Abstract
Metabolism and immunity are inextricably linked both to each other and to organism-wide function, allowing mammals to adapt to changes in their internal and external environments. In the modern context of obesogenic diets and lifestyles, however, these adaptive responses can have deleterious consequences. In this review, we discuss the pleiotropic actions of inflammation and insulin resistance in metabolic homeostasis and disease. An appreciation of the adaptive context in which these responses arose is useful for understanding their pathogenic actions in disease.
Introduction
Humans have evolutionarily confronted three primary killers: starvation, infection, and predation. Through modern agriculture, hygiene, and our relatively recent elevation to top predator status, we have made remarkable progress in mitigating these only to find new, evolutionarily novel threats taking their place, principally cardiovascular disease, diabetes, and cancer. Unmasked by our successes against more ancient challenges, these modern diseases represent a rapidly increasing share of human morbidity and mortality in both relative and absolute terms and threaten the gains in life expectancy already achieved. In recent years, obesity has emerged as the driving force behind these disturbing trends. From 1980 to 2008 alone, the number of overweight individuals worldwide doubled to more than half a billion people, eclipsing the number of underweight individuals for the first time in history and driving the obesity-attributable death rate to ~3 million per year (1). More poignantly, even a spare handful of extra pounds in midlife is associated with a 20–40% increase in all-cause mortality, obesity with an ~100% increase, and morbid obesity with an ~300% increase (2). Despite such chilling numbers, the true effect of obesity is still likely to be understated.
Notwithstanding its catastrophic consequences, obesity’s importance went long unappreciated because it acts less by overt effect than by promoting/exacerbating cardiovascular disease, diabetes, and cancer, among other diseases. Indeed, the mechanistic links between obesity and better-established pathologies have been hotly investigated over the past two decades. This review primarily explores the cellular and molecular connections between chronic low-grade inflammation, insulin resistance, and obesity-induced metabolic disease. We begin by summarizing our current mechanistic understanding of obesity-induced insulin resistance and then discuss the importance of the histologic and evolutionary context within which it arises. Specifically, we present the argument that key mediators of obesity-induced metabolic disease, such as insulin resistance and inflammation, are evolutionarily conserved adaptive traits with maladaptive effects in the modern obesogenic environment.
Obesity-induced insulin resistance
The fundamental characteristic of obesity is chronic imbalance between caloric intake and energy expenditure, resulting in the storage of excess nutrients in white adipose tissue (WAT) (3). In lean individuals, professional metabolic tissues, such as WAT, liver, and skeletal muscle, readily buffer excess nutrients by storing them as triglycerides and glycogen. With chronic over-nutrition, however, the storage capacity of professional metabolic tissues is eventually exceeded. This causes intracellular buffering mechanisms within dedicated nutrient-storing cells to break down and excess nutrients to overflow into physiologic compartments ill-equipped for substrate handling. Consequently, both professional metabolic and bystander tissues are exposed to super-physiologic levels of metabolic substrates, resulting in cell-intrinsic and -extrinsic dysfunction (4, 5). The primary cell-intrinsic dysfunctions include lipid dysregulation (e.g. accumulation of intracellular diacylglycerols, saturated fatty acids, and ceramides), abnormal intracellular protein modification, mitochondrial dysfunction/oxidative stress, and endoplasmic reticulum/membrane stress. Ectopic lipid deposition, abnormal extracellular protein modification (e.g. hemoglobin A1c, advanced glycation end-products), and adipokine dysregulation represent the major cell-extrinsic pathways (Fig. 1). With persistent imbalance between energy intake and expenditure, these processes escalate and eventually lead to adipocyte death, as is observed in obese WAT (6).
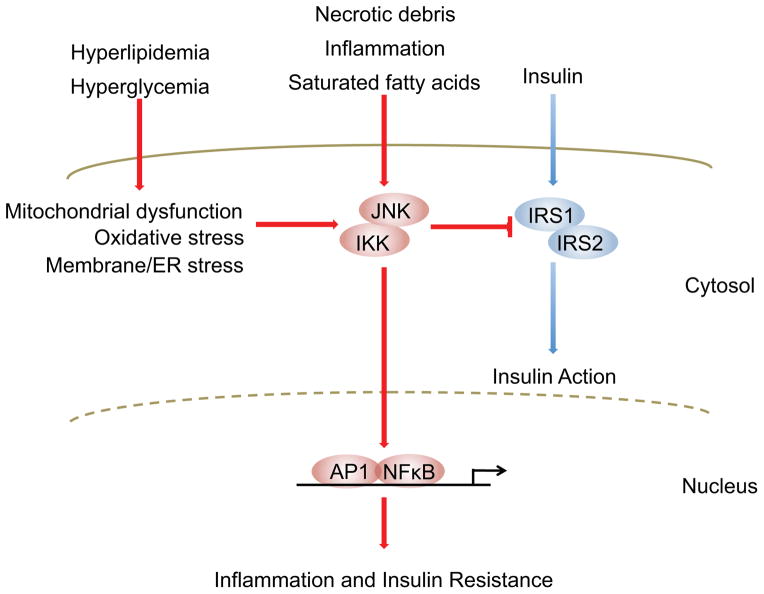
Figure 1. Inflammatory signaling pathways link nutrient excess to insulin resistance.
