Abstract
To provide information for public health policy on mosquito nets in the Amazon region of Colombia, we conducted landing catches to estimate Anopheles species composition and biting activity. Two hundred twenty person-nights of catches were done in seven locations over a period of 14 mo. A total of 1,780 Anopheles mosquitoes were caught (8.1 per person-night). Among the nine species found, An. oswaldoi Peryassú was the most common (776 mosquitoes, 44%), followed by An. darlingi Root s.l. (498, 28%). An. oswaldoi was the most common species collected outdoors, where its biting rate dropped steadily from a peak of >15 bites/person-night at the start of the night (1800–1900 hours) to ≈2 bites/person-night before dawn. An. darlingi was the most common species collected indoors, with a biting rate of ≈3–4 bites/person-night until about midnight, when the rate dropped below 1 bite/person-night, before showing a secondary peak before dawn. Sixty-four mosquito nets were analyzed by the technique of high-performance liquid chromatography (HPLC) for levels of deltamethrin (DM). All but two (62) of these were reported by their owners to have been impregnated with insecticide, and 53 were found by HPLC to have deltamethrin. However, one half (32) of the nets had concentrations <4 mg/m2 and therefore were likely to have been inadequately protective. An inverse association was found between the reported time between washes and deltamethrin concentration. These findings show a need for additional protection from mosquitoes when not inside nets, as well as for more effective impregnation, possibly through wash-resistant insecticide formulation.
Keywords: Anopheles nyssorhynchus spp., mosquito abundance, mosquito nets, malaria vectors
Malaria remains one of the most serious human public health problems, with 3.2 billion people in 107 countries and territories at risk in 2004 (Roll Back Malaria et al. 2005). Despite major efforts in drug treatment and vector control, malaria incidence has generally increased over the last 30 yr, largely because of socioeconomic underdevelopment and drug and insecticide resistance (Takken 2002). Insecticide-treated nets (ITNs) have become a mainstay of malaria control since the mid-1980s (World Health Organization 1993, Lengeler 2004) and reduce transmission by killing or repelling mosquitoes before they can take a blood meal from the occupants (Kroeger et al. 1995, 1997; Santos et al. 1999; Takken 2002).
In areas of malaria transmisssion where sustained vector control is needed, ITNs are the principal strategy for malaria prevention (Roll Back Malaria et al. 2005), having replaced indoor residual spraying in many countries (Takken 2002). However, their uptake in Latin America has been slow (Kroeger et al. 2002). Mosquito contact with insecticide-impregnated bed nets may affect biting behavior and host preference; for example, reducing biting in rooms where impregnated bed nets have been installed (Zimmerman and Voorham 1997, Mathenge et al. 2001). These factors can affect the longevity and vectorial capacity of the vector population as a whole (Magesa et al. 1991).
Malaria transmission occurs in nine countries of the region that share the Amazon rainforest (Roll Back Malaria et al. 2005). In Colombia, Amazonas state (departamento) is considered at high risk of malaria (Ministerio de Protección Social 2003), although the annual incidence varies substantially, e.g., in relation to the El Niño phenomenon. Malaria vectors in the region are Anopheles mosquitoes of the Nyssorhynchus subgenus (Faran and Linthicum 1981). Species include those of the An. darlingi Root complex, which favors lowland rural areas close to rivers and is considered the main vector in the Amazonas state of Colombia (Domínguez 1999, Pérez et al. 1999). However, little quantitative information about anopheline abundance and biting patterns in the area is available, and this makes it difficult to assess the maximum potential of ITNs for reducing malaria transmission. To provide data for the mosquito net impregnation program of the Amazonas state health services, we studied the species composition of mosquitoes and their feeding behavior over time between indoor, peridomestic, and outdoor areas. We also related levels of residual insecticide in mosquito nets in routine use to frequency of washing and insecticide impregnation.
Materials and Methods
Study Area
The climate and vegetation are typical of tropical rain forests. As well as the Amazon itself, rivers in the area include the Putumayo, Loretoyaco, and Cothue (Fig. 1). There are two rainy seasons per year, the first extending from January to April and the second from November to December (Domínguez 1999, Franky and Zárate 2001).
Fig. 1.
Map of the study area. Modified from Alexander et al. (2005), with permission of the American Journal of Tropical Medicine and Hygiene.
