bacterial parasite Pasteuria ramosa we show how easily an interaction can shift from a severe interaction, i.e. when host fitness declines substantially with each unit of parasite growth, to a tolerable relationship by changing only simple environmental variables: temperature and food availability. We explored the evolutionary and epidemiological implications of such a shift by modelling pathogen evolution and disease spread under different levels of infection severity, and find that environmental shifts that promote tolerance ultimately result in populations harbouring more parasitized individuals. We also find that the opportunity for selection, as indicated by the variance around traits, varied considerably with the environmental treatment. Thus our results suggest two mechanisms that could underlie co-evolutionary hot- and coldspots: spatial variation in tolerance and spatial variation in the opportunity for selection.
Keywords: co-evolution, context-dependent, virulence, parasitism, tolerance
Introduction
The outcome of interspecific interactions is frequently context-dependent (Thompson 1994, 2005). For example, in natural plant communities, variation in physical and biological factors is known to lead to a continuum of interspecific interactions, moving from facilitation to competition as environmental stress decreases (Callaway and Walker 1997; Callaway et al. 2002). Similar shifts between mutualism and antagonism are also known (e.g. Michalakis et al. 1992; Thompson and Fernandez 2006; Fellous and Salvaudon 2009; Ryan and Kohler 2010), especially when changes in abiotic and biotic conditions render mutualistic partnerships too costly and antagonism more profitable (Sachs and Simms 2006; Kiers et al. 2010). Parasitism is the ultimate case of antagonistic exploitation: parasites (or pathogens) gain fitness by growing within hosts and extracting host resources, which reduces host health often causing death. The severity of this exploitation (commonly called virulence) is also recognized to be context-dependent (Michalakis et al. 1992; Fellous and Salvaudon 2009; Wolinska and King 2009). This was perhaps first formalized in the “disease triangle” (McNew 1960; Scholthof 2006), a conceptual framework that explicitly acknowledges infection outcomes as the product of three factors: host genetics, parasite genetics, and the environmental conditions both experience during infection. It is the epidemiological and evolutionary consequences of such context-dependent parasitism that we address in this article.
There is currently a vast body of empirical evidence demonstrating the pervasiveness of environment-dependent infection (reviewed in Lazzaro and Little 2009; Wolinska and King 2009). While many of these studies impose similar environmental treatments during experimental infection, they often differ with regard to the traits they measure and, consequently, the conclusions that may be drawn from them. Many studies considered the effect of environmental treatments (often food level or temperature) on the probability or severity of infection (see Wolinska and King 2009 and references therein). The severity of infection in such studies is frequently gauged by the magnitude of the change in the mean of host traits (such as longevity or fecundity) under different infection conditions (e.g. Blanford et al. 2003; Bedhomme et al. 2004; Restif and Kaltz, 2006; Tops et al. 2009). These effects usually reflect environment-induced changes in host condition or resistance (e.g. Jokela et al. 1999; Brown et al. 2000; Lazzaro and Little 2008) and / or shifts in the optimal conditions for parasite growth (Thomas and Blanford 2003; Fels and Kaltz, 2006; Tseng 2006; Little et al. 2007; Seppälä et al. 2008; Allen and Little in press). Other studies have gone further and included several genotypes of hosts or parasites, thereby uncovering genotype-specific responses to environmental conditions (Mitchell et al. 2005; Lambrechts at al. 2006a; Laine 2007; Lazzaro et al. 2008; Vale et al. 2008). Apart from analyzing changes in the mean severity of infection, such studies lend insight into potential mechanisms for the maintenance of polymorphism due to environment-dependent selection (Byers 2005; Laine and Tellier 2008; Wolinska and King 2009). In the few cases where host and parasite genotypes have been studied simultaneously (Tétard-Jones et al. 2007; Vale and Little 2009) environmental effects on host-parasite specificity have been explored. Given the role of host-parasite specificity in fostering co-evolution (Hamilton, 1993; Lambrechts et al. 2006b), this type of experiment begins to address the role of environmental variation on speeding up or tempering co-evolutionary dynamics, an idea formalized by the Geographic Mosaic Theory of Co-evolution (Thompson 1994, 2005).
A common aspect in the above studies is that host or parasite traits are considered independently of each other. However, a clear implication of the disease triangle, which is supported by the experimental evidence mentioned above, is that host and parasite traits measured during infection depend on the interaction between the host, the parasite, and their abiotic environment. Taking host mortality under infection as an example, while it can be directly caused by parasite population growth, it is also certainly influenced by the host’s ability to withstand the damage caused by this growth, termed tolerance (reviewed in Raberg et al. 2009). Studying tolerance is therefore not possible by analyzing changes in mean host traits under infection, but instead it requires measuring the reduction in host health per unit of parasite population growth, that is, the per parasite virulence (Little et al. 2010). Thus, the trait of interest is not some measure of host health or parasite growth per se, but rather the relationship between them.
Knowledge of the relationship between host health and parasite growth is thus essential for elucidating the evolutionary trajectories of both interactors. Under parasitism we should expect increased parasite population growth to cause a greater reduction in host health. This fundamental description of parasitism is embodied in theory on parasite evolution (Anderson and May 1982; Bull 1994; Frank 1996; reviewed in Alizon et al. 2009) and generally makes the assumption that higher within-host parasite population growth leads to increased host death rate (usually termed virulence in the literature) and therefore a shorter infectious period leading to less parasite transmission. Understanding parasite evolution is therefore only possible by analysing the relationships between host health and parasite growth, and parasite induced mortality is the most frequent indicator of host health in the literature (Anderson and May 1982; Frank 1996).
The relationship between parasite population growth and host health has been studied in a number of host-parasite systems, notably in laboratory studies of rodent malaria (Mackinnon and Read 1999; Mackinnon and Read 2004), and in natural populations of monarch butterflies and a protozoan parasite (de Roode and Altizer 2010; de Roode et al. 2008). In these systems, parasites that grow faster during infection (for example certain genotypes may show a higher intrinsic rate of growth), also produce a higher number of transmission stages and reduce host health more severely than parasites that grow more slowly. Other studies however have not detected the expected relationship between parasite population growth and host health (Little et al. 2008; Sacristan et al. 2005; Salvaudon et al. 2007). In these experiments, parasite genotypes with higher growth either did not reduce host health more than slower growing genotypes (Little et al. 2008; Sacristan et al. 2005), or they only reduced the health of some host genotypes (Salvaudon et al. 2007). These examples may reflect the inherent difficulty of obtaining accurate measures of parasite population growth and host health, but also highlight that, at least in some conditions, the burden of parasitism may not clearly manifest (Michalakis et al. 1992; Lipsitch and Moxon 1997, Fellous and Salvaudon 2009).