Insulin’s presence at the cell surface is transduced to cytoplasmic and nuclear responses by tyrosine phosphorylation of insulin receptor substrate (IRS)-1 and -2. Serine phosphorylation of these same proteins by Jun N-terminal kinases (JNK) and inhibitor of nuclear factor κB (NF-κB) kinases (IKK), however, potently inhibits insulin signaling. Many diverse cell-intrinsic and -extrinsic sequelae of chronic nutrient excess activate these signaling pathways, directly linking overfeeding to insulin resistance. Furthermore, JNK and IKK activation triggers inflammatory cytokine production, further activating JNK/IKK in an autocrine and paracrine manner and reinforcing insulin resistance. Abbreviations: ER, endoplasmic reticulum; AP-1, activator protein-1.
Obesity-induced cellular dysfunction activates a diverse range of stress-responsive and counter-regulatory signaling pathways, including activation of JNK, IKKβ, IRE-1, mTOR, ERKs, PKCθ, SOCS proteins, and PKR (4, 5, 7–11). Although a detailed discussion is beyond the scope of this review, these pathways collaborate to produce two metabolically important effects. First, each pathway converges upon and inhibits insulin signaling pathways, primarily through serine phosphorylation of IRS (insulin receptor substrate) proteins, which blunts insulin action in stressed target tissues and stems the influx of nutrients into already overwhelmed cells (Fig. 1). Second, these signals converge on two main inflammatory signaling pathways, JNK and IKKβ, to initiate, support, and augment an inflammatory response within metabolic tissues (Figs. 1 and 2). In parallel with these actions, dysregulated nutrient intake can also bypass cellular stress responses entirely and trigger inflammatory activation through a variety of mechanisms, including triggering of innate immune receptors (e.g. saturated fatty acids-fetuin A ligation of Toll like receptor 4) (12), increased gut-derived lipopolysaccharide (LPS) translocation, and intestinal dysbiosis (13).
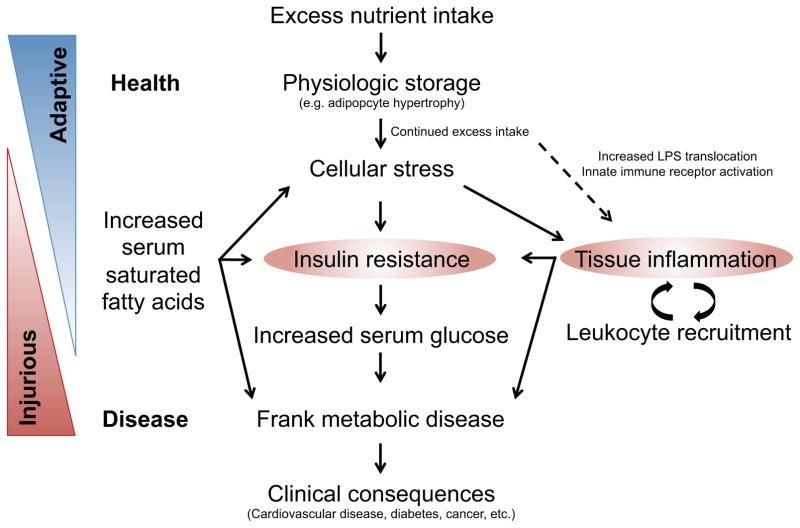
Figure 2. Inflammation and insulin resistance are central to obesity-induced metabolic disease.
Under conditions of acute intake-expenditure imbalance, metabolic tissues store excess nutrients for future use. With chronic imbalance, physiologic storage capacity is exceeded, activating cellular stress signaling pathways that attempt to stem further nutrient influx by inhibiting insulin signaling and promoting inflammation. In adaptive obesity, such as is seen in hibernators, nutrient excess is time-limited with eventual resumption of physiologic normality before tissue damage can occur. In obesity-induced metabolic disease, however, continued nutrient imbalance drives this process forward, leading to chronic inflammation and insulin resistance and, ultimately, to diabetes, cardiovascular disease, and other overtly pathologic consequences.
Despite utilizing similar pathways and mediators, the inflammatory response in obesity differs significantly in duration and intensity from that observed in the more familiar setting of infection. For instance, infectious inflammation involves short-lived, high-amplitude responses, whereas metabolic inflammation, like other chronic inflammatory conditions, smolders at low levels for years-to-decades. While the underlying mechanisms contributing to these differences are not entirely clear, it seems likely that hormonal or epigenetic programming may permanently reassign certain systemic and tissue-specific parameters, such as body weight and leukocyte activation, in obesity (14).
