Abstract
Rhizosphere priming is the change in decomposition of soil organic matter (SOM) caused by root activity. Rhizosphere priming plays a crucial role in soil carbon (C) dynamics and their response to global climate change. Rhizosphere priming may be affected by soil nutrient availability, but rhizosphere priming itself can also affect nutrient supply to plants. These interactive effects may be of particular relevance in understanding the sustained increase in plant growth and nutrient supply in response to a rise in atmospheric CO2 concentration. We examined how these interactions were affected by elevated CO2 in two similar semiarid grassland field studies. We found that an increase in rhizosphere priming enhanced the release of nitrogen (N) through decomposition of a larger fraction of SOM in one study, but not in the other. We postulate that rhizosphere priming may enhance N supply to plants in systems that are N limited, but that rhizosphere priming may not occur in systems that are phosphorus (P) limited. Under P limitation, rhizodeposition may be used for mobilization of P, rather than for decomposition of SOM. Therefore, with increasing atmospheric CO2 concentrations, rhizosphere priming may play a larger role in affecting C sequestration in N poor than in P poor soils.
Keywords: 15N tracer, microbial mining, N:P stoichiometry, nutrient competition, preferential substrate utilization, progressive nitrogen limitation, root exudates
Introduction
Rhizosphere priming is the change in soil organic matter (SOM) decomposition caused by plant root activity that is often associated with rhizodeposition (Kuzyakov, 2002). A substantial fraction of net carbon assimilation goes into the soil as rhizodeposition. Estimates of how much C is allocated to rhizodeposition vary widely among plant species, with plant age, soil type, and nutrient availability, and are on average between 11 and 17% of net fixed C (Nguyen, 2003; Jones et al., 2009). Rhizodeposition is an important energy source for the microbial production of extra-cellular enzymes that break down SOM (Schimel and Weintraub, 2003; Blagodatskaya and Kuzyakov, 2008; Averill and Finzi, 2011). The subsequent change in SOM decomposition (i.e., the rhizosphere priming effect) is usually measured by comparing the CO2 produced from SOM in a soil with and without plants. Often, CO2 produced from SOM in planted soil is greater than in the unplanted or fallow soil, and is referred to as a positive priming effect (Kuzyakov, 2002). However, smaller CO2 production in planted compared to unplanted soil, or a negative priming effect, has also been observed (Cheng, 1996; Bader and Cheng, 2007). Although rhizosphere priming effects have frequently been reported in a variety of soil-plant systems, the mechanisms behind these effects remain unclear (Kuzyakov, 2010).
Effects of soil nutrient availability on rhizosphere priming
The direction and magnitude of rhizosphere priming have been related to soil nutrient availability. Plants and microbes require C and nutrients within specific boundaries and at the same time affect the relative availability of C and nutrients in their immediate environment, the rhizosphere. Because of this close interdependence between C and nutrients, the nutrient status of the soil is an important factor for rhizosphere priming. Several hypotheses have been proposed explaining the relationship between rhizosphere priming and soil nutrient availability (Figure 1). First, in soils of low nutrient availability, inputs of energy-rich carbon compounds from roots may be used for the production of extra-cellular enzymes that can release nutrients locked in SOM (Asmar et al., 1994; Brzostek et al., 2012). Under these conditions, microbes may use root exudates to release nutrients thereby meeting their nutrient requirement. This has also been referred to as the microbial mining hypothesis (Craine et al., 2007; Fontaine et al., 2011). Because rhizosphere priming effects are usually measured through changes in CO2 production, microbial mining for nutrients associated with rhizosphere priming should only relate to nutrients released through oxidation of SOM accompanied by the production CO2. Much of the N in humified SOM is released through oxidation (biological mineralization). While some organic N compounds (proteins, amino acids, amino sugars) do not need to be oxidized for the N to be utilized, the C skeletons of these compounds are often catabolized by microbes thereby producing CO2. On the other hand, organic P is mostly released through hydrolysis without CO2 production (biochemical mineralization, McGill and Cole, 1981). Therefore, the microbial mining hypothesis may be more important for N than for P.
Figure 1.
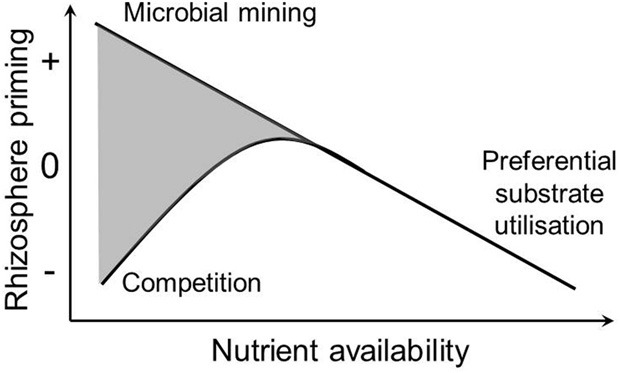
Hypothetical relationship between soil nutrient availability and rhizosphere priming. Three nutrient-centered hypotheses are illustrated: Microbial mining: microbes utilize rhizodeposition to mine for nutrients in SOM thereby causing a positive rhizosphere priming effect when nutrient availability is low; Preferential substrate utilization: microbes switch from decomposing SOM to utilizing rhizodeposition when nutrient availability is high; Competition: microbes compete for nutrients with plants causing a negative rhizosphere priming effect because microbial growth and decomposition are nutrient limited. Both positive and negative rhizosphere priming can occur under low nutrient availability (gray area).
Second, in soils of high nutrient availability, a negative priming effect may occur. Under conditions of high nutrient availability, there is less need for microbes to mine nutrients, but instead, microbes may switch from decomposing recalcitrant SOM to utilizing labile root exudates for their carbon and energy requirements (Blagodatskaya et al., 2007; Guenet et al., 2010). As a result, decomposition of SOM could decrease with inputs of root exudates. This has been referred to as the preferential substrate utilization hypothesis (Cheng, 1999) (Figure 1).
A third nutrient-centered mechanism that has been proposed for explaining negative rhizosphere priming effects is when plants and microbes compete for the same nutrients. When plants remove nutrients from the soil through uptake they may reduce microbial decomposition (Dijkstra et al., 2010b; Pausch et al., 2013). The resulting negative rhizosphere priming effect may be stronger when nutrient availability is already low and limiting both plant and microbial growth (competition hypothesis, Cheng, 1999) (Figure 1). Production of rhizodeposits may also be a strategy for slow-growing plant species to lower soil N availability thereby outcompeting neighboring fast-growing plant species (Meier et al., 2009).
Low soil nutrient conditions can invoke both positive and negative rhizosphere priming (Figure 1). Clearly, there is a need to better understand why rhizodeposition in soils with low nutrient conditions sometimes result in enhanced microbial mining for nutrients (and a positive rhizosphere priming effect) and at other times in enhanced competition for nutrients inducing reduced microbial activity (and a negative rhizosphere priming effect). Several explanations may be involved in observations of positive and negative priming effects, including soil microbial community effects, quality and stoichiometry of the root exudates, and the relative availability of N and P, while none of these explanations are mutually exclusive.