Parasite growth diverts a considerable amount of host resources that would otherwise be available for host maintenance and reproduction (Ebert et al. 2004; Hall et al. 2009), so one possible explanation for variable relationships between parasite population growth and host health is that under some conditions the conflict over limited resources is less severe (Salvaudon et al. 2007; Hall et al. 2009), and hosts are better able to tolerate parasites (Raberg et al. 2009). This could arise if resource abundance varies (Hall et al. 2009), or if some environmental conditions (e.g. temperature) change the rate of resource utilization. Despite ample evidence of genotype-by-environment effects on resistance to infection (reviewed in Lazzaro and Little 2009; Wolinska and King 2009), the possibility that environmental conditions can promote tolerance by shifting the relationship between parasite growth and host health has not been explored.
Here we study such environment-mediated tolerance to infection with experimental studies, complimented by mathematical modelling to probe its consequences on disease spread and pathogen evolution. This work incorporates statistical methodology that is often neglected in the host-parasite literature, but is appropriate for analysing relationships between infection-related traits where cause and effect is uncertain.
Methods
Experiment
Below we provide brief summary of the experimental protocol used for measuring parasitism under food and temperature variation. Detailed experimental methods can be found in online Appendix A. Twelve replicates of each eight host genotypes were maintained in standard lab conditions for three generations. Following a split-jar design, offspring from each replicate jar were split into different treatments and exposed to sympatric parasite spores under high or low food treatments chemostat-grown Chlorella vulgaris microalgae (High food - Absλ=665 = 1.5, approximately 1.5-2 ml per jar; Low food Absλ=665 = 0.3, approximately 0.3 - 0.5 ml per jar), at three temperatures (15°C, 20°C, and 25°C). Infections were carried out on 5-day old female Daphnia, by adding a fixed number of spores to each jar. After exposure, hosts were transferred to clean medium, and their infection status, fecundity, and mortality were recorded daily. All infected hosts were followed until their death to gain precise measures of parasite lifetime transmission potential (LTP), by counting the number of spores produced on the day of death of each infected host using a Cell Counter (Casy, Model TT).
Data analysis
Ultimately our aim was to study the covariance between traits, in particular between parasite lifetime transmission potential (LTP) and the variables that estimate host fitness when they are infected. Before modelling the among-trait correlation structure, however, we first fitted univariate models for each of the three response variables, with food and temperature as explanatory covariates (factors) as well as genotype (as an 8 level factor) and all first order interactions of these terms. The purpose of these univariate analyses was to determine whether trait expression varies with experimental food or temperature treatment. In the absence of such effects we would not expect the correlation among traits to vary with treatment either. Hence, while it is not the principle goal of this study, these univariate analyses also provide a direct test for genetic (i.e. among-genotype) variance in response variables, and for genotype-by-environment (i.e. treatment) effects).
We then fitted a series of multivariate linear models to test the sensitivity of the relationship between parasite transmission (LTP) and host fitness, estimated as both host mortality and host fecundity, to the environmental variables of food treatment and temperature. Since the causal relationships between LTP and host fitness are unknown we used correlations rather than regression to describe the among-trait associations (see Graham et al. in press). Firstly, we defined food treatment-specific subtraits (LTP, mortality and fecundity measured under low and high food treatments respectively) and estimated the correlation structure among them using a six-trait model. Genotype and temperature were included as explanatory covariates (factors) on each subtrait. This analysis yielded estimates of trait variances and among-trait covariances (for traits measured under the same food treatment) that were rescaled to correlations. These correlations should be interpreted as describing the relationships among traits after correcting for variation due to genotype and/or temperature.
To test the statistical significance of each correlation we compared the model likelihood of that of a reduced model in which the relevant correlation was constrained to equal 0 using a likelihood ratio test. Then to test our specific hypothesis that the LTP-host fitness correlations differ across food treatments we then compared the likelihood of the full model to one in which we constrained either rLTP-mortality or rLTP-fecundity as appropriate to be equal in the two food treatments. Finally, since the variance in relative fitness (i.e. fitness/mean fitness) sets an upper limit for selection (the opportunity for selection; see main text), we also tested for differences in this parameter (after conditioning on genotype and temperature) across food treatments. This was done by dividing each observation of our fitness traits (LTP, mortality and fecundity) by the corresponding within-treatment mean before refitting the full model as described above. We then compared the likelihood of this model to that obtained when the variances of relative LTP, mortality or fecundity were constrained to be equal across food treatments. This provides an explicit test of whether the opportunity for selection through each measure of fitness differs with treatment.
An identical approach was then used to investigate the effect of temperature on host-parasite relationships. Since three temperature regimes (15°C, 20°C and 25°C) were imposed the full multivariate model was specified for nine response variables, with covariates of genotype and food treatment specified on each response. In all other respects the analyses were conducted as described above. All models were solved using restricted maximum likelihood using ASReml V2.0. To facilitate the use of bivariate analysis in other types of interspecific interactions beyond parasitism, we have included code for ASReml V2.0 (Appendix C), along with the original data we present here in (Appendix D).
Modelling
We modelled the evolutionary dynamics of our system using an adaptive dynamics analysis (Geritz et al. 1998) of a modified Susceptible-Infected (SI) model (Anderson and May 1982) that captures the infection biology of D. magna and P. ramosa. We studied parasite evolution under different relationships between growth rate and mortality, as reflected in the experimental data, by considering the stability of the equilibrium of the resident and the rare mutant under these conditions (see Online Appendix B for details of model and analysis).
Results and Discussion
Environmental effects on individual traits
We first tested the effect of host genotype, food and temperature on the number of transmission spores produced on the day of host death, host offspring production, and mortality using a univariate analysis of each response variable (Table 1). We found that host genotype affected host offspring production, but had no direct effect on either parasite population growth or mortality rate. Food levels affected all measured traits and for offspring production this depended on the host genotype (Food x genotype interaction). Temperature had similar effects on host mortality and on the number of transmission spores produced which in this case depended completely on the host genotype involved in the infection (Temperature × genotype interaction).
Table 1.