Inflammation: a link between obesity and metabolic disease
Inflammatory activation within metabolic tissues potentiates insulin resistance and metabolic disease by three principal means (Fig. 2) (15). First, inflammatory signaling pathways, like cellular stress-induced and counter-regulatory cascades, inhibit insulin signaling through direct inhibitory serine phosphorylation of IRS proteins by JNK and IKKβ (9). Second, secreted inflammatory mediators (e.g. the chemokines Ccl2, Ccl5, and Ccl8, which are produced by lipid-engorged adipocytes) recruit circulating leukocytes (e.g. Ly6CHi monocytes) to stressed tissue to augment the inflammatory signaling and tissue remodeling capacity of tissue-resident cells (15). Third, secreted inflammatory mediators communicate insulin resistance systemically as well as locally to recruited leukocytes, thereby biasing them towards an inflammatory phenotype (11, 15). Although the first effect involves professional nutrient handling cells, such as adipocytes, hepatocytes, and skeletal myocytes, the latter two establish a stable, feed-forward signaling loop in which tissue-resident and recruited leukocytes sustain and augment both local and systemic inflammation and insulin resistance.
As might be expected, activation of this inflammatory circuit is accompanied by shifts in leukocyte populations and activation status that have profound effects on systemic metabolic parameters. In obesity, for example, macrophages increase from ~10% of all adipose tissue cells to over 50%, shift from an even to a clustered topographic distribution (primarily due to the appearance of necrotic adipocytes), and swap an immunoregulatory M2 phenotype (CD206+, Arg1+, CD301+) for a pro-inflammatory, M1 bias (CD11c+, NOS2+, TNFα+) (16–19). Adipose tissue-associated lymphocytes undergo a similar reorganization with the small T helper 2 (TH2)/regulatory T cell (Treg)-dominated repertoire associated with lean individuals giving way to a much larger and more inflammatory TH1/CD8-dominated population in the obese (20–22). Furthermore, interleukin (IL)-4-expressing eosinophils resident in lean WAT are displaced by waves of ingressing neutrophils, mast cells, and B cells in obese individuals (23–25).
The marked shift in leukocyte population represents a key mechanistic link in the progression from overfeeding-related cellular stress to metabolic dysregulation to frank disease (26). In lean mice, alternative M2 macrophages, Tregs, eosinophils, and invariant natural killer T cells collaborate to maintain an insulin-sensitive, tolerogenic immune environment (17, 20, 23, 27–31) (Fig. 3). Functional depletion of any of these leukocyte lineages disrupts this collaborative effort, destabilizing the anti-inflammatory environment, negatively affecting adipocyte insulin signaling, and exacerbating the deleterious effects of high fat diets. Supplementation of any one of these cellular constituents has the opposite effect. Through the mechanisms discussed above, obesity reorganizes the leukocyte landscape into an insulin resistant, pro-inflammatory milieu in which the tolerogenic leukocyte network is disrupted and replaced with inflammatory M1 macrophages, CD8+ T cells, and TH1 cells (Fig. 3). Functional depletion of any one of these lineages weakens this inflammatory influence, lessening the effects of obesogenic diets, whereas their supplementation exacerbates inflammation and disease (21, 22, 32). Furthermore, even interventions that prevent or augment recruitment of new leukocytes to WAT without influencing the existing leukocyte populations (e.g. abrogation or amplification of the Ccl2-Ccr2 chemotactic axis) can dramatically modulate obesity-associated insulin resistance (33, 34).
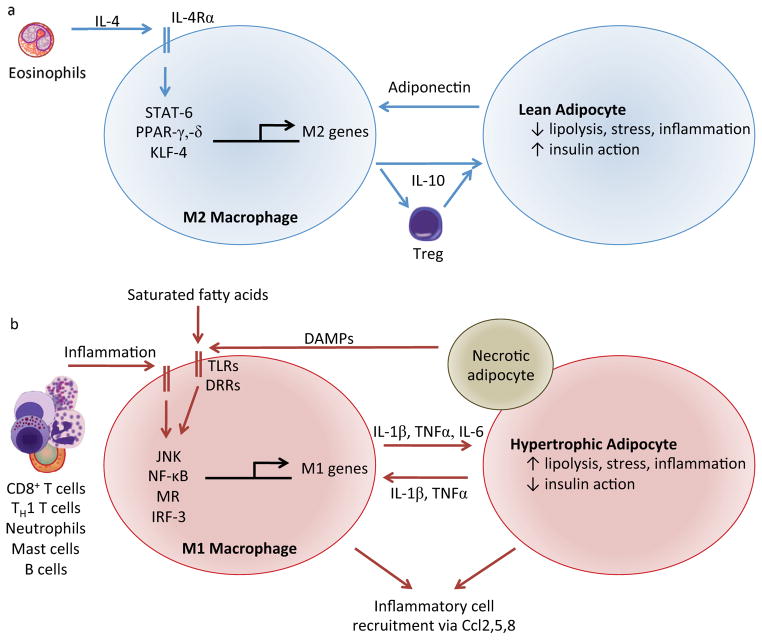
Figure 3. Lean and obese adipose tissues are associated with distinct macrophage phenotypes.