First, dominance of one group of microbes over the other could potentially determine whether rhizodeposition results in negative or positive rhizosphere priming under low nutrient conditions. Microbes vary tremendously in their ability to decompose SOM. While rhizodeposition may primarily increase growth and activity of fast growing microbes (r-strategists), a proportion of the rhizodeposition may be utilized by slow growing microbes decomposing recalcitrant organic matter (K-strategists), particularly when nutrient availability is low (Fontaine et al., 2003). Fungi, gram-negative and gram-positive bacteria have all been associated with enhanced SOM decomposition with increased rhizodeposition and input of other labile C (Nottingham et al., 2009; Bird et al., 2011; Fontaine et al., 2011; Garcia-Pausas and Paterson, 2011). Bacteria may be more sensitive to competition for nutrients with plants than fungi because fungal hyphae have a greater ability to explore the soil (Otten et al., 2001), and therefore fungi may escape competition for nutrients with plants. Furthermore, mycorrhizae, a special group of fungi that grow in direct association with plants, may supply nutrients directly to plants in return for plant C, thereby reducing nutrient competition between plants and mycorrhizae (Koide, 1991).
If the soil microbial community is dominated by bacteria that are activated close to the root, then the microbial competition hypothesis (or preferential substrate utilization hypothesis under high nutrient availability) may prevail resulting in negative rhizosphere priming. These bacteria would be dominated by r-strategists and utilize fresh exudates (Dorodnikov et al., 2009). On the other hand, if the soil microbial community is dominated by fungi, supply of plant C may stimulate fungi to mine for nutrients further away from the roots resulting in positive rhizosphere priming. These fungal decomposers act more like K-strategists, and may be saprotrophic or mycorrhizal (Talbot et al., 2008).
Second, the direction and magnitude of rhizosphere priming under low nutrient conditions may depend on the type of organic compounds released by plants. Plant roots release a myriad of organic compounds, not only from root exudation, but also from mucilage, as sloughed cells via mechanical abrasion, and from root death (Jones et al., 2004, 2009). Because these compounds have different stoichiometric and energetic properties, rhizodeposition may have variable effects on priming (Mary et al., 1993; Hamer and Marschner, 2005; Kuzyakov and Bol, 2006). Although many of these compounds often showed idiosyncratic priming effects, root exudates that generate more alkalinity during their decomposition or contain more N have been shown to cause greater priming (Rukshana et al., 2012; Drake et al., 2013).
Third, contrasting rhizosphere priming effects under low nutrient availability may occur because priming also depends on soil properties such as total C content and texture (Zhang et al., 2013), mineralogy (Rasmussen et al., 2007), pH (Blagodatskaya and Kuzyakov, 2008; Luo et al., 2011), and heavy metal concentration (Ohm et al., 2011). Here we propose that the contrasting effects of rhizosphere priming under low nutrient availability can also be related to the relative availability of N and P in the soil.
N and P cycling and their role in rhizosphere priming
As discussed above, the supply of N to plants and microbes in soils predominantly occurs through oxidation of organic matter whereby N is mineralized. Phosphorus can also be released from SOM, but in soils with low organic P, inorganic sources are an important source for P supply (Walker and Syers, 1976). For instance, in calcareous soils much of the soil P is contained in calcium phosphates and the supply of P is regulated by precipitation/dissolution equilibria with P in soil solution (Lajtha and Bloomer, 1988; Tunesi et al., 1999). Similarly, in many acidic soils, P is bound to Al and Fe oxides, and the supply of P to plants is controlled by adsorption/desorption processes (Sanyal and Datta, 1991). Furthermore, much of the organic P is present in soil as monoesters and diesters (Doolette and Smernik, 2011). The P in these bonds can be released by hydrolysis with the help of phosphatase enzymes (without causing CO2 production, Nannipieri et al., 2011), rather than through oxidation of organic matter (causing CO2 production). Therefore, the supply of N and P to plants is decoupled in many soil types (McGill and Cole, 1981).
Terrestrial ecosystems are frequently limited by P (Elser et al., 2007; Harpole et al., 2011). Microbes in particular have a high P requirement relative to N, where microbial N:P ratios are often lower than the plant or SOM N:P ratios from the same system (Cleveland and Liptzin, 2007). Microbial activity and growth can be limited by P, which has mostly been observed in highly weathered tropical soils (Cleveland et al., 2002; Ehlers et al., 2010), but also in a calcareous (Raiesi and Ghollarata, 2006), peat (Amador and Jones, 1993), and boreal forest soils (Giesler et al., 2002).
Whether the input of C compounds via rhizodeposition results in altered SOM decomposition may depend on whether microbial activity is N or P limited. In soils where microbes are more limited by N than by P, root exudates could be utilized to mine for N through enhanced SOM decomposition. On the other hand, in soils where microbes are more limited by P than by N, root exudates are not needed by microbes for releasing N from SOM, but instead, could be used to mobilize P from inorganic or organic sources. Root exudates could be utilized by microbes to produce phosphatase extracellular enzymes releasing P through hydrolysis (Dakora and Phillips, 2002). Increased levels of microbial biomass and phosphatase extracellular enzymes have been observed in the rhizosphere (Chen et al., 2002), but it is unclear to what degree phosphatase extracellular enzymes are produced by plants or microbes (George et al., 2011). Root exudates can also directly increase P mobilization by increasing desorption and solubilisation from mineral surfaces through ligand exchange and dissolution (Dakora and Phillips, 2002; George et al., 2011). We hypothesise that microbial N limitation results in rhizosphere priming, while microbial P limitation does not (Figure 2). After discussing the effects of rhizosphere priming on nutrient availability, we will illustrate this hypothesis with examples of rhizosphere priming effects that were observed under elevated atmospheric CO2 concentration.
Figure 2.
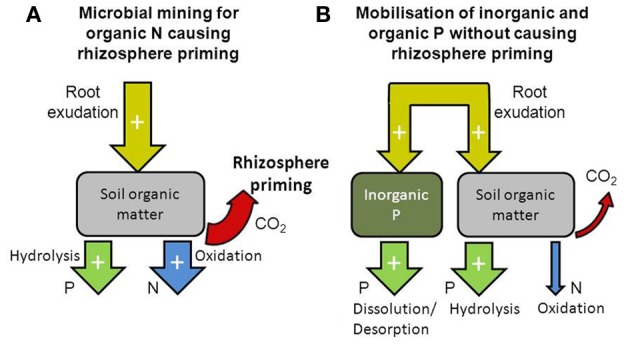
Diagram illustrating how the availability of nitrogen and phosphorus in the soil can influence rhizosphere priming. When nitrogen availability is low, microbes utilize rhizodeposition to mine for nitrogen locked in organic matter thereby increasing rhizosphere priming and release of nitrogen (through oxidation of SOM) and phosphorus [mostly through hydrolysis of P-esters in SOM, (A)]. When phosphorus availability is low, rhizodeposition is utilized to mobilize phosphorus from inorganic and organic sources (through dissolution/desorption and hydrolysis, respectively) thereby increasing the release of phosphorus without affecting rhizosphere priming (B).