Univariate analysis of host genotype, food and temperature effects.
| Response | Source of variation | Numerator DF |
F | p-value |
|---|---|---|---|---|
|
|
||||
| LTP | Food | 2 | 525.03 | <0.001 |
| Temperature | 2 | 0.34 | 0.715 | |
| Host genotype | 7 | 1.20 | 0.305 | |
| Food × Temperature | 2 | 2.97 | 0.054 | |
| Food × Genotype | 7 | 1.86 | 0.078 | |
| Temperature × Genotype | 14 | 2.02 | 0.018 | |
|
|
||||
| Fecundity | Food | 2 | 848.28 | <0.001 |
| Temperature | 2 | 77.96 | <0.001 | |
| Host genotype | 7 | 4.60 | <0.001 | |
| Food × Temperature | 2 | 36.86 | <0.001 | |
| Food × Genotype | 7 | 2.23 | 0.034 | |
| Temperature × Genotype | 14 | 0.81 | 0.659 | |
|
|
||||
| Mortality | Food | 2 | 1611.75 | <0.001 |
| Temperature | 2 | 190.03 | <0.001 | |
| Host genotype | 7 | 1.54 | 0.155 | |
| Food × Temperature | 2 | 0.28 | 0.757 | |
| Food × Genotype | 7 | 1.72 | 0.107 | |
| Temperature × Genotype | 14 | 1.21 | 0.270 | |
Conditional F tests of food, temperature and host genotype effects on response variable of parasite growth (LTP, the lifetime transmission potential), host fecundity and host mortality. Results are from univariate linear models of each response variable and the denominator degrees of freedom is 195 in all cases. Note that, in contrast to the bivariate analyses which follow, Host Genotype here is a fixed effect and thus differences among genotypes can not be used to make inferences about a wider hypothetical source population; significant differences refer only to the genotypes studied.
Relationships between traits
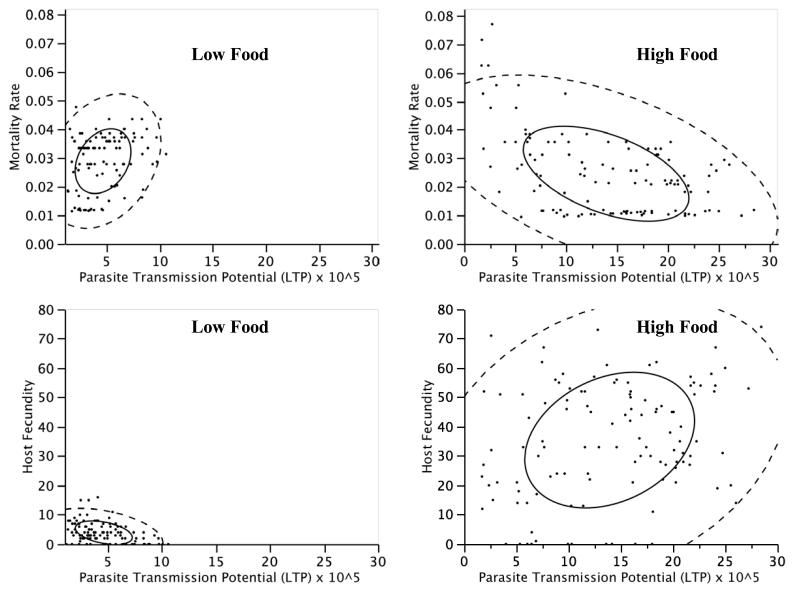
We were especially interested in understanding how these environmental treatments affected the severity of parasitism, as inferred from the relationship between parasite population growth (measured as the lifetime transmission potential, LTP) and host health. We found that these relationships were modified by both food and temperature variation (Table 2). Greater parasite growth can cause greater harm (i.e. increased mortality and decreased fecundity), especially to hosts in the low food treatment, but with abundant food, this is often not the case (Figure 1). It was unexpected that the relationship between host health and parasite population growth might even be positive, but it appears that with abundant resources both host and parasite are able to simultaneously prosper, and parasites grow with little impact on host health (see also Krist et al. 2004). We interpret this as the outcome of reduced conflict for shared resources between hosts and parasites under high food (Hall et al. 2009). From a parasite perspective, well-fed hosts are good resources (Ebert et al. 2004), and thus when hosts are doing well, parasites also benefit. We emphasize, however, that a positive relationship between host health and parasite growth does not equate with mutualism in this case, because our analysis only included infected hosts, and regardless of environmental treatment there is always a cost of being infected relative to uninfected hosts (see for example Vale et al. 2008).
Table 2.
Context-dependent parasitism.
| Food |
Temperature |
||||
|---|---|---|---|---|---|
| Low | High | 15°C | 20°C | 25°C | |
| LTP-Mortality | −0.06 (0.09) |
−0.61 (0.06) *** |
−0.48 (0.11) |
−0.75 (0.05) |
−0.51 (0.09) * |
| LTP-Fecundity | −0.26 (0.09) |
0.16 (0.09) ** |
0.03 (0.12) |
0.44 (0.09) |
0.14 (0.12) * |
Numbers are correlation coefficients (r) and their standard errors (in parenthesis) that describe how the relationship between host mortality or fecundity and parasite lifetime transmission potential (LTP) is affected by food levels and by different temperatures, after accounting for the variation introduced by host genotype and the other effect (i.e. food after controlling for temperature (Figure 1) or temperature after controlling for food (Figure2)).
p < 0.0001,
p < 0.01,
p <0.05. See Methods for statistical details.
Figure 1. Food-dependent parasitism.
Relationships between host fitness traits and parasite lifetime transmission potential (LTP). Upper panels: the relationship between parasite LTP and host mortality rate under low or high food availability, after accounting for the effects of host genotype and temperature. Lower panels, the relationship between parasite LTP and host fecundity under low or high food availability, after accounting for the effects of host genotype and temperature. Shown are density ellipses that include 50% (solid lines) or 95% of all data points.
That host and parasite traits are in greater conflict under low food (i.e. resource competition is acting) is not unlike trade-offs between life-history traits of single species, which are more readily detected under resource-limited environments (Mckean et al. 2008; Sgrò and Hoffmann 2004). The ‘trade-off’ we study is different in that it is between host and parasite fitness traits, that is, between two different species, but it is also brought about by competition for limited resources. It is precisely this conflict that results in the trade-off between host and parasite fitness expected under parasitism (Bull 1994).
