Abstract
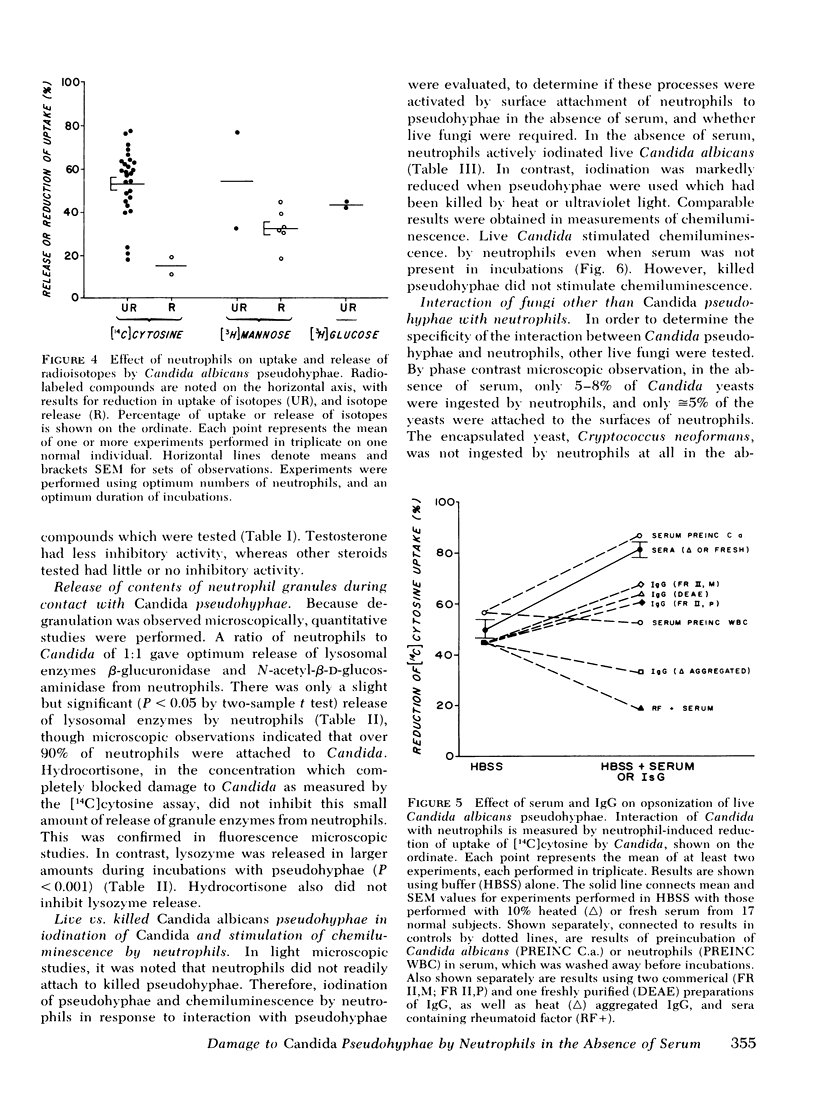
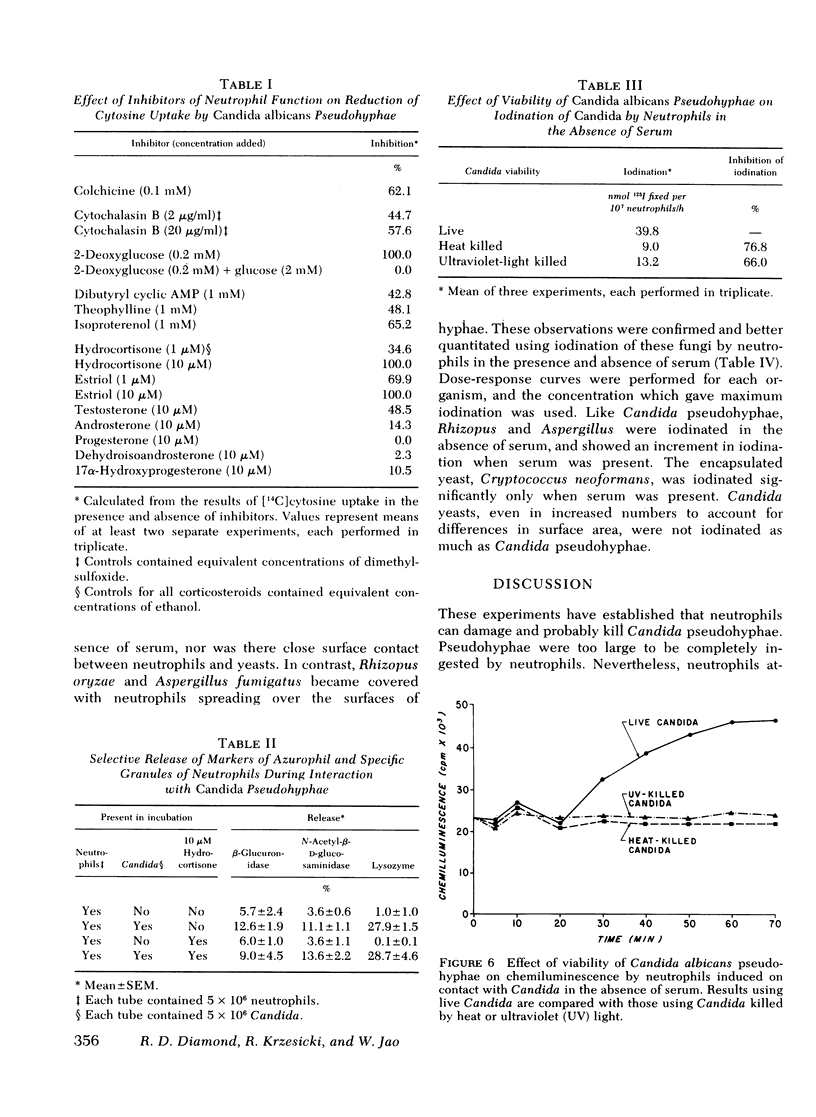
Large forms of Candida are characteristically present in invasive lesions and are often cleared by host defenses. Therefore, an in vitro system was developed to study interactions between leukocytes and pseudohyphae. By light, phase contrast, and electron microscopic observations, in the absence of serum, neutrophils attached to and spread over the surfaces of partially ingested pseudohyphae, which then appeared damaged. Using a new assay which measured neutrophil-induced inhibition of uptake of [14C]cytosine by Candida, damage to Candida in the absence of serum was 53.04±2.96% by neutrophils from 27 normal subjects. With serum, damage to Candida increased because of opsonization by low levels of anti-Candida immunoglobulin G in normal sera. Damage to Candida was inhibited by colchicine, cytochalasin B, and 2-deoxyglucose, which interfered with spreading of neutrophils over the surfaces of Candida. Dibutyryl cyclic AMP, theophylline, and isoproterenol also inhibited damage to Candida. Hydrocortisone was inhibitory in levels (10 μM) achievable with pharmacologic doses in man. Light, fluorescence, and electron microscopy indicated that neutrophils degranulated after contact with Candida. Quantitative studies revealed only a minimal increase in specific release of lysosomal enzymes from azurophil granules, but much greater release of lysozyme from specific granules. Candida activated neutrophil oxidative microbicidal mechanisms, as shown by iodination of Candida by neutrophils, and chemiluminescence from neutrophils interacting with Candida. Unlike live Candida, killed Candida did not induce chemiluminescence, were not iodinated, and did not attach to neutrophils by microscopy. Like Candida pseudohyphae, contact between neutrophils and hyphal forms of Aspergillus and Rhizopus occurred in the absence of serum. This did not occur with Cryptococcus neoformans, an encapsulated yeast, and was low with Candida yeasts. These findings indicate that neutrophils can recognize and attach to Candida pseudohyphae, then damage the Candida. This may represent a general reaction between neutrophils and large forms of fungi. Though the size of the organisms precludes complete ingestion, neutrophil oxidative microbicidal mechanisms are activated, and preferential release of contents of specific granules appears to occur.