Abstract
Mechanisms were studied that might explain the attachment and damage to Candida albicans pseudohyphae by neutrophils in the absence of serum. Attachment of neutrophils to pseudo hyphae was inhibited by Candida mannans (1-10 mg/ml), but not by mannose, dextran, chitin, conconavalin A, or highly charged polyamino acids. Contact was also inhibited by pretreatment of Candida before incubation with neutrophils with chymotrypsin, but not trypsin or several inhibitors of proteases. Similar results were obtained with pretreatment of neutrophils, except that trypsin was inhibitory. When pseudohyphae were killed with ultraviolet light, proteinpolysaccharide complexes of mol wt <10,000 were released which appeared to bind to the surfaces of neutrophils and inhibit contact between neutrophils and Candida, as well as other fungi.
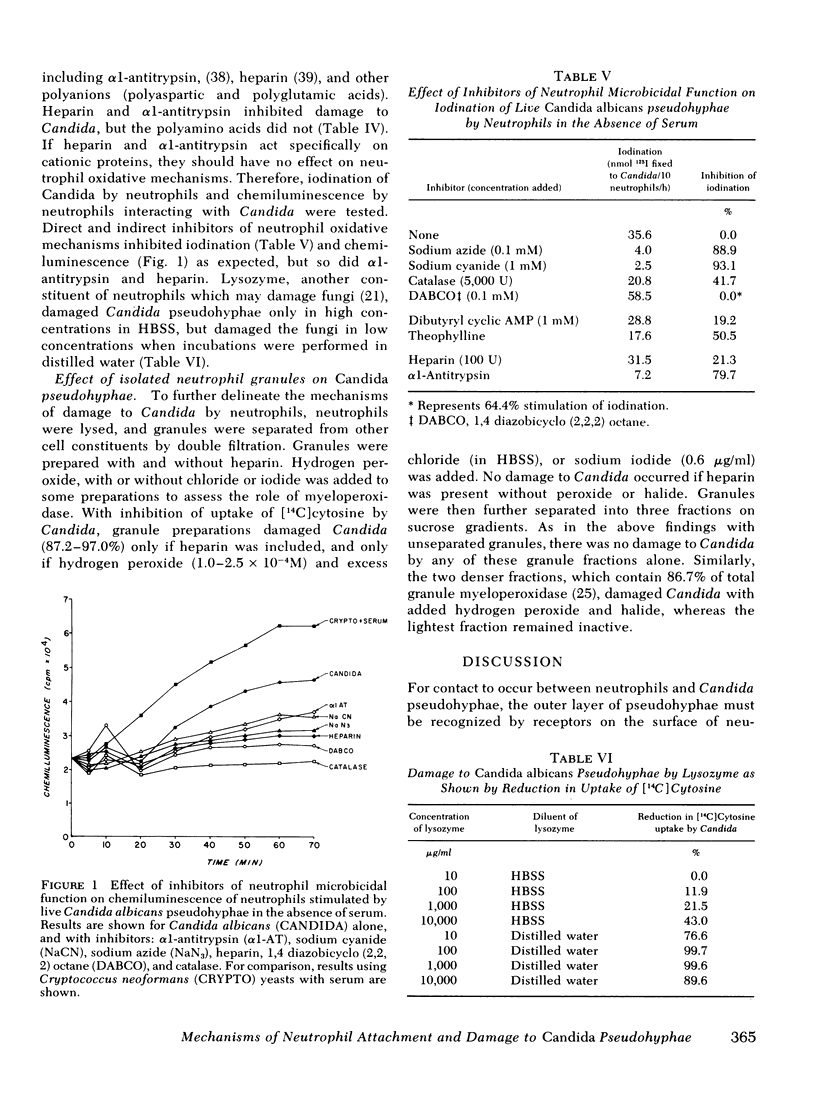
Damage to Candida by neutrophils was inhibited by agents known to act on neutrophil oxidative microbicidal mechanisms, including sodium cyanide, sodium azide, catalase, superoxide dismutase, and 1, 4 diazobicyclo (2, 2, 2) octane, a singlet oxygen quencher. Neutrophils from a patient with chronic granulomatous disease did not damage Candida at all. However, the hydroxyl radical scavengers mannitol and benzoate were not inhibitory. Cationic proteins and lactoferrin also did not appear to play a major role in this system. Low concentrations of lysozyme which did not damage Candida in isotonic buffer solutions damaged pseudohyphae in distilled water. Isolated neutrophil granules damaged pseudohyphae only with added hydrogen peroxide and halide, and damage occurred only with granule fractions known to contain myeloperoxidase. These findings suggest that neutrophils recognized a molecule on the Candida surface which has a chymotrypsin sensitive protein component, and which may be liberated from the cell surface upon death of organism. The neutrophil receptors for Candida appear to be sensitive to trypsin and chymotrypsin. Damage to Candida by neutrophils occurred primarily by oxidative mechanisms, including the production of superoxide and hydrogen peroxide interacting with myeloperoxidase and halide, as well as singlet oxygen, but did not appear to involve hydroxyl radical. Lysozyme might have an accessory role, under some conditions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Murrmann S. K., Davis J., Johnston R. B., Jr The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Invest. 1975 Sep;56(3):571–576. doi: 10.1172/JCI108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., De Duve C., Masson P. L., Heremans J. F. Association of lactoferrin with specific granules in rabbit heterophil leukocytes. J Exp Med. 1970 Mar 1;131(3):559–570. doi: 10.1084/jem.131.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L. Some interrelations of neutrophil chemotaxis, lysosomal enzyme secretion, and phagocytosis as revealed by synthetic peptides. Am J Pathol. 1976 Nov;85(2):385–394. [PMC free article] [PubMed] [Google Scholar]