Rhizosphere priming effects on nutrient availability
While some research has been done on how rhizosphere priming is affected by nutrient availability, particularly N availability, considerably less is known regarding the consequences of rhizosphere priming for nutrient cycling and plant nutrient uptake. From an evolutionary perspective it could be argued that the loss of expensive energy-rich carbon compounds into the soil through root exudation should result in some benefit to the plant. Does the stimulation of SOM decomposition through rhizosphere priming result in increased nutrient availability to plants or does it only result in increased microbial nutrient immobilization? There are only a limited number of studies that have tried to tackle this question.
Positive priming effects should be associated with net N mineralization, but Cheng (2009) observed that the amount of net mineral N released from the accelerated SOM decomposition associated with the rhizosphere priming effect was much lower than the expected amount based on the C:N ratio of the SOM. This suggests that much of the N associated with the increase in SOM decomposition remained in the microbial biomass or was returned to the organic soil pool. On the other hand, a range of positive rhizosphere priming effects were observed among six different soil type-plant species combinations that also resulted in substantial increases in net N mineralization (Dijkstra et al., 2009). Except for one soil-plant combination, rhizosphere priming effects were positively related to gross N mineralization and plant N uptake, suggesting that rhizosphere priming not only enhanced microbial mining for N, but also enhanced the release of N for plant uptake. An increase in net N mineralization with root exudation could also occur because of microbial grazing by protozoa (Clarholm, 1985) creating a microbial loop. According to this microbial loop hypothesis, increased microbial growth in response to root exudation causes increased microbial immobilization of N, which in return is released for plant uptake after grazing by protozoa or nematodes.
However, there appears to be a fine balance between how rhizosphere priming affects microbial mineralization and immobilization. Microbial immobilization increased more than gross N mineralization with increased root exudation of three species of tree seedlings so that net N mineralization was reduced at high rates of root exudation (Bengtson et al., 2012). In modeling and field experiments Drake et al. (2013), simulated exudates and showed that adding C alone enhanced SOM decomposition, but adding C and N together stimulated SOM decomposition and N mineralization significantly more than C alone. These results suggest that both quantity and quality of root exudation have important consequences for the release of N through rhizosphere priming.
Rhizosphere priming under elevated atmospheric [CO2]
Atmospheric CO2 concentrations have increased by more than 35% during the last 150 years and will continue to rise in the future (Forster et al., 2007), causing large impacts on C cycling in terrestrial ecosystems (Heimann and Reichstein, 2008). The immediate plant response to an increase in atmospheric CO2 is often an increase in photosynthesis and net primary production (Amthor, 1995). Several studies have indicated that elevated CO2 also increases rhizodeposition (Darrah, 1996; Pendall et al., 2004; Fransson and Johansson, 2010), and could potentially increase rhizosphere priming. Indeed, increased loss of soil C or mineral-associated organic matter under elevated CO2 has been associated with greater rhizosphere priming (Carney et al., 2007; Hofmockel et al., 2011b).
An increase in SOM decomposition caused by rhizosphere priming under elevated CO2 may also affect N cycling, and this has important ramifications for long-term responses of terrestrial ecosystems to elevated CO2. Plant growth in most terrestrial ecosystems is N limited (Vitousek and Howarth, 1991). It has been suggested that without external input of N, elevated CO2 will reduce N availability to plants in the long-term and that therefore plant growth responses to elevated CO2 cannot be sustained (Luo et al., 2004). A crucial component of this concept of Progressive N Limitation (PNL) is the expectation that N availability is reduced under elevated CO2 because of increased plant N uptake and immobilization in long-lived plant biomass, and because of increased microbial N immobilization associated with increased C inputs into the soil. Indeed, elevated CO2 often reduces N availability in the soil (Díaz et al., 1993; Gill et al., 2002; Reich et al., 2006). Tree growth in a temperate forest increased during the first 6 years in response to elevated CO2, but this effect disappeared after 11 years (Norby et al., 2010). It was suggested that a decline in soil N availability constrained the plant growth responses to elevated CO2 in the long-term thereby providing support for the PNL concept.
However, PNL has not always been observed (Luo et al., 2006) and rhizosphere priming may be one of the mechanisms responsible for the lack of PNL. Tree growth in a temperate forest in North Carolina was still higher after 10 years of elevated CO2 (McCarthy et al., 2010). Moreover, plant N uptake remained higher under elevated CO2, which appears to have caused the sustained increase in tree growth in response to elevated CO2 in this study (Drake et al., 2011). While some of this extra N may have been taken up from deeper soil layers (Finzi et al., 2006), it was also shown that elevated CO2 enhanced root exudation and N release from SOM decomposition through rhizosphere priming (Phillips et al., 2011, 2012) Increased rhizosphere priming and plant N uptake under elevated CO2 has also been observed in several other studies (Martín-Olmedo et al., 2002; de Graaff et al., 2009; Hofmockel et al., 2011a). These results suggest that enhanced rhizosphere priming could delay or at least alleviate PNL under elevated CO2.
Contrasting elevated CO2 effects on N cycling in semiarid grasslands
Sustained increases in N cycling and plant N uptake under elevated CO2 were also observed in a semiarid grassland in Colorado, USA (King et al., 2004; Dijkstra et al., 2008). Enhanced N cycling and microbial mining for N under elevated CO2 was illustrated with a 15N tracer study. In this field experiment 15N (as 15NH4 15NO3) was added as a tracer to the soil and followed into aboveground plant biomass collected in plots exposed to ambient and elevated CO2 using open top chambers, up to 5 years after the pulse addition (OTC experiment). The 15N label in plant tissue, expressed as a fraction of total aboveground plant tissue N, decreased with time (Dijkstra et al., 2008) (Figure 3A). The decrease of the 15N label was explained by ongoing mineralization of unlabeled N in the soil that progressively diluted the 15N label in the available N pool to plants (Dijkstra, 2009). This dilution of the 15N label in the plant was faster under elevated CO2, suggesting that mineralization of unlabeled N from SOM was enhanced under elevated CO2. Rhizodeposition was also greater under elevated CO2 in this system (Pendall et al., 2004). These combined results suggest that elevated CO2 may have increased rhizosphere priming through microbial mining for N and subsequent release of N for plant uptake.
Figure 3.