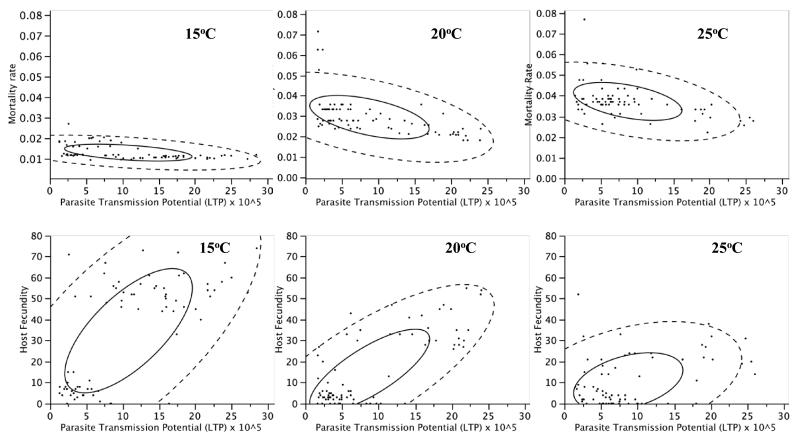
Temperature (Figure 2) caused similar alterations, and these may also be due to variation in the level of conflict over resources between host and parasite. Pasteuria ramosa growth is reduced at 15°C, which should reduce the competition for resources and therefore also the detrimental effects of parasitism (Vale et al. 2008). However, temperature also affects Daphnia physiology, with higher temperatures leading to accelerated development (Mitchell et al. 2005), and perhaps greater demands for food. Figure 2 illustrates how such demands for food might differ across temperatures: at both 15°C and 20°C two clear clusters are visible when plotting host fecundity against parasite population growth, corresponding to the low and high food treatments. However, the overall correlation still changes with temperature (Figure 2; Table 2), meaning that after taking into account any possible effects of food (and genotype) the relationship between host health and parasite growth is still significantly altered by temperature. Whatever the basis of changes in the relationship between host and parasite fitness with temperature, variation in temperature does not affect host and parasite equally, with consequences for the degree of conflict and the fitness of both interactors. As a result, the relationship between parasite population growth and host health varies significantly (Table 2, Figure 2).
Figure 2. Temperature-dependent parasitism.
Relationships between host fitness traits and parasite lifetime transmission potential (LTP). Upper panels: the relationship between parasite LTP and host mortality rate at 15°C or 20°C or 25°C, after accounting for the effects of host genotype and food. Lower panels, the relationship between parasite LTP and host fecundity at 15°C or 20°C or 25°C, after accounting for the effects of host genotype and food. Shown are density ellipses that include 50% (solid lines) or 95% of all data points.
Our use of bivariate analyses and correlations differed from some previous studies which have used regression-based statistics to study relationships between traits (e.g. Mackinnon & Read 1999; Salvaudon et al. 2007; Raberg et al 2007; de Roode et al. 2008, 2010; Ryan and Kohler 2010). For comparison we have included such an analysis (see Appendix D) and the main result remains: food and temperature change the relationship between host health and parasite population growth. However, our reason for favoring a bivariate analysis is twofold. First, when parasite density is not experimentally controlled (as here), it is measured with error that is typically unaccounted for by regression, leading to underestimation of the magnitude of the slope (Sokal & Rohlf 1995), and an overestimation of tolerance. Second, Model II regression which is often used for these purposes (see for example Legendre and Legendre 1998) assumes a cause-effect relationship between parasite density (the independent variable) and host fitness (the response), but this may not be so (see Graham et al. in press). Thus, there is merit in choosing to treat both parasite density and host fitness as response variables in a bivariate analysis (Graham et al. in press). We would hope that future work on interspecific interactions will measure between-species relationships of traits and analyze them using similar bivariate approaches that do not implicitly assume direct causality.
Parasite evolution and epidemiology
The changing of a parasitic interaction from a severe state to one where hosts tolerate parasites, has considerable epidemiological, evolutionary, and co-evolutionary consequences (Best et al. 2008; Lively 2009; Miller et al. 2006). For example, theoretical work has indicated that increases in host tolerance, even when leading to the evolution of less harmful parasites, tends to ultimately increase disease prevalence and the overall amount of mortality a population suffers (Miller et al. 2006): this is the “tragedy of tolerance”. This conclusion holds for an obligate killer such as P. ramosa. We modelled parasite evolution (see Online Appendix B for model details) and considered different forms of the relationship between parasite population growth (λ) and host mortality rate (α) (Figure 3): a positive relationship (i.e. harshly parasitic), an essentially flat relationship, and one where the relationship is slightly negative.
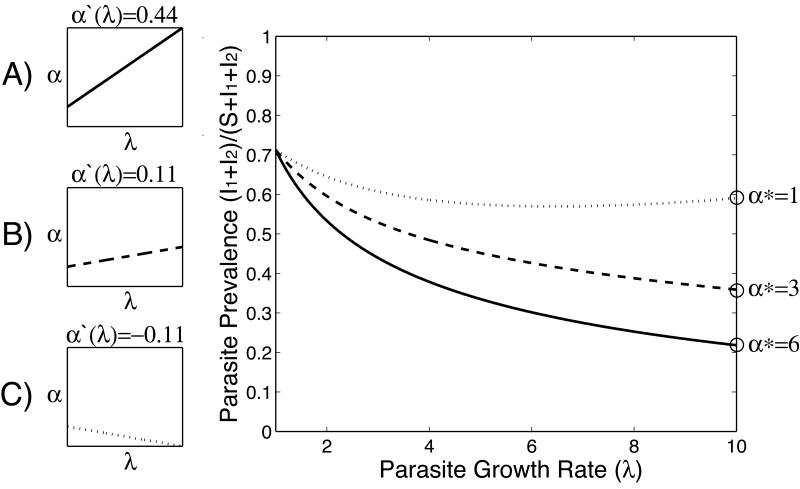
Figure 3. Disease prevalence after context-dependent evolution.
Graph showing how disease prevalence varies with parasite growth rate (λ) for three different α-λ relationships that qualitatively mirror our empirical results. A) the relationship between parasite growth and mortality is positive and relatively steep (α(λ) >> 0, solid line); B) the relationship between parasite growth and mortality is weakly positive (α(λ) > 0, dashed line); and C) the relationship between parasite growth and mortality is weakly negative (α(λ) < 0, dotted line). As the relationship becomes flatter and then negative (i.e. moving from A to C), the mortality of infected hosts is reduced at any level of parasite growth, meaning infected hosts survive for longer and disease prevalence increases. In each case, the parasite will evolve to maximize its growth rate (λ*=10; see Figure B1). The open circles denote the level of evolved mortality for each α-λ relationship at this maximum growth rate. Again, as the relationship becomes flatter and then negative (A to C), the stable level of evolved mortality rate (α*) is reduced. I1, I2 and S are categories of infected (I1+I2) and uninfected susceptible (S) hosts, see Online Appendix B for details.