- Berlin R. D. Effect of concanavalin A on phagocytosis. Nat New Biol. 1972 Jan 12;235(54):44–45. doi: 10.1038/newbio235044a0. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Olsson I., Klebanoff S. J. Cytotoxicity for tumor cells of cationic proteins from human neutrophil granules. J Cell Biol. 1976 Sep;70(3):719–723. doi: 10.1083/jcb.70.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. S., Pappagianis D. Lysozyme-enhanced killing of Candida albicans and Coccidioides immitis by amphoteracin B. Sabouraudia. 1974 Nov;12(3):329–340. [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. G., Richardson M. D., Odds F. C., Holland K. T. Relevance of antigenicity of Candida albicans growth phases to diagnosis of systemic candidiasis. Br Med J. 1973 Oct 13;4(5884):86–87. doi: 10.1136/bmj.4.5884.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green I., Kirkpatrick C. H., Dale D. C. Lactoferrin--specific localization in the nuclei of human polymorphonuclear neutrophilic leukocytes. Proc Soc Exp Biol Med. 1971 Sep;137(4):1311–1317. doi: 10.3181/00379727-137-35779. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. H., Rodey G. E., Good R. A., Chilgren R. A., Quie P. G. Defective candidacidal capacity of polymorphonuclear leukocytes in chronic granulomatous disease of childhood. J Pediatr. 1969 Aug;75(2):300–303. doi: 10.1016/s0022-3476(69)80403-8. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Green I., Rich R. R., Schade A. L. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J Infect Dis. 1971 Dec;124(6):539–544. doi: 10.1093/infdis/124.6.539. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Iodination by human polymorphonuclear leukocytes: a re-evaluation. J Lab Clin Med. 1977 Mar;89(3):675–686. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Kolarova N., Masler L., Sikl D. Cell wall glycopeptides of Candida albicans serotypes A and B. Biochim Biophys Acta. 1973 Nov 11;328(1):221–227. doi: 10.1016/0005-2795(73)90348-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrer R. I. Antifungal effects of peroxidase systems. J Bacteriol. 1969 Aug;99(2):361–365. doi: 10.1128/jb.99.2.361-365.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Functional aspects of a second mechanism of candidacidal activity by human neutrophils. J Clin Invest. 1972 Oct;51(10):2566–2572. doi: 10.1172/JCI107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister H., Heymer B., Schäfer H., Haferkamp O. Role of Candida albicans in granulomatous tissue reactions. I. In vitro degradation of C. albicans and immunospecificity of split products. J Infect Dis. 1977 Feb;135(2):224–234. doi: 10.1093/infdis/135.2.224. [DOI] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Musson R. A., Becker E. L. The role of an activatable esterase in immune-dependent phagocytosis by human neutrophils. J Immunol. 1977 Apr;118(4):1354–1365. [PubMed] [Google Scholar]