The field work was done between October 2002 and December 2003 in rural areas of the Leticia, Puerto Nariño, and Tarapacá districts of Amazonas state in southeastern Colombia, near the borders with Brazil and Peru (see Fig. 1). Leticia district has ≈32,450 inhabitants, according to the 2005 national census (http://www.dane.gov.co); 71% of these are in the main settlement (also called Leticia) situated on the frontier with Brazil and Peru (4°12′55″ S, 69°56′26″ W) (Domínguez 1999). The average temperature is 26.4°C, average rainfall is 3,250 mm, and relative humidity is >78% (Domínguez 1999). Puerto Nariño district has an estimated population of 6,836; 26% of these are in the main settlement, which is a village, also called Puerto Nariño, located on the Loretoyaco River tributary of the Amazon River (3°77′57″ S, 70°38′45″ W). The center of the village is inundated during the rainy season. The average temperature is 25.5°C, average rainfall is 2,990 mm, and relative humidity is >75% (Domínguez 1999). Tarapacá district is north of Leticia on the rivers Putumayo and Cothue with an estimated population of 2,407. The average temperature is 26.1°C, average rainfall is 3,016 mm, and relative humidity is >78% (Domínguez 1999). In the main settlement, also called Tarapacá (2°87′66″ S, 69°74′47″ W), houses near the river are inundated during the first rainy season.
In Leticia district, an average of 109 cases of malaria per year were reported between 1999 and 2003, with the corresponding numbers for Puerto Nariño and Tarapacá districts being 70 and 255, respectively. Using the estimated 2005 populations as a denominator, these correspond to annual incidences of 0.3, 1.0, and 10.6%, respectively. The ratio of Plasmodium vivax to falciparum cases is roughly 2:1 (Alexander et al. 2005). Malaria control is undertaken by the department of health and consists of case management and impregnation of nets with insecticide. Although most nets have been bought in stores, impregnation is done free with deltamethrin (DM), either as a 2.5% suspension concentrate or tablets (K-Othrine 25 SC or K-O Tab; Aventis CropScience Colombia, Bogotá, Colombia).
In the three districts, the dominant ethnic groups are Ticunas and, to a lesser extent, Huitotos. Both ethnic groups are mainly fishers, woodcutters, and farmers (Franky and Zárate 2001). People rise early, often before dawn. Men typically go straight to pursue one of the above-mentioned occupations, whereas women do domestic tasks, such as cooking and washing. After breakfasting together, adults of both sexes go to work and children to school. People are usually indoors and in bed by 2100 hours. Most of the rural houses have wood walls and thatched or corrugated iron roofs. Permanent breeding sites allow anopheline larval development throughout the year.
Mosquito Collection
In Leticia, catches were done in Kilometro 14 (4°21′17″ S, 69°94′87″ W) on the fringe of the town. In Puerto Nariño, catches were done in the villages of Tipisca (3°47′05″ S, 70°17′07″ W) and San Juan del Soco (4°11′54″ S, 69°57′57″ W). In Tarapacá, they were done in the villages of Puerto Huila (2°54′45″ S, 69°43′38″ W), Caña Brava (3°03′15″ S, 70°03′29″ W), and Santa Lucia (2°46′37″ S, 69°46′11″ W) (Franky and Zárate 2001). These locations were chosen because of their relatively high incidence of malaria. Collections were done in and around houses from which cases had been enrolled to a related case-control study (Alexander et al. 2005). In that study, the overall proportion of controls who reported sleeping under a net the previous night was 96% (in Leticia, the proportion was 54/71 or 76%, in Puerto Nariño it was 31/31 or 100%, and in Tarapacá, it was 848/870 or 97%). Almost all nets in the area are of nylon.
To study the diel profile of mosquito behavior, human landing was assumed to result from seeking of blood meals. The human landing rate was therefore used as an estimate of biting rate. Adult anopheline mosquitoes were collected by teams consisting of Secretaríá de Salud del Amazonas personnel and local residents, after the latter had been trained in the use of oral aspirators, and in the differentiation of anopheline from other mosquitoes. Collections were made from 1800 to 0600 hours simultaneously in each of three areas: 1) indoor, 2) peridomestic (outside the house but within a distance of 10 m), and 3) outdoor (at least 10 m from the house). Peridomestic areas were flat, well trodden, and generally open to the sky, except for occasional fruit trees. Outdoor areas were typically forested or, sometimes, on narrow paths leading to cultivated areas. The collectors acted as human baits, with their legs exposed from knee to ankle. Collectors were moved between stations on a shift system. Mosquitoes were caught with oral aspirators when they landed to feed and transferred to tubes labeled by collection hour and area. Female mosquitoes were identified using morphological keys (Faran 1980, Faran and Linthicum 1981). We did not determine the mosquitoes malaria infection status.
Environmental Data
The following environmental variables were recorded hourly during the mosquito catches: temperature (maximum and minimum), presence or absence of rain, presence or absence of wind (sufficient to move leaves on plants), cloudy sky (estimated as the number of eighths of the sky covered), and light source (presence or absence of moonlight, sunlight, or artificial light). Relative humidity (%) was measured nonsystematically.