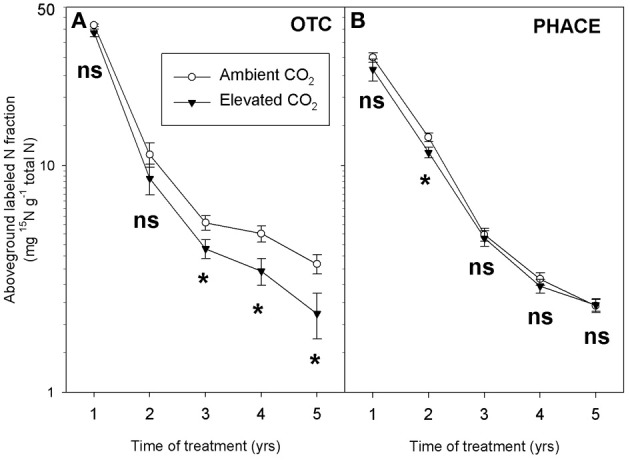
Labeled N fractions (expressed per total amount of N) in aboveground biomass over time in the OTC (A) and PHACE (B) experiment. A 15N label was added to the soil in the Spring of year 1 and aboveground biomass was sampled at peak biomass in July in the following 5 years. For each year, CO2 treatment effects were tested with ANOVA (ns: not significant, *P < 0.05).
We conducted a similar experiment in the Prairie Heating And CO2 Enrichment (PHACE) experiment in Wyoming, USA (Dijkstra et al., 2010a; Morgan et al., 2011). The northern mixed prairie vegetation of the PHACE experiment is similar to that of the shortgrass steppe of the OTC experiment in Colorado with Bouteloua gracilis (a warm season C4 grass), Pascopyrum smithii and Hesperostipa comata (two cool-season C3 grasses) being the dominant species comprising 80% or more of the aboveground biomass at both sites, although net primary productivity is greater at PHACE than at OTC. Free Air CO2 Enrichment technology was used in the PHACE experiment to increase the CO2 concentration to 600 ppm, which is lower than the elevated CO2 treatment in the OTC experiment (720 ppm). Furthermore, a warming treatment (1.5/3°C above ambient during the day/night) using infrared heaters was included in the PHACE experiment in a full factorial design.
As in the OTC experiment, we added a 15N tracer to the plots and followed the 15N label into aboveground biomass during the following years. The 15N label was added by spraying a K15NO3 solution (99 atom% 15N) onto the plots in 2007. As in the OTC experiment the 15N label, expressed as a fraction of the total plant N, decreased with time due to dilution of the label with non-labeled N from mineralization in the soil. However, in contrast to the OTC experiment, the decrease of the 15N label in aboveground biomass was not enhanced under elevated CO2 (Figure 3B). These results suggest that elevated CO2 did not enhance microbial mining and release of unlabeled N in the PHACE experiment.
Does rhizosphere priming under elevated CO2 depend on relative availability of N and P?
Why did these similar semiarid grasslands respond differently to elevated CO2 in terms of its effect on N cycling? A possible explanation is that in the PHACE experiment elevated CO2 did not increase rhizosphere priming of SOM. However, elevated CO2 resulted in larger labile soil C pools, although not in all years (Carrillo et al., 2011). Further, elevated CO2 increased rates of heterotrophic respiration in the PHACE experiment (Pendall et al., 2013). These results suggest that elevated CO2 may have enhanced rhizosphere priming of native soil organic C. However, it is also possible that elevated CO2 enhanced input and cycling of the new and relatively labile soil C only without affecting decomposition of the native soil C pool (e.g., Hungate et al., 1997) (Figure 4). If elevated CO2 increased the cycling of new C without affecting decomposition of native soil organic C, this would not result in enhanced dilution of the 15N in the plant (Dijkstra, 2009). Only an increase in the decomposition of native soil organic C (due to rhizosphere priming) would result in enhanced dilution of the 15N label in the plant. Interestingly, (Allard et al., 2006) also found no enhanced plant N uptake under elevated CO2 despite enhanced C cycling.
Figure 4.
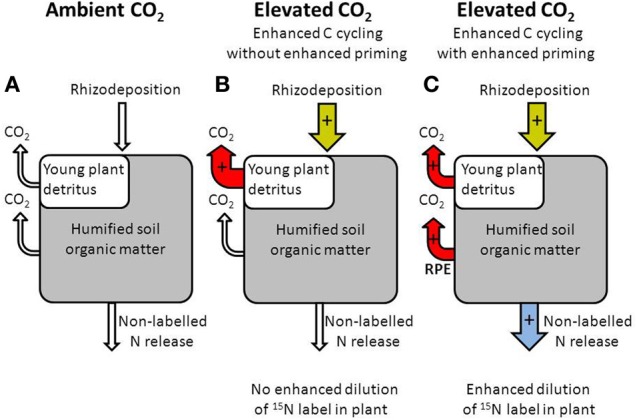
Diagram illustrating the role of rhizodeposition for carbon cycling under ambient (A) and elevated CO2 (B,C). An increase in rhizodeposition under elevated CO2 can enhance the decomposition of young plant detritus only without affecting the decomposition of humified SOM and without affecting the release of non-labeled nitrogen (B), or can enhance the decomposition of plant detritus and humified SOM (rhizosphere priming effect or RPE) thereby increasing release of non-labeled nitrogen (C).
Why then would greater inputs of labile C under elevated CO2 result in enhanced rhizosphere priming of native soil organic C in the OTC experiment, but only result in enhanced cycling of labile C in the PHACE experiment? We postulate that these contrasting effects of elevated CO2 on soil C cycling may have occurred because of differences in the availability and cycling of N and P between the two sites.
As explained above, under conditions of low N availability, root exudates could be used by microbes to improve N supply by enhancing the decomposition of recalcitrant SOM that is relatively rich in N (microbial mining hypothesis), possibly with an extra supply of N-degrading enzymes (Drake et al., 2013). However, as described above, root exudates could also be used for the production of phosphatase extracellular enzymes hydrolyzing organic P (by plants and microbes) and to increase mobilization of P (Dakora and Phillips, 2002; George et al., 2011) without affecting SOM decomposition.
In the PHACE experiment we observed that the availability and plant uptake of P compared to that of N increased under elevated CO2 (Dijkstra et al., 2012). The PHACE experiment was conducted on a calcareous soil high in insoluble calcium phosphates (41% of total soil P was in inorganic form, Dijkstra et al., 2012) that are not directly available to plants. The fixation of P as calcium phosphates may have caused low P availability possibly limiting microbial activity and plant growth. An increase in root exudation under elevated CO2 in this system may have increased P dissolution and mobilization without affecting net N release from native SOM. We have limited data on the availability of P compared to N in the soil of the OTC experiment. While soil P availability was similarly low at both sites (between 4 and 11 mg P kg−1 soil in the OTC experiment and between 4 and 7 mg P kg−1 soil in the PHACE experiment using 0.5 M NaHCO3 extractions), a semiarid grassland similar to the grassland used in the OTC experiment strongly responded to N fertilization (Lauenroth et al., 1978). This suggests that plants and microbes may have been more limited by N than by P in the OTC experiment.