- Odds F. C., Hierholzer J. C. Purification and properties of a glycoprotein acid phosphatase from Candida albicans. J Bacteriol. 1973 Apr;114(1):257–266. doi: 10.1128/jb.114.1.257-266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Microbicidal mechanisms of human granulocytes: synergistic effects of granulocyte elastase and myeloperoxidase or chymotrypsin-like cationic protein. Infect Immun. 1976 Dec;14(6):1276–1283. doi: 10.1128/iai.14.6.1276-1283.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Pearlman D. S., Ward P. A., Becker E. L. The requirement of serine esterase function in complement-dependent erythrophagocytosis. J Exp Med. 1969 Oct 1;130(4):745–764. doi: 10.1084/jem.130.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querinjean P., Masson P. L., Heremans J. F. Molecular weight, single-chain structure and amino acid composition of human lactoferrin. Eur J Biochem. 1971 Jun 11;20(3):420–425. doi: 10.1111/j.1432-1033.1971.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Reiss E., Stone S. H., Hasenclever H. F. Serological and cellular immune activity of peptidoglucomannan fractions of Candida albicans cell walls. Infect Immun. 1974 May;9(5):881–890. doi: 10.1128/iai.9.5.881-890.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Stossel T. P. Myeloperoxidase-mediated iodination by granulocytes. Intracellular site of operation and some regulating factors. J Clin Invest. 1974 May;53(5):1207–1215. doi: 10.1172/JCI107667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMMERS D. F., GROLLMAN A. P., HASENCLEVER H. F. POLYSACCHARIDE ANTIGENS OF CANDIDA CELL WALL. J Immunol. 1964 Mar;92:491–499. [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Henson P., Holmes B., Good R. A. An in vitro study of exocytosis of neutrophil granule enzymes in chronic granulomatous disease neutrophils. J Immunol. 1974 Apr;112(4):1383–1386. [PubMed] [Google Scholar]
- Venge P., Olsson I. Cationic proteins of human granulocytes. VI. Effects on the complement system and mediation of chemotactic activity. J Immunol. 1975 Dec;115(6):1505–1508. [PubMed] [Google Scholar]
- Venge P., Olsson I., Odeberg H. Cationic proteins of human granulocytes. V. Interaction with plasma protease inhibitors. Scand J Clin Lab Invest. 1975 Dec;35(8):737–744. doi: 10.3109/00365517509095805. [DOI] [PubMed] [Google Scholar]
- Weiner M. H., Yount W. J. Mannan antigenemia in the diagnosis of invasive Candida infections. J Clin Invest. 1976 Nov;58(5):1045–1053. doi: 10.1172/JCI108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West B. C., Rosenthal A. S., Gelb N. A., Kimball H. R. Separation and characterization of human neutrophil granules. Am J Pathol. 1974 Oct;77(1):41–66. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C. Recognition and response in mononuclear and granular phagocytes. Clin Exp Immunol. 1976 Sep;25(3):355–366. [PMC free article] [PubMed] [Google Scholar]