Determination of Levels of Deltamethrin on Nets Using High-performance Liquid Chromatography
Nets for this assay were obtained from a subset of malaria cases and controls enrolled in the related case-control study (Alexander et al. 2005). The study goal was explained to each person, and they were asked about the use, time since impregnation, and washing frequency of the net. Its material, color, and condition, in terms of holes and tears, were recorded. The sample was usually taken from the middle of one side of the net and was a square of side 15 cm. Each net used for these analyses was replaced by a new impregnated net. Sixty-five nets were collected this way. The study did not replace or modify the nets of the remaining study participants or residents of the area.
The net samples were sent to the London School of Hygiene and Tropical Medicine for analyses using Dionex Summit HPLC equipment and software (Camberly, Surrey, United Kingdom). Deltamethrin was extracted from the nets with acetonitrile as the solvent, and samples were separated by injecting onto an Acclaim C18 120Å (250 by 4.6 mm; Dionex) column eluting with water/acetonitrile (90:10%; vol:vol) at a flow rate of 2 ml/min and passed through the photodiode array detector (PDA-100; Dionex) set at 275 nm. The authenticity of the detected peaks was determined by comparison of retention time, spectral extraction at 275 nm, and spiking the sample with commercially available standard of DM. A calibration curve of DM was generated by Chromeleon (Dionex) using known amounts of the standard (0–0.4 μg/ml) in acetonitrile injected onto the column. From this curve, the amount of DM in the nets was determined. Approximate doses of insecticide per square meter were calculated from the quantities detected in each of four 25-cm2 replicates (Yates et al. 2005). The amount of DM on the nets was expressed as milligrams per square meter.
Statistical Analysis
The results of the landing catches are expressed as biting rates per person-night, with night defined as the 12-h period from 1800 to 0600 hours. Collections that lasted <12 h, for example, because of interruption by rain, were not included in the analysis. Nevertheless, because of varying numbers of catchers within a night, the numbers of person-nights are not always integers. Most of the analysis presented is descriptive. However, to assess the effect on biting rate of environmental factors such as wind (present or absent), degree of cloud (eighths of the sky covered), and light source near to the point of catches, we used generalized linear models of the negative binomial distribution family (Wilson and Grenfell 1997). For this regression analysis, the response variable was the number of mosquitoes caught, with one record per hour per location. The logarithmic link function was used, with the log-number of catchers in any hour as an offset, so the analysis yields ratios of mean biting rates. To relate deltamethrin density on nets to impregnation and washing history, we used generalized linear models of the gamma family. Analysis was done with S-PLUS and STATA software.
Ethical approval was granted by the Instituto Nacional de Salud (Nacional Institute of Health) of Colombia.
Results
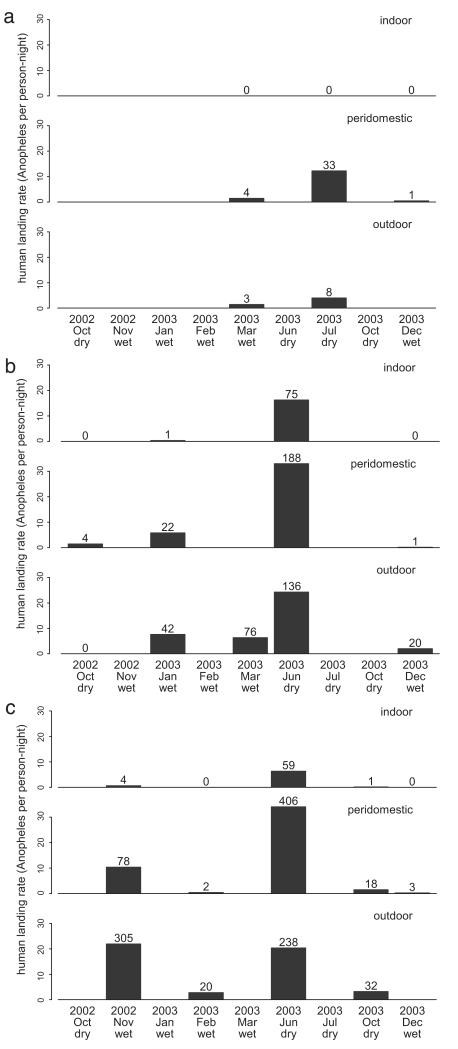
Over the three districts, a total of 1,780 anophelines were caught over 220 person-nights between October 2002 and December 2003 (Fig. 2). Biting rates varied considerably over the duration of the study. In the rural villages of Puerto Nariño and Tarapacá districts, the highest biting rates in the three catching areas (indoors, peridomestically, and outdoors) were generally in June 2003. These rates reached >30 bites/person-night in the peridomestic areas. Rates were very much lower in some months, such as December 2003. There was little biting in the Kilometro 14 settlement, on the outskirts of Leticia town, although comparability with other districts is limited by the low overlap of the catches’ timing. Overall, catches were greater outdoors and peridomestically than indoors (11, 10, and 2.2 bites/person-night, respectively). The most common species were An. oswaldoi Peryassú, (43.6%) and An. darlingi s.l. (28.0%; Table 1). An. oswaldoi was the most common species outdoors, whereas An. darlingi was hardly caught there. The reverse was true indoors, whereas both species were caught in the peridomestic area (Fig. 3).