We propose that contrasting effects of elevated CO2 on 15N dilution in plant biomass in the OTC and PHACE experiments were due to differences in N and P availability to microbial activity and plant growth. In the N limited semiarid grassland, where the OTC experiment was conducted (Lauenroth et al., 1978), increased root exudation under elevated CO2 resulted in a greater rhizosphere priming thereby enhancing SOM decomposition and mineralization of N, and possibly P. The enhanced N mineralization from native SOM then resulted in enhanced dilution of the 15N label in the plant (Figure 2). On the other hand, in the PHACE experiment where P availability was low (Dijkstra et al., 2012) and that may have limited microbial activity and plant growth more than N, the increase in root exudation under elevated CO2 may have increased the dissolution/desorption and mobilization of P, more so than enhancing decomposition of native SOM. As a result, the dilution of the 15N label in aboveground plant biomass with time was unaffected by elevated CO2. Others have also suggested that enhanced rhizodeposition under elevated CO2 may be utilized for mobilizing P, rather than for enhancing SOM decomposition (Cardon, 1996; Lloyd et al., 2001).
Conclusion
Several studies have suggested that elevated CO2 can enhance SOM decomposition through increased rhizosphere priming effects (Cheng, 1999; Paterson et al., 2008; Phillips et al., 2011). An increase in rhizosphere priming has important implications for long-term C sequestration in soils under elevated CO2 and how this feedbacks to the global climate. However, the magnitude and direction of the rhizosphere priming effect may strongly depend on the relative availability of N and P in the soil (Bradford et al., 2008; Milcu et al., 2011; Sullivan and Hart, 2013). Although rhizosphere priming is influenced by several factors, we argue that the relative availability of N and P has to be considered in understanding how perturbations such as climate change affect rhizosphere priming and soil C sequestration.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was supported by the US Department of Agriculture Agricultural Research Service Climate Change, Soils and Emissions Program, USDA-CSREES Soil Processes Program (Grant no. 2008-35107-18655), US Department of Energy's Office of Science (BER), through the Terrestrial Ecosystem Science program and the Western Regional Center of the National Institute for Climatic Change Research at Northern Arizona University, by NSF (DEB# 1021559), and by the Australian Research Council (FT100100779).
References
- Allard V., Robin C., Newton P. C. D., Lieffering M., Soussana J. F. (2006). Short and long-term effects of elevated CO2 on Lolium perenne rhizodeposition and its consequences on soil organic matter turnover and plant N yield. Soil Biol. Biochem. 38, 1178–1187 10.1016/j.soilbio.2005.10.002 [DOI] [Google Scholar]
- Amador J., Jones R. D. (1993). Nutrient limitations on microbial respiration in peat soils with different total phosphorus content. Soil Biol. Biochem. 25, 793–801 10.1016/0038-0717(93)90125-U [DOI] [Google Scholar]
- Amthor J. S. (1995). Terrestrial higher-plant response to increasing atmospheric [CO2] in relation to the global carbon cycle. Glob. Change Biol. 1, 243–274 10.1111/j.1365-2486.1995.tb00025.x [DOI] [Google Scholar]
- Asmar F., Eiland F., Nielsen N. E. (1994). Effect of extracellular-enzyme activities on solubilization rate of soil organic nitrogen. Biol. Fertil. Soils 17, 32–38 10.1007/BF00418669 [DOI] [Google Scholar]
- Averill C., Finzi A. (2011). Plant regulation of microbial enzyme production in situ. Soil Biol. Biochem. 43, 2457–2460 10.1016/j.soilbio.2011.09.002 [DOI] [Google Scholar]
- Bader N. E., Cheng W. (2007). Rhizosphere priming effect of Populus fremontii obscures the temperature sensitivity of soil organic carbon respiration. Soil Biol. Biochem. 39, 600–606 10.1016/j.soilbio.2006.09.009 [DOI] [Google Scholar]
- Bengtson P., Barker J., Grayston S. J. (2012). Evidence of a strong coupling between root exudation, C and N availability, and stimulated SOM decomposition caused by rhizosphere priming effects. Ecol. Evol. 2, 1843–1852 10.1002/ece3.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird J. A., Herman D. J., Firestone M. K. (2011). Rhizosphere priming of soil organic matter by bacterial groups in a grassland soil. Soil Biol. Biochem. 43, 718–725 10.1016/j.soilbio.2010.08.010 [DOI] [Google Scholar]
- Blagodatskaya E., Kuzyakov Y. (2008). Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol. Fertil. Soils 45, 115–131 10.1007/s00374-008-0334-y [DOI] [Google Scholar]
- Blagodatskaya E. V., Blagodatsky S. A., Anderson T. H., Kuzyakov Y. (2007). Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl. Soil Ecol. 37, 95–105 10.1016/j.apsoil.2007.05.002 [DOI] [Google Scholar]
- Bradford M. A., Fierer N., Reynolds J. F. (2008). Soil carbon stocks in experimental mesocosms are dependent on the rate of labile carbon, nitrogen and phosphorus inputs to soils. Funct. Ecol. 22, 964–974 10.1111/j.1365-2435.2008.01404.x [DOI] [Google Scholar]
- Brzostek E. R., Greco A., Drake J. E., Finzi A. C. (2012). Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 10.1007/s10533-012-9818-9. [DOI] [Google Scholar]
- Cardon Z. G. (1996). Influence of rhizodeposition under elevated CO2 on plant nutrition and soil organic matter. Plant Soil 187, 277–288 10.1007/BF00017093 [DOI] [Google Scholar]
- Carney K. M., Hungate B. A., Drake B. G., Megonigal J. P. (2007). Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc. Natl. Acad. Sci. U.S.A. 104, 4990–4995 10.1073/pnas.0610045104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo Y., Pendall E., Dijkstra F. A., Morgan J. A., Newcomb J. M. (2011). Response of soil organic matter pools to elevated CO2 and warming in a semi-arid grassland. Plant Soil 347, 339–350 10.1007/s11104-011-0853-4 [DOI] [Google Scholar]
- Chen C. R., Condron L. M., Davis M. R., Sherlock R. R. (2002). Phosphorus dynamics in the rhizosphere of perennial ryegrass (Lolium perenne L.) and radiata pine (Pinus radiata D. Don). Soil Biol. Biochem. 34, 487–499 10.1016/S0038-0717(01)00207-3 [DOI] [Google Scholar]