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F. Sequential degranulation of the two types of polymorphonuclear leukocyte granules during phagocytosis of microorganisms. J Cell Biol. 1973 Aug;58(2):249–264. doi: 10.1083/jcb.58.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P. Fungal infections complicating acute leukemia. J Chronic Dis. 1966 Jun;19(6):667–687. doi: 10.1016/0021-9681(66)90066-x. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lehrer R. I., Cline M. J., Melmon K. L. Cyclic 3',5'-adenosine monophosphate in the human lukocyte: synthesis, degradation, andeffects n neutrophil candidacidal activity. J Clin Invest. 1971 Apr;50(4):920–929. doi: 10.1172/JCI106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Chang H. Y., Rodriguez V., Narboni G., Bodey G. P., Luna M. A., Freireich E. J. Causes of death in adults with acute leukemia. Medicine (Baltimore) 1976 May;55(3):259–268. doi: 10.1097/00005792-197605000-00005. [DOI] [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowa N., Taxer S. S., Howard D. H. Germination of Candida albicans induced by proline. Infect Immun. 1976 Mar;13(3):830–835. doi: 10.1128/iai.13.3.830-835.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Allison A. C. Nature of the effector cells responsible for antibody-dependent cell-mediated killing of Cryptococcus neoformans. Infect Immun. 1976 Sep;14(3):716–720. doi: 10.1128/iai.14.3.716-720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. G., Richardson M. D., Odds F. C., Holland K. T. Relevance of antigenicity of Candida albicans growth phases to diagnosis of systemic candidiasis. Br Med J. 1973 Oct 13;4(5884):86–87. doi: 10.1136/bmj.4.5884.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebner J. V., Mills E. L., Gray G. H., Quie P. G. Comparison of phagocytic and chemiluminescence response of human polymorphonuclear neutrophils. J Lab Clin Med. 1977 Jan;89(1):153–159. [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Hurley D. L., Fauci A. S. Disseminated candidiasis. i. an experimental model in the guinea pig. J Infect Dis. 1975 May;131(5):516–527. doi: 10.1093/infdis/131.5.516. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Glucocorticosteroid inhibition of nonphagocytic discharge of lysosomal enzymes from human neutrophils. Arthritis Rheum. 1977 Jan-Feb;20(1):73–83. doi: 10.1002/art.1780200114. [DOI] [PubMed] [Google Scholar]