Fig. 2.
Human biting rate by month and season in indoor, peridomestic, and outdoor areas. (a) Leticia district (Kilometro 14), total person-nights: 6.3 indoors, 7.4 peridomestically, and 4.1 outdoors. (b) Puerto Nariño district (sum of Tipisca and San Juan del Soco), total person-nights: 12.9 indoors, 18.3 peridomestically, and 35 outdoors. (c) Tarapacá district (sum of Santa Lucia, Caña Brava, Puerto Huila and Ventura), total person-nights: 45 indoors, 48.9 peridomestically, and 42.1 outdoors. The number above each bar is that of Anopheles caught. In some months, only one or two of the three districts were included.
Table 1. Anopheline species by district, caught indoors (ID), peridomestically (PD), and outdoors (OD).
| Leticia |
Puerto Nariño |
Tarapacá |
Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | PD | OD | ID | PD | OD | ID | PD | OD | ||
| Person-nights of catches | 6.3 | 7.4 | 4.1 | 12.9 | 18.4 | 34.3 | 44.9 | 49.0 | 42.8 | 220.1 |
| An. darlingi | 0 (0) | 23 (3.1) | 0 (0) | 73 (5.7) | 107 (5.8) | 4 (0.1) | 55 (1.2) | 224 (4.6) | 12 (0.3) | 498 (2.3) |
| An. oswaldoi | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 63 (3.4) | 139 (4.0) | 2 (0.04) | 165 (3.4) | 407 (9.5) | 776 (3.5) |
| Others of subgenus Nyssorhynchusa | 0 (0) | 11 (1.5) | 8 (2.0) | 0 (0) | 42 (2.3) | 63 (1.8) | 0 (0) | 30 (0.6) | 41 (1.0) | 195 (0.9) |
| Subgenus Anophelesb | 0 (0) | 4 (0.5) | 3 (0.7) | 3 (0.2) | 3 (0.2) | 68 (2.0) | 7 (0.2) | 88 (1.8) | 135 (3.2) | 311 (1.4) |
| Total | 0 (0) | 38 (5.1) | 11 (2.7) | 76 (5.9) | 215 (12) | 274 (8.0) | 64 (1.4) | 507 (10.3) | 595 (14) | 1780 (8.1) |
For each mosquito or group, each cell shows the no. caught, with the human landing rate per night in parentheses.
An. nueztovari Gabaldon (124 caught), An. triannulatus Neiva and Pinto (45 caught), An. evansae Brthes (15 caught), and An. benarrochi Gabaldon, Cova-Garcia, and Lopez (11 caught).
An. mediopunctatus Theobald (219 caught), An. mattogrossensis Lutz and Neiva (88 caught), and An. peryassui Dyar and Knab (4 caught).
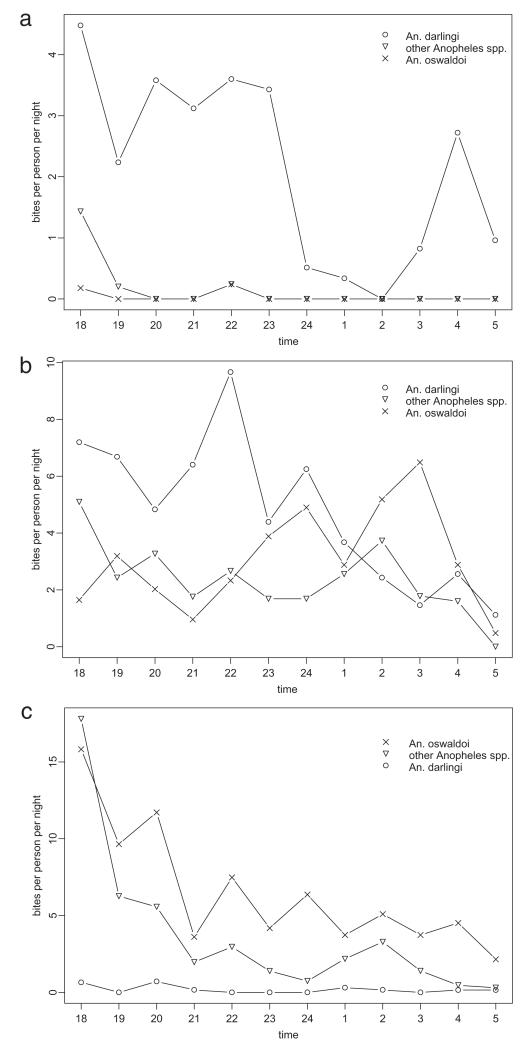
Fig. 3.
Human landing rate by hour (a) indoors, (b) peridomestic, and (c) outdoors.