- Cheng W. (1996). Measurement of rhizosphere respiration and organic matter decomposition using natural 13C. Plant Soil 183, 263–268 10.1007/BF00011441 [DOI] [Google Scholar]
- Cheng W. (1999). Rhizosphere feedbacks in elevated CO2. Tree Physiol. 19, 313–320 10.1093/treephys/19.4-5.313 [DOI] [PubMed] [Google Scholar]
- Cheng W. (2009). Rhizosphere priming effect: Its functional relationships with microbial turnover, evapotranspiration, and C-N budgets. Soil Biol. Biochem. 41, 1795–1801 10.1016/j.soilbio.2008.04.018 [DOI] [Google Scholar]
- Clarholm M. (1985). Interactions of bacteria, protozoa and plants leading to mineralization of soil nitrogen. Soil Biol. Biochem. 17, 181–187 10.1016/0038-0717(85)90113-0 [DOI] [Google Scholar]
- Cleveland C. C., Liptzin D. (2007). C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85, 235–252 10.1007/s10533-007-9132-0 [DOI] [Google Scholar]
- Cleveland C. C., Townsend A. R., Schmidt S. K. (2002). Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5, 680–691 10.1007/s10021-002-0202-9 [DOI] [Google Scholar]
- Craine J. M., Morrow C., Fierer N. (2007). Microbial nitrogen limitation increases decomposition. Ecology 88, 2105–2113 10.1890/06-1847.1 [DOI] [PubMed] [Google Scholar]
- Dakora F. D., Phillips D. A. (2002). Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245, 35–47 10.1023/A:1020809400075 [DOI] [Google Scholar]
- Darrah P. R. (1996). Rhizodeposition under ambient and elevated CO2 levels. Plant Soil 187, 265–275 10.1007/BF0001709215091762 [DOI] [Google Scholar]
- de Graaff M.-A., Van Kessel C., Six J. (2009). Rhizodeposition-induced decomposition increases N availability to wild and cultivated wheat genotypes under elevated CO2. Soil Biol. Biochem. 41, 1094–1103 10.1016/j.soilbio.2009.02.015 [DOI] [Google Scholar]
- Díaz S., Grime J. P., Harris J., McPherson E. (1993). Evidence of a feedback mechanism limiting plant response to elevated carbon dioxide. Nature 364, 616–617 10.1038/364616a0 [DOI] [Google Scholar]
- Dijkstra F. A. (2009). Modeling the flow of 15N after a 15N pulse to study long-term N dynamics in a semiarid grassland. Ecology 90, 2171–2182 10.1890/08-1172.1 [DOI] [PubMed] [Google Scholar]
- Dijkstra F. A., Bader N. E., Johnson D. W., Cheng W. (2009). Does accelerated soil organic matter decomposition in the presence of plants increase plant N availability? Soil Biol. Biochem. 41, 1080–1087 10.1016/j.soilbio.2009.02.013 [DOI] [Google Scholar]
- Dijkstra F. A., Blumenthal D., Morgan J. A., Pendall E., Carrillo Y., Follett R. F. (2010a). Contrasting effects of elevated CO2 and warming on nitrogen cycling in a semiarid grassland. New Phytol. 187, 426–437 10.1111/j.1469-8137.2010.03293.x [DOI] [PubMed] [Google Scholar]
- Dijkstra F. A., Morgan J. A., Blumenthal D., Follett R. F. (2010b). Water limitation and plant inter-specific competition reduce rhizosphere-induced C decomposition and plant N uptake. Soil Biol. Biochem. 42, 1073–1082 10.1016/j.soilbio.2010.02.026 [DOI] [Google Scholar]
- Dijkstra F. A., Pendall E., Morgan J. A., Blumenthal D. M., Carrillo Y., LeCain D. R., et al. (2012). Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol. 196, 807–815 10.1111/j.1469-8137.2012.04349.x [DOI] [PubMed] [Google Scholar]
- Dijkstra F. A., Pendall E., Mosier A. R., King J. Y., Milchunas D. G., Morgan J. A. (2008). Long-term enhancement of N availability and plant growth under elevated CO2 in a semi-arid grassland. Funct. Ecol. 22, 975–982 10.1111/j.1365-2435.2008.01398.x [DOI] [Google Scholar]
- Doolette A. L., Smernik R. J. (2011). “Soil organic phosphorus speciation using spectroscopic techniques,” in Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling, eds Bünemann E. K., Oberson A., Frossard E. (Heidelberg: Springer; ), 3–36 10.1007/978-3-642-15271-9_1 [DOI] [Google Scholar]
- Dorodnikov M., Blagodatskaya E., Blagodatsky S., Fangmeier A., Kuzyakov Y. (2009). Stimulation of r- vs. K-selected microorganisms by elevated atmospheric CO2 depends on soil aggregate size: research article. FEMS Microbiol. Ecol. 69, 43–52 10.1111/j.1574-6941.2009.00697.x [DOI] [PubMed] [Google Scholar]
- Drake J. E., Darby B. A., Giasson M. A., Kramer M. A., Phillips R. P., Finzi A. C. (2013). Stoichiometry constrains microbial response to root exudation- insights from a model and a field experiment in a temperate forest. Biogeosciences 10, 821–838 10.5194/bg-10-821-2013 [DOI] [Google Scholar]
- Drake J. E., Gallet-Budynek A., Hofmockel K. S., Bernhardt E. S., Billings S. A., Jackson R. B., et al. (2011). Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol. Lett. 14, 349–357 10.1111/j.1461-0248.2011.01593.x [DOI] [PubMed] [Google Scholar]
- Ehlers K., Bakken L. R., Frostegård Å, Frossard E., Bünemann E. K. (2010). Phosphorus limitation in a ferralsol: impact on microbial activity and cell internal P pools. Soil Biol. Biochem. 42, 558–566 10.1016/j.soilbio.2009.11.025 [DOI] [Google Scholar]
- Elser J. J., Bracken M. E. S., Cleland E. E., Gruner D. S., Harpole W. S., Hillebrand H., et al. (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142 10.1111/j.1461-0248.2007.01113.x [DOI] [PubMed] [Google Scholar]
- Finzi A. C., Moore D. J. P., DeLucia E. H., Lichter J., Hofmockel K. S., Jackson R. B., et al. (2006). Progressive nitrogen limitation of ecosystem processes under elevated CO2 in a warm-temperate forest. Ecology 87, 15–25 10.1890/04-1748 [DOI] [PubMed] [Google Scholar]
- Fontaine S., Henault C., Aamor A., Bdioui N., Bloor J. M. G., Maire V., et al. (2011). Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 43, 86–96 10.1016/j.soilbio.2010.09.017 [DOI] [Google Scholar]
- Fontaine S., Mariotti A., Abbadie L. (2003). The priming effect of organic matter: a question of microbial competition? Soil Biol. Biochem. 35, 837–843 10.1016/S0038-0717(03)00123-8 [DOI] [Google Scholar]
- Forster P., Ramaswamy V., Artaxo P., Berntsen T., Betts R., Fahey D. W., et al. (2007). “Changes in atmospheric constituents and in radiative forcing,” in Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L. (Cambridge: Cambridge University Press; ), 129–234 [Google Scholar]
- Fransson P. M. A., Johansson E. M. (2010). Elevated CO2 and nitrogen influence exudation of soluble organic compounds by ectomycorrhizal root systems. FEMS Microbiol. Ecol. 71, 186–196 10.1111/j.1574-6941.2009.00795.x [DOI] [PubMed] [Google Scholar]