In lean adipose tissue (a), eosinophil-derived interleukin (IL)-4 supports alternatively activated M2 macrophages characterized by production of tolerogenic cytokines such as IL-10 and minimal production of inflammatory mediators. This phenotype establishes a tolerogenic immune environment and directly promotes adipocyte insulin sensitivity. In turn, lean adipocytes produce adiponectin, which collaborates with IL-4 signaling to enhance alternative M2 macrophage activation. In obese adipose tissue (b), inflammatory M1 macrophages, activated by the stigmata of chronic nutrient excess, produce pro-inflammatory cytokines and chemokines that exacerbate adipocyte insulin resistance, enhance cellular stress, and recruit additional leukocytes. Adipocytes, in turn, also secrete inflammatory cytokines and saturated fatty acids that, along with signals from necrotic cells, reinforce the inflammatory environment. Abbreviations: STAT, signal transducer and activator of transcription; PPAR, peroxisome proliferator-activated receptor; KLF, Kruppel-like factor; TLRs, Toll-like receptors; DRRs, Danger Recognition Receptors; MR, mineralocorticoid receptor; IRF, interferon regulatory factor; TNF, tumor necrosis factor; Ccl, CC chemokine ligand.
Although WAT, the most structurally dynamic nutrient-storing tissue, demonstrates dramatic alterations in obesity, similar leukocyte shifts take place in other metabolically important organs as well. For example, obesity precedes restructuring of pancreas- and liver-associated leukocyte populations (though the latter occurs without significant change in macrophage number) (28, 35, 36), whereas brain and skeletal muscle acquire inflammatory microenvironments without significant numeric alterations in leukocyte complements (37, 38). Coincident with this shift, the major tissue targets of insulin action begin to advertise the hallmarks of insulin resistance: increased triglyceride lipolysis in WAT, increased insulin production in pancreatic islets, elevated gluconeogenesis, glycogenolysis and lipogenesis in the liver, decreased insulin-stimulated glucose disposal in skeletal muscle, and decreased satiety signaling in the brain. Importantly, shifts in the leukocyte populations occurring in these organs have systemic importance similar in scope to those occurring in adipose tissue. For example, liver-selective abrogation of alternative M2 macrophage activation results in increased obesity and systemic metabolic disease in response to high-fat diet (27, 28), a phenotype similar to that seen in whole-animal abrogation of the alternative M2 program (17, 29, 30, 39). These data support an integral role for resident leukocytes in local, tissue-specific manifestations of metabolic disease and indicate that leukocyte dysregulation in even one tissue bed increases systemic susceptibility to metabolic disease.
Although obesity-induced metabolic disease promotes and exacerbates pathology through numerous disease-specific mechanisms, most pathology ultimately arises from obesity’s characteristic milieu of chronic low-grade inflammation and insulin resistance. For example, obesity has been recognized for decades as an important risk factor for cardiovascular disease; however, only recently has that risk been mechanistically understood as a result of obesity’s accompanying inflammation and insulin resistance (40). Similarly, hyperlipidemia, another well-described contributor to cardiovascular disease, arises as a consequence of inflammatory insulin resistance through increased adipose tissue lipolysis and hepatic lipogenesis. Even the biomechanical dynamics of cardiovascular disease—atherosclerotic plaque formation, remodeling, and rupture—are influenced by the inflammatory milieu (40). Indeed, the efficacy of some anti-hyperlipidemic therapies correlates with their immunomodulatory potency as much as with their lipid-lowering capacity (e.g. statins and salicylates) (41). Given the shared pathophysiology, it is not surprising that individuals with one cardiovascular disease risk factor often have multiple others, an observation that forms the basis for metabolic syndrome.
Insulin resistance as an adaptive trait
As inflammatory insulin resistance underpins much of the overt pathology associated with obesity and excess caloric intake, we have adopted a biased view of this physiology as a maladaptive response to over-feeding. Although the clinical consequences of obesity are undoubtedly grim, three lines of evidence call into question the current view of insulin resistance as an injurious response to mounting adiposity. First, numerous examples exist in which obesity and insulin resistance are divorced, including both lean, insulin resistant (e.g. lipodystrophy and caveolin-1 knock-out mice (42)) and obese, insulin sensitive states (e.g. Fabp4-knock-out mice (43), and cold-adapted mammals (44)). Indeed, the relationship between insulin resistance and obesity in humans is similarly disjointed (45) with many obese individuals exhibiting better insulin sensitivity than expected for their adiposity (46). Moreover, many of the most widely-used insulin sensitizing pharmaceuticals are associated with an increase in adiposity rather than a decrease (e.g. TZD treatment (47)).
Second, insulin resistance develops as an evolutionarily conserved adaptive response in specific physiologic contexts unassociated with obesity. For example, both infection and pregnancy require organisms to reserve priority nutrient access for an emerging metabolic requirement—immune system activation and fetal development, respectively, in this instance. Organisms meet these requirements by decreasing systemic insulin sensitivity (i.e. developing insulin resistance), thereby decreasing nutrient uptake by non-priority tissues and reserving glucose for priority cells. Indeed, the resulting adaptive physiology in both situations closely resembles that which develops in the context of obesity (15, 48).
Lastly, extreme diet-induced obesity, complete with systemic insulin resistance, hyperinsulinemia, and hyperlipidemia, is observed as an evolutionarily conserved, adaptive, and entirely pathology-free response in mammalian hibernators (44). These animals circannually engage in post-reproduction periods of over-feeding and rapidly eat themselves into what in humans would be morbid obesity—some Zapus species, for example, enter hibernation with fat comprising more than 80% of their body weight (49) (for comparison, the threshold for human obesity is ~25% in males, ~32% in females). For most hibernators, autumnal obesity is accompanied by physiologic hallmarks of type 2 diabetes and metabolic syndrome, including decreased insulin sensitivity in primary target tissues and significant elevations in serum insulin, triglyceride, and both total and LDL cholesterol levels (44, 50). Although its exact role is unclear, insulin resistance may function in this context as both a sensor of nutrient stores and as an instructive signal for tissues to switch from glucose to fatty acid metabolism in preparation for hibernation.