In terms of hourly patterns, An. darlingi was caught indoors at a roughly constant rate of ≈3–4 bites/person-night until about midnight, when the rate dropped below 1 bite/person-night, before showing a secondary peak before dawn (Fig. 3). Outdoors, An. oswaldoi dropped steadily from a peak of >15 bites/person-night at the start of the night (1800–1900 hours) to ≈2 bites/person-night before dawn. In the peridomestic area, both species were caught for most of the night, although An. darlingi showed a slight reduction in biting toward dawn, and An. oswaldoi the reverse. In terms of environmental factors (Table 2), An. darlingi was caught in greater abundance when there was light from artificial sources or the moon and when there was less cloud cover. Catches of An. oswaldoi, in contrast, did not show associations with these factors.
Table 2. Human landing rates of An. darlingi and An. oswaldoi by catching location and environmental conditions.
| Person- nights |
An. darlingi
|
An. oswaldoi
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mosquitoes caught (rate) |
Adjusted rate ratio (95% CI) |
P | Mosquitoes (rate) |
Adjusted rate ratio (95% CI) |
P | |||||||
| Location | Indoors | 63.9 | 126 | (2.0) | 1 | 2 | (0.03) | 0.008 | (0.002–0.04) | <0.0001 | ||
| Peridomestic | 74.0 | 354 | (4.8) | 3.0 | (1.75–5.2) | <0.0001 | 228 | (3.1) | 1a | |||
| Outdoors | 80.9 | 16 | (0.2) | 0.21 | (0.10–0.45) | <0.0001 | 546 | (6.7) | 2.1 | (1.3–3.4) | 0.002 | |
| Cloud (eighths of sky) | 0 | 19.8 | 172 | (8.7) | 0.82b | (0.75–0.90) | <0.0001 | 118 | (6.0) | 0.94b | (0.86–1.02) | 0.14 |
| 1–2 | 17.6 | 71 | (4.0) | 48 | (2.7) | |||||||
| 3–4 | 20.8 | 109 | (5.2) | 94 | (4.5) | |||||||
| 5–6 | 35.5 | 51 | (1.4) | 144 | (4.1) | |||||||
| 7–8 | 125.2 | 93 | (0.7) | 372 | (3.0) | |||||||
| Illumination | None | 154.6 | 195 | (1.3) | 1 | 548 | (3.5) | 1 | ||||
| Artificial | 10.8 | 55 | (5.1) | 4.3 | (1.52–12) | 0.006 | 48 | (4.5) | 1.79 | (0.47–6.8) | 0.39 | |
| Moon | 40 | 233 | (5.8) | 3.5 | (1.92–6.4) | <0.0001 | 163 | (4.1) | 0.95 | (0.51–1.8) | 0.86 | |
| Sun | 13.5 | 13 | (1.0) | 1.4 | (0.48–3.9) | 0.56 | 17 | (1.3) | 0.39 | (0.13–1.1) | 0.09 | |
| Wind | Absent | 175.1 | 369 | (2.1) | 1 | 657 | (3.8) | 1 | ||||
| Present | 43.8 | 127 | (2.9) | 2.1 | (1.12–3.9) | 0.02 | 119 | (2.7) | 1.05 | (0.56–2.0) | 0.88 | |
1.3 person-nights were excluded because of missing data on cloud and/or illumination. For each species, the adjusted rate ratios are obtained by including all the speciffied variables in a negative binomial regression model.
For An. oswaldoi, the peridomestic area was chosen as baseline because of the very small no. caught indoors.
Rate ratio for each additional eighth of the sky obscured by cloud.
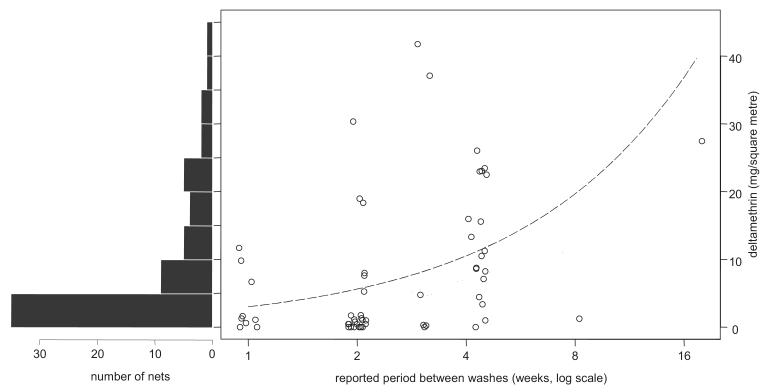
Samples from 64 of the 65 mosquito nets collected were analyzed by HPLC, one sample having been lost. Sixty-two of these were reported by their owners to have been impregnated with insecticide, all of these within the previous 12 mo. Eleven nets tested negative for deltamethrin, including the two that had reportedly never been impregnated. Overall, including those nets with a value of zero, the mean concentration was 8.5 mg/m2, the median was 3.7 mg/m2, and the maximum was 42 mg/m2. One half of the 64 nets had concentrations of at least 4 mg/m2, and 15 (23%) had at least 15 mg/m2 (Fig. 4). An inverse association was found between the reported time between washes and the concentration. A doubling in time between washes was associated with almost a doubling of mean concentration (ratio of means = 1.9, 95% confidence interval: 1.2–2.9, P = 0.005). However, there was substantial variation in concentration about this fitted line (Fig. 4). Only a weak, statistically nonsignificant, negative association was found between the reported time since last impregnation and deltamethrin concentration. A doubling of this time was associated with a ratio of means of 0.87 (95% confidence interval: 0.71–1.07, P = 0.18).