- Kim M. H., Rodey G. E., Good R. A., Chilgren R. A., Quie P. G. Defective candidacidal capacity of polymorphonuclear leukocytes in chronic granulomatous disease of childhood. J Pediatr. 1969 Aug;75(2):300–303. doi: 10.1016/s0022-3476(69)80403-8. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Iodination by human polymorphonuclear leukocytes: a re-evaluation. J Lab Clin Med. 1977 Mar;89(3):675–686. [PubMed] [Google Scholar]
- Laforce F. M., Mills D. M., Iverson K., Cousins R., Everett E. D. Inhibition of leukocyte candidacidal activity by serum from patients with disseminated candidiasis. J Lab Clin Med. 1975 Oct;86(4):657–666. [PubMed] [Google Scholar]
- Land G. A., McDonald W. C., Stjernholm R. L., Friedman L. Factors affecting filamentation in Candida albicans: changes in respiratory activity of Candida albicans during filamentation. Infect Immun. 1975 Jul;12(1):119–127. doi: 10.1128/iai.12.1.119-127.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte candidacidal activity and resistance to systemic candidiasis in patients with cancer. Cancer. 1971 May;27(5):1211–1217. doi: 10.1002/1097-0142(197105)27:5<1211::aid-cncr2820270528>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilien D. L., Spivak J. L., Goldman I. D. Chromate transport in human leukocytes. J Clin Invest. 1970 Aug;49(8):1551–1557. doi: 10.1172/JCI106372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Dismukes W. E., Meade R. H., 3rd, Moses J. M. A new therapeutic approach to Candida infections. A preliminary report. Arch Intern Med. 1972 Aug;130(2):241–245. [PubMed] [Google Scholar]
- Messner R. P., Laxidal T., Quie P. G., Williams R. C., Jr Serum opsonin, bacteria, and polymorphonuclear leukocyte interactions in subacute bacterial endocarditis. Anti-gamma-globulin factors and their interaction with specific opsonins. J Clin Invest. 1968 May;47(5):1109–1120. doi: 10.1172/JCI105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages II. Dissociation of the inhibitory effects of 2-deoxyglucose on phagocytosis and ATP generation. J Exp Med. 1976 Dec 1;144(6):1484–1493. doi: 10.1084/jem.144.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickenberg I. D., Root R. K., Wolff S. M. Leukocytic function in hypogammaglobulinemia. J Clin Invest. 1970 Aug;49(8):1528–1538. doi: 10.1172/JCI106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARRY R. M., Jr, CHANDAN R. C., SHAHANI K. M. A RAPID AND SENSITIVE ASSAY OF MURAMIDASE. Proc Soc Exp Biol Med. 1965 Jun;119:384–386. doi: 10.3181/00379727-119-30188. [DOI] [PubMed] [Google Scholar]