- Garcia-Pausas J., Paterson E. (2011). Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biol. Biochem. 43, 1705–1713 10.1016/j.soilbio.2011.04.016 [DOI] [Google Scholar]
- George T. S., Fransson A. M., Hammond J. P., White P. J. (2011). “Phosphorus nutrition: rhizosphere processes, plant response and adaptation,” in Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling, eds Bünemann E. K., Oberson A., Frossard E. (Heidelberg: Springer; ), 245–271 10.1007/978-3-642-15271-9_10 [DOI] [Google Scholar]
- Giesler R., Petersson T., Högberg P. (2002). Phosphorus limitation in boreal forests: effects of aluminum and iron accumulation in the humus layer. Ecosystems 5, 300–314 10.1007/s10021-001-0073-5 [DOI] [Google Scholar]
- Gill R. A., Polley H. W., Johnson H. B., Anderson L. J., Maherali H., Jackson R. B. (2002). Nonlinear grassland responses to past and future atmospheric CO2. Nature 417, 279–282 10.1038/417279a [DOI] [PubMed] [Google Scholar]
- Guenet B., Neill C., Bardoux G., Abbadie L. (2010). Is there a linear relationship between priming effect intensity and the amount of organic matter input? Appl. Soil Ecol. 46, 436–442 10.1016/j.apsoil.2010.09.006 [DOI] [Google Scholar]
- Hamer U., Marschner B. (2005). Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions. Soil Biol. Biochem. 37, 445–454 10.1016/j.soilbio.2004.07.037 [DOI] [Google Scholar]
- Harpole W. S., Ngai J. T., Cleland E. E., Seabloom E. W., Borer E. T., Bracken M. E. S., et al. (2011). Nutrient co-limitation of primary producer communities. Ecol. Lett. 14, 852–862 10.1111/j.1461-0248.2011.01651.x [DOI] [PubMed] [Google Scholar]
- Heimann M., Reichstein M. (2008). Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451, 289–292 10.1038/nature06591 [DOI] [PubMed] [Google Scholar]
- Hofmockel K. S., Gallet-Budynek A., McCarthy H. R., Currie W. S., Jackson R. B., Finzi A. (2011a). Sources of increased N uptake in forest trees growing under elevated CO2: results of a large-scale 15N study. Glob. Change Biol. 17, 3338–3350 10.1111/j.1365-2486.2011.02465.x [DOI] [Google Scholar]
- Hofmockel K. S., Zak D. R., Moran K. K., Jastrow J. D. (2011b). Changes in forest soil organic matter pools after a decade of elevated CO2 and O3. Soil Biol. Biochem. 43, 1518–1527 10.1016/j.soilbio.2011.03.030 [DOI] [Google Scholar]
- Hungate B. A., Holland E. A., Jackson R. B., Chapin F. S. III, Mooney H. A., Field C. B. (1997). The fate of carbon in grassland under carbon dioxide enrichment. Nature 388, 576–579 10.1038/4155017286825 [DOI] [Google Scholar]
- Jones D., Nguyen C., Finlay R. (2009). Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321, 5–33 10.1007/s11104-009-9925-0 [DOI] [Google Scholar]
- Jones D. L., Hodge A., Kuzyakov Y. (2004). Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 163, 459–480 10.1111/j.1469-8137.2004.01130.x [DOI] [PubMed] [Google Scholar]
- King J. Y., Mosier A. R., Morgan J. A., LeCain D. R., Milchunas D. G., Parton W. J. (2004). Plant nitrogen dynamics in shortgrass steppe under elevated atmospheric carbon dioxide. Ecosystems 7, 147–160 10.1007/s10021-003-0201-5 [DOI] [Google Scholar]
- Koide R. T. (1991). Tansley Review No.29. Nutrient supply, nutrient demand and plant response to mycorrhizal infection. New Phytol. 117, 365–386 10.1111/j.1469-8137.1991.tb00001.x [DOI] [PubMed] [Google Scholar]
- Kuzyakov Y. (2002). Factors affecting rhizosphere priming effects (review). J. Plant Nutr. Soil Sci. 165, 382–396 [Google Scholar]
- Kuzyakov Y. (2010). Priming effects: interactions between living and dead organic matter. Soil Biol. Biochem. 42, 1363–1371 10.1016/j.soilbio.2010.04.003 [DOI] [Google Scholar]
- Kuzyakov Y., Bol R. (2006). Sources and mechanisms of priming effect induced in two grassland soils amended with slurry and sugar. Soil Biol. Biochem. 38, 747–758 10.1016/j.soilbio.2005.06.025 [DOI] [Google Scholar]
- Lajtha K., Bloomer S. H. (1988). Factors affecting phosphate sorption and phosphate retention in a desert ecosystem. Soil Sci. 146, 160–167 10.1097/00010694-198809000-00003 [DOI] [Google Scholar]
- Lauenroth W. K., Dodd J. L., Sims P. L. (1978). The effects of water- and nitrogen-induced stresses on plant community structure in a semiarid grassland. Oecologia 36, 211–222 10.1007/BF00349810 [DOI] [PubMed] [Google Scholar]
- Lloyd J., Bird M. I., Veenendaal E. M., Kruijt B. (2001). “Should phosphorus availability be constraining moist tropical forest responses to increasing CO2 concentrations?,” in Global Biogeochemical Cycles in the Climate System eds Schulze E. D., Heimann M., Harrison S., Holland E., Lloyd J., Prentice I. C., Schimel D. (San Diego, CA: Academic Press; ), 95–114 [Google Scholar]
- Luo Y., Durenkamp M., De Nobili M., Lin Q., Brookes P. C. (2011). Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol. Biochem. 43, 2304–2314 10.1016/j.soilbio.2011.07.020 [DOI] [Google Scholar]
- Luo Y., Field C. B., Jackson R. B. (2006). Does nitrogen constrain carbon cycling, or does carbon input stimulate nitrogen cycling? Ecology 87, 3–4 10.1890/05-0923 [DOI] [Google Scholar]
- Luo Y., Su B., Currie W. S., Dukes J. S., Finzi A., Hartwig U., et al. (2004). Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54, 731–739 10.1641/0006-3568(2004)054[0731:PNLOER]2.0.CO;216634296 [DOI] [Google Scholar]
- Martín-Olmedo P., Rees R. M., Grace J. (2002). The influence of plants grown under elevated CO2 and N fertilization on soil nitrogen dynamics. Glob. Change Biol. 8, 643–657 10.1046/j.1365-2486.2002.00499.x [DOI] [Google Scholar]
- Mary B., Fresneau C., Morel J. L., Mariotti A. (1993). C and N cycling during decomposition of root mucilage, roots and glucose in soil. Soil Biol. Biochem. 25, 1005–1014 10.1016/0038-0717(93)90147-4 [DOI] [Google Scholar]
- McCarthy H. R., Oren R., Johnsen K. H., Gallet-Budynek A., Pritchard S. G., Cook C. W., et al. (2010). Re-assessment of plant carbon dynamics at the Duke free-air CO2 enrichment site: interactions of atmospheric [CO2] with nitrogen and water availability over stand development. New Phytol. 185, 514–528 10.1111/j.1469-8137.2009.03078.x [DOI] [PubMed] [Google Scholar]
- McGill W. B., Cole C. V. (1981). Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 26, 267–286 10.1016/0016-7061(81)90024-0 [DOI] [Google Scholar]