Fig. 4.
Histogram of concentration of deltamethrin on nets and relation with reported frequency of washing. The dashed line shows that a doubling in time between washes was associated with an estimated 1.9-fold increase in mean concentration (95% confidence interval: 1.2–2.9).
Discussion
This study was done to provide information for the local public health service’s malaria control efforts, in particular their mosquito net impregnation program. Although the distribution of our catches between months and sites was not balanced (Fig. 2), the resulting information should nevertheless be an important contribution to these efforts. We found evidence of substantial biting in circumstances when people are not protected by nets: indoors in the early evening and peridomestically (Fig. 3). This suggests a role for topical or combustible repellents, which have been found to be effective against malaria elsewhere in Amazonia (Hill et al. 2007). In our study area, most people (96%) sleep under nets at home. However, it is a limitation of the study that we did not evaluate mosquito abundance and behavior according to number and impregnation status of household nets. The population attributable fraction of malaria caused by recent travel is greater than that caused by failing to use nets (Alexander et al. 2005). For these travelers, both topical repellents and promotion of net use could be useful in malaria prevention.
The two Anopheles species that we caught most frequently—An. darlingi, a species complex, and An. oswaldoi—have been incriminated as malaria vectors (Klein et al. 1991, Branquinho et al. 1996, Galardo et al. 2007). It seems that An. oswaldoi can be a important vector when An. darlingi is not present (Rubio-Palis and Curtis 1992, Quiñones et al. 2006). However, its zoophilic and exophilic behavior, its low susceptibility to Plasmodium spp., and the concomitant presence of An. darlingi suggest a secondary role for A. oswaldoi as a malaria vector in our study area. We found An. darlingi to be endophagic and to seek blood meals indoors until about midnight, with an additional peak before dawn. This is in agreement with some previous studies (Roberts et al. 2002, Harris et al. 2006), although, in general, this species’ activity seems to follow that of humans, consistent with anthrophagy (Charlwood 1996). For example, in a gold mining area of southern Venezuela, it was active throughout the night, concurrent with the miners’ work (Moreno et al. 2007). It is a limitation of this study that we do not have systematic information on human nocturnal behavior to correlate with that of mosquitoes. We caught An. oswaldoi almost exclusively outdoors and peridomestically, consistent with a previous landing catch study in western Venezuela, which found it to be exophilic (Rubio-Palis and Curtis 1992). The Venezuelan study found its peak biting time, both indoors and outdoors, to be ≈1900 hours, which is roughly consistent with our findings outdoors, although peridomestically the peak occurred at ≈0300 hours.
Our previous case-control study showed a protective effect of treated nets versus not using a net but not versus untreated nets (Alexander et al. 2005). From this information and evidence from other studies (Lengeler 2004), we concluded that treated nets merited their place in the local malaria control program, in particular because 39% (including mixed infections) of malaria episodes were associated with Plasmodium falciparum, for which the evidence from other sources is stronger. Almost all of the nets sampled contained some deltamethrin, although only one half had at least 4 mg/m2, which was found to be minimally effective against An. gambiae (knockdown ≥ 75% or mortality ≥ 50%). Less than one quarter (23%) of nets had concentrations of at least 15 mg/m2, which was found to be optimally effective against that species (i.e., with knockdown ≥ 95% or mortality ≥ 80%; Kilian et al. 2008). At the time of the study, almost all (94%) nets in the study area were purchased (Alexander et al. 2005). Only nonimpregnated were available for sale, and the only source of insecticide was the local health services’ program of annual reimpregnation. Hence, the impregnation was likely to have been done to an adequate standard, although we do not know the actual density of deltamethrin that resulted. We found increased frequency of washing to be associated with lower levels of deltamethrin. Washing typically involves scrubbing, sometimes with wood or stones, and hanging the nets vertically to dry in the sun. These two practices may increase the loss of insecticide (World Health Organization 2002), although data from Venezuela suggest that the latter may not be as detrimental as previously thought (Magris Crestini 2004). The sampling method used to obtain the nets was not random but, because they were taken from houses near to those of malaria cases, they probably do not overestimate the general level of impregnation. These results suggest that, in the previous case-control study, the lack of apparent difference between impregnated and nonimpregnated nets may be partly caused by depletion of insecticide and that switching to a wash-resistant formulation could be worthwhile (Yates et al. 2005).