As improving environmental conditions cause the interaction to become increasingly benign (as hosts increasingly tolerate the pathogen), the pathogens evolve to cause less mortality, despite always maximising growth (Figure 3, see also figure B1). Over time, this affects overall infection prevalence (Figure 3), with lower prevalence occurring where environmental conditions dictate that parasitism is more severe. The reason for this is straightforward: when the relationship between parasite growth and mortality is positive and steep, the evolution of parasite growth and mortality rate (Figure B1) strongly prunes infected hosts from the population, decreasing the proportion of hosts with infection (Figure 3). When the relationship between parasite growth and mortality is flat or even weakly negative, i.e. when hosts can tolerate more parasites, the evolution of low host mortality rate (Figure B1) results in infected individuals persisting in the population for longer, increasing the proportion of hosts with infection. Thus, although favourable local conditions would allow local populations to maintain high parasite prevalence these could, ultimately act as ‘transmission hotspots’ i.e. they could be a pathogen source for neighbouring populations where environmental conditions could dictate more severe infections.
A similar situation in which environmental variation alters the relationship between parasite population growth and host fitness is found in medical interventions that, while not preventing infection, reduce pathology (that is, boost tolerance). Some such interventions (most notably of vaccination that lowers the growth rate or toxicity of established parasites) may have the potential to select for faster replicating, and potentially more harmful pathogens (Gandon and Day 2008). While local populations are able to tolerate these pathogens, they present a particular risk for those (e.g. migrants) who come into contact with the disease but are not vaccinated (Gandon et al. 2001). While theoretical predictions for an obligate killer may differ in part from those for the continuously transmitting parasites modelled in vaccination studies, the key point from our empirical data is that tilting the balance towards either harmful or tolerable infections by altering fitness relationships is achieved with rather subtle changes in environmental conditions, and this will have distinct consequences for local, migrant, or neighbouring populations.
Co-evolution
The geographic mosaic theory of co-evolution postulates that spatial variation in environmental conditions in nature can alter the strength of reciprocal selection, resulting in spatial variation in the strength of co-evolution (so-called “hotspots” and “coldspots”(Thompson 1999). By modulating how host and parasite fitness co-vary, the environmental heterogeneity we have studied illustrates possible mechanisms for Geographic Mosaics. First, the steep to flat relationships between parasite population growth and host health reflect strong to weak parasite-mediated selection, respectively, and so are consistent with the notion that coevolutionary hot- and coldspots easily arise with environmentally-mediated variation in the strength of natural selection. However, conditions of stronger parasite mediated selection as indicated by correlations between host and parasite fitness traits do not necessarily correspond to conditions with the greatest variance in key parasitological traits (Table 3). Thus the “opportunity for selection”, calculated as the variance in relative fitness and sets the upper limit for potential selection on phenotype (Arnold and Wade 1984; Crow 1958) also differs significantly across food and temperature treatments, but in complex ways.
Table 3.
Context-dependent opportunity for selection.
| Food |
Temperature |
||||
|---|---|---|---|---|---|
| Low | High | 15°C | 20°C | 25°C | |
| LTP | 0.18 (0.03) |
0.24 (0.03) |
0.19 (0.03) |
0.31 (0.05) |
0.37 (0.07) *** |
| Fecundity | 0.54 (0.08) |
0.13 (0.02) *** |
0.10 (0.02) |
0.33 (0.06) |
0.68 (0.12) *** |
| Mortality’(×10−3) | 0.11 (0.02) |
1.50 (0.20) *** |
0.41 (0.07) |
98.5 (16.3) |
42.7 (0.08) ** |
Numbers are variance in relative fitness (i.e. variance in fitness/mean fitness: the opportunity for selection; see main text) and their standard errors (in parenthesis) for three traits (host fecundity and mortality, and parasite lifetime transmission potential). We tested for differences in these parameters (after conditioning on genotype and temperature) across food and temperature treatments.
p < 0.0001,
p < 0.01
For example, the opportunity for selection on fecundity is greater at high relative to low food, which should lead to accelerated rates of coevolution, as was shown for Pseudomonas bacteria and phage when grown under conditions of high host productivity (Lopez-Pascua and Buckling 2008). However, higher resource environments might not simply translate into greater variance and more efficient selection (e.g. if variance peaks at intermediate productivity (Kassen et al. 2000; Hall and Colegrave 2007). Indeed, in contrast to the opportunity for selection on fecundity, we found that the opportunity for mortality selection was substantially higher at low food (Table 3). Across temperature treatments we found increasing variance in host (fecundity and mortality) and parasite fitness with temperature (Table 3). Thus at low temperatures both a weak parasite fitness-host fitness relationship and lower variance in fitness traits is likely to result in weaker selection; however in the high temperature treatment, even though the covariance is equally flat, the relatively higher variance should translate into a greater opportunity for selection (Tables 2, 3; Figure 2).
The mechanisms that heat up or temper antagonistic interactions are not clear; there remains a need to gain a fuller view of the co-evolutionary dynamics that are driven by tolerance evolution and the new adaptive peaks this presents to parasites. Most tolerance theory omits successive host (Miller et al. 2006) or parasite (Roy and Kirchner 2000) counter-adaptation, and changes in variance that may accompany alterations in the host fitness-parasite fitness relationship have been little studied in fully co-evolutionary models. Further modelling can only benefit from empirical parameterization of parasitism under real-world levels of genetic and environmental variation.
Co-evolutionary interactions may affect a broader range of traits that initially appear to be unrelated to infectious disease. In particular, a parasitic interaction that switches from relatively harmless (as we observe under high food) to severely damaging (as we observe under low food) reflects density-dependent virulence, as resources are depleted at high host densities. Lively (2009) has shown that density dependent virulence could play a key role in the maintenance of sexual reproduction (the Red Queen hypothesis). Essentially, when host densities are low and resources are abundant, infection is tolerable (as in our experimental results), and asexual clones invade sexual populations due to their intrinsic two-fold reproductive advantage. This causes an increase in host density that depletes resources in the environment, and (as in our experimental results) leads to higher infection severity. Recombination in the sexual hosts results in some genotypes that are resistant to infection altogether, making their persistence more likely; clonal asexuals, however, are quickly purged by infection. This leads to a decrease in density, and an increase in per capita resources that again favours asexuals. Hence, in changing the severity of infection by varying resource availability, density-dependent virulence makes the coexistence of sexual and asexual populations more likely. Thus, the sensitivity of parasitism to environmental conditions or resource availability has implications that extend beyond infection, into the most fundamental biological features of organisms, such as their manner of reproduction. Broadly, if we consider that spatial and temporal environmental heterogeneity is common in natural habitats, our understanding of host-parasite interactions, their co-evolution, and our ability to predict parasite evolution, and especially manage it (Ebert and Bull 2003; Read and Mackinnon 2008), must incorporate knowledge of how environmental variation impacts the base nature of parasitism.