Despite meeting clinical criteria for type 2 diabetes and metabolic syndrome, hibernators demonstrate no pathologic consequences of their brief bout with obesity—after shedding their extra fat during the winter fast, animals are able to immediately enter into the reproductive cycle. Nor are the ill-effects of circannual obesity transient—in one Swedish study, atherosclerotic lesions were entirely absent from the major vessels of obese, insulin-resistant hibernators despite marked hyperlipidemia (50). Similarly, obese hibernators fail to develop the smoldering inflammation that characterizes human obesity despite similar metabolic parameters (44, 51). The absence of pathology despite remarkably similar metabolic states suggests that transient obesity and insulin resistance are not necessarily pathologic and may in fact be part of an adaptive, evolutionarily conserved response to excess nutrient storage.
Pregnancy-, sepsis-, and hibernation-associated insulin resistance demonstrate that transient inhibition of insulin signaling can be advantageous in certain contexts. Furthermore, the conservation of these adaptations between organisms as diverse as flies and humans demonstrates that the capacity for insulin resistance is sufficiently advantageous to be conserved through millions of years of evolutionary divergence. This observation, however, is hardly surprising given the evolutionary primacy of infection, starvation, predation, and, above all, reproduction. Indeed, insulin resistance confers evolutionary advantages and enhances organismal fitness in each of these categories: fueling immune function to combat infection, switching hibernator metabolic substrate preference from glucose to lipids to avoid starvation, and reserving metabolic resources for fetal development to optimize reproduction. Insulin resistance is even likely advantageous in predation-driven selection as the “fight-or-flight” response involves antagonism of insulin signaling by the stress-responsive hormones, catecholamines and glucocorticoids (52). In this context, acute inhibition of insulin’s anabolic actions mobilizes stored nutrients to fuel a heightened state of arousal and combat the threat of predation. The pleiotropy and evolutionary importance of variable insulin sensitivity may then explain the diversity of metabolic, inflammatory, hormonal, dietary, and behavioral pathways that influence insulin signaling (Fig. 1).
Importantly, many of these conserved and evolutionarily important pathways are active in obesity-induced metabolic disease, and although much of the resulting physiology appears pathologic, insulin resistance’s full effect remains unclear. For example, one of the consequences of insulin resistance in obesity is to limit further nutrient uptake by over-loaded cells. Certainly nutrient toxicity has consequences worth avoiding, which include necrosis of engorged adipocytes found in obese adipose tissue (6). Without insulin resistance to limit further nutrient uptake, this fate may well extend other adipocytes, decreasing the storage pool available for excess nutrients and establishing a vicious cycle in which fewer and fewer cells are available to shoulder already overwhelming metabolic burdens. After the adipose tissue depots collapse, skeletal myocyte and hepatocyte depots would similarly fail, followed closely by non-professional nutrient storage tissues. Organisms would be able to quite literally eat themselves to death.
Appreciation of this possibility has led some to reconsider the therapeutic potential of insulin-sensitizing treatments as a core approach to obesity-associated metabolic disease (53)—targeting a potentially adaptive response to overfeeding, while moderately effective in reducing its unfortunate long-term consequences, is unlikely to effectively treat the underlying physiologic defect. Rather, effective therapies are more likely to target the fundamental energetic imbalance that underpins the entire state (54).
Adaptive metabolic leukocyte activation
Careful study of diet-induced obesity has identified chronic leukocyte-mediated low-grade inflammation within professional metabolic tissues as the characteristic pathophysiology of metabolic syndrome (11, 15). This focus on obesity, however, has limited our understanding of leukocyte activation to its role in promoting metabolic disease. Nonetheless, leukocytes are normally present in metabolic tissues, where they perform non-redundant, supportive functions (26). In lean WAT, for example, eosinophil-derived IL-4 drives production of IL-10 and other mediators by macrophages (Fig. 3). This phenotype is critical for maintenance of both adipocyte insulin sensitivity (IL-10 directly potentiates insulin signaling in adipocytes (16)) and the general anti-inflammatory timbre of the WAT microenvironment (both directly and through support of adipose tissue resident Tregs) (15). Congruent with these observations, disruption of IL-4 production or signaling in adipose tissue macrophages results in adipocyte dysfunction, insulin resistance, and metabolic disease (17, 23, 27–29). In contrast, augmentation of IL-4 signaling blunts the deleterious effects of high fat diet challenge (23, 55).