Acknowledgments
We are grateful to residents of the study area for help and cooperation with the study. This work was supported financially by the United Kingdom Department for International Development, research project R7829, and the United Kingdom Medical Research Council grant number G7508177 to the Tropical Epidemiology Group. We are grateful to the Gates Malaria Partnership for providing support for the HPLC facility at London School of Hygiene and Tropical Medicine, through an award from the Bill and Melinda Gates Foundation.
References Cited
- Alexander N, Rodríguez M, Pérez L, Caicedo JC, Cruz J, Prieto G, Arroyo JA, Cotacio MC, Suárez M, de la Hoz F, Hall AJ. Case-control study of mosquito nets against malaria in the Amazon region of Colombia. Am. J. Trop. Med. Hyg. 2005;73:140–148. [PubMed] [Google Scholar]
- Branquinho MS, Araujo MS, Natal D, Marrelli MT, Rocha RM, Taveira FA, Kloetzel JK. Anopheles oswaldoi a potential malaria vector in Acre. Brazil. Trans. R. Soc. Trop. Med. Hyg. 1996;90:233. doi: 10.1016/s0035-9203(96)90225-4. [DOI] [PubMed] [Google Scholar]
- Charlwood JD. Biological variation in Anopheles darlingi. Root. Mem. Inst. Oswaldo Cruz. 1996;91:391–398. doi: 10.1590/s0074-02761996000400001. [DOI] [PubMed] [Google Scholar]
- Domínguez C, editor. Departamento del Amazonas: El Hombre y su Medio. Universidad Nacional de Colombia; Leticia, Colombia: 1999. [Google Scholar]
- Faran ME. Mosquito studies (Diptera, Culicidae) XXXIV. A revision of the Albimanus section of the subgenus Nyssorhynchus of Anopheles. Contrib. Am. Entomol. Inst. 1980;15:1–215. [Google Scholar]
- Faran ME, Linthicum KJ. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae) Mosq. Syst. 1981;13:1–81. [Google Scholar]
- Franky CE, Zárate CG, editors. Imani Mundo. Estudios en la Amazonia Colombiana; Unibiblos, Bogotá, Colombia: 2001. [Google Scholar]
- Galardo AK, Arruda M, D’Almeida Couto AA, Wirtz R, Lounibos LP, Zimmerman RH. Malaria vector incrimination in three rural riverine villages in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 2007;76:461–469. [PubMed] [Google Scholar]
- Harris AF, Matias-Arnez A, Hill N. Biting time of Anopheles darlingi in the Bolivian Amazon and implications for control of malaria. Trans. R. Soc. Trop. Med. Hyg. 2006;100:45–47. doi: 10.1016/j.trstmh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Hill N, Lenglet A, Arnez AM, Carneiro I. Plant based insect repellent and insecticide treated bed nets to protect against malaria in areas of early evening biting vectors: double blind randomised placebo controlled clinical trial in the Bolivian Amazon. BMJ. 2007;335:1023. doi: 10.1136/bmj.39356.574641.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian A, Byamukama W, Pigeon O, Atieli F, Duchon S, Phan C. Long-term field performance of a polyester-based long-lasting insecticidal mosquito net in rural Uganda. Malar. J. 2008;7:49. doi: 10.1186/1475-2875-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Lima JB, Tada MS. Comparative susceptibility of anopheline mosquitoes to Plasmodium falciparum in Rondonia, Brazil. Am. J. Trop. Med. Hyg. 1991;44:598–603. doi: 10.4269/ajtmh.1991.44.598. [DOI] [PubMed] [Google Scholar]
- Kroeger A, Mancheno M, Alarcon J, Pesse K. Insecticide-impregnated bed nets for malaria control: varying experiences from Ecuador, Colombia, and Peru concerning acceptability and effectiveness. Am. J. Trop. Med. Hyg. 1995;53:313–323. doi: 10.4269/ajtmh.1995.53.313. [DOI] [PubMed] [Google Scholar]
- Kroeger A, Meyer R, Mancheno M, Gonzalez M, Pesse K. Operational aspects of bednet impregnation for community-based malaria control in Nicaragua, Ecuador, Peru and Colombia. Trop. Med. Int. Health. 1997;2:589–602. doi: 10.1046/j.1365-3156.1997.d01-319.x. [DOI] [PubMed] [Google Scholar]
- Kroeger A, Avinna A, Ordonnez-Gonzalez J, Escandon C. Community cooperatives and insecticide-treated materials for malaria control: a new experience in Latin America. Malar. J. 2002;1:15. doi: 10.1186/1475-2875-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler C. Insecticide-treated bednets and curtains for preventing malaria (Cochrane Review) 1st ed. The Cochrane Library; Wiley; Chichester, United Kingdom: 2004. [DOI] [PubMed] [Google Scholar]