Supplementary Material
Acknowledgements
We thank L. Råberg, M. Stjernman, and D. Shuker for helpful discussion, Phil Wilson for lab assistance, and J. De Roode, J. Jokela and two anonymous reviewers for comments that improved this work. PFV was supported by the GABBA PhD program at the University of Porto, through a PhD studentship from Fundação para a Ciência e Tecnologia, Portugal. TJL is supported by a Wellcome Trust Senior Research Fellowship in Basic Biomedical Sciences. AB was supported by a Natural Environmental Research Council studentship grant.
Footnotes
Elements to appear in full online version: Main Manuscript file, Fig 1-3, Online Appendix A (Detailed experimental methods), Online Appendix B (model).
References
- Allen DE, Little TJ. Dissecting the effect of a heterogeneous environment on the interaction between host and parasite fitness traits. Evolutionary Ecology. 2010 In Press. [Google Scholar]
- Alizon S, Hurford A, Mideo N, Van Baalen M. Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. Journal of Evolutionary Biology. 2009;22:245–259. doi: 10.1111/j.1420-9101.2008.01658.x. [DOI] [PubMed] [Google Scholar]
- Anderson RM, May RM. Coevolution of hosts and parasites. Parasitology. 1982;85(Pt 2):411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Wade MJ. On the measurement of natural and sexual selection. I. Theory. Evolution. 1984;38:709–734. doi: 10.1111/j.1558-5646.1984.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Bedhomme S, Agnew P, Sidobre C, Michalakis Y. Virulence reaction norms across a food gradient. Proceedings of the Royal Society B: Biological Sciences. 2004;271:739–744. doi: 10.1098/rspb.2003.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami F, Regoes R, Ebert D. A quantitative test of the relationship between parasite dose and infection probability across different host–parasite combinations. Proceedings of the Royal Society B: Biological Sciences. 2008;275:853–859. doi: 10.1098/rspb.2007.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best A, White A, Boots M. Maintenance of host variation in tolerance to pathogens and parasites. Proceedings of the National Academy of Science U S A. 2008;105:20786–20791. doi: 10.1073/pnas.0809558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanford S, Thomas MB, Pugh C, Pell JK. Temperature checks the Red Queen? Resistance and virulence in a fluctuating environment. Ecology Letters. 6:2–5. [Google Scholar]
- Bonhoeffer S, Lenski RE, Ebert D. The curse of the pharaoh: the evolution of virulence in pathogens with long living propagules. Proceedings of the Royal Society B: Biological Sciences. 1996;263:715–721. doi: 10.1098/rspb.1996.0107. [DOI] [PubMed] [Google Scholar]
- Bowers RG, Hoyle A, White A, Boots M. The geometric theory of adaptive evolution: trade-off and invasion plots. Journal of Theoreical Biology. 2005;233:363–377. doi: 10.1016/j.jtbi.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Brown MJF, Loosli R, Schmid-Hempel P. Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos. 2000;91(no. 3):421–427. [Google Scholar]
- Bull JJ. Perspective - Virulence. Evolution. 1994;48:1423–1437. doi: 10.1111/j.1558-5646.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Byers DL. Evolution in heterogeneous environments and the potential of maintenance of genetic variation in traits of adaptive significance. Genetica. 2005;123:107–124. doi: 10.1007/s10709-003-2721-5. [DOI] [PubMed] [Google Scholar]
- Callaway RM, Walker LR. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology. 1997;78:1958–1965. [Google Scholar]
- Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ, Michalet R, Paolini L, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417(6891):844–848. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- Carius HJ, Little TJ, Ebert D. Genetic variation in a host-parasite association: potential for coevolution and frequency-dependent selection. Evolution. 2001;55:1136–1145. doi: 10.1111/j.0014-3820.2001.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Chadwick W, Little TJ. A parasite-mediated life-history shift in Daphnia magna. Proceedings of the Royal Society B: Biological Sciences. 2005;272:505–509. doi: 10.1098/rspb.2004.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JF. Some possibilities for measuring selection intensities in man. Hum. Biol. 1958;30:1–13. [PubMed] [Google Scholar]
- Daleo P, Iribarne O. Beyond competition: the stress-gradient hypothesis tested in plant-herbivore interactions. Ecology. 2009 Sep;90(no. 9):2368–2374. doi: 10.1890/08-2330.1. [DOI] [PubMed] [Google Scholar]
- de Mazancourt C, Dieckmann U. Trade-off geometries and frequency-dependent selection. American Naturalist. 2004;164:765–778. doi: 10.1086/424762. [DOI] [PubMed] [Google Scholar]
- Decaestecker E, Gaba S, Raeymaekers JA, Stoks R, Van Kerckhoven L, Ebert D, De Meester L. Host-parasite ‘Red Queen’ dynamics archived in pond sediment. Nature. 2007;450:870–873. doi: 10.1038/nature06291. [DOI] [PubMed] [Google Scholar]
- Decaestecker E, Lefever C, De Meester L, Ebert D. Haunted by the past: Evidence for dormant stage banks of microparasites and epibionts of Daphnia. Limnology and Oceanography. 2004;49:1355–1364. [Google Scholar]
- de Roode JC, Altizer S. Host-parasite genetic interactions and virulence-transmission relationships in natural populations of monarch butterflies. Evolution. 2010;64:502–514. doi: 10.1111/j.1558-5646.2009.00845.x. [DOI] [PubMed] [Google Scholar]
- de Roode JC, Yates AJ, Altizer S. Virulence-transmission trade-offs and population divergence in virulence in a naturally occurring butterfly parasite. Proc Natl Acad Sci U S A. 2008;105:7489–7494. doi: 10.1073/pnas.0710909105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D, Bull JJ. Challenging the trade-off model for the evolution of virulence: is virulence management feasible? Trends in Microbiology. 2003;11:15–20. doi: 10.1016/s0966-842x(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Ebert D, Carius HJ, Little T, Decaestecker E. The evolution of virulence when parasites cause host castration and gigantism. American Naturalist. 2004;164(Suppl 5):S19–32. doi: 10.1086/424606. [DOI] [PubMed] [Google Scholar]