Alternatively activated M2 macrophages are also an indispensable component of the non-shivering thermogenic response of brown adipose tissue (BAT), the sole dedicated thermogenic tissue in mammals (56). Cold exposure elicits this response via hypothalamic stimulation of BAT-innervating efferents of the sympathetic nervous system that, in turn, activate brown adipocytes by releasing catecholamines. Once activated, brown adipocytes oxidize fatty acids and dissipate the resulting mitochondrial proton gradient via uncoupling protein-1, thereby liberating heat (56). Alternative M2 macrophages form an indispensable component of this adrenergic synapse, accounting for ~50% of the total catecholamine content of cold-stimulated brown and white adipose tissues (56). In response to cold exposure, alternative M2 macrophages produce catecholamines, which together with sympathetic efferents, induce the thermogenic program in brown adipocytes while simultaneously inducing lipolysis in white adipocytes (56). Animals lacking alternative M2 macrophages are thus unable to mount an effective thermogenic response or mobilize the fatty acids necessary to support it.
Even within the context of obesity-induced metabolic disease, leukocyte activation can be adaptive. For example, WAT infiltration by inflammatory M1 macrophages is a well-known, necessary component of metabolic disease; however, augmentation of this leukocyte population might be necessary for certain adaptive roles as well. Infiltration of Ly6cHi monocytes/M1-biased macrophages in early obesity seems to be driven by the necrosis/apoptosis of hypertrophic adipocytes (6). Although surrounding parenchymal cells can clear apoptotic debris in most organs, adipocyte death yields large lipid droplets whose uncontrolled lipolysis can be toxic to neighboring cells. Thus, in obese WAT, newly-recruited M1 macrophages would encapsulate and sequester the lipid droplet, and eventually eliminate it (6). Without professional phagocyte intervention, these adipocyte corpses would presumably persist, releasing necrotic cellular debris and free lipids to the detriment of surrounding tissue.
While their intervention is undoubtedly necessary, these phagocytic macrophages demonstrate a M1 bias, presumably in response to necrotic debris, and are thought to be a major source of inflammatory cytokines in obese adipose tissue (16). Disposal of apoptotic cell corpses, however, is not necessarily associated with an inflammatory phenotype (57). Indeed, clearance of apoptotic cells potently suppresses macrophage activation and is strongly associated with a regulatory phenotype not entirely dissimilar from that of lean adipose tissue-associated macrophages. Therapeutic manipulation of the manner in which macrophages dispose of dying adipocytes may thus remove a major pro-inflammatory influence without compromising necessary function.
Integrating tissue architecture with function
The work in obesity, adipose tissue homeostasis, and thermogenesis suggests a close, functional integration of adipocytes and tissue-resident leukocytes in both BAT and WAT. Indeed, the numeric and spatial distribution of these cells within the tissue suggests this would be the case (58). At baseline, adipose tissue-resident leukocyte number and distribution vary little between individuals and are maintained even across species, whereas their depletion results in rapid and precise restoration of the original leukocyte complement without changes in representation/distribution or encroachment by other populations (26). Some adipose tissue-resident leukocytes even display unique, tissue-specific features that distinguish them from cells of similar lineage present elsewhere in the body. For example, WAT-resident Tregs demonstrate a different transcriptional profile characterized by the expression of genes more characteristically associated with neighboring adipocytes including PPARγ, the “master regulator” of adipogenesis (59). These data suggest that resident leukocytes may acquire specific features in support of functional roles particular to the tissue in which they reside.
Despite our focus on it thus far, adipose tissue is not unique in its incorporation of leukocytes. Most tissues, in fact, demonstrate orderly complements of resident leukocytes, suggesting that perhaps tissue-resident leukocytes play integrated roles elsewhere as well. Indeed, like adipose tissue-associated macrophages, Kupffer cells of the liver, sinusoidal macrophages of the spleen, microglia of the brain, and alveolar macrophages of the lung are all phenotypically distinct populations with characteristic gene expression patterns and spatial distributions (60). Although there are well-described functional specializations underlying these phenotypic differences in many tissues (e.g. senescent red blood cell clearance by splenic red pulp macrophages (60) and neural synapse pruning by microglia (61)), the roles of resident leukocytes remain unexplored in others.
Conclusions
The recognition of obesity as a primary source of human disease has engendered fierce interest in metabolic dysfunction and identified inflammatory insulin resistance as its central pathophysiology. Our focus in the matter, however, has been largely on the study of artificial metabolic extremes—high fat diet challenge, lipid infusion, monogenic models, et cetera. These approaches and the simplifications they circumscribe yield valuable insight into human disease; however, they are limited in their ability to describe the complex, non-extreme forms of obesity-induced metabolic disease that dominate the clinical landscape. For example, a genetically-defined caged rodent fed mounds of sugared milk fat and lard over a few months—the basic experimental model of diet-induced obesity—approximates, but will never faithfully recapitulate, an obese human. Our challenge then is to place experimental observations in a more nuanced, relevant context.
First, observations from reductionist disease models, like high fat feeding, must be compared to the disease itself, i.e. to clinical observation and intervention. For example, identification of leptin as a potent regulator of feeding behavior using the ob/ob mouse was popularly hailed as an “obesity cure” and was only placed in proper context by subsequent clinical characterization of obesity-related leptin resistance (53). This argument implies that the current popularity of genetic intervention (e.g. gene knock-outs, floxed alleles, gain-/loss-of-function mutants) must be tempered by careful search for similar aberrations in human cohorts, as exemplified by the recent studies on Gpr120 (62). Moreover, this comparison can also be exploited in the reverse direction. Due to the “trial and error” approach of much clinical research, both therapeutic successes and failures are often instructive, as demonstrated by both the relative success of surgical approaches and failure of pharmaceuticals in the treatment of obesity (63).