- Magesa SM, Wilkes TJ, Mnzava AE, Njunwa KJ, Myamba J, Kivuyo MD, Hill N, Lines JD, Curtis CF. Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. Part 2. Effects on the malaria vector population. Acta Trop. 1991;49:97–108. doi: 10.1016/0001-706x(91)90057-q. [DOI] [PubMed] [Google Scholar]
- Magris Crestini M. Malaria control trial using lambdacyhalothrin treated nets in Yanomami communities in Amazonas state, Venezuela. Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine; University of London, London, United Kingdom: 2004. [Google Scholar]
- Mathenge EM, Gimnig JE, Kolczak M, Ombok M, Irungu LW, Hawley WA. Effect of permethrin-impregnated nets on exiting behavior, blood feeding success, and time of feeding of malaria mosquitoes (Diptera: Culicidae) in western Kenya. J. Med. Entomol. 2001;38:531–536. doi: 10.1603/0022-2585-38.4.531. [DOI] [PubMed] [Google Scholar]
- Ministerio de Protección Social . Malaria: Estrategias para Afrontar una Prioridad en Salud Pública. Ministerio de Protección Social, República de Colombia, Universidad del Valle; Cali, Colombia: 2003. [Google Scholar]
- Moreno JE, Rubio-Palis Y, Paez E, Perez E, Sanchez V. Abundance, biting behaviour and parous rate of anopheline mosquito species in relation to malaria incidence in gold-mining areas of southern Venezuela. Med. Vet. Entomol. 2007;21:339–349. doi: 10.1111/j.1365-2915.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- Pérez L, Suárez M, Murcia L, de la Hoz F, Olano VA, Brochero H, Toro P. La malaria en el Amazonas: conocimientos, prácticas, prevalencia de parasitemia y evaluación entomológica en mayo de 1997. Biomédica. 1999;19:93–102. [Google Scholar]
- Quiñones ML, Ruiz F, Calle DA, Harbach RE, Erazo HF, Linton Y-M. Incrimination of Anopheles (Nyssorhynchus) rangeli and An. (Nys.) oswaldoi as natural vectors of Plasmodium vivax in Southern Colombia. Mem. Inst. Oswaldo Cruz. 2006;101:617–623. doi: 10.1590/s0074-02762006000600007. [DOI] [PubMed] [Google Scholar]
- Roberts DR, Manguin S, Rejmankova E, Andre R, Harbach RE, Vanzie E, Hakre S, Polanco J. Spatial distribution of adult Anopheles darlingi and Anopheles albimanus in relation to riparian habitats in Belize, Central America. J. Vector Ecol. 2002;27:21–30. [PubMed] [Google Scholar]
- Roll Back Malaria. World Health Organization. UNICEF . World malaria report 2005. World Health Organization; Geneva, Switzerland: 2005. [Google Scholar]
- Rubio-Palis Y, Curtis CF. Biting and resting behaviour of anophelines in western Venezuela and implications for control of malaria transmission. Med. Vet. Entomol. 1992;6:325–334. doi: 10.1111/j.1365-2915.1992.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Santos JB, dos Santos F, Macêdo V. Variação da densidade anofélica com o uso de mosquiteiros impregnados com deltametrina em uma área endêmica de malária na Amazônia Brasileira. Cadernos Saude Publ. 1999;15:281–292. doi: 10.1590/s0102-311x1999000200013. [DOI] [PubMed] [Google Scholar]
- Takken W. Do insecticide-treated bednets have an effect on malaria vectors? Trop. Med. Int. Health. 2002;12:1022–1030. doi: 10.1046/j.1365-3156.2002.00983.x. [DOI] [PubMed] [Google Scholar]
- Wilson K, Grenfell BT. Generalized linear modelling for parasitologists. Parasitol. Today. 1997;13:33–38. doi: 10.1016/s0169-4758(96)40009-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Implementation of the global malaria control strategy. Report of a WHO study group on the implementation of the global plan of action for malaria control 1993–2000. World Health Organization; Geneva, Switzerland: 1993. (Technical report series 839). [PubMed] [Google Scholar]
- World Health Organization . Instructions for treatment and use of insecticide-treated mosquito nets. World Health Organization; Geneva, Switzerland: 2002. [Google Scholar]
- Yates A, N′Guessan R, Kaur H, Akogbeto M, Rowland M. Evaluation of KO-Tab 1–2-3: a wash-resistant ‘dip-it-yourself’ insecticide formulation for long-lasting treatment of mosquito nets. Malar. J. 2005;4:52. doi: 10.1186/1475-2875-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RH, Voorham J. Uso de mosquiteros y otros materiales impregnados con insecticida para el contol de la malaria en las Américas. Rev. Panamericana Salud Públ. 1997;2:310–318. [Google Scholar]