- Ebert D, Rainey P, Embley TM, Scholz D. Development, life cycle, ultrastructure and phylogenetic position of Pasteuria ramosa Metchnikoff 1888: Rediscovery of an obligate endoparasite of Daphnia magna Straus. Philosophical Transactions of the Royal Society B. 1996:1689–1701. [Google Scholar]
- Ebert D, Weisser WW. Optimal killing for obligate killers: the evolution of life histories and virulence of semelparous parasites. Proceedings of the Royal Society B: Biological Sciences. 1997;264:985–991. doi: 10.1098/rspb.1997.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fels D, Kaltz O. Temperature-dependent transmission and latency of Holospora undulata, a micronucleus-specific parasite of the ciliate Paramecium caudatum. Proceedings of the Royal Society B: Biological Sciences. 2006;273:1031–1038. doi: 10.1098/rspb.2005.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA. Models of parasite virulence. Quarterly Review Biology. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- Fellous S, Salvaudon L. How can your parasites become your allies? Trends in Parasitology. 2009;2:62–66. doi: 10.1016/j.pt.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Gandon S, Day T. Evidences of parasite evolution after vaccination. Vaccine. 2008;26:C4–C7. doi: 10.1016/j.vaccine.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Gandon S, Mackinnon MJ, Nee S, Read AF. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414:751–756. doi: 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- Geritz SAH, Kisdi E, Meszena G, Metz JAJ. Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evolutionary Ecology. 1998;12:35–57. [Google Scholar]
- Graham AL, Shuker DM, Pollitt L, Auld S, Wilson A, Little TJ. Fitness consequences of immune responses: strengthening the empirical framework for ecoimmunology. Functional Ecology. In Press (Early online version: DOI: 10.1111/j.1365-2435.2010.01777.x) [Google Scholar]
- Hall AR, Colegrave N. How does resource supply affect evolutionary diversification? Proceedings of the Royal Society B: Biological Sciences. 2007;274:73–78. doi: 10.1098/rspb.2006.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SR, Simonis JL, Nisbet RM, Tessier AJ, Caceres CE. Resource ecology of virulence in a planktonic host-parasite system: an explanation using dynamic energy budgets. American Naturalist. 2009;174:149–162. doi: 10.1086/600086. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. Haploid Dynamic Polymorphism in a Host with Matching Parasites: Effects of Mutation/Subdivision, Linkage, and Patterns of Selection. Journal of Heredity. 1993;84:328–338. [Google Scholar]
- Hoyle A, Bowers RG, White A, Boots M. The influence of trade-off shape on evolutionary behaviour in classical ecological scenarios. Journal of Theoretical Biology. 2008;250:498–511. doi: 10.1016/j.jtbi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Jensen KH, Little T, Skorping A, Ebert D. Empirical support for optimal virulence in a castrating parasite. PLoS Biology. 2006;4:1265–1269. doi: 10.1371/journal.pbio.0040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela J, Lively CM, Taskinen J, Peters AD. Effect of starvation on parasite-induced mortality in a freshwater snail ( Potamopyrgus antipodarum) Oecologia. 1999;119:320–325. doi: 10.1007/s004420050792. [DOI] [PubMed] [Google Scholar]
- Kassen R, Buckling A, Bell G, Rainey PB. Diversity peaks at intermediate productivity in a laboratory microcosm. Nature. 2000;406:508–512. doi: 10.1038/35020060. [DOI] [PubMed] [Google Scholar]
- Kiers TE, Palmer TM, Ives AR, Bruno JF, Bronstein JL. Mutualisms in a changing world: an evolutionary perspective. Ecology Letters. 2010;13:1459–1474. doi: 10.1111/j.1461-0248.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- Krist AC, Jokela J, Wiehn J, Lively CM. Effects of host condition on susceptibility to infection, parasite developmental rate, and parasite transmission in a snail-trematode interaction. Journal of Evolutionary Biology. 2004;17:33–40. doi: 10.1046/j.1420-9101.2003.00661.x. [DOI] [PubMed] [Google Scholar]
- Laine AL. Pathogen fitness components and genotypes differ in their sensitivity to nutrient and temperature variation in a wild plant-pathogen association. Journal of Evolutionary Biology. 2007;20:2371–2378. doi: 10.1111/j.1420-9101.2007.01406.x. [DOI] [PubMed] [Google Scholar]
- Laine AL, Tellier A. Heterogeneous selection promotes maintenance of polymorphism in host-parasite interactions. Oikos. 2008;117:1281–1288. [Google Scholar]
- Lambrechts L, Chavatte JM, Snounou G, Koella JC. Environmental influence on the genetic basis of mosquito resistance to malaria parasites. Proceedings of the Royal Society B: Biological Sciences. 2006a;273:1501–1506. doi: 10.1098/rspb.2006.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts L, Fellous S, Koella JC. Coevolutionary interactions between host and parasite genotypes. Trends in Parasitology. 2006b;22:12–16. doi: 10.1016/j.pt.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Lazzaro, Brian P, Flores HA, Lorigan JG, Yourth CP. Genotype-by-environment interactions and adaptation to local temperature affect immunity and fecundity in Drosophila melanogaster. PLoS Pathogens. 2008;4:e1000025. doi: 10.1371/journal.ppat.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro BP, Little TJ. Immunity in a variable world. Philos Trans Biol Sci. 2009;364:15–26. doi: 10.1098/rstb.2008.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Legendre L. Numerical Ecology. Elsevier; 1998. Chapter 10 Interpretation of ecological structures; pp. 481–574. [Google Scholar]
- Lipsitch M, Moxon ER. Virulence and transmissibility of pathogens: what is the relationship? Trends in Microbiology. 1997;5:31–37. doi: 10.1016/S0966-842X(97)81772-6. [DOI] [PubMed] [Google Scholar]
- Little TJ, Birch J, Vale P, Tseng M. Parasite transgenerational effects on infection. Evolutionary Ecology Research. 2007;9:459–469. [Google Scholar]
- Little T, Chadwick W, Watt K. Parasite variation and the evolution of virulence in a Daphnia-microparasite system. Parasitology. 2008;135:6. doi: 10.1017/S0031182007003939. [DOI] [PubMed] [Google Scholar]