Second, disease models and clinical observations must be compared to similar models and clinical observations of health. For example, leukocyte activation is a central pathophysiology in diet-induced obesity; however, the protective effects of WAT- and liver-associated macrophages and WAT-associated T cells in lean, healthy rodents clearly demonstrate that leukocyte activation is context-dependent and explain the otherwise paradoxical exacerbation of metabolic pathology that accompanies complete loss of these lineages. Indeed, these observations have suggested novel therapeutic avenues including pharmacologic skewing of macrophages and lymphocytes towards regulatory phenotypes. In addition, a number of inflammatory pathways that regulate energy expenditure and insulin resistance, such as IKKe, PKR, and Gpr120, might be suitable for therapeutic targeting of obesity-associated metabolic disease (10, 62, 64, 65).
Lastly, disease models and clinical observations must be compared to physiologically similar but pathologically distinct situations. The negative effects of insulin resistance, for instance, are much less clear in the context of pregnancy and infection, whereas the pathology-free “metabolic syndrome” of obese hibernators suggests that our current understanding of causality in human diet-induced obesity is incomplete. Although studies focusing on this particular context are rare, we have already discussed three biological scenarios—pregnancy, infection, and hibernation—in which aspects of obesity-induced metabolic disease manifest and regress. Careful study of how organisms re-establish metabolic normality after these events may provide insight into how we might reestablish the same in obesity-induced metabolic disease.
Our current knowledge of metabolic biology and the basic mechanisms of obesity-induced metabolic disease is impressive. The remarkable progress in this field, however, has largely failed to translate into significant therapeutic advances in our struggle with obesity and metabolic disease, in part due to a failure to place empiric studies in the proper clinical, physiological, and biological context. The challenge going forward then is to provide that context for the wealth of information available in order to identify root pathophysiologies and to appropriately target emerging therapeutics.
Text Box 1. Macrophages.
Macrophages are innate immune cells resident in every tissue in the body, where they participate in a variety of homeostatic functions in addition to host defense. These cells exhibit remarkable functional plasticity and versatility; however, when activated, their phenotypes tend to cluster around two general activation programs. M1, or classical, activation is an inflammatory phenotype characterized by robust expression of pro-inflammatory cytokines (e.g. TNFα, IL-1β, IL-6), type 1-biasing cytokines (e.g. IL-12), and reactive nitrogen species (e.g. nitric oxide). M1 macrophages comprise the primary source for inflammatory cytokines in obese adipose tissue and coordinate inflammatory insulin resistance. M2, or alternative, activation is a tolerogenic phenotype associated with anti-inflammatory cytokines (e.g. IL-10, TGFβ), anti-parasitic responses (e.g. Ym-1, dectin-1, eotaxin), and anabolic functions (e.g. angiogenesis, fibrosis, extracellular matrix remodeling). In contrast to M1 macrophages, these cells are critical for the maintenance of adipocyte insulin signaling and anchor the tolerogenic environment of lean adipose tissue. While all macrophages generally express F4/80 and CD11b, M1 macrophages may be distinguished in vivo by their CD11cHiNos2+TNFα+ immunophenotype, whereas alternative M2 cells are CD206+CD301+Arg1+. Although these basic activation programs represent general phenotypic responses to pathogens, tissue macrophages do not always rigidly adhere to these expression profiles.
Acknowledgments
The author’s work was supported by grants from: NIH (HL076746, DK094641), Larry L. Hillblom Foundation Network Grant, Diabetes Family Fund (UCSF), AHA Innovative Award (12PILT11840038) and an NIH Director’s Pioneer Award (DP1AR064158) to A.C. Due to space limitations, we regret that we are unable to cite all relevant publications on this topic from our colleagues.
Footnotes
The authors declare that they have no competing financial interests.