- Meier C. L., Keyserling K., Bowman W. D. (2009). Fine root inputs to soil reduce growth of a neighbouring plant via distinct mechanisms dependent on root carbon chemistry. J. Ecol. 97, 941–949 10.1111/j.1365-2745.2009.01537.x [DOI] [Google Scholar]
- Milcu A., Heim A., Ellis R., Scheu S., Manning P. (2011). Identification of general patterns of nutrient and labile carbon control on soil carbon dynamics across a successional gradient. Ecosystems 14, 710–719 10.1007/s10021-011-9440-z [DOI] [Google Scholar]
- Morgan J. A., Lecain D. R., Pendall E., Blumenthal D. M., Kimball B. A., Carrillo Y., et al. (2011). C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature 476, 202–205 10.1038/nature10274 [DOI] [PubMed] [Google Scholar]
- Nannipieri P., Giagnoni L., Landi L., Renella G. (2011). “Role of phosphatase enzymes in soil,” in Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling, eds Bünemann E. K., Oberson A., Frossard E. (Heidelberg: Springer; ), 215–243 10.1007/978-3-642-15271-9_9 [DOI] [Google Scholar]
- Nguyen C. (2003). Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23, 375–396 10.1051/agro:2003011 [DOI] [Google Scholar]
- Norby R. J., Warren J. M., Iversen C. M., Medlyn B. E., McMurtrie R. E. (2010). CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl. Acad. Sci. U.S.A. 107, 19368–19373 10.1073/pnas.1006463107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottingham A. T., Griffiths H., Chamberlain P. M., Stott A. W., Tanner E. V. J. (2009). Soil priming by sugar and leaf-litter substrates: a link to microbial groups. Appl. Soil Ecol. 42, 183–190 10.1016/j.apsoil.2009.03.003 [DOI] [Google Scholar]
- Ohm H., Marschner B., Broos K. (2011). Respiration and priming effects after fructose and alanine additions in two copper- and zinc-contaminated Australian soils. Biol. Fertil. Soils 47, 523–532 10.1007/s00374-011-0566-0 [DOI] [Google Scholar]
- Otten W., Hall D., Harris K., Ritz K., Young I. M., Gilligan C. A. (2001). Soil physics, fungal epidemiology and the spread of Rhizoctonia solani. New Phytol. 151, 459–468 10.1046/j.0028-646x.2001.00190.x [DOI] [Google Scholar]
- Paterson E., Thornton B., Midwood A. J., Osborne S. M., Sim A., Millard P. (2008). Atmospheric CO2 enrichment and nutrient additions to planted soil increase mineralisation of soil organic matter, but do not alter microbial utilisation of plant- and soil C-sources. Soil Biol. Biochem. 40, 2434–2440 10.1016/j.soilbio.2008.06.005 [DOI] [Google Scholar]
- Pausch J., Zhu B., Kuzyakov Y., Cheng W. (2013). Plant inter-species effects on rhizosphere priming of soil organic matter decomposition. Soil Biol. Biochem. 57, 91–99 10.1016/j.soilbio.2012.08.029 [DOI] [Google Scholar]
- Pendall E., Heisler-White J. L., Williams D. G., Dijkstra F. A., Carrillo Y., Morgan J. A., et al. (2013). Warming reduces carbon losses from grassland exposed to elevated atmospheric carbon dioxide. PLoS ONE (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendall E., Mosier A. R., Morgan J. A. (2004). Rhizodeposition stimulated by elevated CO2 in a semiarid grassland. New Phytol. 162, 447–458 10.1111/j.1469-8137.2004.01054.x19943173 [DOI] [Google Scholar]
- Phillips R. P., Finzi A. C., Bernhardt E. S. (2011). Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol. Lett. 14, 187–194 10.1111/j.1461-0248.2010.01570.x [DOI] [PubMed] [Google Scholar]
- Phillips R. P., Meier I. C., Bernhardt E. S., Grandy A. S., Wickings K., Finzi A. C. (2012). Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO2. Ecol. Lett. 15, 1042–1049 10.1111/j.1461-0248.2012.01827.x [DOI] [PubMed] [Google Scholar]
- Raiesi F., Ghollarata M. (2006). Interactions between phosphorus availability and an AM fungus (Glomus intraradices) and their effects on soil microbial respiration, biomass and enzyme activities in a calcareous soil. Pedobiologia 50, 413–425 10.1016/j.pedobi.2006.08.001 [DOI] [Google Scholar]
- Rasmussen C., Southard R. J., Horwath W. R. (2007). Soil mineralogy affects conifer forest soil carbon source utilization and microbial priming. Soil Sci. Soc. Am. J. 71, 1141–1150 10.2136/sssaj2006.0375 [DOI] [Google Scholar]
- Reich P. B., Hobbie S. E., Lee T., Ellsworth D. S., West J. B., Tilman D., et al. (2006). Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440, 922–925 10.1038/nature04486 [DOI] [PubMed] [Google Scholar]
- Rukshana F., Butterly C. R., Baldock J. A., Xu J. M., Tang C. (2012). Model organic compounds differ in priming effects on alkalinity release in soils through carbon and nitrogen mineralisation. Soil Biol. Biochem. 51, 35–43 10.1016/j.soilbio.2012.03.022 [DOI] [Google Scholar]
- Sanyal S. K., Datta S. K. (1991). “Chemistry of phosphorus transformations in soil,” in Advances in Soil Science, ed B. A. Stewart (New York, NY: Springer; ), 1–120 [Google Scholar]
- Schimel J. P., Weintraub M. N. (2003). The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol. Biochem. 35, 549–563 10.1016/S0038-0717(03)00015-4 [DOI] [Google Scholar]
- Sullivan B. W., Hart S. C. (2013). Evaluation of mechanisms controlling the priming of soil carbon along a substrate age gradient. Soil Biol. Biochem. 58, 293–301 10.1016/j.soilbio.2012.12.007 [DOI] [Google Scholar]
- Talbot J. M., Allison S. D., Treseder K. K. (2008). Decomposers in disguise: Mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct. Ecol. 22, 955–963 10.1111/j.1365-2435.2008.01402.x [DOI] [Google Scholar]
- Tunesi S., Poggi V., Gessa C. (1999). Phosphate adsorption and precipitation in calcareous soils: the role of calcium ions in solution and carbonate minerals. Nutr. Cycl. Agroecosys. 53, 219–227 10.1023/A:1009709005147 [DOI] [Google Scholar]
- Vitousek P. M., Howarth R. W. (1991). Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13, 87–115 10.1007/BF00002772 [DOI] [Google Scholar]
- Walker T. W., Syers J. K. (1976). The fate of phosphorus during pedogenesis. Geoderma 15, 1–19 10.1016/0016-7061(76)90066-5 [DOI] [Google Scholar]
- Zhang W., Wang X., Wang S. (2013). Addition of external organic carbon and native soil organic carbon decomposition: a meta-analysis. PLoS ONE 8:e54779 10.1371/journal.pone.0054779 [DOI] [PMC free article] [PubMed] [Google Scholar]