- Little TJ, Shuker DM, Colegrave N, Day T, Graham AL. The Coevolution of Virulence: Tolerance in Perspective. PLoS Pathogens. 2010;6:e1001006. doi: 10.1371/journal.ppat.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively CM. The maintenance of sex: host-parasite coevolution with density-dependent virulence. Journal of Evolutionary Biology. 2009;22:2086–2093. doi: 10.1111/j.1420-9101.2009.01824.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Pascua LDC, Buckling A. Increasing productivity accelerates host–parasite coevolution. J Journal of Evolutionary Biology. 2008;21:853–860. doi: 10.1111/j.1420-9101.2008.01501.x. [DOI] [PubMed] [Google Scholar]
- Mackinnon MJ, Read AF. Genetic relationships between parasite virulence and transmission in the rodent malaria Plasmodium chabaudi. Evolution. 1999;53:689–703. doi: 10.1111/j.1558-5646.1999.tb05364.x. [DOI] [PubMed] [Google Scholar]
- Mackinnon MJ, Read AF. Virulence in malaria: an evolutionary viewpoint. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2004;359:965–986. doi: 10.1098/rstb.2003.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckean K, Yourth C, Lazzaro B, Clark A. The evolutionary costs of immunological maintenance and deployment. BMC Evolutioanry Biologoy. 2008;8:76. doi: 10.1186/1471-2148-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew GL. The nature, origin, and evolution of parasitism. In: Horsfall JG, Dimond AE, editors. Plant Pathology: An Advanced Treatise. Academic Press; New York: 1960. pp. 19–69. [Google Scholar]
- Michalakis Y, Olivieri I, Renaud F, Raymond M. Pleiotropic action of parasites - how to be good for the host. Trends in Ecology and Evolution. 1992;7:59–62. doi: 10.1016/0169-5347(92)90108-N. [DOI] [PubMed] [Google Scholar]
- Miller MR, White A, Boots M. The evolution of parasites in response to tolerance in their hosts: the good, the bad, and apparent commensalism. Evolution. 2006;60:945–956. [PubMed] [Google Scholar]
- Mitchell SE, Rogers ES, Little TJ, Read AF. Host-parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution. 2005;59:70–80. [PubMed] [Google Scholar]
- Raberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read AF, Mackinnon MJ. Pathogen evolution in a vaccinated world, Pages 374. In: Stearns SC, Koella JC, editors. Evolution in Health and Disease. Oxford University Press; New York: 2008. [Google Scholar]
- Regoes RR, Hottinger JW, Sygnarski L, Ebert D. The infection rate of Daphnia magna by Pasteuria ramosa conforms with the mass-action principle. Epidemiology and Infection. 2003;131:957–966. doi: 10.1017/s0950268803008793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restif O, Kaltz O. Condition-dependent virulence in a horizontally and vertically transmitted bacterial parasite. Oikos. 2006;114:148–158. [Google Scholar]
- Roy BA, Kirchner JW. Evolutionary dynamics of pathogen resistance and tolerance. Evolution. 2000;54:51–63. doi: 10.1111/j.0014-3820.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Ryan JA, Kohler SL. Virulence is context-dependent in a vertically transmitted aquatic host-microparasite system. International Journal for Parasitology. 2010;40:1665–1673. doi: 10.1016/j.ijpara.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Sachs JL, Simms EL. Pathways to mutualism breakdown. Trends in Ecology and Evolution. 2006;21:585–592. doi: 10.1016/j.tree.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Sacristan S, Fraile A, Malpica JM, Garcia-Arenal F. An analysis of host adaptation and its relationship with virulence in Cucumber mosaic virus. Phytopathology. 2005;95:827–833. doi: 10.1094/PHYTO-95-0827. [DOI] [PubMed] [Google Scholar]
- Salvaudon L, Heraudet V, Shykoff JA. Genotype-specific interactions and the trade-off between host and parasite fitness. BMC Evolutionary Biology. 2007;7:189. doi: 10.1186/1471-2148-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppälä O, Liljeroos K, Karvonen A, Jokela J. Host condition as a constraint for parasite reproduction. Oikos. 2008;117:749–753. [Google Scholar]
- Scholthof KBG. The disease triangle: pathogens, the environment and society. Nature Reviews Microbiology. 2006;5:152–156. doi: 10.1038/nrmicro1596. [DOI] [PubMed] [Google Scholar]
- Sgrò C, Hoffmann A. Genetic correlations, tradeoffs and environmental variation. Heredity. 2004;93:241–248. doi: 10.1038/sj.hdy.6800532. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The Principles and Practices of Statistics in Biological Research. Third Edition W. H. Freeman; 1994. [Google Scholar]
- Tétard-Jones Catherine, Kertesz Michael A, Gallois Patrick, Preziosi Richard F. Genotype-by-genotype interactions modified by a third species in a plant-insect system. American Naturalist. 2007;170:492–499. doi: 10.1086/520115. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Blanford S. Thermal biology in insect-parasite interactions. Trends in Ecology and Evolution. 2003;18:344–350. [Google Scholar]
- Thompson JN, Fernandez CC. Temporal dynamics of antagonism and mutualism in a geographically variable plant-insect interaction. Ecology. 2006;87:103–112. doi: 10.1890/05-0123. [DOI] [PubMed] [Google Scholar]
- Thompson JN. The coevolutionary process. University of Chicago Press; 1994. [Google Scholar]
- Thompson JN. The geographic mosaic of coevolution. University of Chicago Press; 2005. [Google Scholar]
- Tops S, Hartikainen HL, Okamura B. The effects of infection by Tetracapsuloides bryosalmonae (Myxozoa) and temperature on Fredericella sultana (Bryozoa).2009. International Journal for Parasitology. 2009;39:1003–1010. doi: 10.1016/j.ijpara.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Tseng M. Interactions between the parasite’s previous and current environment mediate the outcome of parasite infection. American Naturalist. 2006;168:565. doi: 10.1086/507997. [DOI] [PubMed] [Google Scholar]
- Vale PF, Stjernman M, Little TJ. Temperature dependent costs of parasitism and the maintenance of polymorphism under genotype-by-environment interactions. Journal of Evolutionary Biology. 2008;21:1418–1427. doi: 10.1111/j.1420-9101.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- Vale PF, Little TJ. Measuring parasite fitness under genetic and thermal variation. Heredity. 2009;103:102–109. doi: 10.1038/hdy.2009.54. [DOI] [PubMed] [Google Scholar]
- Wolinska J, King KC. Environment can alter selection in host-parasite interactions. Trends in Parasitology. 2009;25:236–244. doi: 10.1016/j.pt.2009.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.