References
- 1.Finucane MM, et al. Lancet. 2011 Feb 12;377:557. [Google Scholar]
- 2.Adams KF, et al. N Engl J Med. 2006 Aug 24;355:763. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 3.Rosen ED, Spiegelman BM. Nature. 2006;444:847. doi: 10.1038/nature05483. 2006/12/14/print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qatanani M, Lazar MA. Genes Dev. 2007 Jun 15;21:1443. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 5.Samuel VT, Shulman GI. Cell. 2012 Mar 2;148:852. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cinti S, et al. J Lipid Res. 2005 Nov;46:2347. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Hirosumi J, et al. Nature. 2002 Nov 21;420:333. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 8.Yuan M, et al. Science. 2001 Aug 31;293:1673. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 9.Hotamisligil GS. Cell. 2010 Mar 19;140:900. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura T, et al. Cell. 2010 Feb 5;140:338. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olefsky J, Glass C. Annu Rev Physiol. 2010;72:1. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 12.Pal D, et al. Nat Med. 2012 Jul 29; [Google Scholar]
- 13.Tremaroli V, Backhed F. Nature. 2012 Sep 13;489:242. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum M, Kissileff HR, Mayer LE, Hirsch J, Leibel RL. Brain Res. 2010 Sep 2;1350:95. doi: 10.1016/j.brainres.2010.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chawla A, Nguyen KD, Goh YP. Nat Rev Immunol. 2011;11:738. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumeng CN, Bodzin JL, Saltiel AR. J Clin Invest. 2007 Jan;117:175. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odegaard JI, et al. Nature. 2007 Jun 28;447:1116. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisberg SP, et al. J Clin Invest. 2003 Dec;112:1796. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, et al. J Clin Invest. 2003 Dec;112:1821. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feuerer M, et al. Nat Med. 2009 Aug;15:930. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura S, et al. Nat Med. 2009 Aug;15:914. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 22.Winer S, et al. Nat Med. 2009 Aug;15:921. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D, et al. Science. 2011 Apr 8;332:243. [Google Scholar]
- 24.Liu J, et al. Nat Med. 2009 Aug;15:940. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talukdar S, et al. Nat Med. 2012 Sep;18:1407. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odegaard JI, Chawla A. F1000 Biol Rep. 2012;4:13. doi: 10.3410/B4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang K, et al. Cell Metab. 2008 Jun;7:485. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odegaard JI, et al. Cell Metab. 2008 Jun;7:496. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao X, et al. J Clin Invest. 2011 Jul 1;121:2736. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Usher MG, et al. J Clin Invest. 2010 Sep 1;120:3350. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch L, et al. Immunity. 2012 Sep 21;37:574. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patsouris D, et al. Cell Metab. 2008 Oct;8:301. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisberg SP, et al. J Clin Invest. 2006 Jan;116:115. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamei N, et al. J Biol Chem. 2006 Sep 8;281:26602. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 35.Huang W, et al. Diabetes. 2010 Feb;59:347. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eguchi K, et al. Cell Metab. 2012 Apr 4;15:518. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, et al. Cell. 2008 Oct 3;135:61. [Google Scholar]
- 38.Li P, et al. J Biol Chem. 2010 May 14;285:15333. [Google Scholar]
- 39.Hevener AL, et al. J Clin Invest. 2007 Jun;117:1658. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocha VZ, Libby P. Nat Rev Cardiol. 2009 Jun;6:399. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 41.Shoelson SE, Lee J, Goldfine AB. J Clin Invest. 2006 Jul;116:1793. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asterholm IW, Mundy DI, Weng J, Anderson RG, Scherer PE. Cell Metab. 2012 Feb 8;15:171. doi: 10.1016/j.cmet.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hotamisligil GS, et al. Science. 1996 Nov 22;274:1377. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 44.Martin SL. Diab Vasc Dis Res. 2008 Jun;5:76. doi: 10.3132/dvdr.2008.013. [DOI] [PubMed] [Google Scholar]
- 45.Bogardus C, et al. J Clin Invest. 1984 Mar;73:800. doi: 10.1172/JCI111274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samocha-Bonet D, Chisholm DJ, Tonks K, Campbell LV, Greenfield JR. Trends Endocrinol Metab. 2012 Mar;23:116. doi: 10.1016/j.tem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Lehrke M, Lazar MA. Cell. 2005 Dec 16;123:993. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 48.Power ML, Schulkin J. Physiol Behav. 2012 Apr 12;106:22. doi: 10.1016/j.physbeh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Cranford JA. Comp Biochem Physiol A Comp Physiol. 1983;74:595. doi: 10.1016/0300-9629(83)90553-4. [DOI] [PubMed] [Google Scholar]
- 50.Arinell K, et al. Clin Transl Sci. 2012 Jun;5:269. doi: 10.1111/j.1752-8062.2011.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mominoki K, et al. Comp Biochem Physiol A Mol Integr Physiol. 2005 Dec;142:472. doi: 10.1016/j.cbpa.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Hall JE. In: Guyton and Hall Textbook of Medical Physiology. Saunders, editor. 2010. p. 12. [Google Scholar]
- 53.Saltiel AR. Cell Metab. 2012 Jun 6;15:798. doi: 10.1016/j.cmet.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Tseng YH, Cypess AM, Kahn CR. Nat Rev Drug Discov. 2010 Jun;9:465. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricardo-Gonzalez RR, et al. Proc Natl Acad Sci U S A. 2010 Dec 28;107:22617. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen KD, et al. Nature. 2011 Dec 1;480:104. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravichandran KS. Immunity. 2011 Oct 28;35:445. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gordon S, Taylor PR. Nat Rev Immunol. 2005 Dec;5:953. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 59.Cipolletta D, et al. Nature. 2012 Jun 28;486:549. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gautier EL, et al. Nat Immunol. 2012 Sep 30;13:1118. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tremblay ME, Lowery RL, Majewska AK. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ichimura A, et al. Nature. 2012 Mar 15;483:350. [Google Scholar]
- 63.O’Brien PE, et al. Ann Intern Med. 2006 May 2;144:625. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 64.Chiang SH, et al. Cell. 2009 Sep 4;138:961. [Google Scholar]
- 65.Oh DY, et al. Cell. 2010 Sep 3;142:687. [Google Scholar]