- Preisler H. D., Hasenclever H. F., Levitan A. A., Henderson E. S. Serologic diagnosis of disseminated candidiasis in patients with acute leukemia. Ann Intern Med. 1969 Jan;70(1):19–30. doi: 10.7326/0003-4819-70-1-19. [DOI] [PubMed] [Google Scholar]
- Quie P. G., Chilgren R. A. Acute disseminated and chronic mucocutaneous candidiasis. Semin Hematol. 1971 Jul;8(3):227–242. [PubMed] [Google Scholar]
- Root R. K., Stossel T. P. Myeloperoxidase-mediated iodination by granulocytes. Intracellular site of operation and some regulating factors. J Clin Invest. 1974 May;53(5):1207–1215. doi: 10.1172/JCI107667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose H. D., Varkey B. Deep mycotic infection in the hospitalized adult: a study of 123 patients. Medicine (Baltimore) 1975 Nov;54(6):499–507. doi: 10.1097/00005792-197511000-00004. [DOI] [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschdjian C. L., Toni E. F., Hsu K. C., Seelig M. S., Cuesta M. B., Kozinn P. J. Immunofluorescence studies of candida in human reticuloendothelial phagocytes: implications for immunogenesis and pathogenesis of systemic candidiasis. Am J Clin Pathol. 1971 Jul;56(1):50–58. doi: 10.1093/ajcp/56.1.50. [DOI] [PubMed] [Google Scholar]
- WOOLLEN J. W., HEYWORTH R., WALKER P. G. Studies on glucosaminidase. 3. Testicular N-acetyl-beta-glucosaminidase and N-acetyl-beta-galactosaminidase. Biochem J. 1961 Jan;78:111–116. doi: 10.1042/bj0780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M. H., Yount W. J. Mannan antigenemia in the diagnosis of invasive Candida infections. J Clin Invest. 1976 Nov;58(5):1045–1053. doi: 10.1172/JCI108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. C., Bennett J. E., Geelhoed G. W., Levine A. S. Fungemia with compromised host resistance. A study of 70 cases. Ann Intern Med. 1974 May;80(5):605–612. doi: 10.7326/0003-4819-80-5-605. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Effects of cytochalasin B on polymorphonuclear leucocyte locomotion, phagocytosis and glycolysis. Exp Cell Res. 1972 Aug;73(2):383–393. doi: 10.1016/0014-4827(72)90062-6. [DOI] [PubMed] [Google Scholar]